The Predictive Role of Artificial Intelligence-Based Chest CT Quantification in Patients with COVID-19 Pneumonia
Abstract
:1. Introduction
2. Materials and Methods
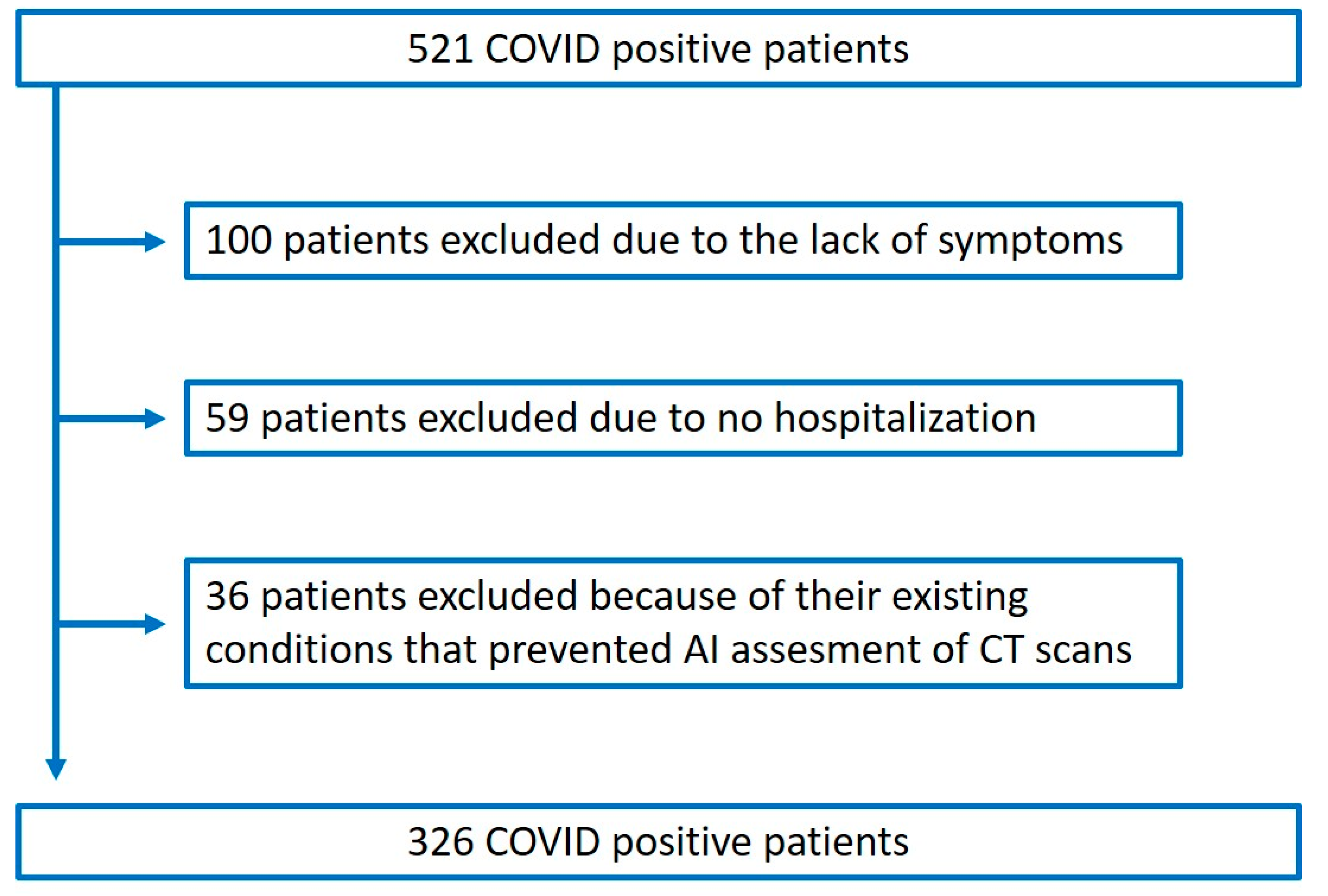
2.1. Patient Selection and Data Collection
2.2. Outcome Definition
2.3. CT Acquisition Protocol and Image Reconstruction
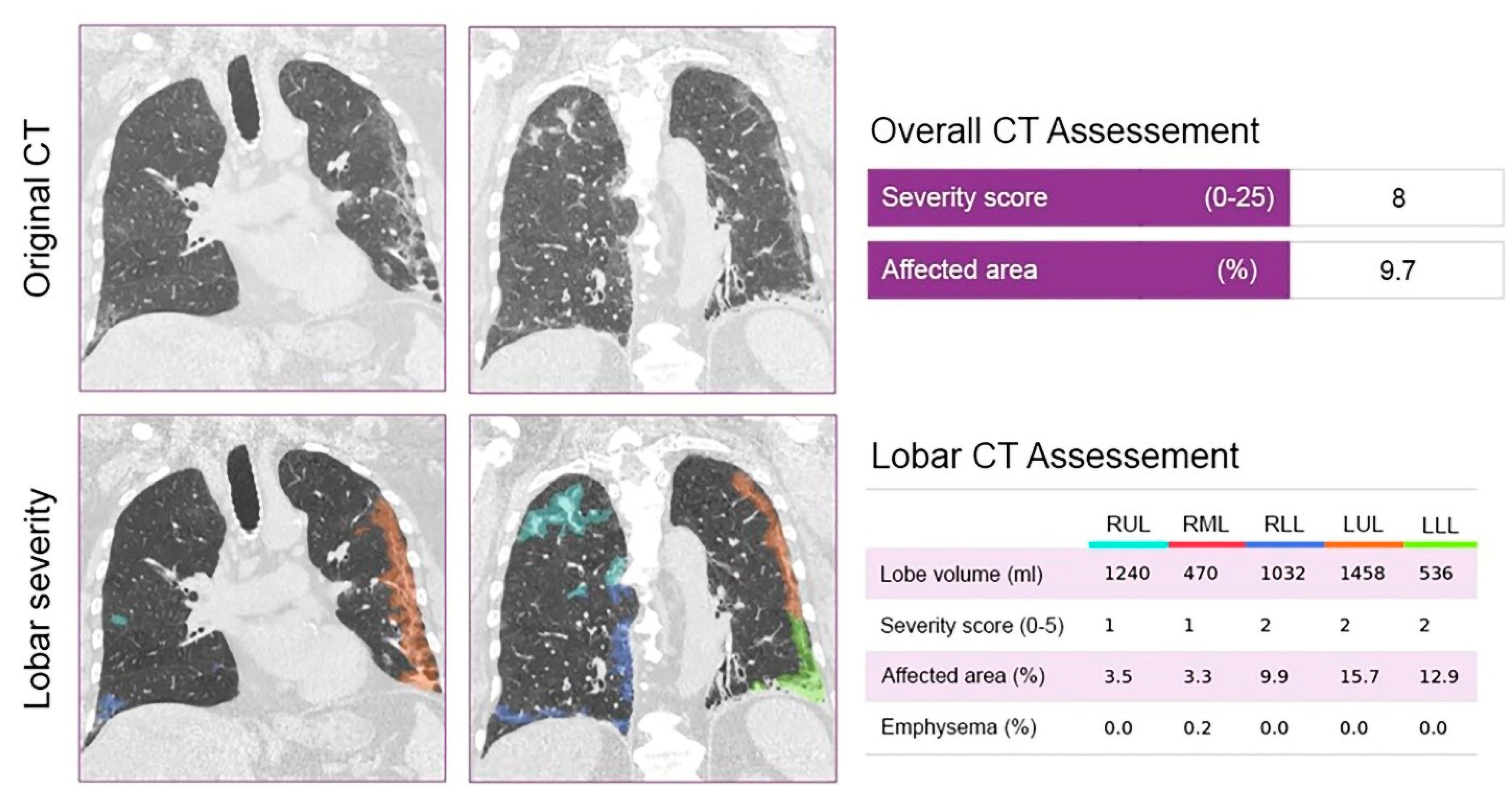
2.4. CT Image Analysis
2.5. Statistical Analysis
2.6. Ethical Approval
3. Results
3.1. Patient Characteristics and Symptoms
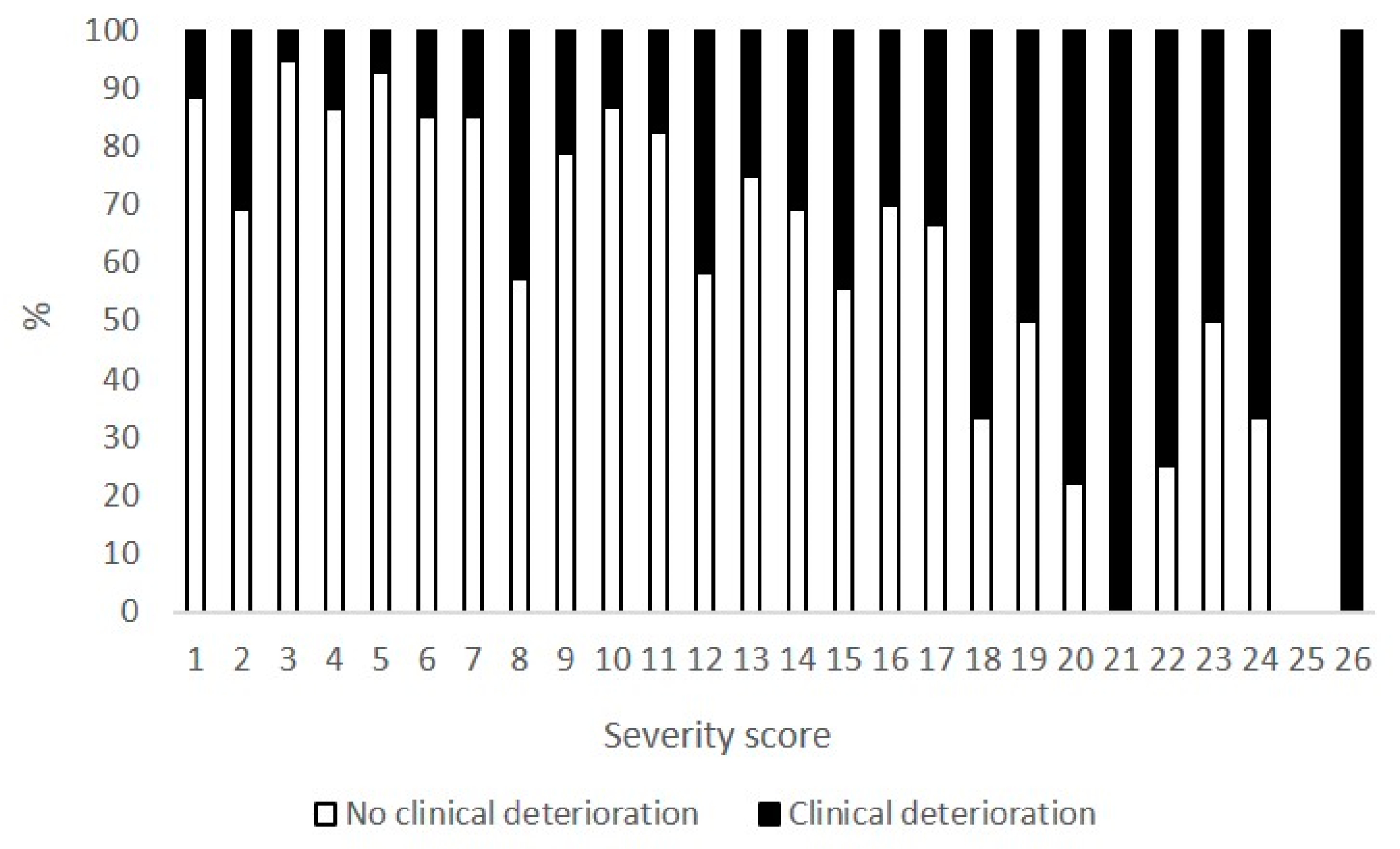
3.2. AI-Based CT Quantification
3.3. Predictors of Adverse Outcome
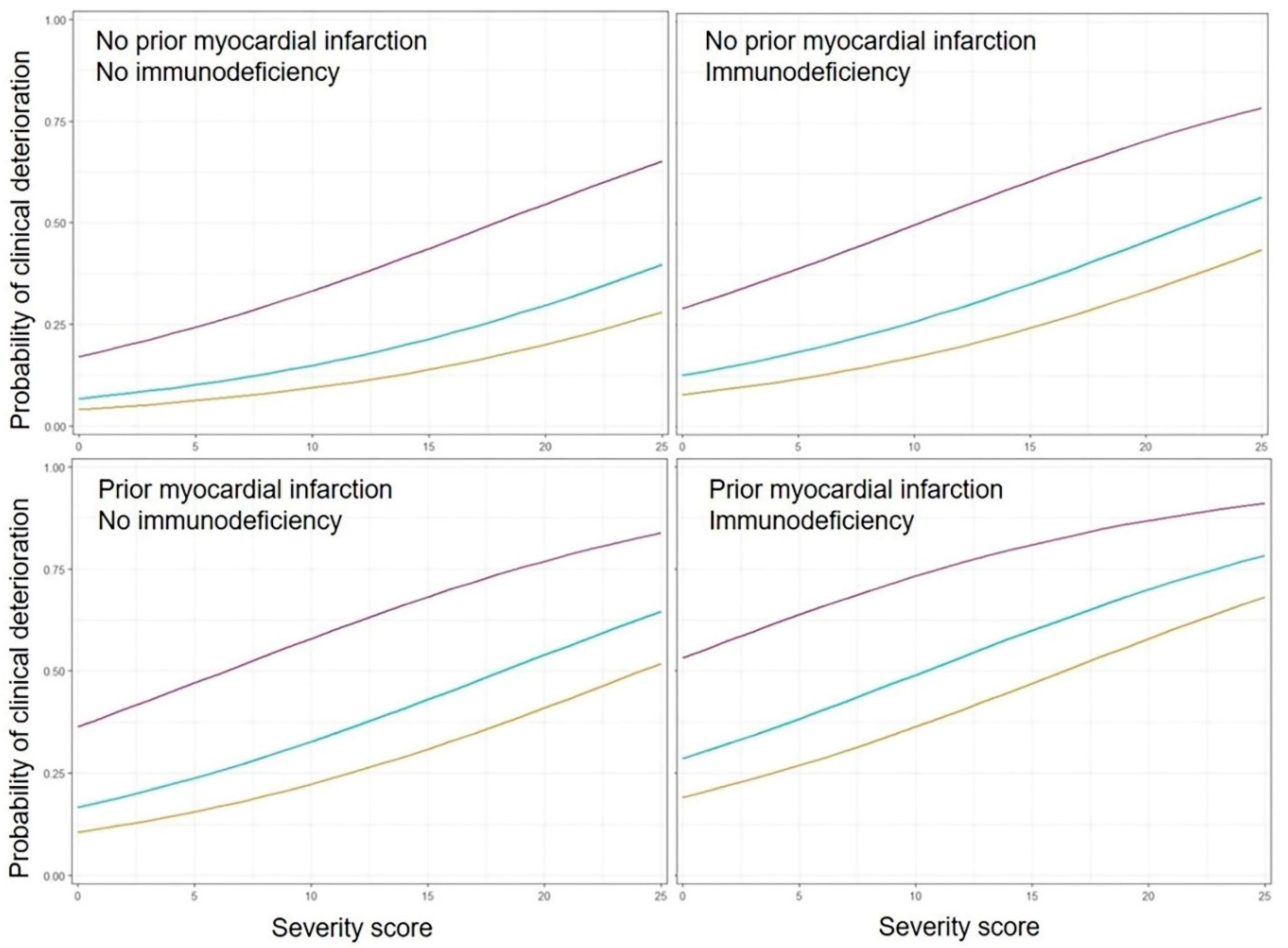
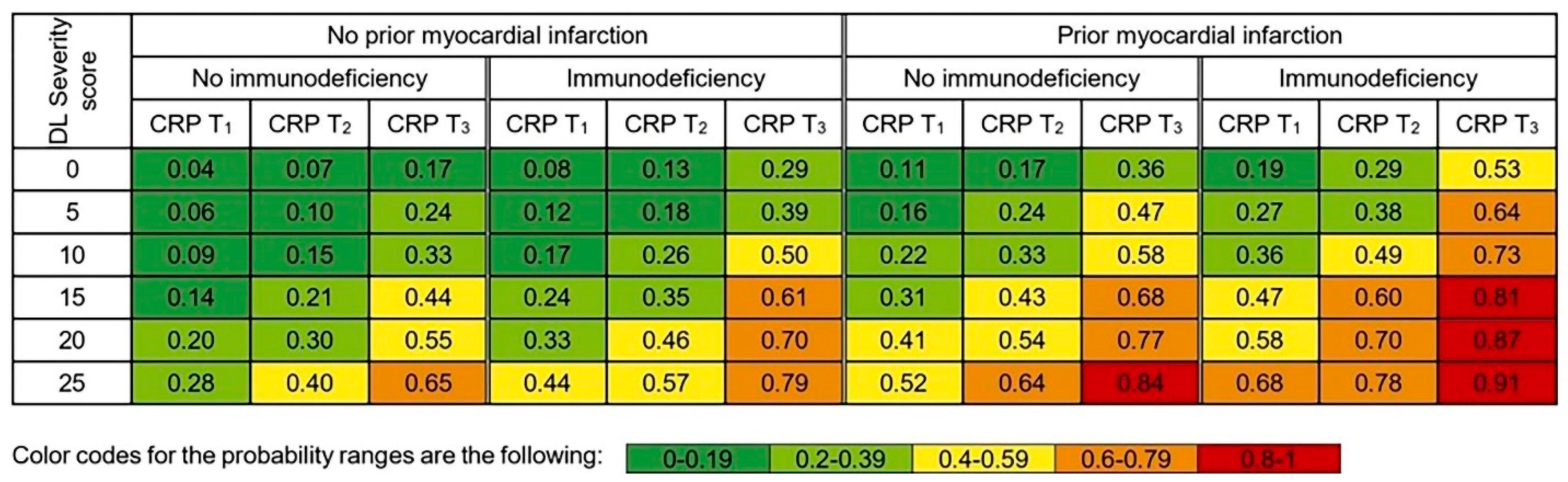
3.4. Personalized Risk Probabilities
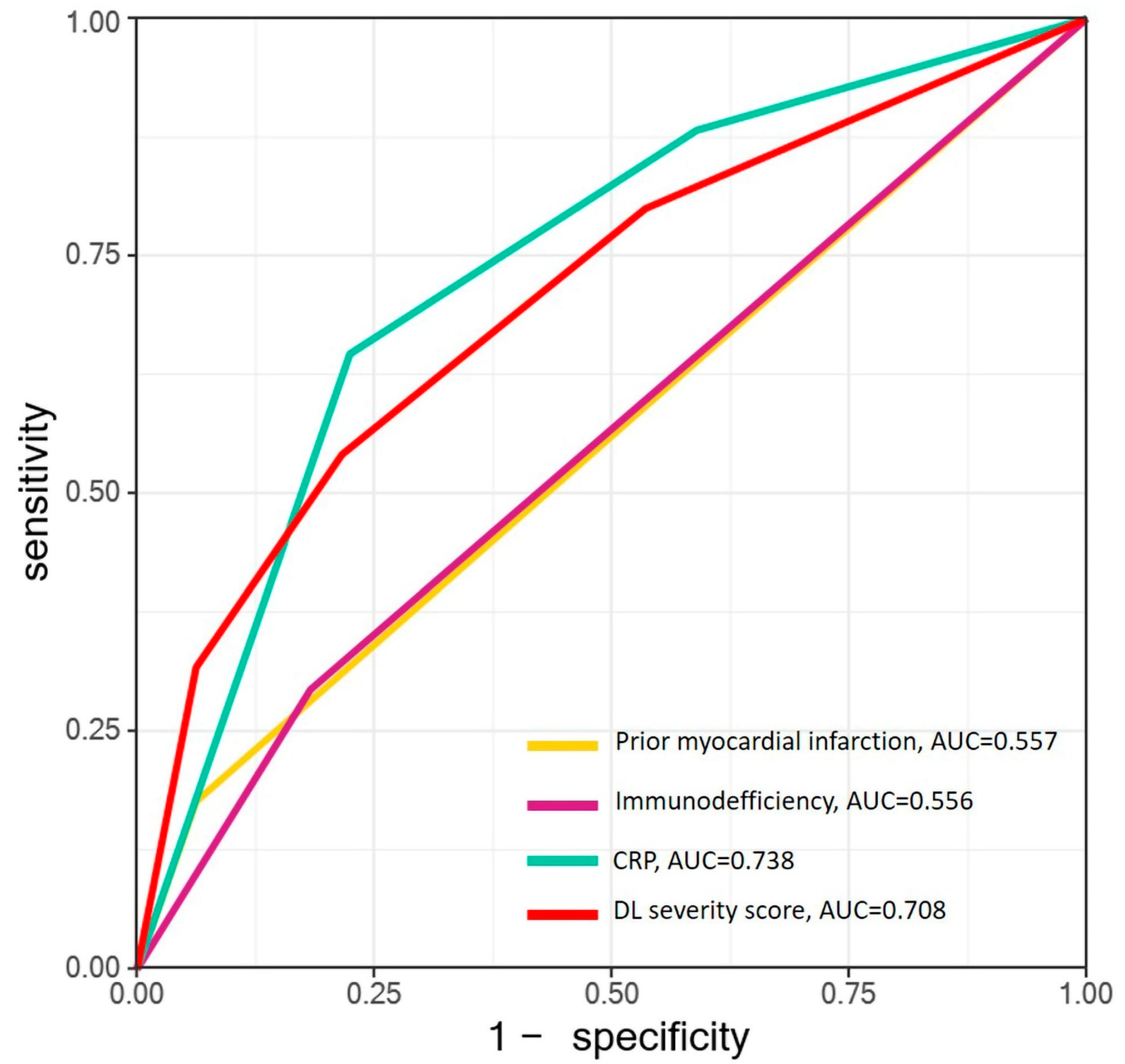
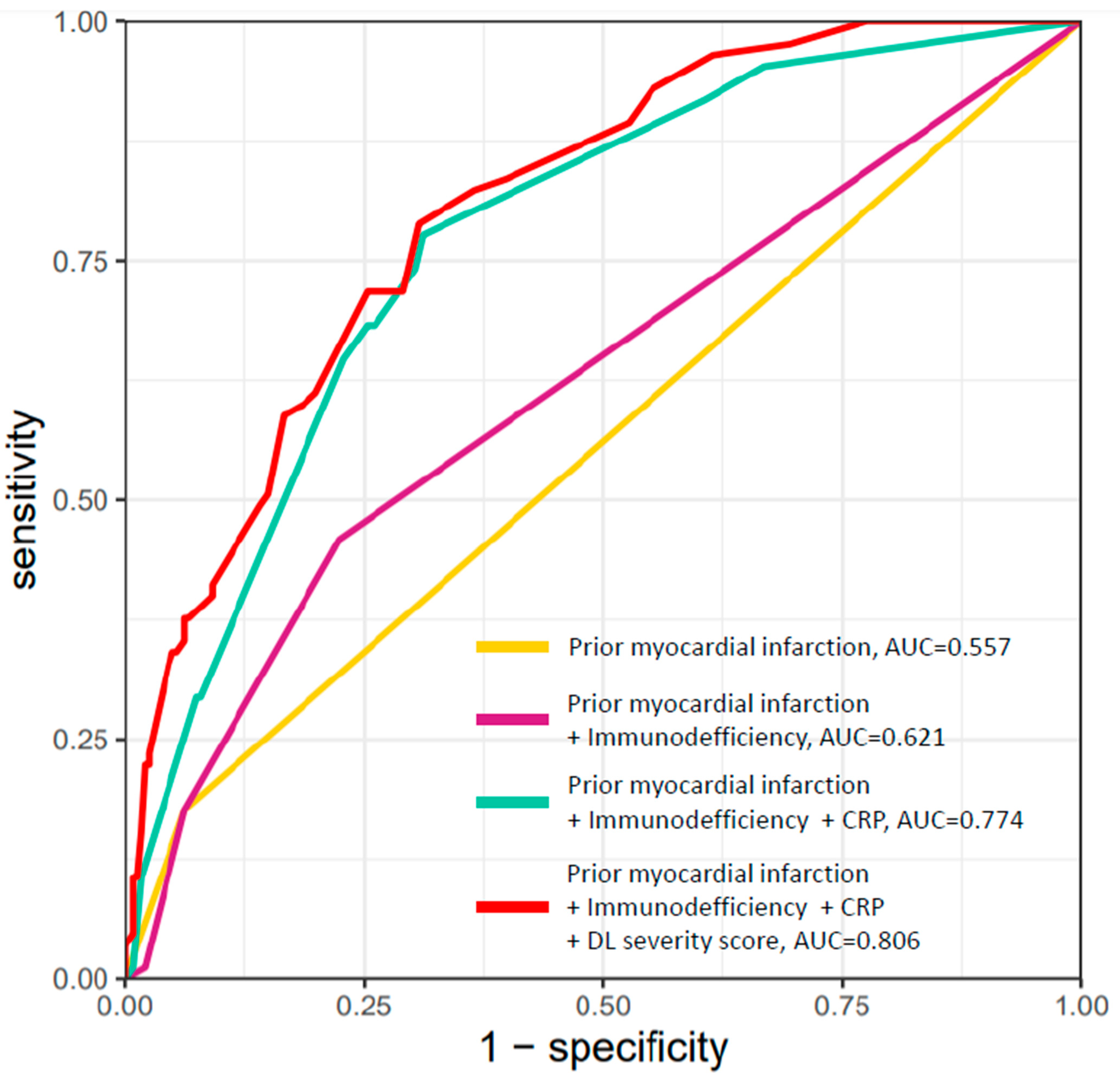
3.5. Receiver Operating Characteristic (ROC) Curves
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Kang, S.J.; Jung, S.I. Age-Related Morbidity and Mortality among Patients with COVID-19. Infect. Chemother. 2020, 52, 154–164. [Google Scholar] [CrossRef]
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Merkely, B.; Szabo, A.J.; Kosztin, A.; Berenyi, E.; Sebestyen, A.; Lengyel, C.; Merkely, G.; Karady, J.; Varkonyi, I.; Papp, C.; et al. Novel coronavirus epidemic in the Hungarian population, a cross-sectional nationwide survey to support the exit policy in Hungary. Geroscience 2020, 42, 1063–1074. [Google Scholar] [CrossRef]
- Abbasi, B.; Akhavan, R.; Ghamari Khameneh, A.; Zandi, B.; Farrokh, D.; Pezeshki Rad, M.; Feyzi Laein, A.; Darvish, A.; Bijan, B. Evaluation of the relationship between inpatient COVID-19 mortality and chest CT severity score. Am. J. Emerg. Med. 2021, 45, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Ozel, M.; Aslan, A.; Arac, S. Use of the COVID-19 Reporting and Data System (CO-RADS) classification and chest computed tomography involvement