Feasibility of Sodium and Amide Proton Transfer-Weighted Magnetic Resonance Imaging Methods in Mild Steatotic Liver Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. APTw Imaging Optimization
2.3. Image Acquisition
2.4. Histology
2.5. Extraction of Parametric MRI Measures
2.6. Statistical Analysis
3. Results
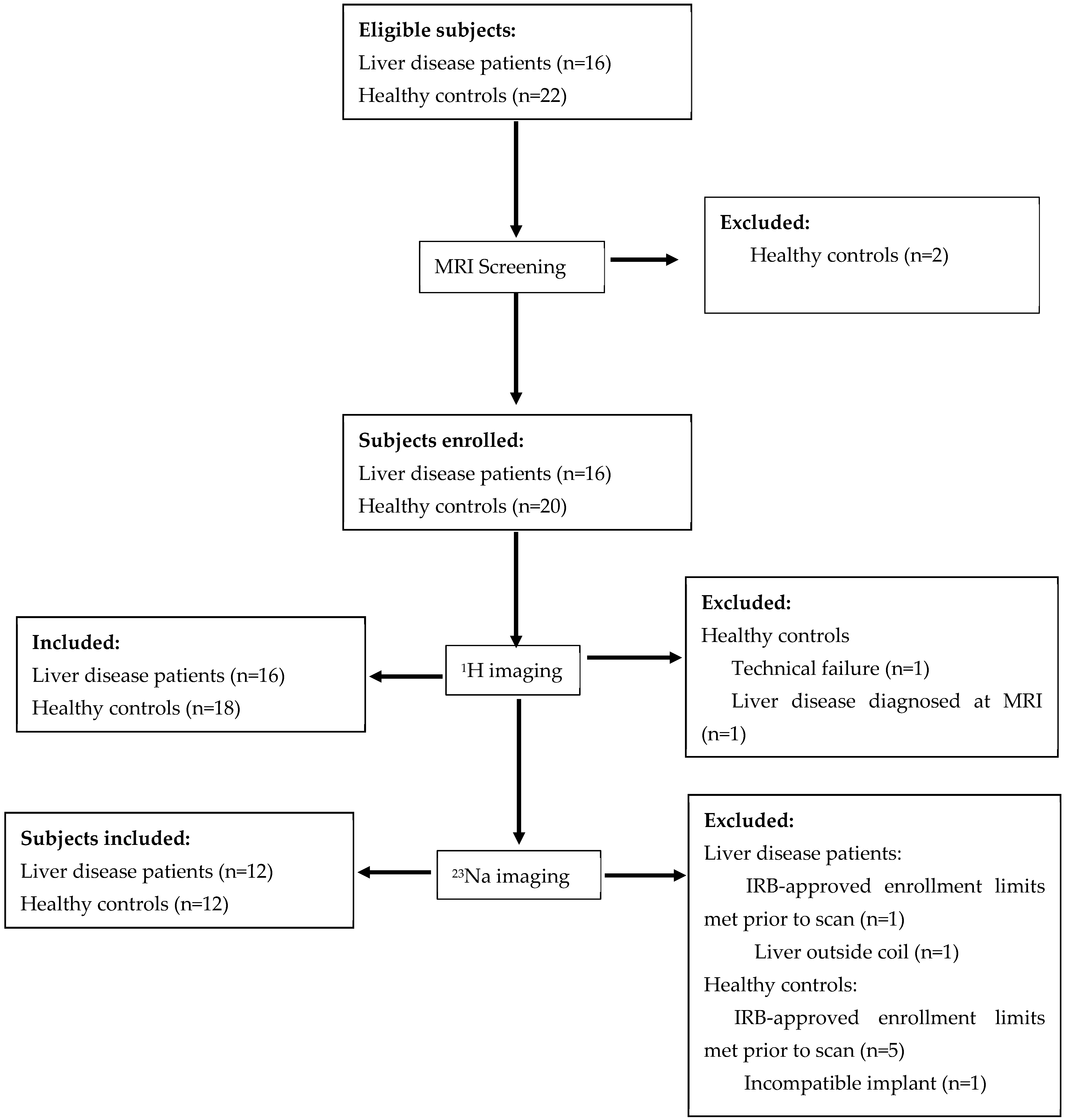
3.1. Study Population
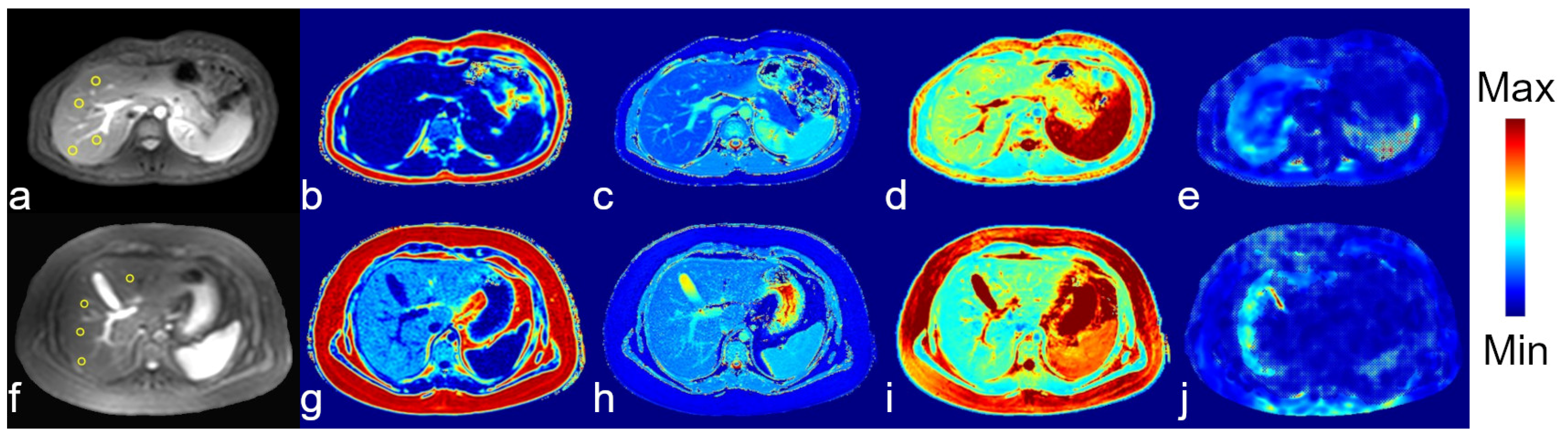
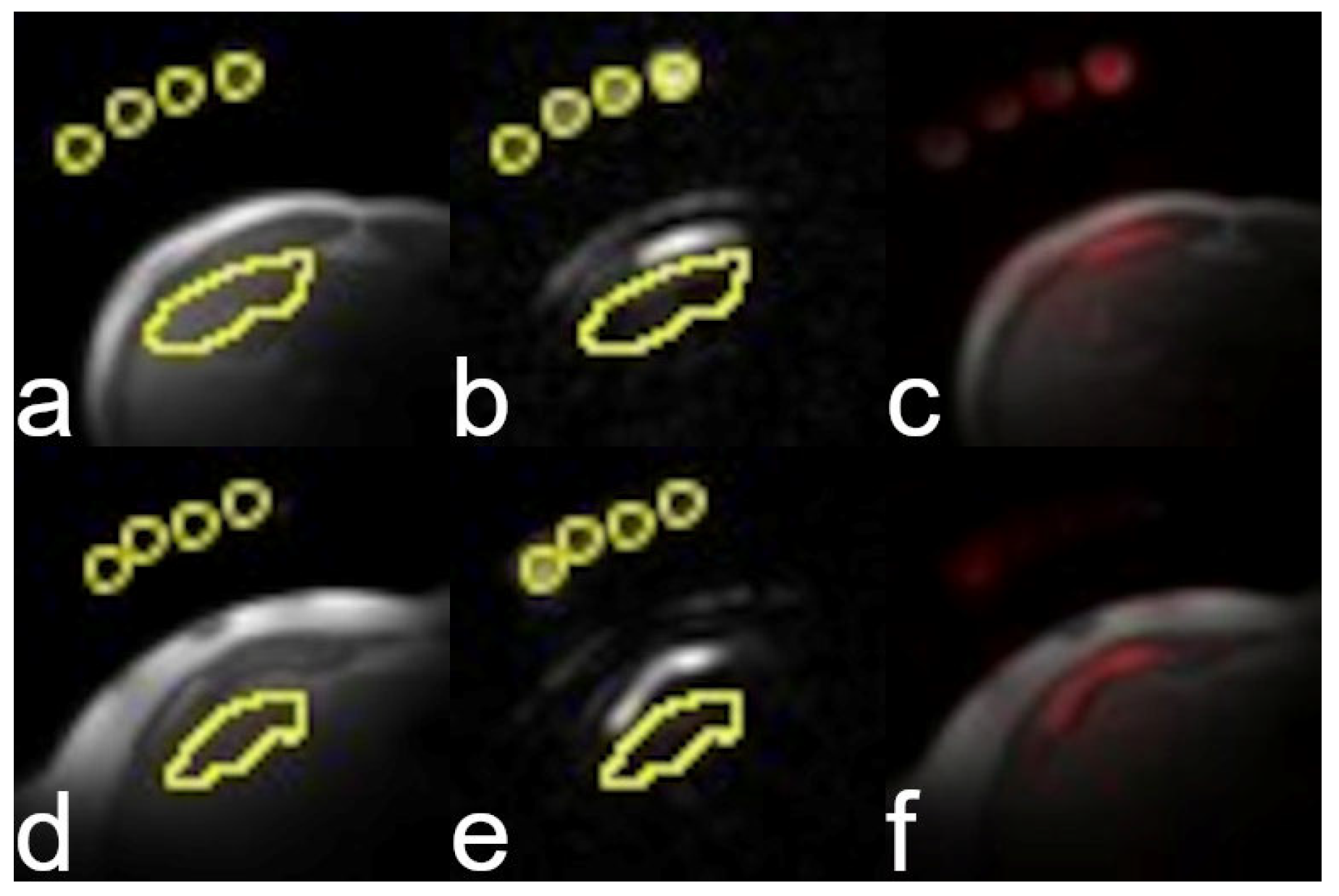
3.2. Image Quality
3.3. Patients vs. Healthy Controls
3.4. Associations Between MRI Measurements
3.5. Associations Between MRI Measurements and Clinical Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MRI | Magnetic resonance imaging |
| APTw | Amide proton transfer-weighted |
| MTR | Magnetization transfer ratio |
| SI | Signal intensity |
| RF | Radiofrequency |
| ROI | Region of interest |
| PDFF | Proton density fat fraction |
| ALT | Alanine transaminase |
| AST | Aspartate aminotransferase |
| NAS | Nonalcoholic fatty liver disease activity score |
| Fib4 | Fibrosis-4 score |
| TSC | Tissue sodium content |
| BMI | Body mass index |
References
- Stal, P. Liver fibrosis in non-alcoholic fatty liver disease-diagnostic challenge with prognostic significance. World J. Gastroenterol. 2015, 21, 11077–11087. [Google Scholar] [CrossRef]
