The Combination of Stent and Antiplatelet Therapy May Be Responsible of Parenchymal Magnetic Susceptibility Artifacts after Endovascular Procedure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Antiplatelet and Anticoagulation Therapy
2.3. Imaging and Clinical Follow-up
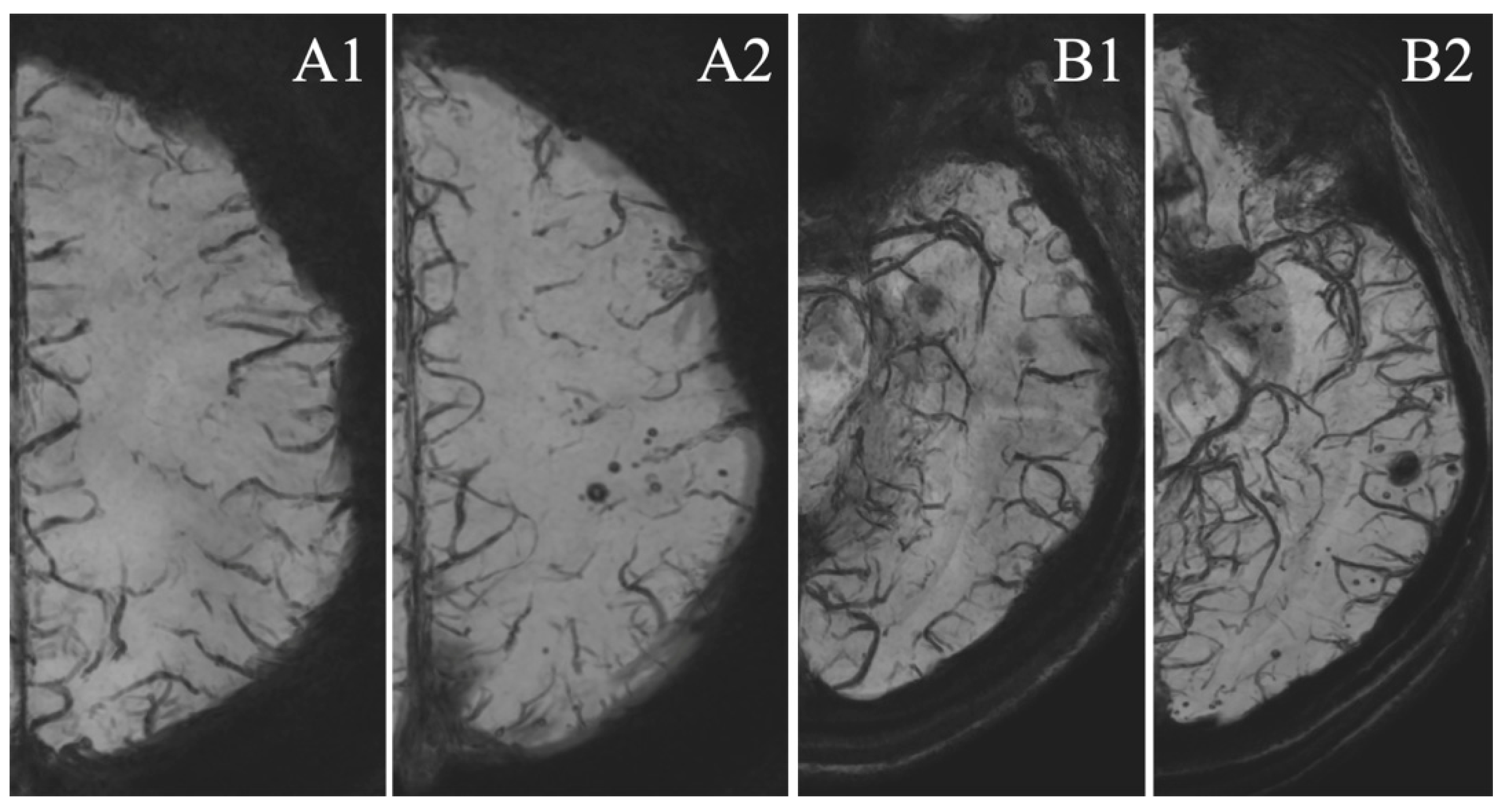
2.4. MRI Sequences
2.5. Reading
2.6. Statistics
3. Results
3.1. Population and Follow-Up
3.2. Interobserver Agreement
3.3. Clinical Outcome
3.4. MSA Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Molyneux, A.J.; Kerr, R.S.; Yu, L.M.; Clarke, M.; Sneade, M.; Yarnold, J.A.; Sandercock, P. International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005, 366, 809–817. [Google Scholar]
- Johnston, S.C.; Dowd, C.F.; Higashida, R.T.; Lawton, M.T.; Duckwiler, G.R.; Gress, D.R. CARAT Investigators: Predictors of rehemorrhage after treatment of ruptured intracranial aneurysms: The Cerebral Aneurysm Rerupture After Treatment (CARAT) study. Stroke 2008, 39, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Naidech, A.M.; Janjua, N.; Kreiter, K.T.; Ostapkovich, N.D.; Fitzsimmons, B.F.; Parra, A.; Commichau, C.; Connolly, E.S.; Mayer, S.A. Predictors and impact of aneurysm rebleeding after subarachnoid hemorrhage. Arch. Neurol. 2005, 62, 410–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalouhi, N.; Tjoumakaris, S.; Starke, R.M.; Gonzalez, L.F.; Randazzo, C.; Hasan, D.; McMahon, J.F.; Singhal, S.; Moukarzel, L.A.; Dumont, A.S.; et al. Comparison of flow diversion and coiling in large unruptured intracranial saccular aneurysms. Stroke 2013, 44, 2150–2154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çinar, C.; Bozkaya, H.; Oran, I. Endovascular treatment of cranial aneurysms with the pipeline flow-diverting stent: Preliminary mid-term results. Endovascular treatment of cranial aneurysms with the pipeline flow-diverting stent: Preliminary mid-term results. Diagn. Interv. Radiol. 2013, 19, 154–164. [Google Scholar] [PubMed]
- Fischer, S.; Vajda, Z.; Perez, M.A.; Schmid, E.; Hopf, N.; Bäzner, H.; Henkes, H. Pipeline embolization device (PED) for neurovascular reconstruction: Initial experience in the treatment of 101 intracranial aneurysms and dissections. Neuroradiology 2012, 4, 369–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becske, T.; Kallmes, D.F.; Saatci, I.; McDougall, C.G.; Szikora, I.; Lanzino, G.; Moran, C.J.; Woo, H.H.; Lopes, D.K.; Berez, A.L.; et al. Pipeline for uncoilable or failed aneurysms: Results from a multicenter clinical trial. Radiology 2013, 267, 858–868. [Google Scholar] [CrossRef]
- Nelson, P.K.; Lylyk, P.; Szikora, I.; Wetzel, S.G.; Wanke, I.; Fiorella, D. The pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am. J. Neuroradiol. 2011, 32, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Klisch, J.; Turk, A.; Turner, R.; Woo, H.H.; Fiorella, D. Very late thrombosis of flow-diverting constructs after the treatment of large fusiform posterior circulation aneurysms. AJNR Am. J. Neuroradiol. 2011, 32, 627–632. [Google Scholar] [CrossRef] [Green Version]
