Improved Image Quality for Static BLADE Magnetic Resonance Imaging Using the Total-Variation Regularized Least Absolute Deviation Solver
Abstract
1. Introduction
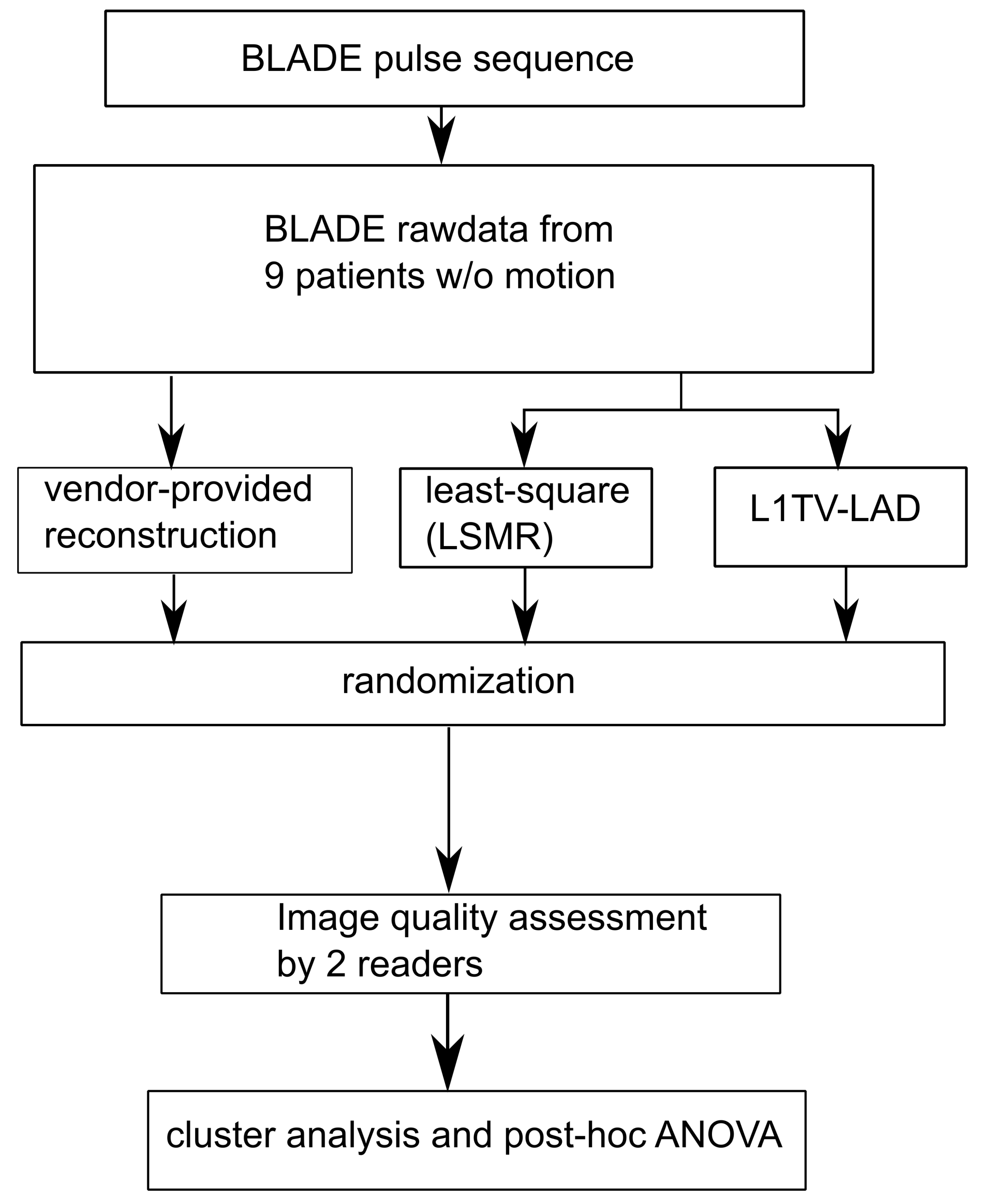
2. Materials and Methods
2.1. Mathematical Background
2.2. Reconstruction of In Vivo Rawdata
2.3. Image Reconstruction Based on Tensor Solvers
2.4. Radiologists’ Evaluation
3. Results
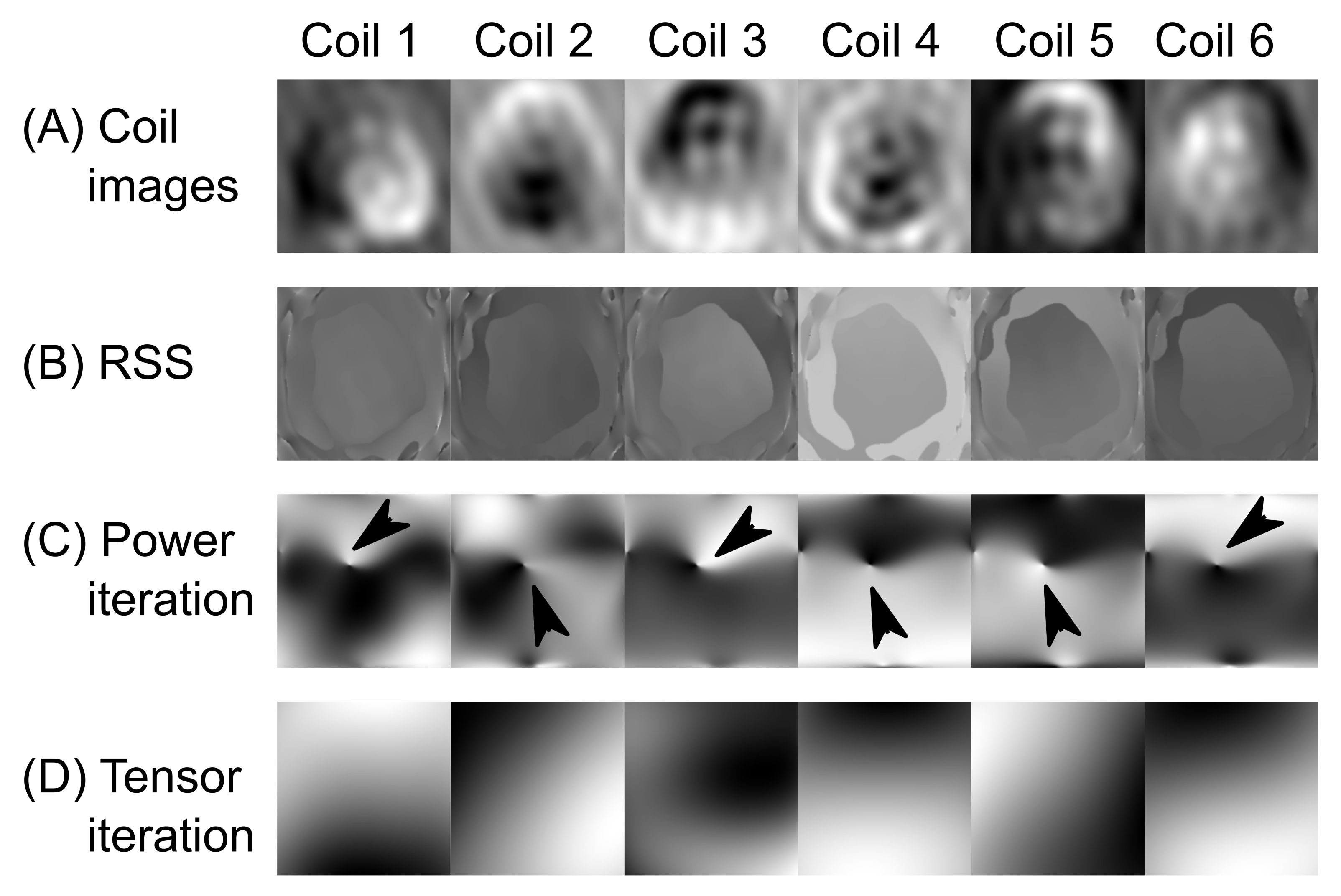
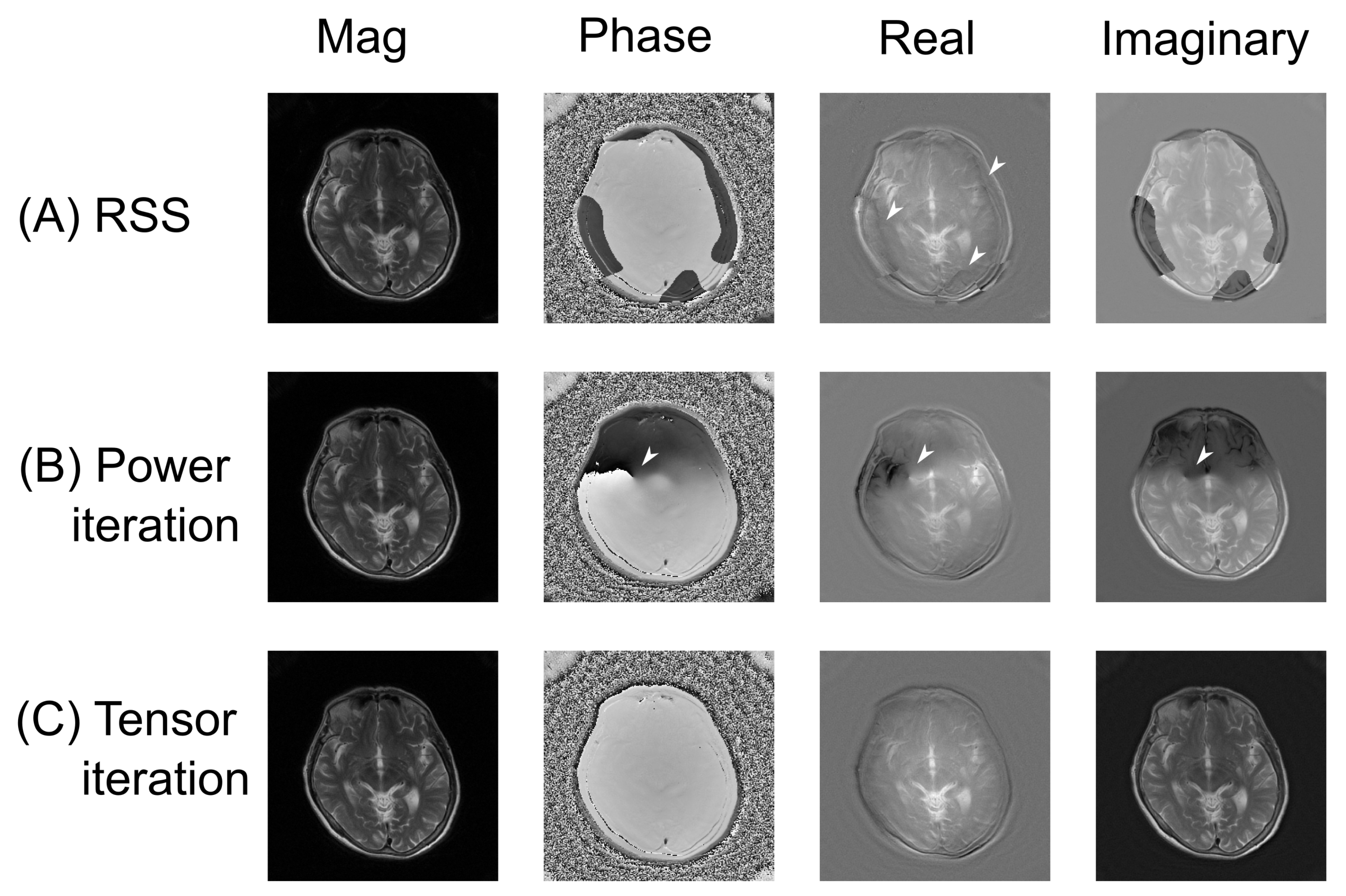
3.1. Coil Sensitivity Profiles
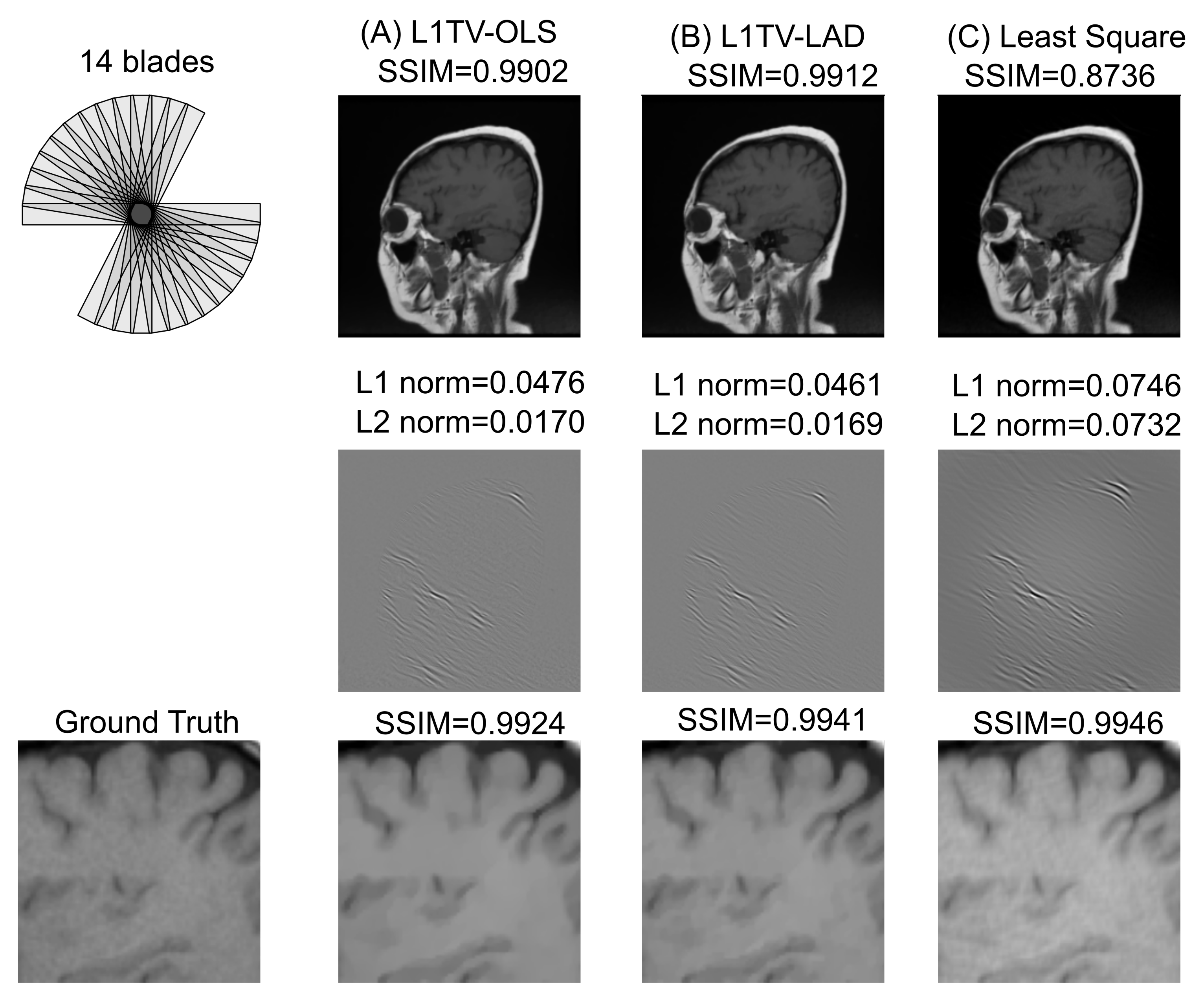
3.2. Numerical Evaluation of the Image Quality
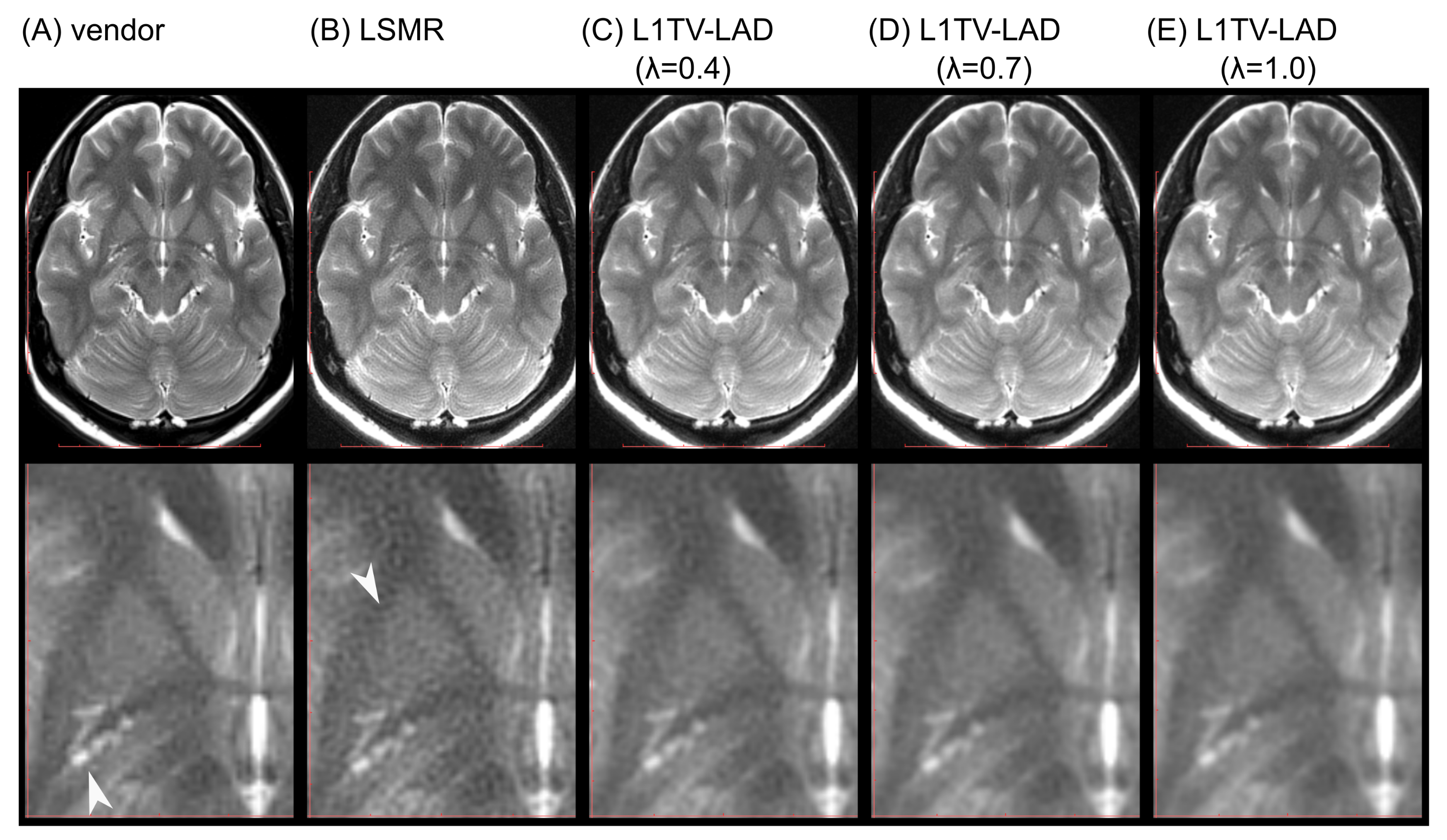
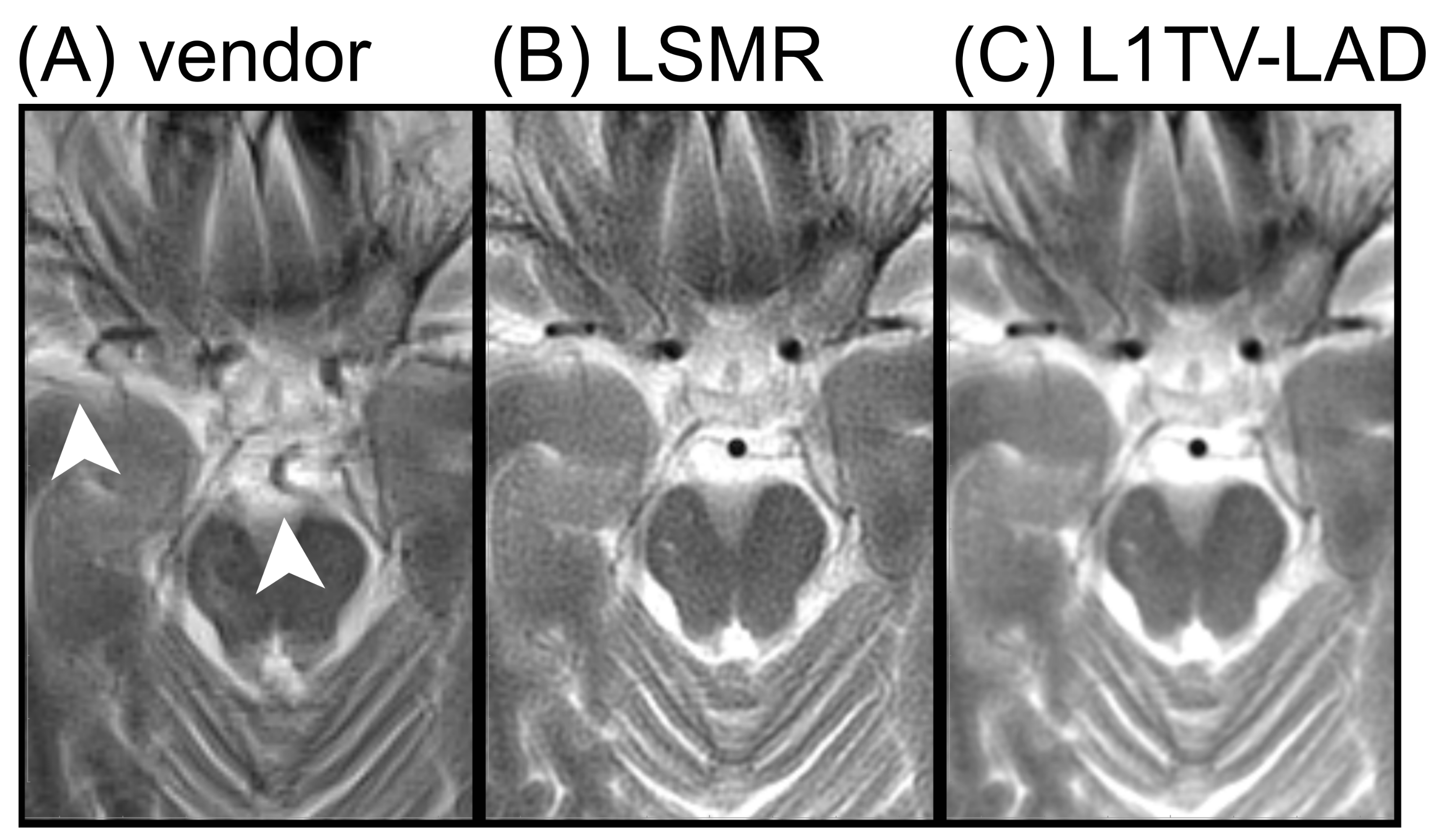
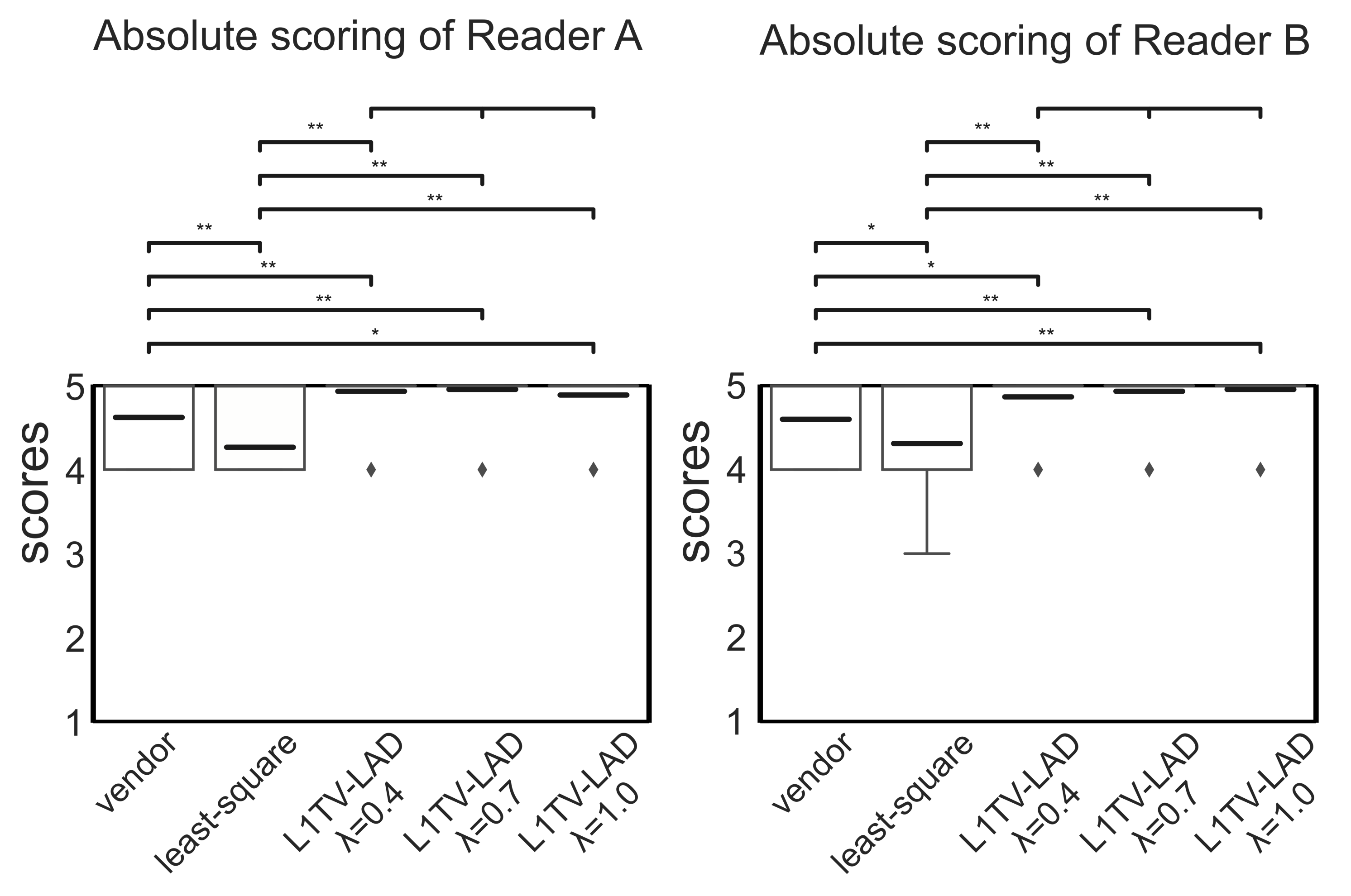
3.3. Clinical Evaluation of the Image Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | Auto-Calibration Signal |
| ANOVA | Analysis of variance |
| CNR | Contrast-to-noise ratio |
| DICOM | Digital Imaging and Communications in Medicine |
| ESPIRiT | Eigenvalue Approach to Autocalibrating Parallel MRI approach |
| ICC | Intraclass correlation coefficient |
| LAD | Least absolute deviation |
| MRI | Magnetic resonance imaging |
| OLS | Ordinary least squares |
| PROPELLER | Periodically Rotated Overlapping ParallEL Lines with Enhanced Reconstruction |
| RSS | Root-sum squared |
| SENSE | SENSitivity Encoding |
| SSIM | Structural Similarity |
| T2 FLAIR | T2 FLuid Attenuated Inversion Recovery |
Appendix A. Parallel MRI Encoding Using the Indexed Tensor Notation
Appendix B. Parallel Imaging Reconstruction
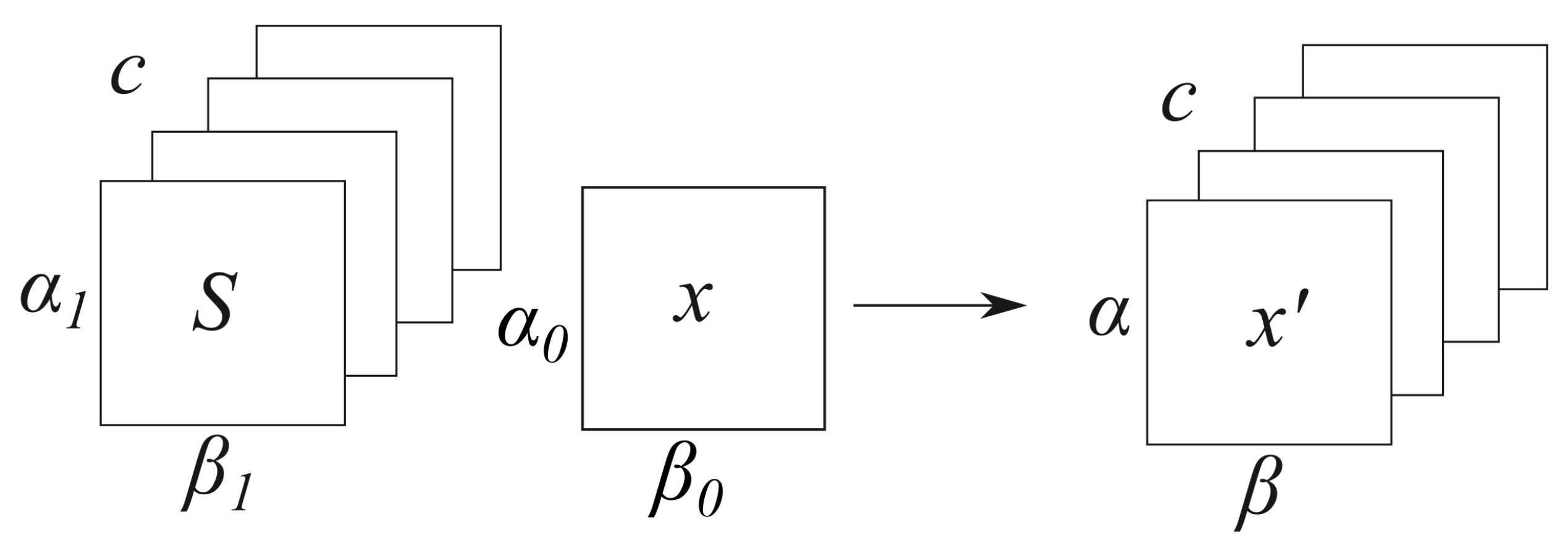
Appendix C. Optimization Algorithms
References
- Pipe, J.G. Motion correction with PROPELLER MRI: Application to head motion and free-breathing cardiac imaging. Magn. Reson. Med. 1999, 42, 963–969. [Google Scholar] [CrossRef]
- Finkenzeller, T.; Wendl, C.; Lenhart, S.; Stroszczynski, C.; Schuierer, G.; Fellner, C. BLADE sequences in transverse T2-weighted MR imaging of the cervical spine. Cut-off for artefacts? In Rofo: Advances in the Field of X-Ray Radiation and Imaging Processes; Thieme Publishers: Stuttgart, Germany, 2015; Volume 36, pp. 102–108. [Google Scholar]