score (CT-IS) in COVID-19 pneumonia. Radiol. Med. 2021, 126, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Lessmann, N.; Sanchez, C.I.; Beenen, L.; Boulogne, L.H.; Brink, M.; Calli, E.; Charbonnier, J.P.; Dofferhoff, T.; van Everdingen, W.M.; Gerke, P.K.; et al. Automated Assessment of COVID-19 Reporting and Data System and Chest CT Severity Scores in Patients Suspected of Having COVID-19 Using Artificial Intelligence. Radiology 2021, 298, E18–E28. [Google Scholar] [CrossRef]
- Luo, X.; Zhou, W.; Yan, X.; Guo, T.; Wang, B.; Xia, H.; Ye, L.; Xiong, J.; Jiang, Z.; Liu, Y.; et al. Prognostic Value of C-Reactive Protein in Patients With Coronavirus 2019. Clin. Infect. Dis. 2020, 71, 2174–2179. [Google Scholar] [CrossRef]
- Wang, G.; Wu, C.; Zhang, Q.; Wu, F.; Yu, B.; Lv, J.; Li, Y.; Li, T.; Zhang, S.; Wu, C.; et al. C-Reactive Protein Level May Predict the Risk of COVID-19 Aggravation. Open Forum Infect. Dis. 2020, 7, ofaa153. [Google Scholar] [CrossRef]
- Wang, L. C-reactive protein levels in the early stage of COVID-19. Med. Mal. Infect. 2020, 50, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, S.R.; Ho, A.; Pius, R.; Buchan, I.; Carson, G.; Drake, T.M.; Dunning, J.; Fairfield, C.J.; Gamble, C.; Green, C.A.; et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ 2020, 370, m3339. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Covassin, N.; Fan, Z.; Singh, P.; Gao, W.; Li, G.; Kara, T.; Somers, V.K. Association Between Hypoxemia and Mortality in Patients With COVID-19. Mayo Clin. Proc. 2020, 95, 1138–1147. [Google Scholar] [CrossRef]
- Wang, X.; Fang, X.; Cai, Z.; Wu, X.; Gao, X.; Min, J.; Wang, F. Comorbid Chronic Diseases and Acute Organ Injuries Are Strongly Correlated with Disease Severity and Mortality among COVID-19 Patients: A Systemic Review and Meta-Analysis. Research 2020, 2020, 2402961. [Google Scholar] [CrossRef] [Green Version]
