Abstract
It is important to evaluate the radiation eye dose (3 mm dose equivalent, Hp (3)) received by physicians during computed tomography fluoroscopy (CTF)-guided biopsy, as physicians are close to the source of scattered radiation. In this study, we measured the radiation eye dose in Hp (3) received by one physician during CTF in a timeframe of 18 months using a direct eye dosimeter, the DOSIRISTM. The physician placed eye dosimeters above and under their lead (Pb) eyeglasses. We recorded the occupational radiation dose received using a neck dosimeter, gathered CT dose-related parameters (e.g., CT-fluoroscopic acquisition number, CT-fluoroscopic time, and CT-fluoroscopic mAs), and performed a total of 95 procedures during CTF-guided biopsies. We also estimated the eye dose (Hp (3)) received using neck personal dosimeters and CT dose-related parameters. The physician eye doses (right and left side) received in terms of Hp (3) without the use of Pb eyeglasses for 18 months were 2.25 and 2.06 mSv, respectively. The protective effect of the Pb eyeglasses (0.5 mm Pb) on the right and left sides during CTF procedures was 27.8 and 37.5%, respectively. This study proved the existence of significant correlations between the eye and neck dose measurement (right and left sides, R2 = 0.82 and R2 = 0.55, respectively) in physicians. In addition, we found significant correlations between CT-related parameters, such as CT-fluoroscopy mAs, and radiation eye doses (right and left sides, R2 = 0.50 and R2 = 0.52, respectively). The eye dose of Hp (3) received in CTF was underestimated when evaluated using neck dosimeters. Therefore, we suggest that the physician involved in CTF use a direct eye dosimeter such as the DOSIRIS for the accurate evaluation of their eye lens dose.
1. Introduction
The International Commission on Radiological Protection (ICRP) adopted the new recommendation of reducing the occupational eye lens dose limit from 150 mSv/year to 20 mSv/year, averaged over 5 years—where the maximum dose should not exceed 50 mSv in any year [1]. The International Atomic Energy Agency (IAEA) has also embraced this new eye lens dose limit for medical workers [2]. Moreover, many countries have used this limit in their regulations. In Japan, the new eye lens dose limit set by the ICRP was adopted in April 2021. Therefore, it is important to evaluate occupational eye doses and eye protection methods [3,4,5].
Computed tomography fluoroscopy (CTF) is one of the main methods used in minimally invasive image-guided procedures for the neck, chest, abdomen, and musculoskeletal system. Additionally, it could be useful to directly assess three-dimensional (3D) data in real time as a guidance tool during various CTF-guided interventions. Thus, CTF-guided biopsy has many advantages with regard to improving performance and procedure times compared to ultrasonography (US) [6,7,8,9,10], and these procedures are being used more frequently. On the other hand, the main disadvantage of CTF-guided interventions is the high radiation exposure of the patient and physician compared with the exposure experienced during conventional CT-guided interventions. During CTF procedures, physicians are situated close to the radiation scattered from patients. Thus, the occupational exposure during CTF is a critical issue for medical staff, especially for the physician involved [11,12,13,14,15,16,17,18]. Many reports have examined the occupational dose received by the eye lenses in various X-ray examinations such as interventional radiology (IR) procedures [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. However, few studies have discussed the radiation eye dose at 3 mm dose equivalent (Hp (3)) experienced by physicians during CTF-guided biopsy, although there has been a phantom study [41].
Therefore, this study aimed to clarify the occupational eye dose received during CTF-guided biopsies using a direct eye dosimeter. The eye dose measured using direct eye dosimeters was compared to the eye dose estimated using neck dosimeters. Furthermore, we used direct eye dosimeters to evaluate whether lead (Pb) eyeglasses adequately protected the eyes of physicians performing CTF procedures.
2. Materials and Methods
2.1. Subjects
This study was conducted at Tohoku University Hospital (Sendai, Japan) from July 2018 to December 2019 (18 months). One physician performed all CTF procedures on patients undergoing 95 procedures using an IR-CT system (Aquilion LB; Toshiba, Otawara, Japan). During these CTF procedures, the physician always wore a protective apron (0.35 mm Pb equivalent), neck guard (0.25 mm Pb equivalent), and Pb eyeglasses (0.5 mm Pb equivalent), as shown in Figure 1a. The X-ray conditions in CT-fluoroscopy were a 120 kV-p tube voltage, 20 mA tube current (median value), 0.5 sec rotation time, and 4 mm or 2 mm slice thickness × 3 cross-sections. During CTF-guided biopsy, the physician was positioned close to the right or left side of the patient.