- Szikora, I.; Berentei, Z.; Kulcsar, Z.; Marosfoi, M.; Vajda, Z.S.; Lee, W.; Berez, A.; Nelson, P.K. Treatment of intracranial aneurysms by functional reconstruction of the parent artery: The Budapest experience with the pipeline embolization device. AJNR Am. J. Neuroradiol. 2010, 31, 1139–1147. [Google Scholar] [CrossRef] [Green Version]
- Leung, G.K.; Tsang, A.C.; Lui, W.M. Pipeline embolization device for intracranial aneurysm: A systematic review. Clin. Neuroradiol. 2012, 22, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Chalouhi, N.; Zanaty, M.; Jabbour, P.M.; Starke, R.M.; Tjoumakaris, S.I.; Rosenwasser, R.H.; Gonzalez, L.F. Intracerebral hemorrhage after pipeline embolization: Management of antiplatelet agents and the case for point-of-care testing—Case reports and review of literature. Clin. Neurol. Neurosurg. 2014, 124, 21–24. [Google Scholar] [CrossRef]
- Brinjikji, W.; Lanzino, G.; Cloft, H.J.; Siddiqui, A.H.; Kallmes, D.F. Risk Factors for Hemorrhagic Complications following Pipeline Embolization Device Treatment of Intracranial Aneurysms: Results from the International Retrospective Study of the Pipeline Embolization Device. AJNR Am. J. Neuroradiol. 2015, 36, 2308–2313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouchaud, A.; Brinjikji, W.; Lanzino, G.; Cloft, H.J.; Kadirvel, R.; Kallmes, D.F. Delayed hemorrhagic complications after flow diversion for intracranial aneurysms: A literature overview. Neuroradiology 2016, 58, 171–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakae, R.; Nagaishi, M.; Kawamura, Y.; Tanaka, Y.; Hyodo, A.; Suzuki, K. Microhemorrhagic transformation of ischemic lesions on T2*-weighted magnetic resonance imaging after Pipeline embolization device treatment. J. Neurosurg. 2018, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, B.J.; Memon, S.; Hope, J.K. Prospective Study of Early MRI Appearances following Flow-Diverting Stent Placement for Intracranial Aneurysms. AJNR Am. J. Neuroradiol. 2015, 36, 943–948. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.S.; Chan, Y.L.; Liu, J.Y.; Gao, S.; Lam, W.W. Asymptomatic microbleeds as a risk factor for aspirin-associated intracerebral hemorrhages. Neurology 2003, 60, 511–538. [Google Scholar] [CrossRef]
- Vernooij, M.W.; Haag, M.D.; van der Lugt, A.; Hofman, A.; Krestin, G.P.; Stricker, B.H.; Breteler, M.M. Use of antithrombotic drugs and the presence of cerebral microbleeds: The Rotterdam Scan Study. Arch. Neurol. 2009, 66, 714–720. [Google Scholar] [CrossRef] [Green Version]
- Lauer, A.; van Veluw, S.J.; William, C.M.; Charidimou, A.; Roongpiboonsopit, D.; Vashkevich, A.; Ayres, A.; Martinez-Ramirez, S.; Gurol, E.M.; Biessels, G.J.; et al. Microbleeds on MRI are associated with microinfarcts on autopsy in cerebral amyloid angiopathy. Neurology 2016, 87, 1488–1492. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Ye, H.; Wang, J.; Wang, J.; Wang, Y. Antiplatelet Therapy, Cerebral Microbleeds, and Intracerebral Hemorrhage: A Meta-Analysis. Stroke 2018, 49, 1751–1754. [Google Scholar] [CrossRef]
