Pulsed Arterial Spin Labeling and Segmented Brain Volumetry in the Diagnostic Evaluation of Frontotemporal Dementia, Alzheimer’s Disease and Mild Cognitive Impairment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Structural MRI Acquisition
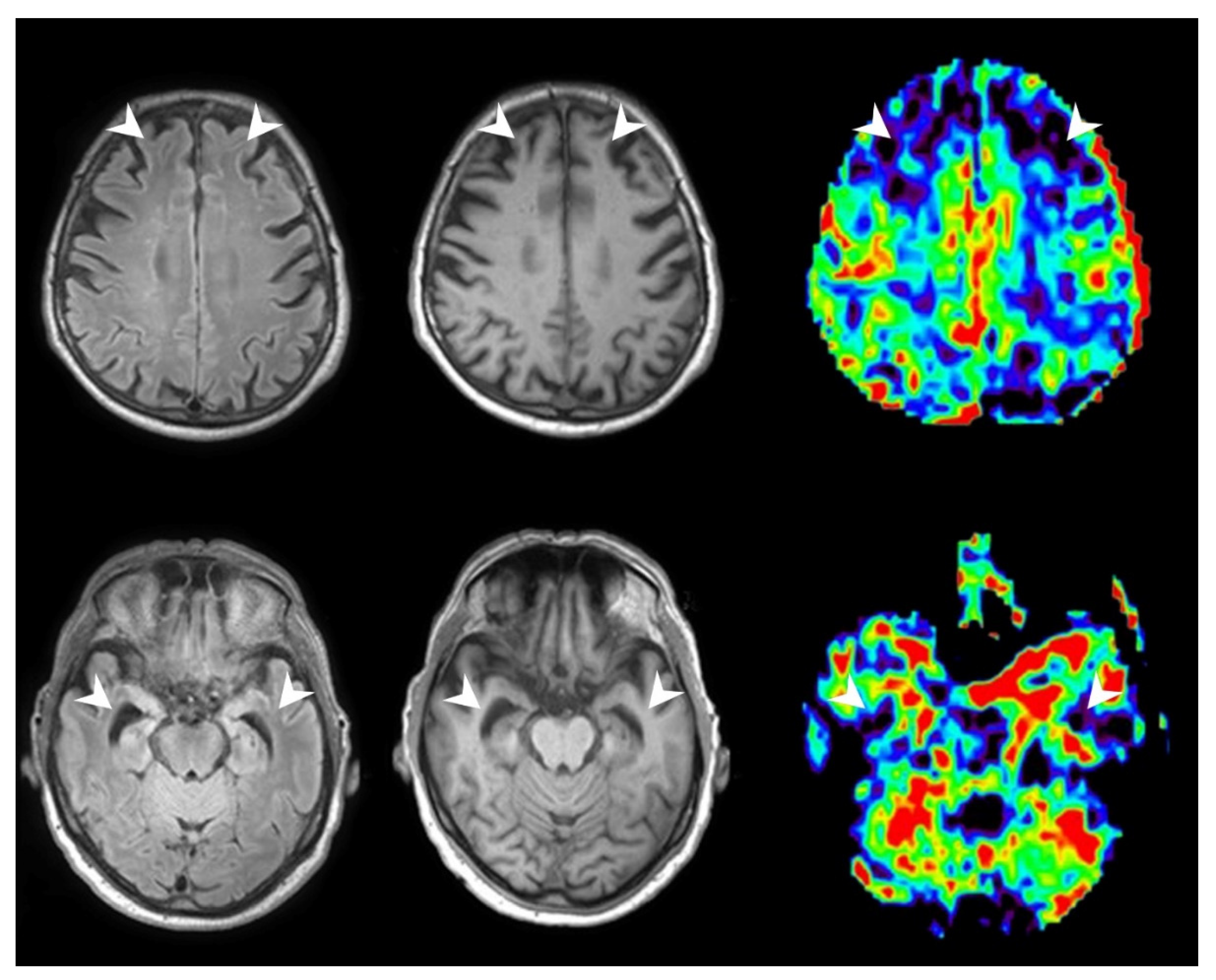
2.4. pASL-Perfusion Image Acquisition
2.5. Brain Volumetry Analysis
2.6. pASL Perfusion Data Analysis
2.7. Statistical Analysis
3. Results
3.1. Results of Brain Regions with Significant Atrophy
3.2. Results of Statistical Correlation between Brain-Volume and pASL
3.2.1. Patients with FTD
3.2.2. Patients with MCI
3.2.3. Patients with AD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACR | anterior corona radiata |
| AD | Alzheimer’s disease |
| AG | angular gyrus |
| ASL | arterial spin labeling |
| bvFTD | behavioral-variant frontotemporal dementia |
| CBF | cerebral blood flow |
| CGH | hippocampal cingulum |
| CI | claustrum complex |
| CP | cerebral peduncle |
| EC | external capsule |
| FDG-PET | fluorodeoxyglucose (18F)– positron emission tomography |
| FTD | frontotemporal dementia |
| GP | globus pallidus |
| ICP | inferior cerebellar peduncle |
| IFGO | pars orbitalis of the inferior frontal gyrus |
| IFO | inferior fronto-occipital fasciculus |
| IFWMO | pars orbitalis of the inferior frontal white matter |
| LFOWM | whiter matter of the lateral fronto-orbital gyrus |
| Mammillary | mammillary body |
| MCI | mild cognitive impairment |
| MFG-DPFC | middle frontal gyrus (dorsal prefrontal cortex) |
| MFOG | middle fronto-orbital gyrus |
| MMSE | Mini-Mental State Examination |
| MRI | magnetic resonance imaging |
| MTG | middle temporal gyrus |
| MTG-pole | pole of the middle temporal gyrus |
| MTWM | white matter of the middle temporal gyrus |
| nfvPPA | non-fluent variant primary progressive aphasia |
| NucAccumbens | Nucleus accumbens |
| pASL | pulsed arterial spin labeling |
| PCR | posterior corona radiata |
| PHG | parahippocampal gyrus |
| PPA | primary progressive aphasia |
| rCBF | relative cerebral blood flow |
| RG | gyrus rectus |
| RGWM | white matter of the gyrus rectus |
| rostralWM-ACC | white matter of the rostral anterior cingulate gyrus |
| SCP | superior cerebellar peduncle |
| SCRSFG-pole | superior corona radiatapole of the superior frontal gyrus |
| Snigra | substantia nigra |
| SOG | superior occipital gyrus |
| SS | sagittal stratum (includes inferior longitudinal fasciculus and inferior fronto-occipital fasciculus) |
| STG | superior temporal gyrus |
| STG-pole | pole of the superior temporal gyrus |
| STWM | white matter of the superior temporal gyrus |