- Hendren, N.S.; de Lemos, J.A.; Ayers, C.; Das, S.R.; Rao, A.; Carter, S.; Rosenblatt, A.; Walchok, J.; Omar, W.; Khera, R.; et al. Association of Body Mass Index and Age With Morbidity and Mortality in Patients Hospitalized With COVID-19: Results From the American Heart Association COVID-19 Cardiovascular Disease Registry. Circulation 2021, 143, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Son, K.J.; Han, C.H.; Jung, J.Y.; Park, S.C. Impact of comorbid asthma on severity of coronavirus disease (COVID-19). Sci. Rep. 2020, 10, 21805. [Google Scholar] [CrossRef]
- Akbariqomi, M.; Hosseini, M.S.; Rashidiani, J.; Sedighian, H.; Biganeh, H.; Heidari, R.; Moghaddam, M.M.; Farnoosh, G.; Kooshki, H. Clinical characteristics and outcome of hospitalized COVID-19 patients with diabetes: A single-center, retrospective study in Iran. Diabetes Res. Clin. Pract. 2020, 169, 108467. [Google Scholar] [CrossRef] [PubMed]
- Ciceri, F.; Castagna, A.; Rovere-Querini, P.; De Cobelli, F.; Ruggeri, A.; Galli, L.; Conte, C.; De Lorenzo, R.; Poli, A.; Ambrosio, A.; et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin. Immunol. 2020, 217, 108509. [Google Scholar] [CrossRef]
- Gupta, S.; Hayek, S.S.; Wang, W.; Chan, L.; Mathews, K.S.; Melamed, M.L.; Brenner, S.K.; Leonberg-Yoo, A.; Schenck, E.J.; Radbel, J.; et al. Factors Associated With Death in Critically Ill Patients With Coronavirus Disease 2019 in the US. JAMA Intern. Med. 2020, 180, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.R.; Gold, J.A.W.; Natarajan, P.; Rossow, J.; Neblett Fanfair, R.; da Silva, J.; Wong, K.K.; Browning, S.D.; Bamrah Morris, S.; Rogers-Brown, J.; et al. Predictors at admission of mechanical ventilation and death in an observational cohort of adults hospitalized with COVID-19. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Cai, Y.; Liu, T.; Shen, B.; Yang, F.; Cao, S.; Liu, X.; Xiang, Y.; Zhao, Q.; et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur. Heart J. 2020, 41, 2070–2079. [Google Scholar] [CrossRef]
