‘Back-and-Forth Stomach’ CT Imaging Findings of a Pathophysiologic Entity Causing Acute Gastric Volvulus
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Pathophysiology of Acute Gastric Volvulus
4.1.1. Association between GV and PH
4.1.2. Terminological Inconsistencies: Upside-Down Stomach, Chronic GV, Organoaxial GV
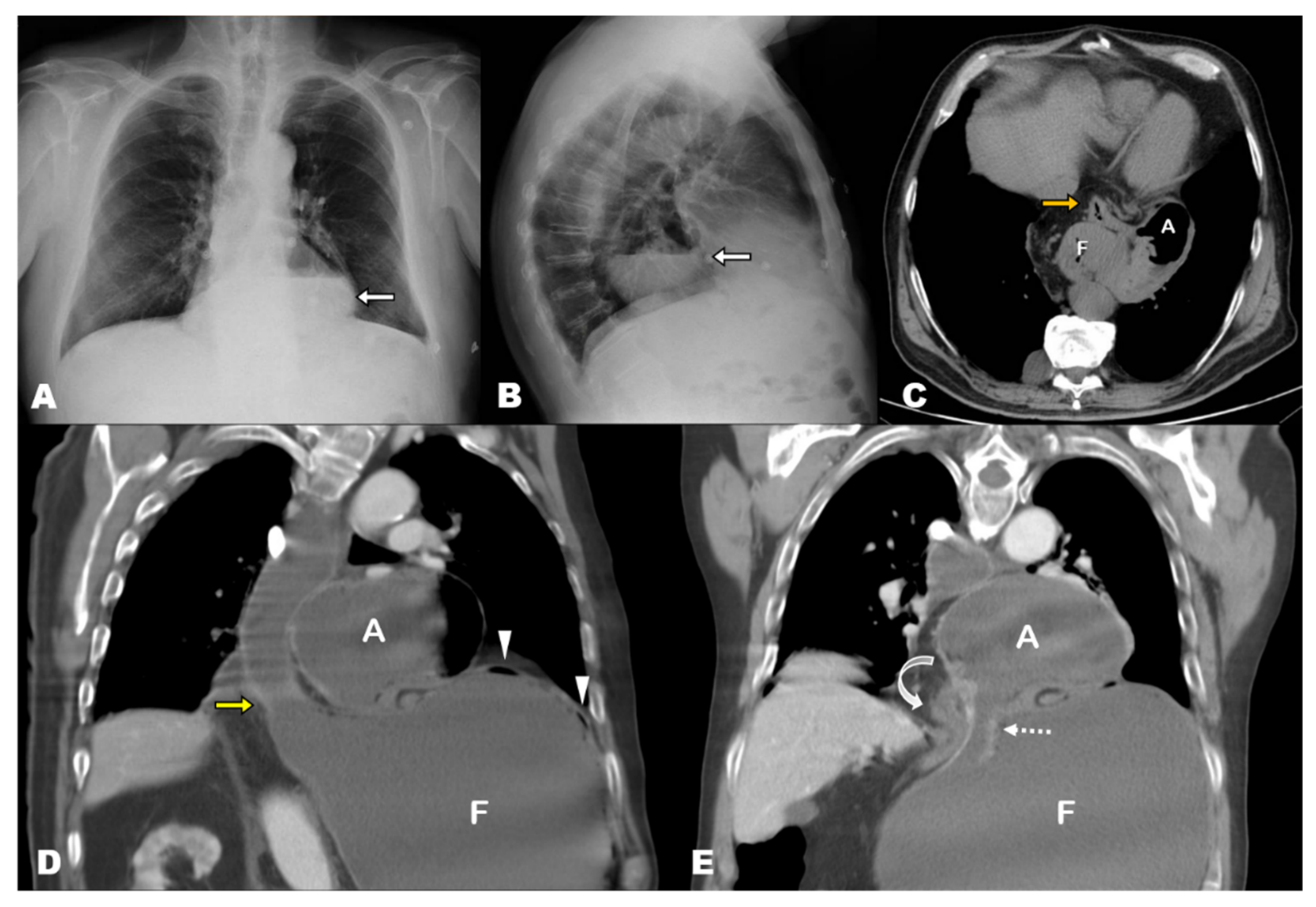
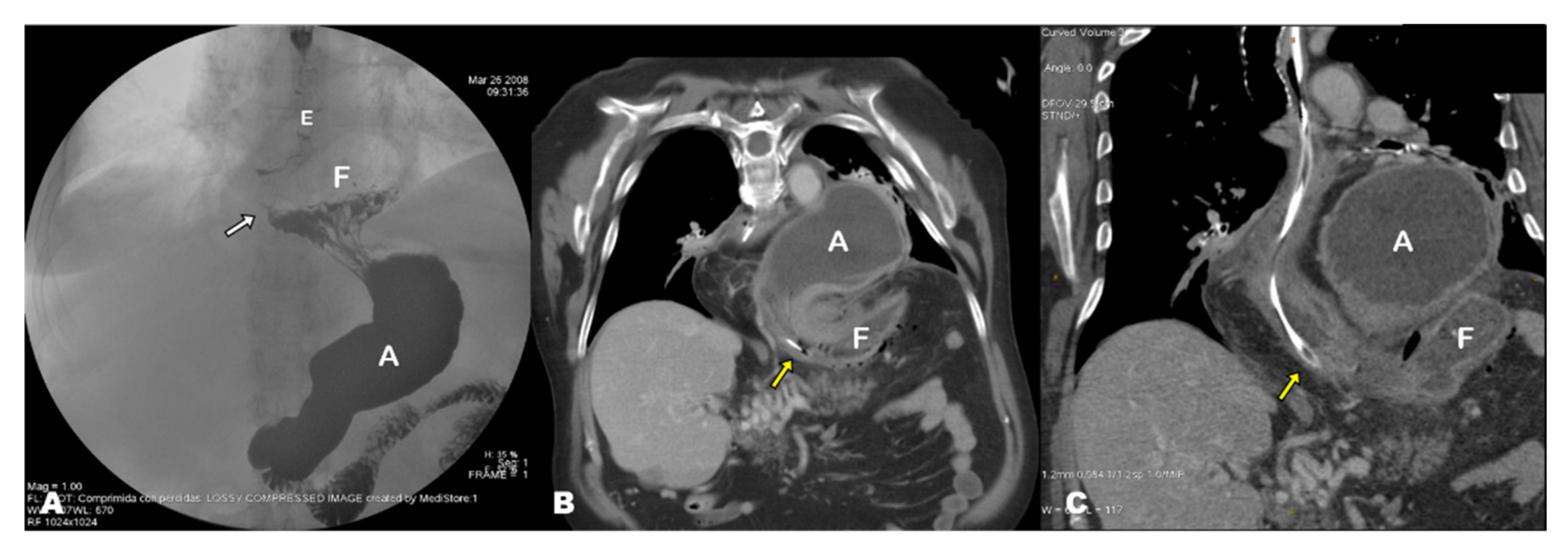
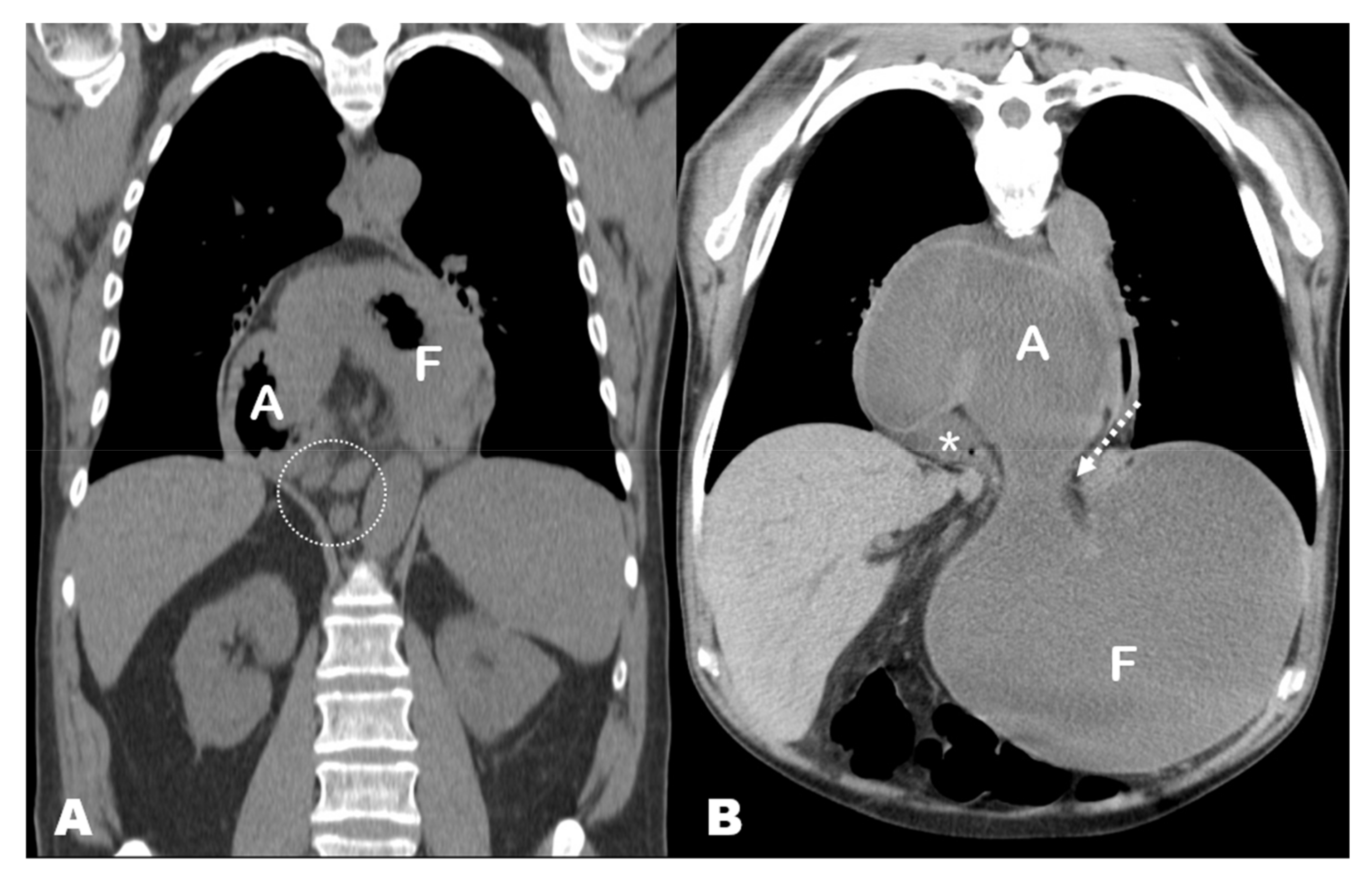
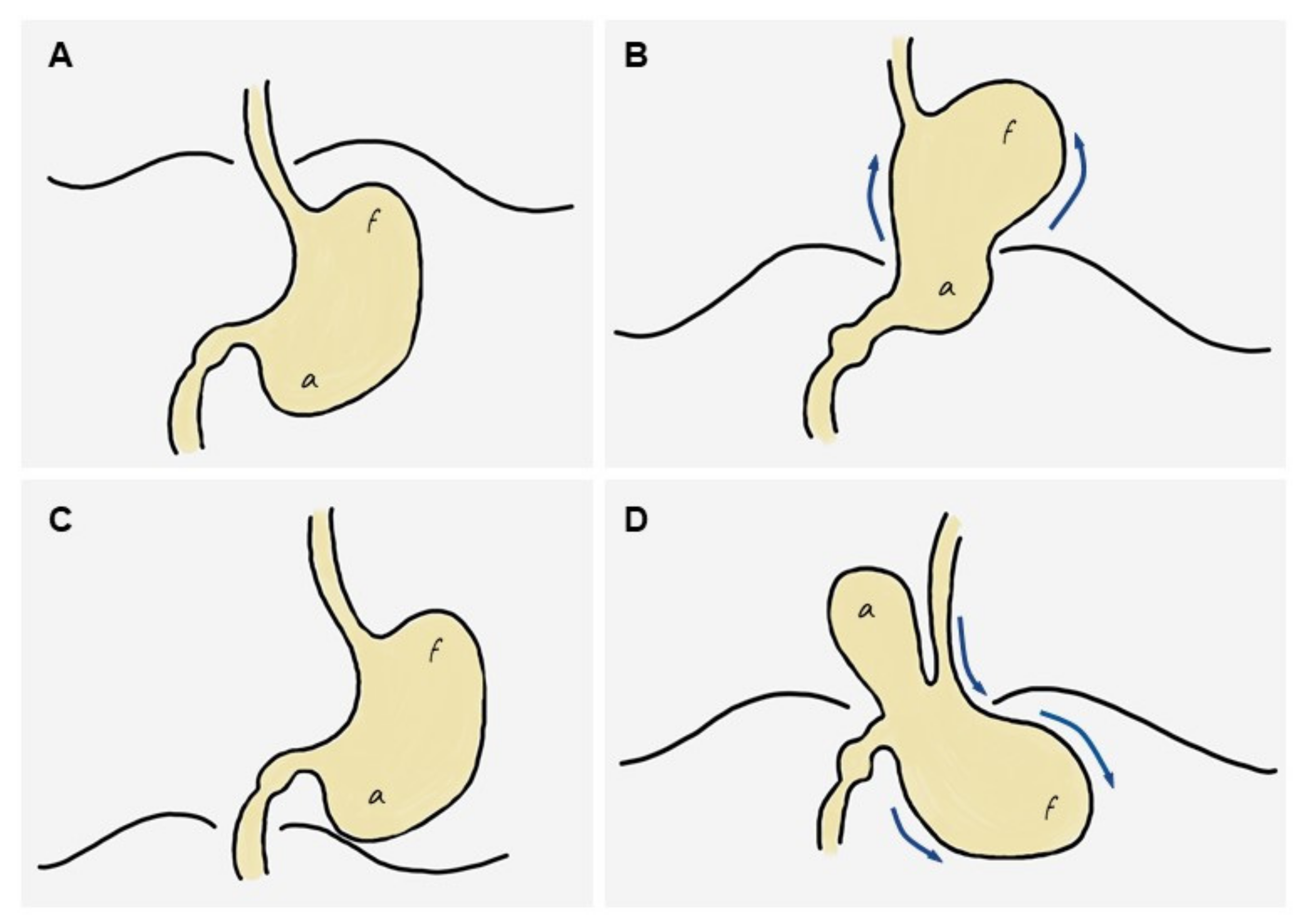
4.1.3. Pathophysiology of GV: The ‘Back-and-Forth Stomach’
4.2. Clinical Features, Laboratory Abnormalities and Outcomes of GV in Our Series
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okeny, P.K.; Abbassi, O.; Warsi, A. Second-look laparostomy for perforated gangrenous gastric volvulus to prevent total gastrectomy. BMJ Case Rep. 2018, 2018, bcr2017223060. [Google Scholar] [CrossRef] [PubMed]
- Rashid, F.; Thangarajah, T.; Mulvey, D.; Larvin, M.; Iftikhar, S.Y. A review article on gastric volvulus: A challenge to diagnosis and management. Int. J. Surg. 2010, 8, 18–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, C.M.; Anderson, J.S.; Hara, A.K.; Carenza, J.W.; Menias, C.O. Volvulus of the gastrointestinal tract: Appearances at multimodality imaging. Radiographics 2009, 29, 1281–1293. [Google Scholar] [CrossRef]
- Millet, I.; Orliac, C.; Alili, C.; Guillon, F.; Taourel, P. Computed tomography findings of acute gastric volvulus. Eur. Radiol. 2014, 24, 3115–3122. [Google Scholar] [CrossRef] [PubMed]
- Berti, A. Singolare attortigliamento dell’esofago col duodeno sequito da rapida morte. Gazz. Med. Ital. 1866, 9, 139–141. [Google Scholar]
- Mazaheri, P.; Ballard, D.H.; Neal, K.A.; Raptis, D.A.; Shetty, A.S.; Raptis, C.A.; Mellnick, V.M. CT of gastric volvulus: Interobserver reliability, radiologists’ accuracy, and imaging findings. Am. J. Roentgenol. 2019, 212, 103–108. [Google Scholar] [CrossRef]