| subcallosalWM-ACC | white matter of the subcallosal anterior cingulate gyrus |
| svPPA | semantic-variant primary progressive aphasia |
References
- Blennow, K.; Hampel, H.; Weiner, M.W.; Zetterberg, H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 131–144. [Google Scholar] [CrossRef]
- Riphagen, J.M.; Ramakers, I.H.; Freeze, W.M.; Pagen, L.H.; Hanseeuw, B.J.; Verbeek, M.M.; Verhey, F.R.; Jacobs, H. Linking APOE-ε4, blood-brain barrier dysfunction, and inflammation to Alzheimer’s pathology. Neurobiol. Aging 2020, 85, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Gordon, M.L.; Goldberg, T.E. Cerebral blood flow measured by arterial spin labeling MRI at resting state in normal aging and Alzheimer’s disease. Neurosci. Biobehav. Rev. 2017, 72, 168–175. [Google Scholar] [CrossRef]
- Wolk, D.A.; Detre, J.A. Arterial spin labeling MRI: An Emerging Biomarker for Alzheimer’s Disease and Other Neuro-degenerative Conditionsv. Curr. Opin. Neurol. 2012, 25, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, P.; Li, Y.; Fan, H.; Su, P.; Peng, S.-L.; Park, D.C.; Rodrigue, K.M.; Jiang, H.; Faria, A.V.; et al. ASL-MRICloud: Towards a comprehensive online tool for ASL data analysis. NMR Biomed. 2018, 32, e4051. [Google Scholar] [CrossRef]
- Mori, S.; Kolasny, A.; Center for Imaging Science, Laboratory for Brain Anatomical MRI, Center for Brain Imaging, AnatomyWorks, LLC. Available online: https://braingps.mricloud.org/docs/tutorials/download_results/reports_to_use.html (accessed on 21 November 2021).
- Mori, S.; Kolasny, A.; Center for Imaging Science, Laboratory for Brain Anatomical MRI, Center for Brain Imaging, Anatomy Works, LLC. Available online: https://braingps.mricloud.org/docs/tutorials/download_results/granularity_levels.html (accessed on 21 November 2021).
- Alsop, D.C.; Detre, J.A.; Golay, X.; Günther, M.; Hendrikse, J.; Hernandez-Garcia, L.; Lu, H.; MacIntosh, B.; Parkes, L.M.; Smits, M.; et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Reson. Med. 2015, 73, 102–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothman, K.J. No adjustments are needed for multiple comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef] [Green Version]
- Chao, L.L.; Buckley, S.T.; Kornak, J.; Schuff, N.; Madison, C.; Yaffe, K.; Miller, B.L.; Kramer, J.H.; Weiner, M.W. ASL Perfusion MRI Predicts Cognitive Decline and Conversion From MCI to Dementia. Alzheimer Dis. Assoc. Disord. 