- Li, Q.; Chen, L.; Li, Q.; He, W.; Yu, J.; Chen, L.; Cao, Y.; Chen, W.; Di, W.; Dong, F.; et al. Cancer increases risk of in-hospital death from COVID-19 in persons <65 years and those not in complete remission. Leukemia 2020, 34, 2384–2391. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.; You, H.; Wu, M.; Zheng, Q.; et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020, 10, 783–791. [Google Scholar] [CrossRef]
- Madjid, M.; Vela, D.; Khalili-Tabrizi, H.; Casscells, S.W.; Litovsky, S. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries: Clues to the triggering effect of acute infections on acute coronary syndromes. Tex. Heart Inst. J. 2007, 34, 11–18. [Google Scholar] [PubMed]
- Jee, J.; Stonestrom, A.J.; Devlin, S.; Nguyentran, T.; Wills, B.; Narendra, V.; Foote, M.B.; Lumish, M.; Vardhana, S.A.; Pastores, S.M.; et al. Oncologic immunomodulatory agents in patients with cancer and COVID-19. Sci. Rep. 2021, 11, 4814. [Google Scholar] [CrossRef] [PubMed]
- Jee, J.; Foote, M.B.; Lumish, M.; Stonestrom, A.J.; Wills, B.; Narendra, V.; Avutu, V.; Murciano-Goroff, Y.R.; Chan, J.E.; Derkach, A.; et al. Chemotherapy and COVID-19 Outcomes in Patients With Cancer. J. Clin. Oncol. 2020, 38, 3538–3546. [Google Scholar] [CrossRef]
- Mortensen, R.F. C-reactive protein, inflammation, and innate immunity. Immunol. Res. 2001, 24, 163–176. [Google Scholar] [CrossRef]
- Coster, D.; Wasserman, A.; Fisher, E.; Rogowski, O.; Zeltser, D.; Shapira, I.; Bernstein, D.; Meilik, A.; Raykhshtat, E.; Halpern, P.; et al. Using the kinetics of C-reactive protein response to improve the differential diagnosis between acute bacterial and viral infections. Infection 2020, 48, 241–248. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Chassagnon, G.; Vakalopoulou, M.; Battistella, E.; Christodoulidis, S.; Hoang-Thi, T.N.; Dangeard, S.; Deutsch, E.; Andre, F.; Guillo, E.; Halm, N.; et al. AI-driven quantification, staging and outcome prediction of COVID-19 pneumonia. Med. Image Anal. 2021, 67, 101860. [Google Scholar] [CrossRef]
- Murphy, K.; Smits, H.; Knoops, A.J.G.; Korst, M.; Samson, T.; Scholten, E.T.; Schalekamp, S.; Schaefer-Prokop, C.M.; Philipsen, R.; Meijers, A.; et al. COVID-19 on Chest Radiographs: A Multireader Evaluation of an Artificial Intelligence System. Radiology 2020, 296, E166–E172. [Google Scholar] [CrossRef]
- Li, L.; Qin, L.; Xu, Z.; Yin, Y.; Wang, X.; Kong, B.; Bai, J.; Lu, Y.; Fang, Z.; Song, Q.; et al. Using Artificial Intelligence to Detect COVID-19 and Community-acquired Pneumonia Based on Pulmonary CT: Evaluation of the Diagnostic Accuracy. Radiology 2020, 296, E65–E71. [Google Scholar] [CrossRef] [PubMed]
| All Patients (n = 326) | No Clinical Deterioration (n = 241) | Clinical Deterioration (n = 85) | p Value | |
|---|---|---|---|---|
| Age (years) | 66.7 ± 15.3 | 65.5 ± 15.6 | 70.0 ± 14.1 | 0.016 |
| Male, n (%) | 170 (52.1) | 126 (52.3) | 44 (51.7) | 1.000 |
| BMI (kg/m2) | 29.5 ± 6.5 | 29.9 ± 6.5 | 27.7 ± 6.0 | 0.126 |
| Hypertension, n (%) | 226 (69.3) | 161 (66.8) | 65 (76.5) | 0.127 |
| Diabetes, n (%) | 101 (31.0) | 73 (30.3) | 28 (32.3) | 0.750 |
| Dyslipidemia, n (%) | 71 (21.8) | 52 (21.6) | 19 (22.4) | 1.000 |
| Smoking ever, n (%) | 80 (24.5) | 57 (12.7) | 23 (27.1) | 0.630 |
| Prior myocardial infarction, n (%) | 30 (9.2) | 15 (6.2) | 15 (17.6) | 0.004 |
| Heart failure, n (%) | 55 (16.9) | 40 (16.6) | 15 (17.6) | 0.957 |
| Chronic lung disease, n (%) | 63 (19.3) | 45 (18.7) | 18 (21.2) | 0.732 |
| Impaired kidney function, n (%) | 45 (13.8) | 27 (11.2) | 18 (21.2) | 0.035 |
| Immunodeficiency, n (%) | 69 (21.2) | 44 (18.3) | 25 (29.4) | 0.044 |