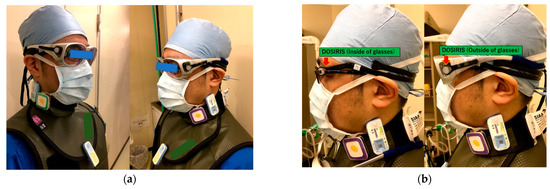
Figure 1.
(a) The physician wore a protective apron (0.35 mm Pb equivalent), neck guard (0.25 mm Pb equivalent), and Pb glasses during CTF, as well as (b) eye dosimeters (DOSIRIS) above and under the Pb eyeglasses.
2.2. Dosimetry
For the measurement of the radiation eye dose in terms of Hp (3) received by the physician during CTF for 18 months, we used the Hp (3) direct eye dosimeter, the DOSIRISTM (IRSN, France). The eye dosimeter (DOSIRIS) consisted of a thermoluminescent sensor (7LiF: Mg, Ti). We conducted the radiation dose reading of the eye dosimeter every month at Chiyoda-Technol. The physician wore eye dosimeters on the lateral side of the right and left eyes. In addition, for the evaluation of the protective effect of Pb eyeglasses, we placed Hp (3) eye dosimeters inside and outside of them (Figure 1b) for 9 months, from April 2019 to December 2019. The protective effect was evaluated using Equation (1):
For the measurement of the neck dose at Hp (3) received by the physician during CTF, we also used a radiophotoluminesence glass personal dosimeter, the Glass Badge (Chiyoda-Technol, Tokyo, Japan). The glass badge consisted of silver-activated phosphate. Dose calibration for the Hp (3) neck dose was performed at Chiyoda-Technol. The physician neck dose received during CTF was monitored outside of the neck guard to estimate the eye dose received using glass badges (right and left), as shown in Figure 1a. Additionally, we evaluated the correlation between the eye dose and neck dose at Hp (3) to determine whether the eye doses were estimated by using neck glass batches. Moreover, we recorded CT dose-related parameters (acquisition numbers, fluoroscopy time, mAs, CT dose index (CTDI), dose length product (DLP), etc.) to estimate the eye dose received from performing 95 consecutive procedures involving CTF-guided biopsy.
2.3. Statical Analysis
Liner regression was used to evaluate the correlations between the radiation doses recorded by the neck dosimeter and eye dosimeter, as well as the correlations between the radiation doses recorded by the internal dosimeter and external dosimeter. Determination coefficient analysis was used to evaluate whether the CT dose-related parameters were linearly related to occupational doses (eye and neck doses).
All statistical analysis were performed based on the JMP Pro 16 software (SAS Institute Inc., Cary, NC, USA). We defined the statistical significance as p < 0.05.
3. Results
Table 1 summarizes the results of our 18-month study on patients undergoing a total of 95 procedures involving CTF-guided biopsies. The averages ± SDs of the CT-fluoroscopic acquisition numbers, CT-fluoroscopic time, and CT-fluoroscopic mAs were 26.2 ± 15.5, 20.0 ± 11.8 s, and 464.1 ± 327.4, respectively. Regarding the standing positions of the physicians performing the 95 CTF procedures, 49 were on the right and 46 were on the left.

Table 1.
Summary of our 18-month study on patients undergoing CTF-guided biopsies (95 procedures).
3.1. Physician Dose
The total physician eye doses (right and left) received in terms of Hp (3) when not wearing Pb glasses for 18 months in CTF procedures (95 procedures) were 2.25 and 2.06 mSv, respectively. Additionally, the total neck doses (right and left) received by physicians in terms of Hp (3) without Pb glasses for 18 months were 1.16 and 1.02 mSv, respectively (Table 2). The neck dose of Hp (3) tended to underestimate the eye dose. The right eye doses for inside and outside the Pb eyeglasses for 9 months (47 procedures) were 0.52 and 0.72 mSv, respectively. Additionally, the left eye doses for the inside and outside of Pb eyeglasses were 0.50 and 0.80 mSv, respectively. Therefore, the protective effect of Pb eyeglasses on the right and left sides was 27.8 and 37.5%, respectively (Table 3).