2010, 24, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Bocchetta, M.; Gordon, E.; Cardoso, M.J.; Modat, M.; Ourselin, S.; Warren, J.D.; Rohrer, J.D. Thalamic atrophy in frontotemporal dementia—Not just a C9orf72 problem. NeuroImage Clin. 2018, 18, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Grundman, M.; Sencakova, D.; Jack, C.R.; Petersen, R.C.; Kim, H.T.; Schultz, A.; Weiner, M.F.; De Carli, C.; DeKosky, S.T.; van Dyck, C.; et al. Brain MRI hippocampal volume and prediction of clinical status in a mild cognitive impairment trial. J. Mol. Neurosci. 2002, 19, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Alladi, S.; Xuereb, J.; Bak, T.; Nestor, P.; Knibb, J.; Patterson, K.; Hodges, J.R. Focal cortical presentations of Alzheimer’s disease. Brain 2007, 130, 2636–2645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, H.J.; Gorno–Tempini, M.L.; Goldman, W.P.; Perry, R.J.; Schuff, N.; Weiner, M.; Feiwell, R.; Kramer, J.H.; Miller, B.L. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology 2002, 58, 198–208. [Google Scholar] [CrossRef] [Green Version]
- Bang, J.; Spina, S.; Miller, B.L. Frontotemporal dementia. Lancet 2015, 386, 1672–1682. [Google Scholar] [CrossRef] [Green Version]
- Mann, D.M.A.; South, P.W. The topographic distribution of brain atrophy in frontal lobe dementia. Acta Neuropathol. 1993, 85, 334–340. [Google Scholar] [CrossRef]
- Carlson, N.R. Physiology of Behavior; Pearson: Upper Saddle River, NJ, USA, 2012; p. 364. ISBN 978-0205239399. [Google Scholar]
- Galton, C.J.; Gomez-Anson, B.; Antoun, N.; Scheltens, P.; Patterson, K.; Graves, M.; Sahakian, B.J.; Hodges, J.R. Temporal lobe rating scale: Application to Alzheimer’s disease and frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 2001, 70, 165–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.E.; Khazenzon, A.M.; Trujilo, A.J.; Guo, C.C.; Yokoyama, J.S.; Sha, S.J.; Takada, L.T.; Karydas, A.M.; Block, N.R.; Coppola, G.; et al. Altered network connectivity in frontotemporal dementia with C9orf72 hexanucleotide repeat expansion. Brain 2014, 137, 3047–3060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halabi, C.; Halabi, A.; Dean, D.L.; Wang, P.-N.; Boxer, A.L.; Trojanowski, J.Q.; DeArmond, S.J.; Miller, B.L.; Kramer, J.H.; Seeley, W.W. Patterns of Striatal Degeneration in Frontotemporal Dementia. Alzheimer Dis. Assoc. Disord. 2013, 27, 74–83. [Google Scholar] [CrossRef] [Green Version]
- Viñas-Guasch, N.; Wu, Y.J. The role of the putamen in language: A meta-analytic connectivity modeling study. Brain Struct. Funct. 2017, 222, 3991–4004. [Google Scholar] [CrossRef]
- Bede, P.; Omer, T.; Finegan, E.; Chipika, R.H.; Iyer, P.M.; Doherty, M.; Vajda, A.; Pender, N.; McLaughlin, R.; Hutchinson, S.; et al. Connectivity-based characterisation of subcortical grey matter pathology in frontotemporal dementia and ALS: A multimodal neuroimaging study. Brain Imaging Behav. 2018, 12, 1696–1707. [Google Scholar] [CrossRef]