| SpO2 (%) | 95 (92–97) | 95 (93–97) | 92 (87–96) | <0.001 |
| All Patients (n = 326) | No Clinical Deterioration (n = 241) | Clinical Deterioration (n = 85) | p Value | |
|---|---|---|---|---|
| Lymphopaenia, n (%) (n = 240, 85) | 145 (44.6) | 100 (41.7) | 45 (52.9) | 0.095 |
| White blood cell count (G/L) (n = 241,84) | 6.76 (4.91–9.30) | 6.27 (4.68–8.48) | 7.97 (5.89–11.37) | <0.001 |
| Elevated liver enzymes, n (%) (n = 230, 80) | 193 (59.2) | 137 (59.6) | 56 (70.0) | 0.127 |
| LDH (U/L) (n = 219, 74) | 275.0 (204.0–398.0) | 241.0 (192.5–339.5) | 448.5 (286.0–627.5) | <0.001 |
| CRP (mg/L) (n = 241, 85) | 82.5 (28.5–139.4) | 62.8 (20.1–107.9) | 140.4 (87.6–226.7) | <0.001 |
| Ferritin (ng/L) (n = 213, 72) | 557.0 (304.0–1004.0) | 683.6 (298.0–859.0) | 835.5 (406.8–1308.2) | <0.001 |
| D-dimer (μg/mL) (n = 192, 72) | 1.17 (0.62–2.62) | 0.92 (0.58–1.68) | 2.50 (1.41–4.24) | <0.001 |
| Prothrombin time (sec) (n = 196, 67) | 9.0 (8.5–9.6) | 9.0 (8.5–9.5) | 9.2 (8.6–9.9) | 0.140 |
| High sensitivity troponin T (ng/L) (n = 194, 71) | 15.0 (7.0–36.0) | 12.0 (6.0–27.0) | 36.0 (19.0–74.0) | 0.144 |
| Creatine-kinase (U/L) (n = 176, 60) | 80.0 (39.8–201.2) | 72.0 (40.8–162.8) | 113.0 (35.0–378.8) | 0.071 |
| All Patients (n = 326) | No Clinical Deterioration (n = 241) | Clinical Deterioration (n = 85) | p Value | |
|---|---|---|---|---|
| Lobe volume (mL) | ||||
| Right upper lobe | 773.0 (585.8–925.2) | 788.5 (628.5–942.0) | 688.0 (541.5–908.5) | 0.017 |
| Right middle lobe | 374.0 (287.0–492.0) | 387.0 (292.0–500.0) | 342.0 (265.2–481.2) | 0.064 |
| Right lower lobe | 792.0 (611.8–1023.0) | 806.0 (629.0–1044.0) | 748.0 (578.0–981.5) | 0.177 |
| Left upper lobe | 966.0 (774.0–1215.0) | 990.0 (796.2–1231.2) | 895.0 (725.0–1177.0) | 0.029 |
| Left lower lobe | 763–0 (565.5–991.0) | 786.0 (589.0–2138.0) | 690.5 (518.2–861.5) | 0.016 |
| Affected area (%) | ||||