- Hu, H.H.; McAllister, A.S.; Jin, N.; Lubeley, L.J.; Selvaraj, B.; Smith, M.; Krishnamurthy, R.; Zhou, K. Comparison of 2D BLADE turbo gradient-and spin-echo and 2D spin-Echo Echo-planar diffusion-weighted brain MRI at 3 T: Preliminary experience in children. Acad. Radiol. 2019, 26, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Sheikh-Sarraf, M.; Nougaret, S.; Forstner, R.; Kubik-Huch, R.A. Patient preparation and image quality in female pelvic MRI: recommendations revisited. Eur. Radiol. 2020, 30, 5378–5383. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.S.; Choi, Y.H.; Cheon, J.E.; Kim, W.S.; Kim, I.O. Application of T1-weighted BLADE sequence to abdominal magnetic resonance imaging of young children: A comparison with turbo spin echo sequence. Acta Radiol. 2020, 61, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Jaimes, C.; Kirsch, J.E.; Gee, M.S. Fast, free-breathing and motion-minimized techniques for pediatric body magnetic resonance imaging. Pediatr. Radiol. 2018, 48, 1197–1208. [Google Scholar] [CrossRef]
- Heiss, R.; Grodzki, D.M.; Horger, W.; Uder, M.; Nagel, A.M.; Bickelhaupt, S. High-performance low field MRI enables visualization of persistent pulmonary damage after COVID-19. Magn. Reson. Imaging 2021, 76, 49–51. [Google Scholar] [CrossRef]
- Vertinsky, A.T.; Rubesova, E.; Krasnokutsky, M.V.; Bammer, S.; Rosenberg, J.; White, A.; Barnes, P.D.; Bammer, R. Performance of PROPELLER relative to standard FSE T2-weighted imaging in pediatric brain MRI. Pediatr. Radiol. 2009, 39, 1038–1047. [Google Scholar] [CrossRef]
- Czyzewska, D.; Sushentsev, N.; Latoch, E.; Slough, R.A.; Barrett, T. T2-PROPELLER compared to T2-FRFSE for image quality and lesion detection at prostate MRI. Can. Assoc. Radiol. J. 2021, 08465371211030206. [Google Scholar] [CrossRef]
- Tamhane, A.A.; Anastasio, M.A.; Gui, M.; Arfanakis, K. Iterative image reconstruction for PROPELLER-MRI using the nonuniform fast fourier transform. J. Magn. Reson. Imaging 2010, 32, 211–217. [Google Scholar] [CrossRef]
- Boyd, S.; Parikh, N.; Chu, E.; Peleato, B.; Eckstein, J. Distributed Optimization and Statistical Learning via the Alternating Direction Method of Multipliers; Now Publishers Inc.: Hanover, MA, USA, 2011. [Google Scholar]