- Domínguez-Vivero, C.; Wu, L.; Lee, S.; Manoochehri, M.; Cines, S.; Brickman, A.M.; Rizvi, B.; Chesebro, A.; Gazes, Y.; Fallon, E.; et al. Structural Brain Changes in Pre-Clinical FTD MAPT Mutation Carriers. J. Alzheimers Dis. 2020, 75, 595–606. [Google Scholar] [CrossRef]
- Fennema-Notestine, C.; McEvoy, L.K.; Hagler, D.J.; Jacobson, M.W.; Dale, A.M.; The Alzheimer’s Disease Neuroimaging Initiative. Structural neuroimaging in the detection and prognosis of pre-clinical and early AD. Behav. Neurol. 2009, 21, 3–12. [Google Scholar] [CrossRef]
- Anderson, N.D. State of the science on mild cognitive impairment (MCI). CNS Spectr. 2019, 24, 78–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allman, J.M.; Hakeem, A.; Erwin, J.M.; Nimchinsky, E.; Hof, P. The Anterior Cingulate Cortex. Ann. N. Y. Acad. Sci. 2001, 935, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Cera, N.; Esposito, R.; Cieri, F.; Tartaro, A. Altered Cingulate Cortex Functional Connectivity in Normal Aging and Mild Cognitive Impairment. Front. Neurosci. 2019, 13, 857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Wahlund, L.-O.; Svensson, L.; Winblad, B.; Julin, P. Cingulate cortex hypoperfusion predicts Alzheimer’s disease in mild cognitive impairment. BMC Neurol. 2002, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Kier, E.L.; Staib, L.H.; Davis, L.M.; Bronen, R.A. MR Imaging of the Temporal Stem: Anatomic Dissection Tractography of the Uncinate Fasciculus, Inferior Occipitofrontal Fasciculus, and Meyer’s Loop of the Optic Radiation. Am. J. Neuroradiol. 2004, 25, 677–691. [Google Scholar] [PubMed]
- Chen, H.; Huang, L.; Li, H.; Qian, Y.; Yang, D.; Qing, Z.; Luo, C.; Li, M.; Zhang, B.; Xu, Y. Microstructural disruption of the right inferior fronto-occipital and inferior longitudinal fasciculus contributes to WMH-related cognitive impairment. CNS Neurosci. Ther. 2020, 26, 576–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baloyannis, S.J.; Mavroudis, I.; Baloyannis, I.S.; Costa, V. Mammillary Bodies in Alzheimer’s Disease: A Golgi and Electron Microscope Study. Am. J. Alzheimer’s Dis. Other Dement. 2015, 31, 247–256. [Google Scholar] [CrossRef]
- Vann, S.D. Re-evaluating the role of the mammillary bodies in memory. Neuropsychologia 2010, 48, 2316–2327. [Google Scholar] [CrossRef]
- Scheltens, P.; Blennow, K.; Breteler, M.M.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Jones, B.F.; Barnes, J.; Uylings, H.B.; Fox, N.; Frost, C.; Witter, M.P.; Scheltens, P. Differential Regional Atrophy of the Cingulate Gyrus in Alzheimer Disease: A Volumetric MRI Study. Cereb. Cortex 2005, 16, 1701–1708. [Google Scholar] [CrossRef]