Table 2.
Total physician doses (eye and neck) received in 18 months during CTF procedures (95 procedures).

Table 3.
Protective effect of Pb eyeglasses used for 9 months during CTF procedures (47 procedures).
3.2. Relationship between the Eye Dosimeter and Neck Dosimeter or CT-Related Parameters
We proved the existence of significant correlations between the eye and neck dosimeter measurements (right side: R2 = 0.82; left side: R2 = 0.55) monthly in CTF for 95 procedures (Figure 2). Figure 3 shows the correlations between the eye dosimeter measurements (right side: R2 = 0.55; left side: R2 = 0.69) inside and outside the Pb eyeglasses, taken monthly for 47 procedures involving CTF-guided biopsies.
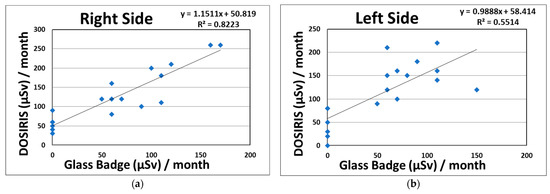
Figure 2.
Relationship between the eye dosimeter (DOSIRIS, Hp (3)) and neck dosimeter (Glass Badge, Hp (3)), taken monthly during CTF-guided biopsies (95 procedures). (a) Right side dosimeter; (b) left side dosimeter.
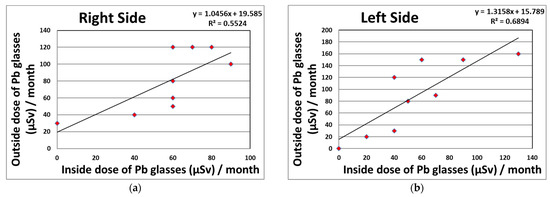
Figure 3.
Relationship between the eye dosimeter measurements (DOSIRIS, Hp (3)) inside and outside the Pb eyeglasses, taken monthly during CTF-guided biopsies (47 procedures). (a) Right side dosimeter; (b) left side dosimeter.
Table 4 shows the significant correlations between the physician doses (eye and neck) received and the CT dose-related parameters—especially CT-fluoroscopic mAs (right eye dose: R2 = 0.50; left eye dose: R2 = 0.52; right neck dose: R2 = 0.75; and left neck dose: R2 = 0.59). Meanwhile, there were no significant correlations between the physician doses and other CT-related parameters, such as CTDI vol. and DLP.

Table 4.
Determination coefficient (R2) between physician doses and CT dose-related parameters (95 procedures).
4. Discussion
Evaluation of radiation exposure received by patients in medicine is very important [42,43,44,45,46,47,48,49,50,51]. Furthermore, occupational radiation protection in medical workers is a critical problem [52,53,54]. The ICRP suggested that tissue reactions of the eyes (e.g., cataracts) can occur at lower radiation doses than those examined in previous epidemiological research [1]. The IAEA recommends wearing a dosimeter as close to the eye as possible so that the lens dose of the eye can be measured the most accurately [55]. ICRP Publication 103 recommends that Hp (3) should be used to measure the equivalent dose given to the lens of the eye [56]. The largest groups of workers who may be affected by lowering the lens dose limit of the eye are employed in the medical sector and are involved in CTF-guided interventional procedures [55,57]. Therefore, medical workers, during CTF-guided biopsies, should be to assess the eye dose and eye protection [6,7,8,9,10,11,12,13,14,15,16,17,18,41,58].
In this study, we revealed that the total eye doses on the right and left sides in terms of Hp (3) received by a physician without Pb eyeglasses for 18 months during CTF procedures were 2.25 and 2.06 mSv, respectively. It was expected that the eye dose limit (20 mGy/year) would not be exceeded. Regarding the total neck doses (right and left), the physician doses were 1.16 and 1.02 mSv, respectively. As shown in Table 2 and Figure 2, the radiation eye dose received by physicians involved in CTF procedures was underestimated by approximately two-fold when using neck badge measurements compared with the direct eye dosimeter measurements carried out using DOSIRIS, although the correlation between the eye and neck dosimeter measurements was high (right side: R2 = 0.82; left sides: R2 = 0.55). This may have been because the angular dependences of the DOSIRIS show better dose responses at almost all angles than that of the neck badge [59]. Hence, we suggest that for the direct evaluation of eye doses received by physicians, an eye dosimeter such as the DOSIRIS should be used. In addition, physicians attending the CTF are advised to place the direct dosimeter as close as possible to the eyes in order to assess accurate radiation doses.