- Shams, S.; Martola, J.; Cavallin, L.; Granberg, T.; Shams, M.; Aspelin, P.; Wahlund, L.O.; Kristoffersen-Wiberg, M. SWI or T2*: Which MRI sequence to use in the detection of cerebral microbleeds? The Karolinska Imaging Dementia Study. AJNR Am. J. Neuroradiol. 2015, 36, 1089–1095. [Google Scholar] [CrossRef] [Green Version]
- Hahnemann, M.L.; Ringelstein, A.; Sandalcioglu, I.E.; Goericke, S.; Moenninghoff, C.; Wanke, I.; Forsting, M.; Sure, U.; Schlamann, M. Silent embolism after stent-assisted coiling of cerebral aneurysms: Diffusion-weighted MRI study of 75 cases. J. Neurointerv. Surg. 2014, 6, 461–465. [Google Scholar] [CrossRef]
- Brooks, N.P.; Turk, A.S.; Niemann, D.B.; Aagaard-Kienitz, B.; Pulfer, K.; Cook, T. Frequency of thromboembolic events associated with endovascular aneurysm treatment: Retrospective case series. J. Neurosurg. 2008, 108, 1095–1100. [Google Scholar] [CrossRef] [Green Version]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Johns, G.S.; Taussky, P.; Tawk, R.G.; Miller, D.A.; Freeman, W.D.; Hanel, R.A. Clopidogrel Resistance by P2Y12 Platelet Function Testing in Patients Undergoing Neuroendovascular Procedures Incidence of Ischemic and Hemorrhagic Complications. J. Vasc. Interv. Neurol. 2013, 6, 26–34. [Google Scholar]
- Lindholm, D.; Varenhorst, C.; Cannon, C.P.; Harrington, R.A.; Himmelmann, A.; Maya, J.; Husted, S.; Steg, P.G.; Cornel, J.H.; Storey, R.F.; et al. Ticagrelor vs. clopidogrel in patients with non-ST-elevation acute coronary syndrome with or without revascularization: Results from the PLATO trial. Eur. Heart J. 2014, 35, 2083–2093. [Google Scholar] [CrossRef]
- Heller, R.S.; Dandamudi, V.; Lanfranchi, M.; Malek, A.M. Effect of antiplatelet therapy on thromboembolism after flow diversion with the pipeline embolization device. J. Neurosurg. 2013, 119, 1603–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, J.P.; Chow, M.; O’Kelly, C.; Marotta, B.; Spears, J.; Montanera, W.; Fiorella, D.; Marotta, T. Delayed ipsilateral parenchymal hemorrhage following flow diversion for the treatment of anterior circulation aneurysms. AJNR Am. J. Neuroradiol. 2012, 33, 603–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, A.H.; Wenderoth, J. Cerebral hyperperfusion after flow diversion of large intracranial aneurysms. J. Neurointerv. Surg. 2013, 5, e48. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.C.; Deshmukh, V.R.; Albuquerque, F.C.; Fiorella, D.; Nixon, R.R.; Heck, D.V.; Barnwell, S.L.; McDougall, C.G. Histopathological assessment of fatal ipsilateral intraparenchymal hemorrhages after the treatment of supraclinoid aneurysms with the Pipeline Embolization Device. J. Neurosurg. 2014, 120, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Poels, M.M.; Vernooij, M.W.; Ikram, M.A.; Hofman, A.; Krestin, G.P.; van der Lugt, A.; Breteler, M.M. Prevalence and risk factors of cerebral microbleeds: An update of the Rotterdam scan study. Stroke 2010, 41, S103–S106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristics | S-Group (n = 46) | C-Group (n = 46) | p-Value |
|---|---|---|---|
