Characterization of Cardiopulmonary Interactions and Exploring Their Prognostic Value in Acute Bronchiolitis: A Prospective Cardiopulmonary Ultrasound Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design, Setting and Study Population
2.2. Research Intervention
2.2.1. Pulmonary Status Assessment
2.2.2. Cardiac Status Assessment
2.3. Follow-Up, Data Collection and Outcome Measures
2.4. Statistics
3. Results
3.1. Baseline Characteristics
3.2. Cardiopulmonary Interactions in Bronchiolitis
3.3. Relationship of Cardiac and Respiratory Variables with a Severe Course of the Disease
4. Discussion
4.1. Pulmonary Status in Acute Bronchiolitis
4.2. Cardiac Status in Acute Bronchiolitis
4.3. Cardiopulmonary Interactions in Acute Bronchiolitis
4.4. Predictive Accuracy of Cardiopulmonary Ultrasound for Adverse Outcomes in Acute Bronchiolitis
4.5. An Integrative Approach for Severity Assessment of Acute Bronchiolitis
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bozzola, E.; Ciarlitto, C.; Guolo, S.; Brusco, C.; Cerone, G.; Antilici, L.; Schettini, L.; Piscitelli, A.L.; Vittucci, A.C.; Cutrera, R.; et al. Respiratory Syncytial Virus Bronchiolitis in Infancy: The Acute Hospitalization Cost. Front. Pediatr. 2021, 8, 594898. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, L.J.; Straney, L.; Gelbart, B.; Alexander, J.; Franklin, D.; Beca, J.; Whitty, J.A.; Ganu, S.; Wilkins, B.; Slater, A.; et al. Burden of disease and change in practice in critically ill infants with bronchiolitis. Eur. Respir. J. 2017, 49, 1601648. [Google Scholar] [CrossRef]
- Pinsky, M.R. Cardiopulmonary Interactions: Physiologic Basis and Clinical Applications. Ann. Am. Thorac. Soc. 2018, 15, S45–S48. [Google Scholar] [CrossRef] [PubMed]
- Bronicki, R.A. Cardiopulmonary Interactions in Children with Heart Failure. Curr. Cardiol. Rev. 2016, 12, 104–106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siraj, S.; Stark, W.; McKinley, S.D.; Morrison, J.M.; Sochet, A.A. The bronchiolitis severity score: An assessment of face validity, construct validity, and interobserver reliability. Pediatr. Pulmonol. 2021, 56, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Basile, V.; Di Mauro, A.; Scalini, E.; Comes, P.; Lofù, I.; Mostert, M.; Tafuri, S.; Manzionna, M.M. Lung ultrasound: A useful tool in diagnosis and management of bronchiolitis. BMC Pediatr. 2015, 15, 63. [Google Scholar] [CrossRef]