- Ebertoux, M.; O’Callaghan, C.; Eflanagan, E.; Hodges, J.R.; Hornberger, M. Fronto-Striatal Atrophy in Behavioral Variant Frontotemporal Dementia and Alzheimer’s Disease. Front. Neurol. 2015, 6, 147. [Google Scholar] [CrossRef]
- Looi, J.C.L.; Svensson, L.; Lindberg, O.; Zandbelt, B.B.; Ostberg, P.; Orndahl, E.; Wahlund, L.O. Putaminal volume in frontotemporal lobar degeneration and Alzheimer disease: Differential volumes in dementia subtypes and controls. AJNR Am. J. Neuroradiol. 2009, 30, 1552–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, R.; McKeith, I.; Ballard, C.; O’Brien, J. Volumetric MRI study of the caudate nucleus in patients with dementia with Lewy bodies, Alzheimer’s disease, and vascular dementia. J. Neurol. Neurosurg. Psychiatry 2002, 72, 406–407. [Google Scholar] [CrossRef]
- Lehmann, M.; Koedam, E.L.; Barnes, J.; Bartlett, J.; Ryan, N.S.; Pijnenburg, Y.A.; Barkhof, F.; Wattjes, M.P.; Scheltens, P.; Fox, N.C. Posterior cerebral atrophy in the absence of medial temporal lobe atrophy in pathologically-confirmed Alzheimer’s disease. Neurobiol. Aging 2012, 33, 627.e1–627.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karas, G.B.; Scheltens, P.; Rombouts, S.A.R.B.; van Schijndel, R.; Klein, M.; Jones, B.; van der Flier, W.; Vrenken, H.; Barkhof, F. Precuneus atrophy in early-onset Alzheimer’s disease: A morphometric structural MRI study. Neuroradiology 2007, 49, 967–976. [Google Scholar] [CrossRef] [Green Version]
- Shi, F.; Liu, B.; Zhou, Y.; Yu, C.; Jiang, T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer’s disease: Meta-analyses of MRI studies. Hippocampus 2009, 19, 1055–1064. [Google Scholar] [CrossRef]
- Tosun, D.; Schuff, N.; Rabinovici, G.D.; Ayakta, N.; Miller, B.L.; Jagust, W.; Kramer, J.; Weiner, M.M.; Rosen, H.J. Diagnostic utility of ASL-MRI and FDG-PET in the behavioral variant of FTD and AD. Ann. Clin. Transl. Neurol. 2016, 3, 740–751. [Google Scholar] [CrossRef]
- Yu, J.; Lee, T.M. The longitudinal decline of white matter microstructural integrity in behavioral variant frontotemporal dementia and its association with executive function. Neurobiol. Aging 2019, 76, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Binnewijzend, M.A.A.; Kuijer, J.P.; Benedictus, M.R.; van der Flier, W.M.; Wink, A.M.; Wattjes, M.P.; Van Berckel, B.N.M.; Scheltens, P.; Barkhof, F. Cerebral Blood Flow Measured with 3D Pseudocontinuous Arterial Spin-labeling MR Imaging in Alzheimer Disease and Mild Cognitive Impairment: A Marker for Disease Severity. Radiology 2013, 267, 221–230. [Google Scholar] [CrossRef]