- Chau, B.; Dufel, S. Gastric volvulus. Emerg. Med. J. 2007, 24, 446–447. [Google Scholar] [CrossRef]
- Verde, F.; Hawasli, H.; Johnson, P.T.; Fishman, E.K. Gastric volvulus: Unraveling the diagnosis with MPRs. Emerg. Radiol. 2019, 26, 221–225. [Google Scholar] [CrossRef]
- Cavanagh, Y.; Carlin, N.; Yuridullah, R.; Shaikh, S. Acute Gastric Volvulus Causing Splenic Avulsion and Hemoperitoneum. Case Rep. Gastrointest. Med. 2018, 2018, 2961063. [Google Scholar] [CrossRef] [Green Version]
- Coulier, B.; Ramboux, A. Acute obstructive gastric volvulus diagnosed by helical CT. JBR-BTR 2002, 85, 43. [Google Scholar]
- Singham, S.; Sounness, B. Mesenteroaxial volvulus in an adult: Time is of the essence in acute presentation. Biomed. Imaging Interv. J. 2009, 5, e18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shivanand, G.; Seema, S.; Srivastava, D.N.; Pande, G.; Sahni, P.; Prasad, R.; Ramachandra, N. Gastric volvulus: Acute and chronic presentation. Clin. Imaging 2003, 27, 265–268. [Google Scholar] [CrossRef]
- Von Haberer, H. Volvulus des Magens bei Carcinom. Dtsch. Z. Chir. 1912, 115, 497–532. [Google Scholar] [CrossRef]
- Singleton, A. Chronic gastric volvulus. Radiology 1940, 34, 53–61. [Google Scholar] [CrossRef]
- Carter, R.; Brewer, L.A.; Hinshaw, D.B. Acute gastric volvulus. A study of 25 cases. Am. J. Surg. 1980, 140, 99–106. [Google Scholar] [CrossRef]
- Wastell, C.; Ellis, H. Volvulus of the stomach a review with a report of 8 cases. Br. J. Surg. 1971, 58, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Al-Balas, H.; Hani, M.B.; Omari, H.Z. Radiological features of acute gastric volvulus in adult patients. Clin. Imaging 2010, 34, 344–347. [Google Scholar] [CrossRef]