As shown in Table 3 and Figure 3, the protective effects of Pb eyeglasses on the right and left sides were 27.8 and 37.5%, respectively. Using protective eyeglasses can further reduce the lens dose received by the physician. However, several studies carried out during IR have reported that the protection provided by radiation protective eyeglasses in clinical settings was approximately 50–60% [24,27,31]. In our results, we found lower effects than those seen in other studies, although the eye dosimeter measurements (DOSIRIS, Hp (3)) clarified the correlations between the inside and outside of the Pb eyeglasses of 0.5 mm Pb equivalent (right side: R2 = 0.55; left sides: R2 = 0.69), taken monthly for 47 CTF procedures. This may have been because the distribution of scattered radiation in CTF is almost the same in the height direction regardless of the protection used [58].
As shown in Table 4, we showed the existence of significant correlations between the physician doses (eye and neck dose) and CT dose-related parameters—especially CT-fluoroscopic mAs (right eye dose: R2 = 0.50; left eye dose: R2 = 0.52; right neck dose: R2 = 0.75; and left neck dose: R2 = 0.59). Thus, we recommend minimizing the CT-fluoroscopic acquisition numbers and lowering the CT-fluoroscopic mAs to reduce the physician doses. In addition, it could be useful to estimate physician doses from CT dose-related parameters such as CT fluoroscopic mAs.
In summary, our study measured the occupational eye doses at Hp (3) received by physicians working in CTF procedures for 18 months. We evaluated the protective effect of Pb eyeglasses with 0.5 mm Pb equivalent. We also estimated the radiation eye doses received by physicians using personal neck dosimeters. The eye lens doses recorded during CTF using neck dosimeters were underestimated by approximately two-fold compared with direct eye dosimeter measurements carried out using the DOSIRIS. Therefore, we suggest that physicians involved in CTF use a direct eye dosimeter (e.g., DOSIRIS) to accurately assess the lens dose of the eye.
Our study has some limitations. This study evaluated the physician doses only at our institution using the same CT. For the CT-fluoroscopic X-ray conditions, we used our hospital protocol. In addition, all CTF procedures were performed by one physician. Therefore, our results might be different for physicians who require different lengths of time to complete procedures, as well as those who need to be at a longer or shorter distance from the intervention site.
5. Conclusions
This study investigated the eye doses associated with CTF-guided biopsies received by physicians using a direct eye dosimeter at our hospital. To reduce the eye dose, we recommend that the physician wear Pb eyeglasses. The eye dose in terms of Hp (3) received in CTF was underestimated when evaluated using neck dosimeters. Therefore, to correctly assess the eye dose at Hp (3), it is essential to use direct eye dosimeters such as DOSIRIS.
Author Contributions
Conceptualization, Y.I. and K.C.; methodology, Y.I.; software, S.H.; validation, S.H., M.W., and K.C.; formal analysis, Y.I.; investigation, Y.I.; resources, M.W.; data curation, K.C.; writing—original draft preparation, Y.I.; writing—review and editing, K.C.; visualization, Y.I.; supervision, S.H.; project administration, S.H.; funding acquisition, S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by a Grant-in-Aid for Scientific Research (18K12127, 19K12860 and 21K12748) from the Japan Society for the Promotion of Science.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Tohoku University Graduate School of Medicine, Japan (IRB approval number: 2020-1-1165).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not Applicable.