- Bron, E.E.; Steketee, R.; Houston, G.; Oliver, R.; Achterberg, H.C.; Loog, M.; van Swieten, J.C.; Hammers, A.; Niessen, W.; Smits, M.; et al. Diagnostic classification of arterial spin labeling and structural MRI in presenile early stage dementia. Hum. Brain Mapp. 2014, 35, 4916–4931. [Google Scholar] [CrossRef]
- Verfaillie, S.C.J.; Adriaanse, S.M.; Binnewijzend, M.A.A.; Benedictus, M.R.; Ossenkoppele, R.; Wattjes, M.P.; Pijnenburg, Y.A.L.; van der Flier, W.M.; Lammertsma, A.A.; Kuijer, J.P.A.; et al. Cerebral Perfusion and Glucose Metabolism in Alzheimer’s Disease and Frontotemporal Dementia: Two Sides of the Same coin? Eur. Radiol. 2015, 25, 3050–3059. [Google Scholar] [CrossRef] [Green Version]
- Anazodo, U.C.; Finger, E.; Kwan, B.Y.M.; Pavlosky, W.; Warrington, J.C.; Guenther, M.; Prato, F.S.; Thiessen, J.D.; St Lawrence, K.S. Using simultaneous PET/MRI to compare the accuracy of diagnosing frontotemporal dementia by arterial spin labelling MRI and FDG-PET. Neuroimage Clin. 2017, 17, 405–414. [Google Scholar] [CrossRef]
- Putcha, D.; Brickhouse, M.; O’Keefe, K.; Sullivan, C.; Rentz, D.; Marshall, G.; Dickerson, B.; Sperling, R. Hippocampal Hyperactivation Associated with Cortical Thinning in Alzheimer’s Disease Signature Regions in Non-Demented Elderly Adults. J. Neurosci. 2011, 31, 17680–17688. [Google Scholar] [CrossRef]
- Killiany, R.J.; Moss, M.B.; Albert, M.S.; Sandor, T.; Tieman, J.; Jolesz, F. Temporal Lobe Regions on Magnetic Resonance Imaging Identify Patients With Early Alzheimer’s Disease. Arch. Neurol. 1993, 50, 949–954. [Google Scholar] [CrossRef]
- Lehéricy, S.; Baulac, M.; Chiras, J.; Piérot, L.; Martin, N.; Pillon, B.; Deweer, B.; Dubois, B.; Marsault, C. Amygdalohippocampal MR volume measurements in the early stages of Alzheimer disease. Am. J. Neuroradiol. 1994, 15, 929–937. [Google Scholar] [PubMed]
- Laakso, M.; Soininen, H.; Partanen, K.; Helkala, E.-L.; Hartikainen, P.; Vainio, P.; Hallikainen, M.; Hänninen, T.; Sr, P.J.R. Volumes of hippocampus, amygdala and frontal lobes in the MRI-based diagnosis of early Alzheimer’s disease: Correlation with memory functions. J. Neural Transm. Park Dis. Dement. Sect. 1995, 9, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Wolters, F.J.; Zonneveld, H.I.; Hofman, A.; Van Der Lugt, A.; Koudstaal, P.J.; Vernooij, M.W.; Ikram, M.A. Cerebral Perfusion and the Risk of Dementia. Circulation 2017, 136, 719–728. [Google Scholar] [CrossRef] [Green Version]
- Wirth, M.; Binette, A.P.; Brunecker, P.; Köbe, T.; Witte, A.V.; Flöel, A. Divergent regional patterns of cerebral hypoperfusion and gray matter atrophy in mild cognitive impairment patients. J. Cereb. Blood Flow Metab. 2017, 37, 814–824. [Google Scholar] [CrossRef] [PubMed]