- Teague, W.J.; Ackroyd, R.; Watson, D.I.; Devitt, P.G. Changing patterns in the management of gastric volvulus over 14 years. Br. J. Surg. 2000, 87, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Al Daoud, F.; Daswani, G.S.; Perinjelil, V.; Nigam, T. Acute Organoaxial gastric volvulus: A massive problem with a twist-case report. Int. J. Surg. Case Rep. 2017, 41, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Tanner, N.C. Chronic and recurrent volvulus of the stomach with late results of “colonic displacement”. Am. J. Surg. 1968, 4, 505–515. [Google Scholar] [CrossRef]
- Culver, G.J.; Pirson, H.S.; Bean, B.C. Mechanism of Obstruction in Para-Esophageal Diaphragmatic Hernias. JAMA J. Am. Med. Assoc. 1962, 181, 933–938. [Google Scholar] [CrossRef]
- Gerson, D.E.; Lewicki, A.M. Intrathoracic stomach: When does it obstruct? Radiology 1976, 119, 257–264. [Google Scholar] [CrossRef]
- Umemura, A.; Suto, T.; Fujiwara, H.; Ikeda, K.; Nakamura, S.; Hayano, M.; Nitta, H.; Takahara, T.; Hasegawa, Y.; Katagiri, H.; et al. Cardiopulmonary impairments caused by a large hiatal hernia with organoaxial gastric volvulus showing upside-down stomach: A case report. Am. J. Case Rep. 2019, 20, 1530–1535. [Google Scholar] [CrossRef]
- Dalgaard, J. Volvulus of the stomach. Acta Clin. Scand. 1952, 103, 131–153. [Google Scholar]
- Gryglewski, A.; Kuta, M.; Pasternak, A.; Opach, Z.; Walocha, J.; Richter, P. Hiatal hernia with upside-down stomach. Management of acute incarceration: Case presentation and review of literature. Folia Med. Crac. 2016, 56, 61–66. [Google Scholar]
- Schiergens, T.S.; Thomas, M.N.; Hüttl, T.P.; Thasler, W.E. Management of acute upside-down stomach. BMC Surg. 2013, 13, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakran, N.; Nevo, H.; Hadar, N.; Raziel, A.; Hershko, D. Laparoscopic Repair of a Large Paraesophageal Hernia with Migration of the Stomach into the Mediastinum Creating an Upside-Down Stomach. Case Rep. Surg. 2017, 2017, 7428195. [Google Scholar] [CrossRef] [PubMed]