| Total | 6.8 (1.9–22.1) | 5.6 (1.5–16.6) | 21.0 (6.2–45.0) | <0.001 |
| Right upper lobe | 2.7 (0.2–17.3) | 1.2 (0.1–10.0) | 13.2 (1.5–45.7) | <0.001 |
| Right middle lobe | 1.9 (0.1–12.2) | 1.3 (0.0–7.9) | 12.3 (0.9–37.4) | <0.001 |
| Right lower lobe | 12.9 (2.8–40.6 | 9.9 (1.9–27.4) | 40.1 (12.3–63.1) | <0.001 |
| Left upper lobe | 2.0 (0.1–15.8) | 1.4 (0.1–9.1) | 8.9 (1.1–34.8) | <0.001 |
| Left lower lobe | 9.4 (1.5–37.3) | 5.9 (0.9–26.8) | 28.9 (4.8–60.6) | <0.001 |
| All Patients (n = 326) | No Clinical Deterioration (n = 241) | Clinical Deterioration (n = 85) | p Value | |
|---|---|---|---|---|
| Severity score | ||||
| Total | 7.0 (4.0–12.0) | 6.0 (3.0–10.0) | 11.0 (7.0–17.3) | <0.001 |
| Right upper lobe | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | 2.0 (1.0–3.3) | <0.001 |
| Right middle lobe | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | 2.0 (1.0–3.0) | <0.001 |
| Right lower lobe | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 3.0 (1.0–4.0) | <0.001 |
| Left upper lobe | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | 2.0 (1.0–3.0) | <0.001 |
| Unadjusted Analysis | Model 1: Clinical Parameters | Model 2: Clinical + AI-Based CT Parameters | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| Age | 1.02 | 1.00–1.04 | 0.022 | 1.00 | 0.98–1.03 | 0.765 | 1.01 | 0.99–1.03 | 0.477 |
| Male | 0.98 | 0.60–1.61 | 0.935 | ||||||
| BMI | 0.94 | 0.87–1.02 | 0.146 | ||||||
| Hypertension | 1.61 | 0.93–2.91 | 0.098 | ||||||
| Diabetes | 1.13 | 0.66–1.91 | 0.650 | ||||||
| Dyslipidemia | 1.05 | 0.57–1.87 | 0.882 | ||||||
| Smoking ever | 1.20 | 0.67–2.09 | 0.531 | ||||||
| Prior myocardial infarction | 3.23 | 1.50–7.00 | 0.003 | 3.31 | 1.37–8.12 | 0.008 | 2.81 | 1.12–7.04 | 0.027 |
| Heart failure | 1.08 | 0.55–2.03 | 0.824 | ||||||
| Chronic lung disease | 1.17 | 0.62–2.13 | 0.615 | ||||||