- La Regina, D.P.; Bloise, S.; Pepino, D.; Iovine, E.; Laudisa, M.; Cristiani, L.; Nicolai, A.; Nenna, R.; Mancino, E.; Di Mattia, G.; et al. Lung ultrasound in bronchiolitis. Pediatr. Pulmonol. 2021, 56, 234–239. [Google Scholar] [CrossRef]
- Di Mauro, A.; Ammirabile, A.; Quercia, M.; Panza, R.; Capozza, M.; Manzionna, M.M.; Laforgia, N.; Mauro, D. Acute Bronchiolitis: Is There a Role for Lung Ultrasound? Diagnostics 2019, 9, 172. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, M.; Perez-Reviriego, A.A.; Castellano-Martinez, A.; Cascales-Poyatos, H.M. The Assessment of Myocardial Strain by Cardiac Imaging in Healthy Infants with Acute Bronchiolitis: A Systematic Review and Meta-Analysis. Diagnostics 2020, 10, 382. [Google Scholar] [CrossRef]
- Le Coz, J.; Orlandini, S.; Titomanlio, L.; Rinaldi, V.E. Point of care ultrasonography in the pediatric emergency department. Ital. J. Pediatr. 2018, 44, 87. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Karakitsos, D. Integrating lung ultrasound in the hemodynamic evaluation of acute circulatory failure (the fluid administration limited by lung sonography protocol). J. Crit. Care 2012, 27, 533.e11–533.e19. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; De Rose, C.; Ferro, V.; Morello, R.; Musolino, A.; Valentini, P. Lung Ultrasound to detect cardiopulmonary interactions in acutely ill children. Pediatr. Pulmonol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S.L.; Lieberthal, A.S.; Meissner, H.C.; Alverson, B.K.; Baley, J.E.; Gadomski, A.M.; Johnson, D.W.; Light, M.J.; Maraqa, N.F.; Mendonca, E.A.; et al. Clinical Practice Guideline: The Diagnosis, Management, and Prevention of Bronchiolitis. Pediatrics 2014, 134, e1474–e1502. [Google Scholar] [CrossRef]
- Balaguer, M.; Alejandre, C.; Vila, D.; Esteban, E.; Carrasco, J.L.; Cambra, F.J.; Jordan, I. Bronchiolitis Score of Sant Joan de Déu: Brosjod Score, validation and usefulness. Pediatr. Pulmonol. 2017, 52, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef]
- Bueno, M.; Sainz, T.; Alba, M.; Del Rosal, T.; Mendez-Echevarría, A.; Echevarria, R.; Tagarro, A.; Ruperez-Lucas, M.; Herrreros, M.L.; Latorre, L.; et al. Lung ultrasound for prediction of respiratory support in infants with acute bronchiolitis: A cohort study. Pediatr. Pulmonol. 2019, 54, 873–880. [Google Scholar] [CrossRef]