- Katkhouda, N.; Mavor, E.; Achanta, K.; Friedlander, M.H.; Grant, S.W.; Essani, R.; Mason, R.J.; Foster, M.; Mouiel, J. Laparoscopic repair of chronic intrathoracic gastric volvulus. Surgery 2000, 128, 784–790. [Google Scholar] [CrossRef]
- Collet, D.; Luc, G.; Chiche, L. Management of large para-esophageal hiatal hernias. J. Visc. Surg. 2013, 150, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Andolfi, C.; Jalilvand, A.; Plana, A.; Fisichella, P.M. Surgical Treatment of Paraesophageal Hernias: A Review. J. Laparoendosc. Adv. Surg. Technol. A 2016, 26, 778. [Google Scholar] [CrossRef]
- Dellaportas, D.; Papaconstantinou, I.; Nastos, C.; Karamanolis, G.; Theodosopoulos, T. Large Paraesophageal Hiatus Hernia: Is Surgery Mandatory? Chirurgia 2018, 113, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Chiechi, M.V.; Hamrick-Turner, J.; Abbitt, P.L. Gastric herniation and volvulus: CT and MR appearance. Gastrointest. Radiol. 1992, 17, 99–101. [Google Scholar] [CrossRef]
- Lee, H.Y.; Park, J.H.; Kim, S.G. Chronic gastric volvulus with laparoscopic gastropexy after endoscopic reduction: A case report. J. Gastric Cancer 2015, 15, 147–150. [Google Scholar] [CrossRef] [Green Version]
- Palanivelu, C.; Rangarajan, M.; Shetty, A.R.; Senthilkumar, R. Laparoscopic suture gastropexy for gastric volvulus: A report of 14 cases. Surg. Endosc. Other Interv. Technol. 2007, 21, 863–866. [Google Scholar] [CrossRef]
- Jacob, C.E.; LoPasso, F.P.; Zilberstein, B.; Bresciani, C.J.C.; Kuga, R.; Cecconello, I.; Gama-Rodrigues, J.J. Gastric volvulus: A review of 38 cases. ABCD Arq. Bras. Cir. Dig. 2009, 22, 96–100. [Google Scholar] [CrossRef]