- Ekstedt, M.; Hagstrom, H.; Nasr, P.; Fredrikson, M.; Stal, P.; Kechagias, S.; Hultcrantz, R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015, 61, 1547–1554. [Google Scholar] [CrossRef]
- Guo, Y.C.; Lu, L.G. Antihepatic Fibrosis Drugs in Clinical Trials. J. Clin. Transl. Hepatol. 2020, 8, 304–312. [Google Scholar] [CrossRef]
- Schuppan, D.; Ashfaq-Khan, M.; Yang, A.T.; Kim, Y.O. Liver fibrosis: Direct antifibrotic agents and targeted therapies. Matrix Biol. 2018, 68–69, 435–451. [Google Scholar] [CrossRef]
- Caravan, P.; Das, B.; Dumas, S.; Epstein, F.H.; Helm, P.A.; Jacques, V.; Koerner, S.; Kolodziej, A.; Shen, L.; Sun, W.C.; et al. Collagen-targeted MRI contrast agent for molecular imaging of fibrosis. Angew. Chem. Int. Ed. Engl. 2007, 46, 8171–8173. [Google Scholar] [CrossRef]
- Welker, K.M.; Joyner, D.; Kam, A.W.; Liebeskind, D.S.; Saindane, A.M.; Segovis, C.; Yahyavi-Firouz-Abadi, N.; Jordan, J.E. State of Practice: ASNR Statement on Gadolinium-Based Contrast Agent Use in Patients with Chronic Kidney Disease. AJNR Am. J. Neuroradiol. 2025, 46, 227–230. [Google Scholar] [CrossRef]