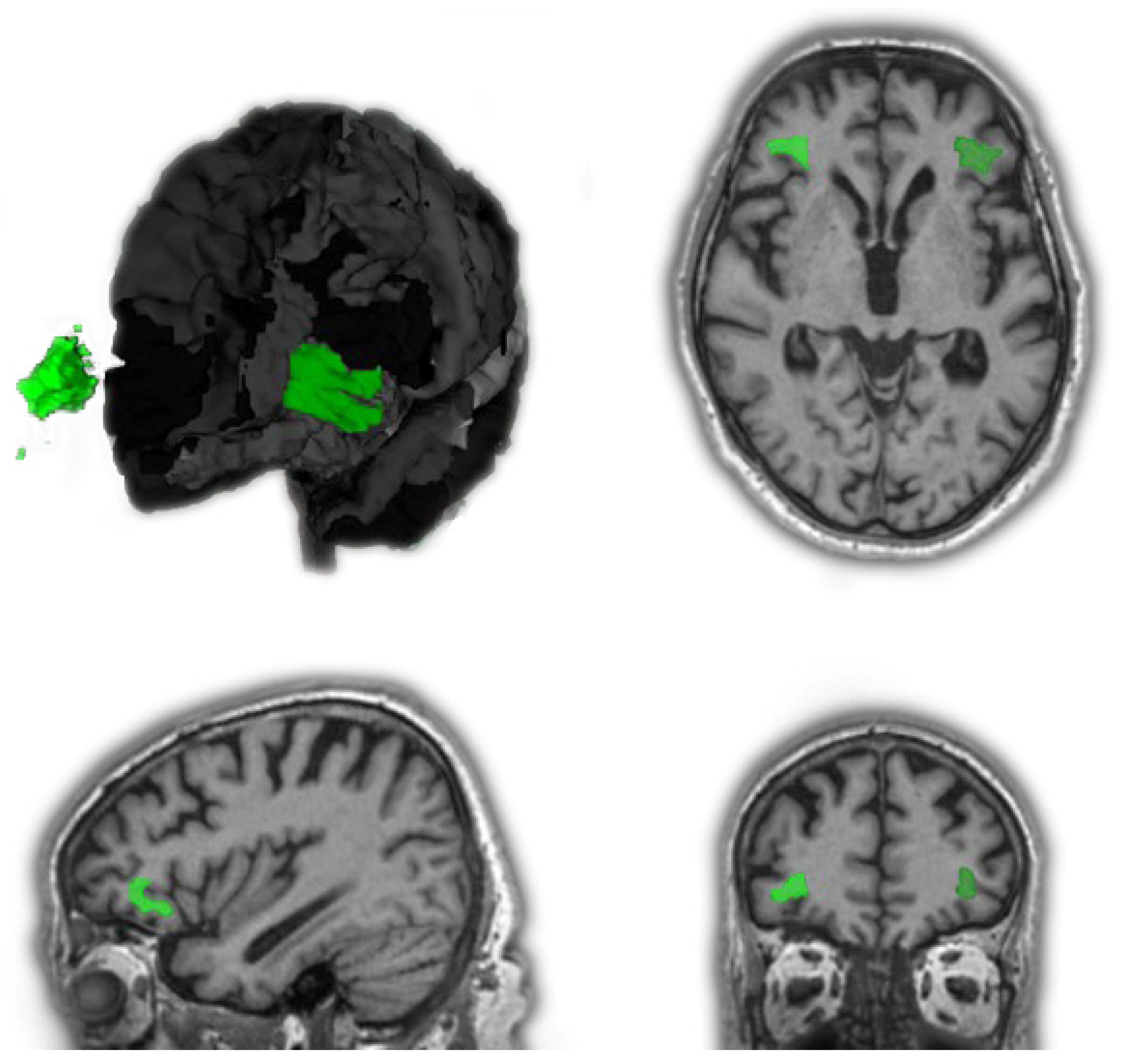
| CO | AD | FTD | MCI | |
|---|---|---|---|---|
| No. | 19 | 5 | 5 | 7 |
| Sex (M:F) | 7:12 | 4:1 | 1:4 | 4:3 |
| Age (years) | 57.1 (46–78) | 63.6 (50–77) | 69.8 (62–79) | 63 (54–78) |
| MMSE | 30 | 22.4 (10–27) | 24.6 (14–30) | 27.4 (25–29) |
| Brain Region of Interest | Mean Volume in FTD (in mm3) | Mean Volume in CO (in mm3) | Level of Significance (p) |
|---|---|---|---|
| MFG-DPFC left | 9525.6 | 11,480.0 | 0.053 |
| MFG-DPFC-right | 9763.6 | 11,947.1 | 0.031 |
| IFGO left | 2755.0 | 3347.1 | 0.023 |
| IFGO right | 2062.2 | 2622.6 | 0.002 |
| MFOG right | 3534.4 | 4028.0 | 0.041 |
| RG left | 3770.2 | 4625.9 | 0.026 |
| RG right | 3747.6 | 4691.5 | 0.006 |
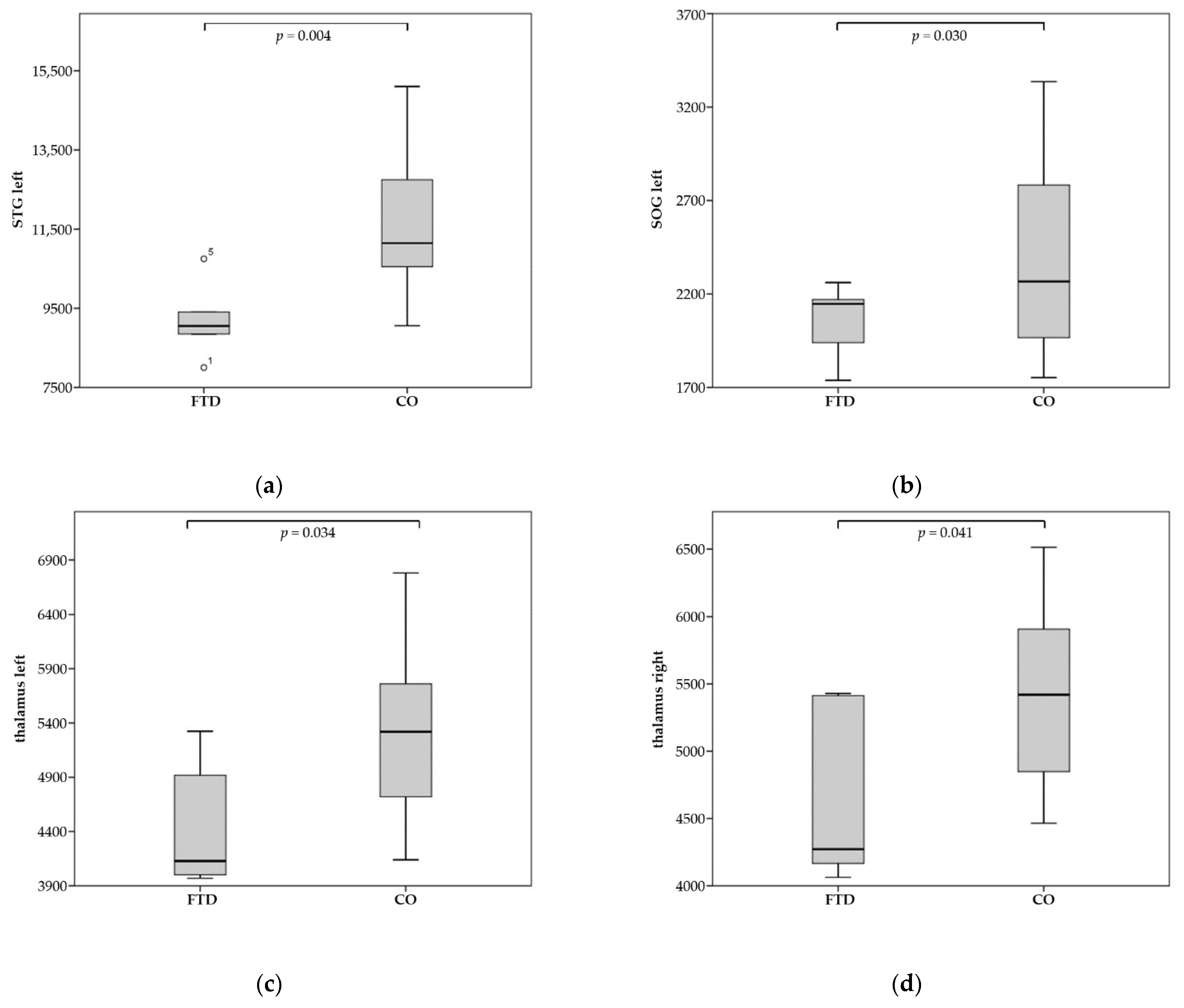
| STG left | 9212.4 | 11,653.5 | 0.004 |
| STG right | 8948.2 | 10,913.4 | 0.004 |
| STG-pole left | 4168.4 | 5227.8 | 0.013 |
| AG left | 5866.8 | 6770.3 | 0.048 |
| SOG left | 2051.2 | 2395.6 | 0.030 |
| Insula left | 5104.8 | 6127.6 | 0.038 |
| Insula right | 5202.0 | 6141.0 | 0.024 |
| Amygdala left | 1071,8 | 1497,9 | 0.000 |
| Amygdala right | 1217.2 | 1630.7 | 0.006 |
| Hippocampus left | 2868.4 | 3442.0 | 0.022 |
| Putamen left | 2886.0 | 3684.6 | 0.001 |
| Putamen right | 3152.0 | 3876.3 | 0.002 |
| GP left | 1124.2 | 1340.4 | 0.025 |
| Thalamus left | 4468.4 | 5237.5 | 0.034 |
| Thalamus right | 4668.8 | 5348.7 | 0.041 |
| NucAccumbens right | 604.6 | 730.8 | 0.049 |
| Snigra left | 261.0 | 318.8 | 0.026 |
| Snigra right | 210.8 | 256.3 | 0.048 |
| CP left | 1536.2 | 1858.2 | 0.026 |
| CP right | 1623.4 | 1916.5 | 0.050 |
| ICP left | 631.4 | 761.0 | 0.036 |
| SCR left | 13,355.8 | 15,739.9 | 0.044 |
| PCR left | 5040.6 | 6124.4 | 0.013 |
| PCR right | 4243.2 | 5156.5 | 0.036 |
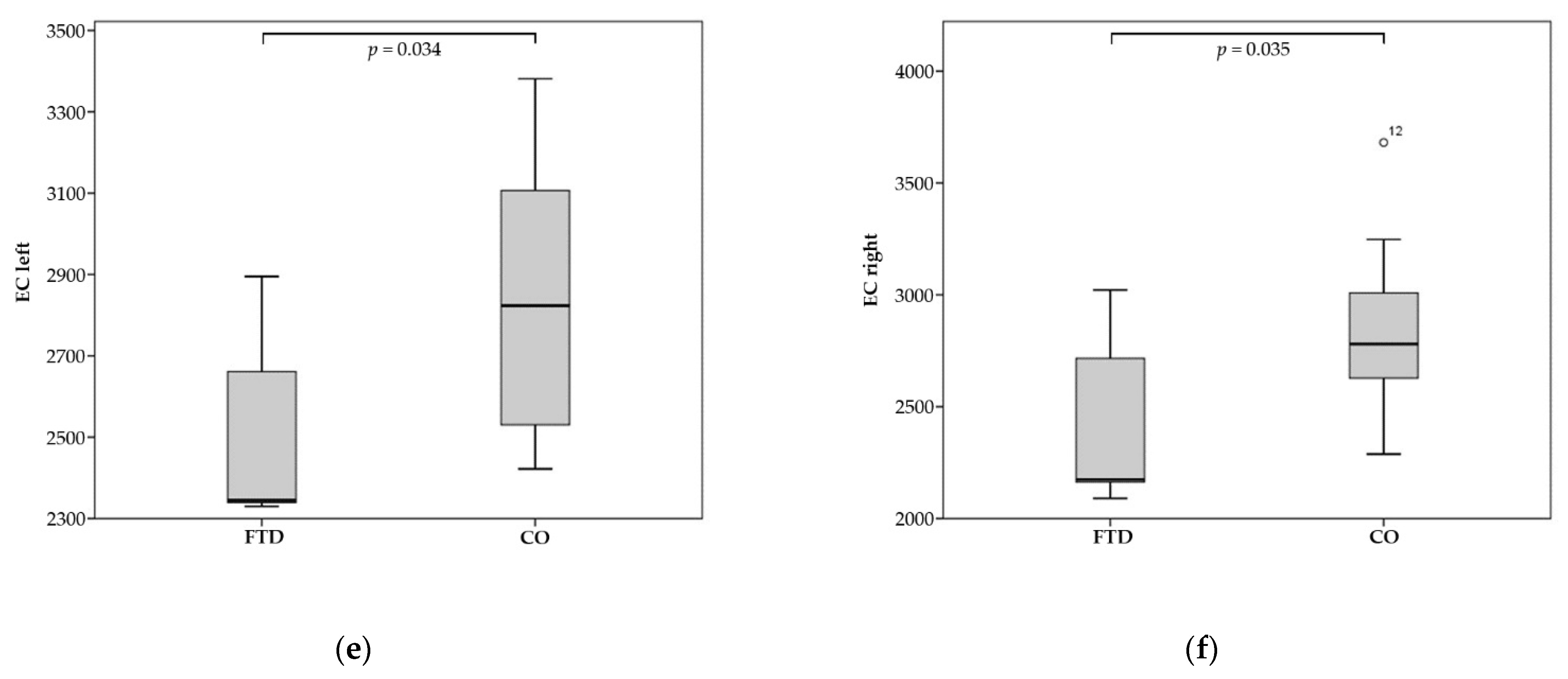
| EC left | 2514.0 | 2862.1 | 0.034 |
| EC right | 2433.0 | 2821.2 | 0.035 |
| IFO left | 1534.8 | 1765.8 | 0.024 |
| IFO right | 1561.2 | 1787.8 | 0.024 |
| SS right | 3210.8 | 4012.9 | 0.009 |
| CI right | 264.2 | 374.7 | 0.003 |
| Basal Forebrain left | 462.6 | 536.5 | 0.044 |
| IFWMO left | 1818.8 | 2283.6 | 0.008 |
| IFWMO right | 1543.2 | 1881.6 | 0.010 |
| Caudate-tail left | 273.4 | 338.1 | 0.030 |
| Caudate-tail right | 253.8 | 347.0 | 0.022 |
| Fimbria right | 16.4 | 36.5 | 0.011 |
| Brain Region of Interest | Mean Volume in MCI (in mm3) | Mean Volume in CO (in mm3) | Level of Significance (p) |
|---|---|---|---|
| SFG-pole left | 2000.3 | 2290.2 | 0.039 |
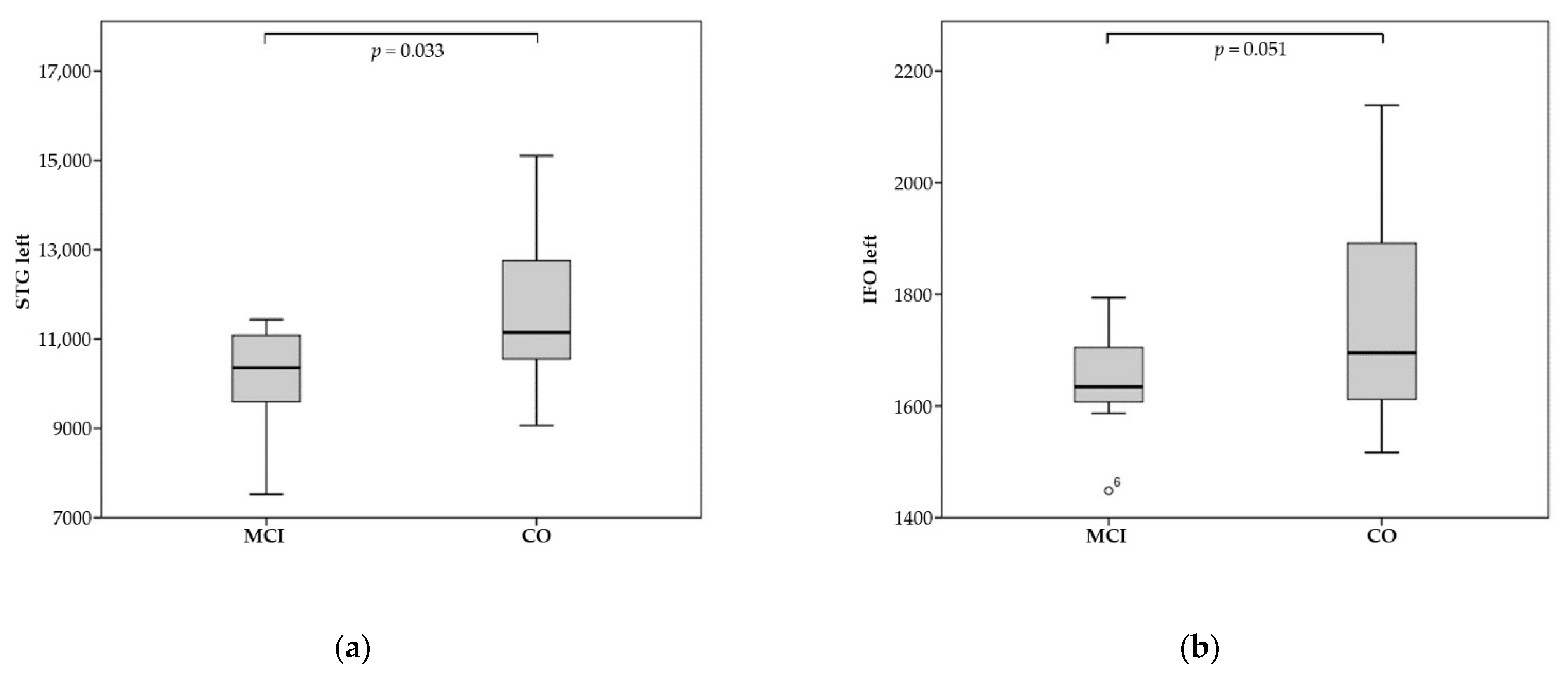
| STG left | 10,094.4 | 11,653.5 | 0.033 |