- Woon, C.Y.L.; Chung, A.Y.F.; Low, A.S.C.; Wong, W.K. Delayed diagnosis of intermittent mesenteroaxial volvulus of the stomach by computed tomography: A case report. J. Med. Case Rep. 2008, 2, 343. [Google Scholar] [CrossRef] [Green Version]
- Godshall, D.; Mossallam, U.; Rosenbaum, R. Gastric volvulus: Case report and review of the literature. J. Emerg. Med. 1999, 17, 837–840. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Ha, C.-Y.; Lee, Y.-J.; Choi, S.-K.; Hong, S.-C.; Jung, E.-J.; Ju, Y.-T.; Jeong, C.-Y.; Ha, W.-S. Acute gastric volvulus treated with laparoscopic reduction and percutaneous endoscopic gastrostomy. J. Korean Surg. Soc. 2013, 85, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Schrag, S.P.; Sharma, R.; Jaik, N.P.; Seamon, M.J.; Lukaszczyk, J.J.; Martin, N.D.; Hoey, B.A.; Stawicki, S.P. Complications related to percutaneous endoscopic gastrostomy (PEG) tubes. A comprehensive clinical review. J. Gastrointest. Liver Dis. 2007, 16, 407–418. [Google Scholar] [PubMed]
- Naim, H.J.; Smith, R.; Gorecki, P.J. Emergent Laparoscopic Reduction of Acute Gastric Volvulus with Anterior Gastropexy. Surg. Laparosc. Endosc. Percutaneous Technol. 2003, 13, 389–391. [Google Scholar] [CrossRef]
- Jamil, L.H.; Huang, B.L.; Kunkel, D.C.; Jayaraman, V.; Soffer, E.E. Successful gastric volvulus reduction and gastropexy using a dual endoscope technique. Case Rep. Med. 2014, 2014, 136381. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Case | Sex | Age | History of HH | Type of HH | Part of the Stomach Herniated Prior to GV | Type of GV |
|---|---|---|---|---|---|---|
| 1 | M | 76 | 10 years [symptomatic] | Sliding [barium swallow] | Fundus | Mesentero-axial (antrum above diaphragm and GEJ) |
| 2 | M | 67 | 1 month [incidental] | Sliding [PET-CT] | Entire stomach | Mesentero-axial (antrum above diaphragm and GEJ) |