- Caiulo, V.A.; Gargani, L.; Caiulo, S.; Fisicaro, A.; Moramarco, F.; Latini, G.; Picano, E. Lung ultrasound in bronchiolitis: Comparison with chest X-ray. Eur. J. Pediatr. 2011, 170, 1427–1433. [Google Scholar] [CrossRef]
- Nir, A.; Lindinger, A.; Rauh, M.; Bar-Oz, B.; Laer, S.; Schwachtgen, L.; Koch, A.; Falkenberg, J.; Mir, T.S. NT-Pro-B-type Natriuretic Peptide in Infants and Children: Reference Values Based on Combined Data from Four Studies. Pediatr. Cardiol. 2009, 30, 3–8. [Google Scholar] [CrossRef]
- Lai, W.W.; Geva, T.; Shirali, G.S.; Frommelt, P.C.; Humes, R.A.; Brook, M.M.; Pignatelli, R.H.; Rychik, J. Guidelines and Standards for Performance of a Pediatric Echocardiogram: A Report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2006, 19, 1413–1430. [Google Scholar] [CrossRef]
- Koestenberger, M.; Apitz, C.; Abdul-Khaliq, H.; Hansmann, G. Transthoracic echocardiography for the evaluation of children and adolescents with suspected or confirmed pulmonary hypertension. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart 2016, 102, ii14–ii22. [Google Scholar] [CrossRef] [PubMed]
- Koestenberger, M.; Ravekes, W.; Everett, A.D.; Stueger, H.P.; Heinzl, B.; Gamillscheg, A.; Cvirn, G.; Boysen, A.; Fandl, A.; Nagel, B. Right Ventricular Function in Infants, Children and Adolescents: Reference Values of the Tricuspid Annular Plane Systolic Excursion (TAPSE) in 640 Healthy Patients and Calculation of z Score Values. J. Am. Soc. Echocardiogr. 2009, 22, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Roberson, D.A. Left Ventricular Tei Index in Children: Comparison of Tissue Doppler Imaging, Pulsed Wave Doppler, and M-Mode Echocardiography Normal Values. J. Am. Soc. Echocardiogr. 2006, 19, 1438–1445. [Google Scholar] [CrossRef]
- Abraham, S.; Weismann, C.G. Left Ventricular End-Systolic Eccentricity Index for Assessment of Pulmonary Hypertension in Infants. Echocardiography 2016, 33, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Liu, X.; Jiang, Q.; Jiao, A.; Jiang, Y. Pulmonary function in infants with respiratory syncytial virus bronchiolitis. Zhonghua Yi Xue Za Zhi 2002, 82, 182–185. [Google Scholar] [PubMed]
- Qi, Y.-Y.; Jiang, G.-L.; Wang, L.-B.; Wan, C.-Z.; Zhang, X.-B.; Qian, L.-L. Lung Function in Wheezing Infants after Acute Lower Respiratory Tract Infection and Its Association with Respiratory Outcome. Chin. Med. J. 2017, 130, 4–10. [Google Scholar] [CrossRef]