Acknowledgments
We thank Mime Endo, Kazuki Otomo, Yuki Murabayashi, Hiroki Ishii, Ko Satsurai, Takafumi Honda, Fumitaka Sato, and Shigeru Tachibana of Tohoku University for their invaluable assistance and data collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- International Commission on Radiolocial Protoection. ICRP Statement on Tissue Reactions/Early and Late Effects of Radiation in Normal Tissues and Organs—Threshold Doses for Tissue Reactions in a Radiation Protection Context; ICRP Publication 118; ICRP: Ottawa, ON, Canada, 2012; Volume 41, pp. 1–322. [Google Scholar]
- International Atomic Energy Agency. Radiation Protection and Safety of Radiation Sources: International Basic Safety Standards. General Safety Requirements Part 3; IAEA: Vienna, Austria, 2014. [Google Scholar]
- Kawauchi, S.; Chida, K.; Hamada, Y.; Tsuruta, W. Lens dose reduction with a bismuth shield in neuro cone-beam computed tomography: An investigation on optimum shield device placement conditions. Radiol. Phys. Technol. 2021, 1–12. [Google Scholar] [CrossRef]
- Kawauchi, S.; Chida, K.; Moritake, T.; Hamada, Y.; Tsuruta, W. Radioprotection of eye lens using protective material in neuro cone-beam computed tomography: Estimation of dose reduction rate and image quality. Phys. Med. 2021, 82, 192–199. [Google Scholar] [CrossRef]
- Ishii, H.; Chida, K.; Satsurai, K.; Haga, Y.; Kaga, Y.; Abe, M.; Inaba, Y.; Zuguchi, M. Occupational eye dose correlation with neck dose and patient-related quantities in interventional cardiology procedures. Radiol. Phys. Technol. 2022, 1–9. [Google Scholar] [CrossRef]
- Silverman, S.G.; Tuncali, K.; Adams, D.F.; Nawfel, R.D.; Zou, K.H.; Judy, P.F. CT fluoroscopy-guided abdominal interventions: Techniques, results, and radiation exposure. Radiology 1999, 212, 673–681. [Google Scholar] [CrossRef]
- Paulson, E.K.; Sheafor, D.H.; Enterline, D.S.; McAdams, H.P.; Yoshizumi, T.T. CT fluoroscopy-guided interventional procedures: Techniques and radiation dose to radiologists. Radiology 2001, 220, 161–167. [Google Scholar] [CrossRef]
- Kim, G.R.; Hur, J.; Lee, S.M.; Lee, H.J.; Hong, Y.J.; Nam, J.E.; Kim, H.S.; Kim, Y.J.; Choi, B.W.; Kim, T.H.; et al. CT fluoroscopy-guided lung biopsy versus conventional CT-guided lung biopsy: A prospective controlled study to assess radiation doses and diagnostic performance. Eur. Radiol. 2011, 21, 232–239. [Google Scholar] [CrossRef]
- Sarti, M.; Brehmer, W.P.; Gay, S.B. Low-dose techniques in CT-guided interventions. Radiographics 2012, 32, 1109–1119. [Google Scholar] [CrossRef]
- Prosch, H.; Stadler, A.; Schilling, M.; Bürklin, S.; Eisenhuber, E.; Schober, E.; Mostbeck, G. CT fluoroscopy-guided vs. multislice CT biopsy mode-guided lung biopsies: Accuracy, complications and radiation dose. Eur. J. Radiol. 2012, 81, 1029–1033. [Google Scholar] [CrossRef]
- Nawfel, R.D.; Judy, P.F.; Silverman, S.G.; Hooton, S.; Tuncali, K.; Adams, D.F. Patient and personnel exposure during CT fluoroscopy-guided interventional procedures. Radiology 2000, 216, 180–184. [Google Scholar] [CrossRef]
- Irie, T.; Kajitani, M.; Itai, Y. CT fluoroscopy-guided intervention: Marked reduction of scattered radiation dose to the physician’s hand by use of a lead plate and an improved I-I device. J. Vasc. Interv. Radiol. 2001, 12, 1417–1421. [Google Scholar] [CrossRef]
- Buls, N.; Pagés, J.; de Mey, J.; Osteaux, M. Evaluation of patient and staff doses during various CT fluoroscopy guided interventions. Health Phys. 2003, 85, 165–173. [Google Scholar] [CrossRef]
- Aviles Lucas, P.; Dance, D.R.; Castellano, I.A.; Vano, E. Estimation of the peak entrance surface air kerma for patients undergoing computed tomography-guided procedures. Radiat. Prot. Dosim. 2005, 114, 317–320. [Google Scholar] [CrossRef]