| 3 | F | 69 | 5 years [symptomatic] | Sliding [CT] | Fundus | Mesentero-axial (antrum above diaphragm and GEJ) |
| 4 | M | 81 | 7 years [incidental] | Sliding [CT] | Entire stomach | Mesentero-axial (antrum above diaphragm and GEJ) |
| 5 | M | 69 | 2 years [symptomatic] | Sliding [CT] | Entire stomach | Mesentero-axial (antrum above diaphragm and GEJ) |
| 6 | F | 85 | 5 years [symptomatic] | Sliding [CT] | Entire stomach | Mesentero-axial (antrum above diaphragm and GEJ) |
| 7 | F | 47 | 4 years [incidental] | Sliding [CR] | At least fundus 1 | Mesentero-axial (antrum above diaphragm and GEJ) |
| Case | Clinical Presentation (GV) | Blood Test Workup (GV) | Complications of GV | Relevant Associated Findings | Treatment | Outcome |
|---|---|---|---|---|---|---|
| 1 | Dark vomits, abdominal pain, food and fluids intolerance, dehydration, tachycardia [100 bpm] | WBC count [16,700/μL], CRP [12.0 mg/L], LDH [968 U/L] | Microperforation of fundus | - | Surgery [partial resection + fundoplication] | Died during post-operative period |
| 2 | Abdominal and lower chest pain, nausea and vomiting, fever [38.5 °C], inability to pass NG tube (Borchardt’s triad) | WBC count [660/μL], CRP [41.4 mg/L], LDH [542 U/L] | - | Lymphadenopathies (lymphoma) | Surgery [partial resection + fundoplication] | Alive (died 7 years later due to lymphoma) |
| 3 | Abdominal and lower chest pain, severe vomiting | WBC count [12,370/μL], CRP [46.9 mg/L], K+ [3 mEq/L] | - | Prostatic tumor | Surgery [fundoplication + cardioplasty] | Alive (4 years follow-up) |
| 4 | Intense abdominal pain, severe vomiting, tachycardia [150 bpm], signs of peritonitis, inability to pass NG tube (Borchardt’s triad) | WBC count [18,730/μL], CRP [2.7 mg/dL] Lactic acid [69.6 mg/dL] K+ [2.9 mEq/L] | Gastric pneumatosis and microperforaation of fundus | Splenic laceration, left hernioplasty | Surgery [partial resection + fundoplication + cardioplasty + splenectomy] | Alive (2 years follow-up) |