- Beck, A.; Teboulle, M. A fast iterative shrinkage-thresholding algorithm for linear inverse problems. SIAM J. Imaging Sci. 2009, 2, 183–202. [Google Scholar] [CrossRef]
- Taylor, A.B.; Hendrickx, J.M.; Glineur, F. Exact worst-case performance of first-order methods for composite convex optimization. SIAM J. Optim. 2017, 27, 1283–1313. [Google Scholar] [CrossRef]
- Zhao, D.; Du, H.; Han, Y.; Mei, W. Compressed sensing MR image reconstruction exploiting TGV and wavelet sparsity. Comput. Math. Methods Med. 2014, 2014, 958671. [Google Scholar] [CrossRef]
- Lin, J.M.; Patterson, A.J.; Chang, H.C.; Gillard, J.H.; Graves, M.J. An iterative reduced field-of-view reconstruction for periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER) MRI. Med. Phys. 2015, 42, 5757–5767. [Google Scholar] [CrossRef]
- Bassett, G., Jr.; Koenker, R. Asymptotic theory of least absolute error regression. J. Am. Stat. Assoc. 1978, 73, 618–622. [Google Scholar] [CrossRef]
- Edgeworth, F.Y. On observations relating to several quantities. Hermathena 1887, 6, 279–285. [Google Scholar]
- Dielman, T.E. Least absolute value regression: Recent contributions. J. Stat. Comput. Simul. 2005, 75, 263–286. [Google Scholar] [CrossRef]
- Li, H.L. Solve least absolute value regression problems using modified goal programming techniques. Comput. Oper. Res. 1998, 25, 1137–1143. [Google Scholar] [CrossRef][Green Version]
- Cerezci, E.; Gokpinar, F. A comparison of three linear programming models for computing least absolute value estimates. Hacet. J. Math. Stat. 2005, 34, 95–102. [Google Scholar]
- Rauhut, H.; Ward, R. Interpolation via weighted L1 minimization. Appl. Comput. Harmon. Anal. 2016, 40, 321–351. [Google Scholar] [CrossRef]
- Wang, L.; Gordon, M.D.; Zhu, J. Regularized least absolute deviations regression and an efficient algorithm for parameter tuning. In Proceedings of the Sixth International Conference on Data Mining (ICDM’06), Hong Kong, China, 18–22 December 2006; pp. 690–700. [Google Scholar]