- Neeman, Z.; Dromi, S.A.; Sarin, S.; Wood, B.J. CT fluoroscopy shielding decreases in scattered radiation for the patient and operator. J. Vasc. Interv. Radiol. 2006, 17, 1999–2004. [Google Scholar] [CrossRef] [Green Version]
- Hohl, C.; Suess, C.; Wildberger, J.E.; Honnef, D.; Das, M.; Mühlenbruch, G.; Schaller, A.; Günther, R.W.; Mahnken, A.H. Dose reduction during CT fluoroscopy: Phantom study of angular beam modulation. Radiology 2008, 246, 519–525. [Google Scholar] [CrossRef]
- Joemai, R.M.; Zweers, D.; Obermann, W.R.; Geleijns, J. Assessment of patient and occupational dose in established and new applications of MDCT fluoroscopy. Am. J. Roentgenol. 2009, 192, 881–886. [Google Scholar] [CrossRef]
- Yamao, Y.; Yamakado, K.; Takaki, H.; Yamada, T.; Kodama, H.; Nagasawa, N.; Nakatsuka, A.; Uraki, J.; Takeda, K. CT-fluoroscopy in chest interventional radiology: Sliding scale of imaging parameters based on radiation exposure dose and factors increasing radiation exposure dose. Clin. Radiol. 2013, 68, 162–166. [Google Scholar] [CrossRef]
- Chida, K.; Kato, M.; Kagaya, Y.; Zuguchi, M.; Saito, H.; Ishibashi, T.; Takahashi, S.; Yamada, S.; Takai, Y. Radiation dose and radiation protection for patients and physicians during interventional procedure. J. Radiat. Res. 2010, 51, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Chida, K.; Morishima, Y.; Inaba, Y.; Taura, M.; Ebata, A.; Takeda, K.; Shimura, H.; Zuguchi, M. Physician-received scatter radiation with angiography systems used for interventional radiology: Comparison among many X-ray systems. Radiat. Prot. Dosim. 2012, 149, 410–416. [Google Scholar] [CrossRef]
- Chida, K.; Kaga, Y.; Haga, Y.; Kataoka, N.; Kumasaka, E.; Meguro, T.; Zuguchi, M. Occupational Dose in Interventional Radiology Procedures. Am. J. Roentgenol. 2013, 200, 138–141. [Google Scholar] [CrossRef]
- Inaba, Y.; Chida, K.; Kobayashi, R.; Kaga, Y.; Zuguchi, M. Fundamental study of a real-time occupational dosimetry system for interventional radiology staff. J. Radiol. Prot. 2014, 34, N65. [Google Scholar] [CrossRef]
- Morishima, Y.; Chida, K.; Watanabe, H. Estimation of the Dose of Radiation Received by Patient and Physician during a Videofluoroscopic Swallowing Study. Dysphagia 2016, 31, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Haga, Y.; Chida, K.; Kaga, Y.; Sota, M.; Meguro, T.; Zuguchi, M. Occupational Eye Dose in Interventional Cardiology Procedures. Sci. Rep. 2017, 7, 569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsubara, K.; Lertsuwunseri, V.; Srimahachota, S.; Krisanachinda, A.; Tulvatana, W.; Khambhiphant, B.; Sudchai, W.; Rehani, M. Eye lens dosimetry and the study on radiation cataract in interventional cardiologists. Phys. Med. 2017, 44, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Vano, E.; Sanchez, R.M.; Fernandez, J.M. Strategies to optimize occupational radiation protection in interventional cardiology using simultaneous registration of patient and staff doses. J. Radiol. Prot. 2018, 38, 1077–1088. [Google Scholar] [CrossRef]
- Kato, M.; Chida, K.; Ishida, T.; Sasaki, F.; Toyoshima, H.; Oosaka, H.; Terata, K.; Abe, Y.; Kinoshita, T. Occupational radiation exposure dose of the eye in department of cardiac arrhythmia physician. Radiat. Prot. Dosim. 2019, 187, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Coppeta, L.; Pietroiusti, A.; Neri, A.; Spataro, A.; Angelis, E.D.; Perrone, S.; Magrini, A. Risk of radiation-induced lens opacities among surgeons and interventional medical staff. Radiol. Phys. Technol. 2019, 12, 26–29. [Google Scholar] [CrossRef]
- Ishii, H.; Chida, K.; Satsurai, K.; Haga, Y.; Kaga, Y.; Abe, M.; Inaba, Y.; Zuguchi, M. A Phantom Study to Determine the Optimal Placement of Eye Dosemeters on Interventional Cardiology Staff. Radiat. Prot. Dosim. 2019, 185, 409–413. [Google Scholar] [CrossRef]