| STG right | 9364.7 | 10,913.4 | 0.009 |
| MTG right | 12,041.7 | 13,891.3 | 0.030 |
| MTG-pole left | 2108.6 | 2460.1 | 0.048 |
| PHG right | 1059.1 | 1208.4 | 0.038 |
| Hippocampus right | 3187.7 | 3510.2 | 0.016 |
| Basal Forebrain left | 240.9 | 277.9 | 0.059 |
| Basal Forebrain right | 225.1 | 277.2 | 0.006 |
| SCP left | 736.0 | 831.8 | 0.011 |
| Pons left | 247.1 | 284.9 | 0.029 |
| IFO left | 1642.7 | 1765.8 | 0.051 |
| Mammillary left | 80.9 | 100.0 | 0.040 |
| LFOWM right | 1523.4 | 1683.6 | 0.002 |
| RGWM left | 1556.4 | 1787.8 | 0.052 |
| STWM right | 7752.7 | 8730.2 | 0.050 |
| rostralWM-ACC left | 65.1 | 141.2 | 0.002 |
| subcallosalWM-ACC right | 60.9 | 88.9 | 0.044 |
| Brain Region of Interest | Mean Volume in AD (in mm3) | Mean Volume in CO (in mm3) | Level of Significance (p) |
|---|---|---|---|
| STG right | 9516.6 | 10,913.4 | 0.056 |
| SOG right | 1916.4 | 2465.6 | 0.008 |
| Hippocampus right | 2873.0 | 3510.2 | 0.025 |
| CGH right | 1182.6 | 1456.6 | 0.036 |
| MTWM right | 7245.6 | 8682.2 | 0.045 |
| Caudate-tail left | 259.4 | 338.1 | 0.013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marterstock, D.C.; Knott, M.F.X.; Hoelter, P.; Lang, S.; Oberstein, T.; Kornhuber, J.; Doerfler, A.; Schmidt, M.A. Pulsed Arterial Spin Labeling and Segmented Brain Volumetry in the Diagnostic Evaluation of Frontotemporal Dementia, Alzheimer’s Disease and Mild Cognitive Impairment. Tomography 2022, 8, 229-244. https://doi.org/10.3390/tomography8010018
Marterstock DC, Knott MFX, Hoelter P, Lang S, Oberstein T, Kornhuber J, Doerfler A, Schmidt MA. Pulsed Arterial Spin Labeling and Segmented Brain Volumetry in the Diagnostic Evaluation of Frontotemporal Dementia, Alzheimer’s Disease and Mild Cognitive Impairment. Tomography. 2022; 8(1):229-244. https://doi.org/10.3390/tomography8010018
Chicago/Turabian StyleMarterstock, Dominique Cornelius, Michael Franz Xaver Knott, Philip Hoelter, Stefan Lang, Timo Oberstein, Johannes Kornhuber, Arnd Doerfler, and Manuel A. Schmidt. 2022. "Pulsed Arterial Spin Labeling and Segmented Brain Volumetry in the Diagnostic Evaluation of Frontotemporal Dementia, Alzheimer’s Disease and Mild Cognitive Impairment" Tomography 8, no. 1: 229-244. https://doi.org/10.3390/tomography8010018
APA StyleMarterstock, D. C., Knott, M. F. X., Hoelter, P., Lang, S., Oberstein, T., Kornhuber, J., Doerfler, A., & Schmidt, M. A. (2022). Pulsed Arterial Spin Labeling and Segmented Brain Volumetry in the Diagnostic Evaluation of Frontotemporal Dementia, Alzheimer’s Disease and Mild Cognitive Impairment. Tomography, 8(1), 229-244. https://doi.org/10.3390/tomography8010018