- Wang, H.; Li, G.; Jiang, G. Robust regression shrinkage and consistent variable selection through the LAD-lasso. J. Bus. Econ. Stat. 2007, 25, 347–355. [Google Scholar] [CrossRef]
- Wang, L. The L1 penalized LAD estimator for high dimensional linear regression. J. Multivar. Anal. 2013, 120, 135–151. [Google Scholar] [CrossRef]
- Wang, Z.; Peterson, B.S. Constrained least absolute deviation neural networks. IEEE Trans. Neural Netw. 2008, 19, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Küstner, T.; Fuin, N.; Hammernik, K.; Bustin, A.; Qi, H.; Hajhosseiny, R.; Masci, P.G.; Neji, R.; Rueckert, D.; Botnar, R.M.; et al. CINENet: Deep learning-based 3D cardiac CINE MRI reconstruction with multi-coil complex-valued 4D spatio-temporal convolutions. Sci. Rep. 2020, 10, 13710. [Google Scholar] [CrossRef] [PubMed]
- König, R.E.; Stucht, D.; Baecke, S.; Rashidi, A.; Speck, O.; Sandalcioglu, I.E.; Luchtmann, M. Phase-Contrast MRI Detection of Ventricular Shunt CSF Flow: Proof of Principle. J. Neuroimaging 2020, 30, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Holme, H.C.M.; Rosenzweig, S.; Ong, F.; Wilke, R.N.; Lustig, M.; Uecker, M. ENLIVE: An efficient nonlinear method for calibrationless and robust parallel imaging. Sci. Rep. 2019, 9, 3034. [Google Scholar] [CrossRef]
- Balbastre, Y.; Acosta-Cabronero, J.; Corbin, N.; Josephs, O.; Ashburner, J.; Callaghan, M.F. A generative approach to estimating coil sensitivities from autocalibration data. In Proceedings of the 27th Annual Meeting of the International Society for Magnetic Resonance in Medicine, Montréal, QC, Canada, 1–16 May 2019. [Google Scholar]
- Chen, Z.; Chen, Y.; Li, D.; Christodoulou, A. A deep learning algorithm for non-Cartesian coil sensitivity map estimation. In Proceedings of the 27th Annual Meeting of the International Society for Magnetic Resonance in Medicine, Montréal, QC, Canada, 1–16 May 2019. [Google Scholar]
- Chen, N.k.; Wu, P.H. The use of Fourier-domain analyses for unwrapping phase images of low SNR. Magn. Reson. Med. 2019, 82, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Perkins, K.; Clifford, B.; Sutton, B.; Liang, Z.P. Deep-SENSE: Learning Coil Sensitivity Functions for SENSE Reconstruction Using Deep Learning. In Proceedings of the 26th Annual Meeting of the International Society for Magnetic Resonance in Medicine, Paris, France, 16–21 June 2018. [Google Scholar]