- Willson, D.F.; Landrigan, C.P.; Horn, S.D.; Smout, R.J. Complications in infants hospitalized for bronchiolitis or respiratory syncytial virus pneumonia. J. Pediatr. 2003, 143, 142–149. [Google Scholar] [CrossRef]
- Caplow, J.; McBride, S.C.; Steil, G.M.; Wong, J. Changes in cardiac output and stroke volume as measured by non-invasive CO monitoring in infants with RSV bronchiolitis. J. Clin. Monit. 2012, 26, 197–205. [Google Scholar] [CrossRef]
- Rodríguez-González, M.; Estepa-Pedregosa, L.; Estalella-Mendoza, A.; Castellano-Martínez, A.; Rodríguez-Campoy, P.; Flores-González, J.C. Early elevated NT-proBNP but not troponin I is associated with severe bronchiolitis in infants. Clin. Chim. Acta 2021, 518, 173–179. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, M.; Perez-Reviriego, A.A.; Castellano-Martinez, A.; Lubian-Lopez, S.; Benavente-Fernandez, I. Left Ventricular Dysfunction and Plasmatic NT-proBNP Are Associated with Adverse Evolution in Respiratory Syncytial Virus Bronchiolitis. Diagnostics 2019, 9, 85. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, M.; Benavente-Fernandez, I.; Castellano-Martinez, A.; Lechuga-Sancho, A.; Lubian-Lopez, S.P. NT-proBNP plasma levels as biomarkers for pulmonary hypertension in healthy infants with respiratory syncytial virus infection. Biomarkers Med. 2019, 13, 605–618. [Google Scholar] [CrossRef]
- Thorburn, K.; Eisenhut, M.; Shauq, A.; Narayanswamy, S.; Burgess, M. Right ventricular function in children with severe respiratory syncytial virus (RSV) bronchiolitis. Minerva Anestesiol. 2011, 77, 46–53. [Google Scholar]
- Eisenhut, M.; Sidaras, D.; Johnson, R.; Newland, P.; Thorburn, K. Cardiac troponin T levels and myocardial involvement in children with severe respiratory syncytial virus lung disease. Acta Paediatr. 2004, 93, 887–890. [Google Scholar] [CrossRef]
- Massolo, A.C.; Cantone, G.V.; Musolino, A.M.C.; Corsini, I.; Patel, N.; Evangelisti, M.; Monaco, F.; Villa, M.P.; Braguglia, A. Myocardial strain on admission predicts disease severity in infants hospitalized for bronchiolitis. Pediatr. Pulmonol. 2020, 55, 1217–1223. [Google Scholar] [CrossRef]
- Kimura, D.; Saravia, J.; Jaligama, S.; McNamara, I.; Vu, L.D.; Sullivan, R.D.; Mancarella, S.; You, D.; Cormier, S.A. New mouse model of pulmonary hypertension induced by respiratory syncytial virus bronchiolitis. Am. J. Physiol. Circ. Physiol. 2018, 315, H581–H589. [Google Scholar] [CrossRef] [PubMed]