| Age (years) | 53.22 [49.69; 56.74] | 55 [51.36; 58.64] | 0.480 |
| Sex (female) | 33 (71.74%) | 33 (71.74%) | 1.000 |
| History of SAH | 20 (43.47%) | 36 (78.26%) | 0.001 |
| Localization | |||
| Anterior complex | 4 (8.70%) | 4 (8.70%) | 0.003 |
| Carotid and MCA | 36 (78.26%) | 22 (47.82%) | |
| Posterior circulation | 6 (13.04%) | 20 (43.48%) | |
| Flow diversion | 23 (50%) | - | |
| Dual antiplatelet therapy duration (days) | 185.1 [172;16; 182.07] | - | |
| Details of the imaging survey | |||
| Number of scan (pretreatment and FU) | 3.57 [3.32; 3.82] | 4.80 [4.56; 5.04] | <0.001 |
| Mean FU duration (days) | 940.19 [919.04; 961.35] | 1095.54 [1094.33; 1096.75] | <0.001 |
| number of M3-6 FU | 46 (100%) | 46 (100%) | |
| number of M12 FU | 40 (86.96%) | 46 (100%) | |
| number of M24 FU | 37 (80.43%) | 46 (100%) | |
| number of M36 FU | 46 (100%) | 46 (100%) |
| S-Group (n = 46) | Non-FD Stent (n = 23) | FD Stent (n = 23) | C-Group (n = 46) | p-Value | |
|---|---|---|---|---|---|
| Mean number of MSA | 8.76 [5.76; 11.76] | 0.78 [0.32; 1.25] | <0.001 | ||
| 3.77 [2.09; 5.45] | 7.78 [3.10; 12.46] | 0.022 | |||
| Appearance of MSA | 36 (78.26%) | 18 (21.74%) | <0.001 | ||
| 19 (82.61%) | 17 (73.91%) | 0.722 | |||
| Territorial appearance of MSA | 34 (94.44%) | 15 (83.33%) | 0.319 | ||
| 18 (94.74%) | 16 (94.11%) | 1.000 |
| MSA Appearance: Multivariate Analysis * | ||||
|---|---|---|---|---|
| OR | 95% CI | p | ||
| S-group | 20.98 | 5.24 | 83.95 | <0.001 |
| Age | 1.04 | 0.98 | 1.11 | 0.165 |
| Sex | 1.68 | 0.47 | 5.98 | 0.423 |
| Follow-up duration | 1.00 | 0.99 | 1.00 | 0.081 |
| Localization | 1.97 | 0.68 | 5.71 | 0.211 |
| Previous Sub-Arachnoid Hemorrhage | 1.36 | 0.34 | 5.37 | 0.662 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourhis-Guizien, F.; Dissaux, B.; Boulouis, G.; Ben Salem, D.; Gentric, J.-C.; Ognard, J. The Combination of Stent and Antiplatelet Therapy May Be Responsible of Parenchymal Magnetic Susceptibility Artifacts after Endovascular Procedure. Tomography 2021, 7, 792-800. https://doi.org/10.3390/tomography7040066
Bourhis-Guizien F, Dissaux B, Boulouis G, Ben Salem D, Gentric J-C, Ognard J. The Combination of Stent and Antiplatelet Therapy May Be Responsible of Parenchymal Magnetic Susceptibility Artifacts after Endovascular Procedure. Tomography. 2021; 7(4):792-800. https://doi.org/10.3390/tomography7040066
Chicago/Turabian StyleBourhis-Guizien, Fanny, Brieg Dissaux, Grégoire Boulouis, Douraied Ben Salem, Jean-Christophe Gentric, and Julien Ognard. 2021. "The Combination of Stent and Antiplatelet Therapy May Be Responsible of Parenchymal Magnetic Susceptibility Artifacts after Endovascular Procedure" Tomography 7, no. 4: 792-800. https://doi.org/10.3390/tomography7040066
APA StyleBourhis-Guizien, F., Dissaux, B., Boulouis, G., Ben Salem, D., Gentric, J.-C., & Ognard, J. (2021). The Combination of Stent and Antiplatelet Therapy May Be Responsible of Parenchymal Magnetic Susceptibility Artifacts after Endovascular Procedure. Tomography, 7(4), 792-800. https://doi.org/10.3390/tomography7040066