- Sriram, A.; Zbontar, J.; Murrell, T.; Zitnick, C.L.; Defazio, A.; Sodickson, D.K. GrappaNet: Combining parallel imaging with deep learning for multi-coil MRI reconstruction. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, Seattle, WA, USA, 14–19 June 2020; pp. 14315–14322. [Google Scholar]
- Lin, J.; Chang, H.; Chao, T.; Tsai, S.; Patterson, A.; Chung, H.; Gillard, J.; Graves, M. L1-LAD: Iterative MRI Reconstruction Using L1 Constrained Least Absolute Deviation. In Proceedings of the Annual Scientific Meeting of ESMRMB, Barcelona, Spain, 19–21 October 2017. [Google Scholar]
- Lin, J.M. Python non-uniform fast Fourier transform (PyNUFFT): An accelerated non-Cartesian MRI package on a heterogeneous platform (CPU/GPU). J. Imaging 2018, 4, 51. [Google Scholar] [CrossRef]
- Tan, Z.; Hohage, T.; Kalentev, O.; Joseph, A.A.; Wang, X.; Voit, D.; Merboldt, K.D.; Frahm, J. An eigenvalue approach for the automatic scaling of unknowns in model-based reconstructions: Application to real-time phase-contrast flow MRI. NMR Biomed. 2017, 30, e3835. [Google Scholar] [CrossRef]
- Uecker, M.; Hohage, T.; Block, K.T.; Frahm, J. Image reconstruction by regularized nonlinear inversion—Joint estimation of coil sensitivities and image content. Magn. Reson. Med. 2008, 60, 674–682. [Google Scholar] [CrossRef]
- Ehses, P.; Huynh, C.; Physics, B.M. Twixtools. 2020. Available online: https://github.com/pehses/twixtools (accessed on 1 October 2021).
- Fong, D.C.L.; Saunders, M. LSMR: An iterative algorithm for sparse least-squares problems. SIAM J. Sci. Comput. 2011, 33, 2950–2971. [Google Scholar] [CrossRef]
- Uecker, M.; Lai, P.; Murphy, M.J.; Virtue, P.; Elad, M.; Pauly, J.M.; Vasanawala, S.S.; Lustig, M. ESPIRiT—An eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn. Reson. Med. 2014, 71, 990–1001. [Google Scholar] [CrossRef]
- Goldstein, T.; Osher, S. The split Bregman method for L1-regularized problems. SIAM J. Imaging Sci. 2009, 2, 323–343. [Google Scholar] [CrossRef]