- Starekova, J.; Pirasteh, A.; Reeder, S.B. Update on Gadolinium-Based Contrast Agent Safety, From the AJR Special Series on Contrast Media. AJR Am. J. Roentgenol. 2024, 223, e2330036. [Google Scholar] [CrossRef]
- Bower, D.V.; Richter, J.K.; von Tengg-Kobligk, H.; Heverhagen, J.T.; Runge, V.M. Gadolinium-Based MRI Contrast Agents Induce Mitochondrial Toxicity and Cell Death in Human Neurons, and Toxicity Increases With Reduced Kinetic Stability of the Agent. Investig. Radiol. 2019, 54, 453–463. [Google Scholar] [CrossRef]
- Erdogan, M.A.; Apaydin, M.; Armagan, G.; Taskiran, D. Evaluation of toxicity of gadolinium-based contrast agents on neuronal cells. Acta Radiol. 2021, 62, 206–214. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef]
- Zhou, J.; Lal, B.; Wilson, D.A.; Laterra, J.; van Zijl, P.C. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn. Reson. Med. 2003, 50, 1120–1126. [Google Scholar] [CrossRef]
- Zaiss, M.; Windschuh, J.; Paech, D.; Meissner, J.E.; Burth, S.; Schmitt, B.; Kickingereder, P.; Wiestler, B.; Wick, W.; Bendszus, M.; et al. Relaxation-compensated CEST-MRI of the human brain at 7T: Unbiased insight into NOE and amide signal changes in human glioblastoma. Neuroimage 2015, 112, 180–188. [Google Scholar] [CrossRef]