| Impaired kidney function | 2.13 | 1.09–4.08 | 0.024 | 2.03 | 0.93–4.41 | 0.074 | 2.15 | 0.96–4.78 | 0.059 |
| Immunodeficiency | 1.87 | 1.05–3.28 | 0.032 | 1.66 | 0.84–3.23 | 0.138 | 2.08 | 1.02–4.25 | 0.043 |
| SpO2 | 0.90 | 0.86–0.94 | <0.001 | 0.94 | 0.89–0.98 | 0.005 | 0.96 | 0.91–1.00 | 0.060 |
| CRP * | 2.25 | 1.76–2.96 | <0.001 | 1.95 | 1.51–2.58 | <0.001 | 1.73 | 1.32–2.33 | <0.001 |
| CT severity score | 1.15 | 1.10–1.20 | <0.001 | 1.08 | 1.02–1.15 | 0.013 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, I.V.; Simon, J.; Nardocci, C.; Kardos, A.S.; Nagy, N.; Abdelrahman, R.-H.; Zsarnóczay, E.; Fejér, B.; Futácsi, B.; Müller, V.; et al. The Predictive Role of Artificial Intelligence-Based Chest CT Quantification in Patients with COVID-19 Pneumonia. Tomography 2021, 7, 697-710. https://doi.org/10.3390/tomography7040058
Szabó IV, Simon J, Nardocci C, Kardos AS, Nagy N, Abdelrahman R-H, Zsarnóczay E, Fejér B, Futácsi B, Müller V, et al. The Predictive Role of Artificial Intelligence-Based Chest CT Quantification in Patients with COVID-19 Pneumonia. Tomography. 2021; 7(4):697-710. https://doi.org/10.3390/tomography7040058
Chicago/Turabian StyleSzabó, István Viktor, Judit Simon, Chiara Nardocci, Anna Sára Kardos, Norbert Nagy, Renad-Heyam Abdelrahman, Emese Zsarnóczay, Bence Fejér, Balázs Futácsi, Veronika Müller, and et al. 2021. "The Predictive Role of Artificial Intelligence-Based Chest CT Quantification in Patients with COVID-19 Pneumonia" Tomography 7, no. 4: 697-710. https://doi.org/10.3390/tomography7040058
APA StyleSzabó, I. V., Simon, J., Nardocci, C., Kardos, A. S., Nagy, N., Abdelrahman, R.-H., Zsarnóczay, E., Fejér, B., Futácsi, B., Müller, V., Merkely, B., & Maurovich-Horvat, P. (2021). The Predictive Role of Artificial Intelligence-Based Chest CT Quantification in Patients with COVID-19 Pneumonia. Tomography, 7(4), 697-710. https://doi.org/10.3390/tomography7040058