- Chen, F.; Taviani, V.; Malkiel, I.; Cheng, J.Y.; Tamir, J.I.; Shaikh, J.; Chang, S.T.; Hardy, C.J.; Pauly, J.M.; Vasanawala, S.S. Variable-density single-shot fast spin-echo MRI with deep learning reconstruction by using variational networks. Radiology 2018, 289, 366–373. [Google Scholar] [CrossRef]
- Carrillo, R.E.; Ramirez, A.B.; Arce, G.R.; Barner, K.E.; Sadler, B.M. Robust compressive sensing of sparse signals: A review. EURASIP J. Adv. Signal Process. 2016, 2016, 108. [Google Scholar] [CrossRef]
- Renieblas, G.P.; Nogués, A.T.; González, A.M.; León, N.G.; Del Castillo, E.G. Structural similarity index family for image quality assessment in radiological images. J. Med. Imaging 2017, 4, 035501. [Google Scholar] [CrossRef]
- Mason, A.; Rioux, J.; Clarke, S.E.; Costa, A.; Schmidt, M.; Keough, V.; Huynh, T.; Beyea, S. Comparison of objective image quality metrics to expert radiologists’ scoring of diagnostic quality of MR images. IEEE Trans. Med. Imaging 2020, 39, 1064–1072. [Google Scholar] [CrossRef]
- Lustig, M.; Donoho, D.L.; Santos, J.M.; Pauly, J.M. Compressed sensing MRI. IEEE Signal Process. Mag. 2008, 25, 72–82. [Google Scholar] [CrossRef]
- Malgouyres, F. Combining total variation and wavelet packet approaches for image deblurring. In Proceedings of the IEEE Workshop on Variational and Level Set Methods in Computer Vision, Vancouver, BC, Canada, 13 July 2001; pp. 57–64. [Google Scholar]
- Donoho, D.; Tanner, J. Observed universality of phase transitions in high-dimensional geometry, with implications for modern data analysis and signal processing. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 4273–4293. [Google Scholar] [CrossRef] [PubMed]
- Donoho, D.L.; Maleki, A.; Montanari, A. Message-passing algorithms for compressed sensing. Proc. Natl. Acad. Sci. USA 2009, 106, 18914–18919. [Google Scholar] [CrossRef] [PubMed]
- Simmross-Wattenberg, F.; Rodríguez-Cayetano, M.; Royuela-del Val, J.; Martín-González, E.; Moya-Sáez, E.; Martín-Fernández, M.; Alberola-López, C. OpenCLIPER: An OpenCL-based C++ Framework for overhead-reduced medical image processing and reconstruction on heterogeneous devices. IEEE J. Biomed. Health Inform. 2018, 23, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Einstein, A.; Perrett, W.; Jeffery, G. The foundation of the general theory of relativity. Ann. Der Phys. 1916, 49, 769–822. [Google Scholar] [CrossRef]