- Vu, L.D.; Saravia, J.; Jaligama, S.; Panday, R.V.B.; Sullivan, R.D.; Mancarella, S.; Cormier, S.A.; Kimura, D. Deficiency in ST2 signaling ameliorates RSV-associated pulmonary hypertension. Am. J. Physiol. Circ. Physiol. 2021, 321, H309–H317. [Google Scholar] [CrossRef]
- Burkett, D.A.; Slorach, C.; Patel, S.S.; Redington, A.N.; Ivy, D.D.; Mertens, L.; Younoszai, A.K.; Friedberg, M.K. Impact of Pulmonary Hemodynamics and Ventricular Interdependence on Left Ventricular Diastolic Function in Children With Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2016, 9, e004612. [Google Scholar] [CrossRef] [PubMed]
- Driessen, M.M.P.; Hui, W.; Bijnens, B.H.; Dragulescu, A.; Mertens, L.; Meijboom, F.J.; Friedberg, M.K. Adverse ventricular–ventricular interactions in right ventricular pressure load: Insights from pediatric pulmonary hypertension versus pulmonary stenosis. Physiol. Rep. 2016, 4, e12833. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.S.; Pinsky, M.R. Heart-lung interactions during mechanical ventilation: The basics. Ann. Transl. Med. 2018, 6, 349. [Google Scholar] [CrossRef] [PubMed]
- Kimura, D.; McNamara, I.F.; Wang, J.; Fowke, J.H.; West, A.N.; Philip, R. Pulmonary hypertension during respiratory syncytial virus bronchiolitis: A risk factor for severity of illness. Cardiol. Young 2019, 29, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Habash, S.; Laser, K.T.; Moosmann, J.; Reif, R.; Adler, W.; Glöckler, M.; Kececioglu, D.; Dittrich, S. Normal values of the pulmonary artery acceleration time (PAAT) and the right ventricular ejection time (RVET) in children and adolescents and the impact of the PAAT/RVET-index in the assessment of pulmonary hypertension. Int. J. Cardiovasc. Imaging 2019, 35, 295–306. [Google Scholar] [CrossRef]
- Ord, H.L.; Griksaitis, M.J. Fifteen-minute consultation: Using point of care ultrasound to assess children with respiratory failure. Arch. Dis. Child.-Educ. Pract. Ed. 2019, 104, 2–10. [Google Scholar] [CrossRef]
- Musolino, A.M.; Buonsenso, D.; Massolo, A.C.; Gallo, M.; Supino, M.C.; Boccuzzi, E. Point of care ultrasound in the paediatric acute care setting: Getting to the ‘heart’ of respiratory distress. J. Paediatr. Child Health 2021, 57, 318–322. [Google Scholar] [CrossRef]