| 5 | Abdominal pain, severe vomiting, inability to pass nasogastric tube (Borchardt’s triad) | WBC count [14,980/μL], CRP [13 mg/L] | - | - | Surgery [cardioplasty + fundoplication + jejunostomy] | Alive (3 years follow-up) |
| 6 | Dark vomits, food and fluid intolerance, tachycardia [100 bpm] | WBC count [9,070/μL], LDH [286 U/L] | - | - | Surgery [cardioplasty + fundoplication] | Alive (2 years follow-up) |
| 7 | Abdominal pain, vomitting, mass in left hypochondrium | WBC count [21,670/μL] CRP [35 mg/L] K+ [2.2 mEq/L] Cl− [76 mEq/L] | Microperforation of fundus | - | Surgery [Hernia repair + fundoplication] | Alive (3 years follow-up) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Láinez Ramos-Bossini, A.J.; Ruiz Carazo, E.; Rabadán Caravaca, M.D. ‘Back-and-Forth Stomach’ CT Imaging Findings of a Pathophysiologic Entity Causing Acute Gastric Volvulus. Tomography 2022, 8, 245-256. https://doi.org/10.3390/tomography8010019
Láinez Ramos-Bossini AJ, Ruiz Carazo E, Rabadán Caravaca MD. ‘Back-and-Forth Stomach’ CT Imaging Findings of a Pathophysiologic Entity Causing Acute Gastric Volvulus. Tomography. 2022; 8(1):245-256. https://doi.org/10.3390/tomography8010019
Chicago/Turabian StyleLáinez Ramos-Bossini, Antonio Jesús, Eduardo Ruiz Carazo, and María Dolores Rabadán Caravaca. 2022. "‘Back-and-Forth Stomach’ CT Imaging Findings of a Pathophysiologic Entity Causing Acute Gastric Volvulus" Tomography 8, no. 1: 245-256. https://doi.org/10.3390/tomography8010019
APA StyleLáinez Ramos-Bossini, A. J., Ruiz Carazo, E., & Rabadán Caravaca, M. D. (2022). ‘Back-and-Forth Stomach’ CT Imaging Findings of a Pathophysiologic Entity Causing Acute Gastric Volvulus. Tomography, 8(1), 245-256. https://doi.org/10.3390/tomography8010019