- Tax, C.M.; Duits, R.; Vilanova, A.; ter Haar Romeny, B.M.; Hofman, P.; Wagner, L.; Leemans, A.; Ossenblok, P. Evaluating contextual processing in diffusion MRI: Application to optic radiation reconstruction for epilepsy surgery. PLoS ONE 2014, 9, e101524. [Google Scholar] [CrossRef] [PubMed]
- Dullemond, K.; Peeters, K. Introduction to Tensor Calculus 1991–2010. Available online: https://www.ita.uni-heidelberg.de/~dullemond/lectures/tensor/tensor.pdf (accessed on 1 October 2021).
- Morozov, O.V.; Unser, M.; Hunziker, P. Reconstruction of large, irregularly sampled multidimensional images. A tensor-based approach. IEEE Trans. Med. Imaging 2010, 30, 366–374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pruessmann, K.P.; Weiger, M.; Scheidegger, M.B.; Boesiger, P. SENSE: Sensitivity encoding for fast MRI. Magn. Reson. Med. 1999, 42, 952–962. [Google Scholar] [CrossRef]
- Luo, T.; Noll, D.C.; Fessler, J.A.; Nielsen, J.F. A GRAPPA algorithm for arbitrary 2D/3D non-Cartesian sampling trajectories with rapid calibration. Magn. Reson. Med. 2019, 82, 1101–1112. [Google Scholar] [CrossRef] [PubMed]

| Score | Overall Image Quality | Noise (SNR) | Tissue Contrast * | Sharpness | Artifacts |
|---|---|---|---|---|---|
| 1 | Non-diagnostic | All structures are noisy | Not all tissues can be separated | No structures are sharp | Severe |
| 2 | Limited | Most structures are noisy | Few tissues can be separated clearly | Few structures are sharp | Moderate |
| 3 | Diagnostic | Several structures are noisy | Several structures can be separated clearly | Several structures are sharp | Mild |
| 4 | Good | A few structures are noisy | Most structures can be separated clearly | Most structures are sharp | Minimal |
| 5 | Excellent | No noticeable noise on any image | All tissues can be separated clearly | All structures are sharp | None |
| Score | Overall Image Quality | Noise (SNR) | Tissue Contrast * | Sharpness | Artifacts |