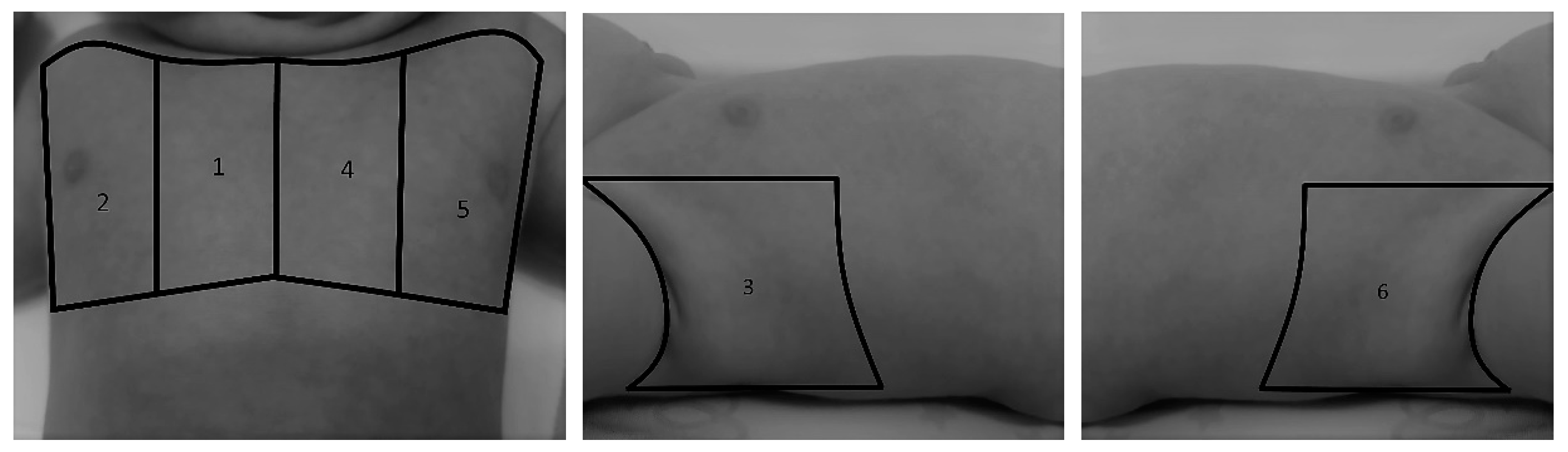

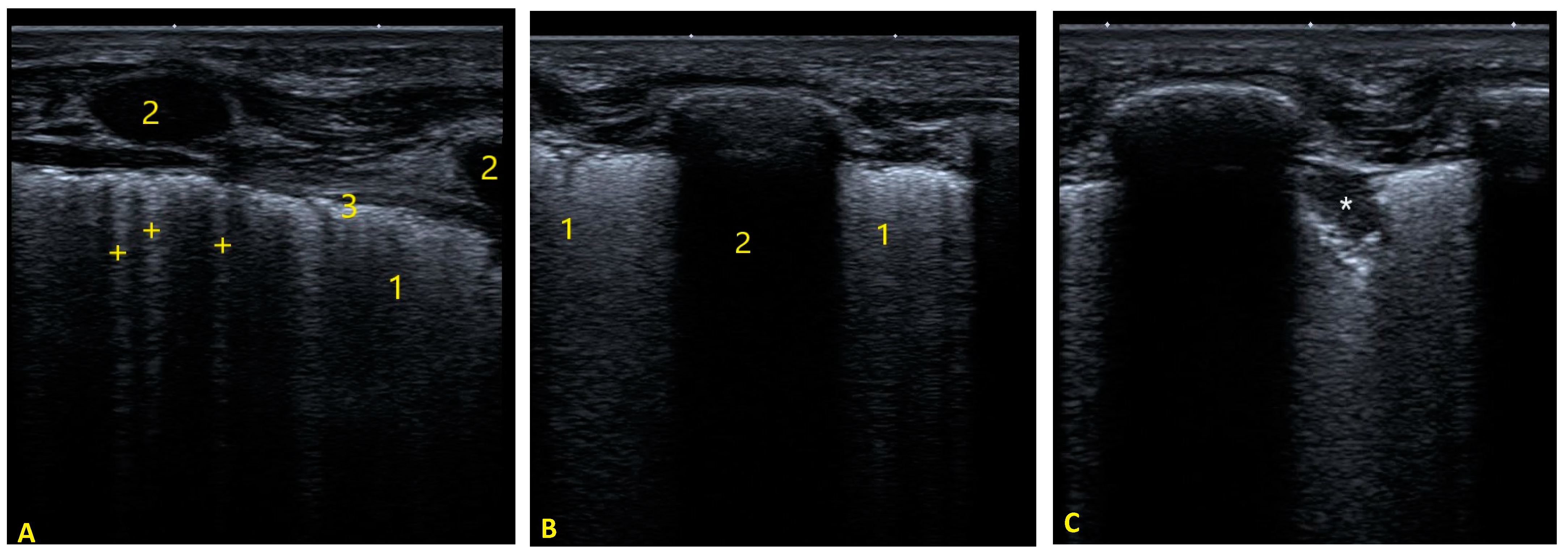

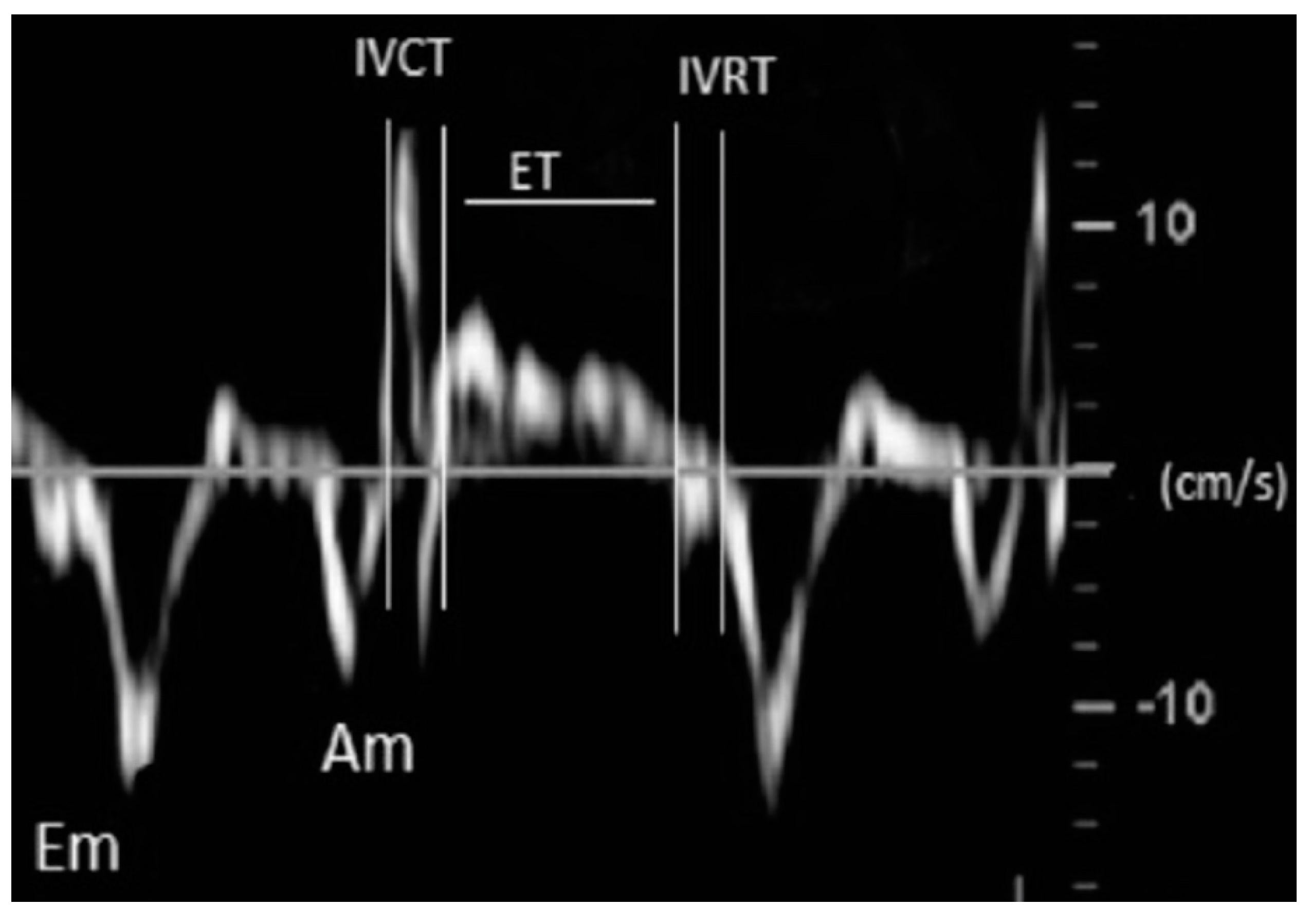
| Echo Parameter | Cardiac Parameter Evaluated | Abnormal Value | Significance |
|---|---|---|---|
| LVEF | LV systolic function | <50% | Myocardial dysfunction |
| TAPSE | RV systolic function | <2 SD for BSA | Myocardial dysfunction |
| LVTX | LV global (systolic and diastolic) function | >0.50 | Myocardial dysfunction |
| RVTX | RV global (systolic and diastolic) function | >0.50 | Myocardial dysfunction |
| TRJG | Pulmonary pressures | >40 mmHg | Pulmonary Hypertension |
| ATET | Pulmonary pressures | <0.29 | Pulmonary Hypertension |
| LVEI | RV systolic pressure | >1.3 | Pulmonary Hypertension |
| RVLV | RV dimension | >0.6 | Dilated RV |
| Patient Number 112 | Results |
|---|---|
| Age (months) * | 1 (0.5–3) |
| Weight (kg) * | 4.6 (3.7–5.8) |
| Gender (male) ^ | 62 (55) |
| Time from onset of symptoms to ED evaluation (days) * | 1 (0–2) |
| RSV positive ^ | 86 (77) |
| Nebulized therapies ^ | 63 (58) |
| Oxygen therapy (nasal canulae) ^ | 56 (50) |
| Duration (days) * | 2 (1–4) |
| HFNC ^ | 3 (2.5) |
| Duration (days) * | 1 (1–2) |
| PICU admission ^ | 36 (32) |
| PICU stay (days) * | 6 (4–9) |
| CPAP/BiPAP ^ | 28 (25) |
| Duration (days) * | 3 (2–5) |
| MV ^ | 6 (5) |
| Duration (days) * | 11 (10–11) |
| LOS hospitalization (days) * | 5 (2–10) |
| Inotropic support | 0 (0) |
| Death or sequel ^ | 0 (0) |
| Patient Number (112) | N (%) |
|---|---|
| Respiratory variables | |
| BROSJOD score > 10 points | 24 (21) |
| pH < 7.30 | 30 (26) |
| pCO2 > 55 cmH20 | 51 (45) |
| LUS > 10 points | 25 (22) |
| Cardiac variables | |
| NT-proBNP > 1121 pg/mL | 57 (51) |
| Myocardial function | |
| LVEF < 50% | 0 (0) |
| TAPSE < 2 Z-score for BSA | 4 (3.5) |
| LVTX > 0.5 | 26 (23) |
| RVTX > 0.5 | 25 (22) |
| Pulmonary pressures | |
| TRJG < 40 mmHg (n = 50) | 11 (22) |
| ATET < 0.29 | 31 (27) |
| LVEI > 1.3 | 31 (27) |
| RVLV > 0.6 | 39 (35) |
| Cardiac Variables | BROSJOD Score | pCO2 | LUS Score | |||
|---|---|---|---|---|---|---|
| CC | p-Value | CC | p-Value | CC | p-Value | |
| NT-proBNP (pg/mL) * | 0.37 | 0.001 | 0.48 | 0.000 | 0.46 | 0.000 |
| LVEF (%) * | 0.01 | Ns. | −0.05 | Ns. | −0.05 | Ns. |
| LVTX ** | 0.25 | 0.006 | 0.39 | 0.000 | 0.33 | 0.000 |
| TAPSE * | −0.10 | Ns. | −0.18 | Ns. | −0.19 | Ns. |