- Mortensen, C.; Chung, J.; Liu, D.; Ho, S.; Legiehn, G.; Machan, L.; Klass, D. Prospective study on total fluoroscopic time in patients undergoing uterine artery embolization: Comparing transradial and transfemoral approaches. Cardiovasc. Interv. Radiol. 2019, 42, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Chida, K.; Ishida, T.; Toyoshima, H.; Yoshida, Y.; Yoshioka, S.; Moroi, J.; Kinoshita, T. Occupational radiation exposure of the eye in neurovascular interventional physician. Radiat. Prot. Dosim. 2019, 185, 151–156. [Google Scholar] [CrossRef]
- Koenig, A.M.; Etzel, R.; Greger, W.; Viniol, S.; Fiebich, M.; Thomas, R.P.; Mahnken, A.H. Protective efficacy of different ocular radiation protection devices: A phantom study. Cardiovasc. Interv. Radiol. 2020, 43, 127–134. [Google Scholar] [CrossRef]
- Haga, Y.; Chida, K.; Sota, M.; Kaga, Y.; Abe, M.; Inaba, Y.; Suzuki, M.; Meguro, T.; Zuguchi, M. Hybrid Operating Room System for the Treatment of Thoracic and Abdominal Aortic Aneurysms: Evaluation of the Radiation Dose Received by Patients. Diagnostics 2020, 10, 846. [Google Scholar] [CrossRef] [PubMed]
- Mihić, M.S.; Pavelić, L.; Kortmiš, M.V.; Šiško, J.; Maltar-Strmečki, N.M.; Prlić, I. 3D-printed eye lens dosemeter holder for use in interventional radiology and interventional cardiology. Radiat. Meas. 2020, 135, 106385. [Google Scholar] [CrossRef]
- Matsubara, K.; Takei, Y.; Mori, H.; Kobayashi, I.; Noto, K.; Igarashi, T.; Suzuki, S.; Akahane, K. A multicenter study of radiation doses to the eye lenses of medical staff performing non-vascular imaging and interventional radiology procedures in Japan. Phys. Med. 2020, 74, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Nakamura, M.; Zuguchi, M.; Chida, K. Development of Novel Real-Time Radiation Systems Using 4-Channel Sensors. Sensors 2020, 20, 2741. [Google Scholar] [CrossRef]
- Dehairs, M.; Marshall, N.M.; Bosmans, H.; Leghissa, M. Radiation protection operators and patients in a hybrid angio-MR suite. Phys. Med. 2020, 74, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Chida, K.; Murabayashi, Y.; Endo, M.; Otomo, K.; Zuguchi, M. An initial investigation of a wireless patient radiation dosimeter for use in interventional radiology. Radiol. Phys. Technol. 2020, 13, 321–326. [Google Scholar] [CrossRef]
- Haga, Y.; Chida, K.; Kimura, Y.; Yamanda, S.; Sota, M.; Kaga, Y.; Abe, M.; Meguro, T.; Zuguchi, M. Radiation eye dose to medical staff during respiratory endoscopy under X-ray fluoroscopy. J. Radiat. Res. 2020, 61, 691–696. [Google Scholar] [CrossRef]
- Matsubara, K.; Yoshida, S.; Hirosawa, A.; Chusin, T.; Furukawa, Y. Characterization of Small Dosimeters Used for Measurement of Eye Lens Dose for Medical Staff during Fluoroscopic Examination. Diagnostics 2021, 11, 150. [Google Scholar] [CrossRef]
- Osanai, M.; Sato, H.; Sato, K.; Kudo, K.; Hosoda, M.; Hosokawa, S.; Kitajima, M.; Tsushima, M.; Fujita, A.; Hosokawa, Y.; et al. Occupational Radiation Dose, Especially for Eye Lens: Hp(3), in Medical Staff Members Involved in Computed Tomography Examinations. Appl. Sci. 2021, 11, 4448. [Google Scholar] [CrossRef]
- Nemoto, M.; Chida, K. Reducing the Breast Cancer Risk and Radiation Dose of Radiography for Scoliosis in Children: A Phantom Study. Diagnostics 2020, 10, 753. [Google Scholar] [CrossRef]