|---|---|---|---|---|---|
| 1 | Much inferior | Much inferior | Much inferior | Much inferior | Much more |
| 2 | Somewhat inferior | Somewhat inferior | Somewhat inferior | Somewhat inferior | Somewhat more |
| 3 | No distinction | No distinction | No distinction | No distinction | No distinction |
| 4 | Somewhat better | Somewhat better | Somewhat better | Somewhat better | Somewhat fewer |
| 5 | Much better | Much better | Much better | Much better | Much fewer |
| CNR | Vendor | Least-Square | L1TV-LAD () | L1TV-LAD () | L1TV-LAD () |
|---|---|---|---|---|---|
| GM | |||||
| WM | |||||
| CSF | |||||
| Thalamus | |||||
| GP | |||||
| CN | |||||
| Putamen |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-C.; Yang, H.-C.; Chen, C.-C.; Harrevelt, S.; Chao, Y.-C.; Lin, J.-M.; Yu, W.-H.; Chang, H.-C.; Chang, C.-K.; Hwang, F.-N. Improved Image Quality for Static BLADE Magnetic Resonance Imaging Using the Total-Variation Regularized Least Absolute Deviation Solver. Tomography 2021, 7, 555-572. https://doi.org/10.3390/tomography7040048
Chen H-C, Yang H-C, Chen C-C, Harrevelt S, Chao Y-C, Lin J-M, Yu W-H, Chang H-C, Chang C-K, Hwang F-N. Improved Image Quality for Static BLADE Magnetic Resonance Imaging Using the Total-Variation Regularized Least Absolute Deviation Solver. Tomography. 2021; 7(4):555-572. https://doi.org/10.3390/tomography7040048
Chicago/Turabian StyleChen, Hsin-Chia, Haw-Chiao Yang, Chih-Ching Chen, Seb Harrevelt, Yu-Chieh Chao, Jyh-Miin Lin, Wei-Hsuan Yu, Hing-Chiu Chang, Chin-Kuo Chang, and Feng-Nan Hwang. 2021. "Improved Image Quality for Static BLADE Magnetic Resonance Imaging Using the Total-Variation Regularized Least Absolute Deviation Solver" Tomography 7, no. 4: 555-572. https://doi.org/10.3390/tomography7040048
APA StyleChen, H.-C., Yang, H.-C., Chen, C.-C., Harrevelt, S., Chao, Y.-C., Lin, J.-M., Yu, W.-H., Chang, H.-C., Chang, C.-K., & Hwang, F.-N. (2021). Improved Image Quality for Static BLADE Magnetic Resonance Imaging Using the Total-Variation Regularized Least Absolute Deviation Solver. Tomography, 7(4), 555-572. https://doi.org/10.3390/tomography7040048