| RVTX * | 0.35 | 0.000 | 0.44 | 0.000 | 0.37 | 0.000 |
| TRJG (mmHg) *, n = 50 | 0.36 | 0.010 | 0.56 | 0.000 | 0.34 | 0.014 |
| ATET * | −0.37 | 0.001 | −0.43 | 0.000 | −0.34 | 0.000 |
| LVEI * | 0.38 | 0.000 | 0.48 | 0.000 | 0.45 | 0.000 |
| RVLV * | 0.26 | 0.005 | 0.30 | 0.002 | 0.32 | 0.000 |
| Variable | Respiratory Support (n = 36; 32%) | No Respiratory Support (n = 76; 68%) | p-Value |
|---|---|---|---|
| Demographic and outcome data | |||
| Age (months) * | 1 (0.5–2.5) | 1 (0.5–3) | Ns. |
| Weight (kg) * | 4.3 (3.5–5.8) | 4.8 (4–6.2) | Ns. |
| Gender (male) ^ | 19 (53) | 43 (56) | Ns. |
| Time of symptoms previous admission (days) * | 1 (0–2) | 1 (0–2) | Ns. |
| RSV positive ^ | 32 (89) | 54 (71) | Ns. |
| Pulmonary assessment | |||
| BROSJOD score (points) * | 11 (9–13) | 6 (4–8) | 0.000 |
| pH ^** | 7.28 (0.07) | 7.34 (0.05) | 0.000 |
| pCO2 (cmH2O) * | 62 (47–80) | 47 (42–54) | 0.000 |
| LUS (points) * | 10 (5–13) | 4 (1–6) | 0.000 |
| Cardiac assessment | |||
| NT-proBNP (pg/mL) * | 3949 (2249–6042) | 722 (309–1461) | 0.000 |
| LVEF (%) ** | 39.7 (2.4) | 39.6 (2.6) | Ns. |
| LVTX ** | 0.60 (0.17) | 0.41 (0.10) | 0.000 |
| TAPSE ** | 14 (2.5) | 15 (2.2) | Ns. |
| RVTX ** | 0.59 (0.12) | 0.42 (0.10) | 0.000 |
| TRJG (mmHg) **, n 50 | 38 (14) | 25 (9.5) | 0.000 |
| ATET ** | 0.28 (0.05) | 0.37 (0.06) | 0.000 |
| LVEI ** | 1.36 (0.21) | 1.1 (0.13) | 0.000 |
| RVLV ** | 0.62 (0.12) | 0.49 (0.10) | 0.000 |
| Cardiac and Respiratory Variables | LOS Hospitalization | PICU Stay | Duration of RS | |||
|---|---|---|---|---|---|---|
| CC | p-Value | CC | p-Value | CC | p-Value | |
| BROSJOD score * | 0.55 | <0.001 | −0.08 | Ns. | 0.47 | <0.001 |
| pH * | −0.34 | <0.001 | −0.21 | Ns. | −0.23 | 0.021 |
| pCO2 * | 0.42 | <0.001 | 0.03 | Ns. | 0.29 | 0.004 |
| LUS * | 0.58 | <0.001 | 0.06 | Ns. | 0.02 | Ns. |
| NT-proBNP * | 0.60 | <0.001 | 0.14 | Ns. | 0.50 | <0.001 |
| LVEF * | 0.01 | Ns. | −0.05 | Ns. | −0.05 | Ns. |
| LVTX * | 0.49 | <0.001 | 0.17 | Ns. | 0.40 | <0.001 |
| TAPSE * | −0.10 | Ns. | −0.18 | Ns. | −0.19 | Ns. |
| RVTX * | 0.58 | <0.001 | 0.23 | Ns. | 0.42 | <0.001 |
| TRJG, n = 50 * | 0.48 | <0.001 | −0.13 | Ns. | 0.31 | 0.024 |
| ATET * | −0.52 | <0.001 | −0.18 | Ns. | −0.45 | <0.001 |
| LVEI * | 0.58 | <0.001 | 0.20 | Ns. | 0.44 | <0.001 |
| RVLV * | 0.54 | <0.001 | 0.21 | Ns. | 0.38 | <0.001 |