- Matsunaga, Y.; Chida, K.; Kondo, Y.; Kobayashi, K.; Kobayashi, M.; Minami, K.; Suzuki, S.; Asada, Y. Diagnostic reference levels and achievable doses for common computed tomography examinations: Results from the Japanese nationwide dose survey. Br. J. Radiol. 2019, 92, 20180290. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Nakamura, M.; Chida, K.; Zuguchi, M. Effectiveness of a novel real-time dosimeter in interventional radiology: A comparison of new and old radiation sensors. Radiol. Phys. Technol. 2018, 11, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Chida, K.; Kato, M.; Inaba, Y.; Kobayashi, R.; Nakamura, M.; Abe, Y.; Zuguchi, M. Real-time patient radiation dosimeter for use in interventional radiology. Phys. Med. 2016, 32, 1475–1478. [Google Scholar] [CrossRef]
- Inaba, Y.; Chida, K.; Kobayashi, R.; Zuguchi, M. A Cross-Sectional Study of the Radiation Dose and Image Quality of X-ray Equipment Used in IVR. J. Appl. Clin. Med. Phys. 2016, 17, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Chida, K.; Kobayashi, R.; Haga, Y.; Zuguchi, M. Radiation Dose of Cardiac IVR X-ray Systems: A Comparison of Present and Past. Acta Cardiol. 2015, 70, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Chida, K.; Inaba, Y.; Morishima, Y.; Taura, M.; Ebata, A.; Yanagawa, I.; Takeda, K.; Zuguchi, M. Comparison of Dose at an Interventional Reference Point Between the Displayed Estimated Value and Measured Value. Radiol. Phys. Technol. 2011, 4, 189–193. [Google Scholar] [CrossRef]
- Chida, K.; Inaba, Y.; Saito, H.; Ishibashi, T.; Takahashi, S.; Kohzuki, M.; Zuguchi, M. Radiation dose of interventional radiology system using a flat-panel detector. Am. J. Roentgenol. 2009, 193, 1680–1685. [Google Scholar] [CrossRef]
- Chida, K.; Inaba, Y.; Masuyama, H.; Yanagawa, I.; Mori, I.; Saito, H.; Maruoka, S.; Zuguchi, M. Evaluating the performance of a MOSFET dosimeter at diagnostic X-ray energies for interventional radiology. Radiol. Phys. Technol. 2009, 2, 58–61. [Google Scholar] [CrossRef]
- Chida, K.; Saito, H.; Otani, H.; Kohzuki, M.; Takahashi, S.; Yamada, S.; Shirato, K.; Zuguchi, M. Relationship between fluoroscopic time, dose-area product, body weight, and maximum radiation skin dose in cardiac interventional procedures. Am. J. Roentgenol. 2006, 186, 774–778. [Google Scholar] [CrossRef]
- Kato, M.; Chida, K.; Munehisa, M.; Sato, T.; Inaba, Y.; Suzuki, M.; Zuguchi, M. Non-Lead Protective Aprons for the Protection of Interventional Radiology Physicians from Radiation Exposure in Clinical Settings: An Initial Study. Diagnostics 2021, 11, 1613. [Google Scholar] [CrossRef]
- Chida, K.; Takahashi, T.; Ito, D.; Shimura, H.; Takeda, K.; Zuguchi, M. Clarifying and visualizing sources of staff-received scattered radiation in interventional procedures. Am. J. Roentgenol. 2011, 197, W900–W903. [Google Scholar] [CrossRef] [PubMed]
- Zuguchi, M.; Chida, K.; Taura, M.; Inaba, Y.; Ebata, A.; Yamada, S. Usefulness of non-lead aprons in radiation protection for physicians performing interventional procedures. Radiat. Prot. Dosim. 2008, 131, 531–534. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Implications for Occupational Radiation Protection of the New Dose Limit for the Lens of the Eye; TECDOC 1731; IAEA: Vienna, Austria, 2013; pp. 1–34. [Google Scholar]
- International Commission on Radiolocial Protection. The 2007 Recommendations of the International Commission on Radiological Protection; ICRP Publication 103; ICRP: Ottawa, ON, Canada, 2007; Volume 37, pp. 1–332. [Google Scholar]
- Martin, C.J. A 20 mSv dose limit for the eye: Sense or no sense? J. Radiol. Prot. 2021, 31, 385. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Hitachi, S.; Watanuki, M.; Chida, K. Occupational Radiation Dose to Eye Lenses in CT-Guided Interventions Using MDCT-Fluoroscopy. Diagnostics 2021, 11, 646. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Haga, Y.; Sota, M.; Inaba, Y.; Chida, K.M. Performance of the DOSIRISTM eye lens dosimeter. J. Radiol. Prot. 2019, 39, N19. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).