| Predictive Variables | OR (95% CI) | AUC (95% CI) | S | Sp | PPV | NPV |
|---|---|---|---|---|---|---|
| BROSJOD > 10 points | 28 (12–125) | 0.87 (0.78–0.93) | 0.91 | 0.61 | 0.91 | 0.76 |
| pH < 7.30 | 3.5 (1.5–8.5) | 0.72 (0.61–0.83) | 0.44 | 0.81 | 0.53 | 0.75 |
| pCO2 > 55 cmH2O | 4.3 (2–10) | 0.74 (0.62–0.86) | 0.69 | 0.65 | 0.49 | 0.82 |
| LUS > 10 points | 6 (2.3–15) | 0.77 (0.67–0.85) | 0.44 | 0.88 | 0.64 | 0.77 |
| NT-proBNP > 1121 pg/mL | 9 (3.3–24) | 0.83 (0.73–0.91) | 0.85 | 0.71 | 0.55 | 0.90 |
| LVTX > 0.5 | 8.5 (3–22) | 0.80 (0.69–0.89) | 0.50 | 0.89 | 0.70 | 0.79 |
| RVTX > 0.5 | 18 (5.8–54) | 0.84 (0.76–0.92) | 0.55 | 0.93 | 0.80 | 0.81 |
| LVEI > 1.3 | 15 (5.5–40) | 0.84 (0.75–0.92) | 0.64 | 0.89 | 0.74 | 0.84 |
| ATET < 0.29 | 7.4 (3–18) | 0.83 (0.75–0.91) | 0.55 | 0.85 | 0.65 | 0.80 |
| RVLV > 0.6 | 8.4 (3.3–21) | 0.77 (0.68–0.86) | 0.61 | 0.84 | 0.64 | 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Gonzalez, M.; Rodriguez-Campoy, P.; Estalella-Mendoza, A.; Castellano-Martinez, A.; Flores-Gonzalez, J.C. Characterization of Cardiopulmonary Interactions and Exploring Their Prognostic Value in Acute Bronchiolitis: A Prospective Cardiopulmonary Ultrasound Study. Tomography 2022, 8, 142-157. https://doi.org/10.3390/tomography8010012
Rodriguez-Gonzalez M, Rodriguez-Campoy P, Estalella-Mendoza A, Castellano-Martinez A, Flores-Gonzalez JC. Characterization of Cardiopulmonary Interactions and Exploring Their Prognostic Value in Acute Bronchiolitis: A Prospective Cardiopulmonary Ultrasound Study. Tomography. 2022; 8(1):142-157. https://doi.org/10.3390/tomography8010012
Chicago/Turabian StyleRodriguez-Gonzalez, Moises, Patricia Rodriguez-Campoy, Ana Estalella-Mendoza, Ana Castellano-Martinez, and Jose Carlos Flores-Gonzalez. 2022. "Characterization of Cardiopulmonary Interactions and Exploring Their Prognostic Value in Acute Bronchiolitis: A Prospective Cardiopulmonary Ultrasound Study" Tomography 8, no. 1: 142-157. https://doi.org/10.3390/tomography8010012
APA StyleRodriguez-Gonzalez, M., Rodriguez-Campoy, P., Estalella-Mendoza, A., Castellano-Martinez, A., & Flores-Gonzalez, J. C. (2022). Characterization of Cardiopulmonary Interactions and Exploring Their Prognostic Value in Acute Bronchiolitis: A Prospective Cardiopulmonary Ultrasound Study. Tomography, 8(1), 142-157. https://doi.org/10.3390/tomography8010012