- Zaiss, M.; Xu, J.; Goerke, S.; Khan, I.S.; Singer, R.J.; Gore, J.C.; Gochberg, D.F.; Bachert, P. Inverse Z-spectrum analysis for spillover-, MT-, and T1 -corrected steady-state pulsed CEST-MRI--application to pH-weighted MRI of acute stroke. NMR Biomed. 2014, 27, 240–252. [Google Scholar] [CrossRef]
- Zimmermann, F.; Korzowski, A.; Breitling, J.; Meissner, J.E.; Schuenke, P.; Loi, L.; Zaiss, M.; Bickelhaupt, S.; Schott, S.; Schlemmer, H.P.; et al. A novel normalization for amide proton transfer CEST MRI to correct for fat signal-induced artifacts: Application to human breast cancer imaging. Magn. Reson. Med. 2020, 83, 920–934. [Google Scholar] [CrossRef]
- Seo, N.; Jeong, H.K.; Choi, J.Y.; Park, M.S.; Kim, M.J.; Chung, Y.E. Liver MRI with amide proton transfer imaging: Feasibility and accuracy for the characterization of focal liver lesions. Eur. Radiol. 2020, 31, 222–231. [Google Scholar] [CrossRef]
- Heo, H.Y.; Zhang, Y.; Burton, T.M.; Jiang, S.; Zhao, Y.; van Zijl, P.C.M.; Leigh, R.; Zhou, J. Improving the detection sensitivity of pH-weighted amide proton transfer MRI in acute stroke patients using extrapolated semisolid magnetization transfer reference signals. Magn. Reson. Med. 2017, 78, 871–880. [Google Scholar] [CrossRef]
- Zhou, J.; Heo, H.Y.; Knutsson, L.; van Zijl, P.C.M.; Jiang, S. APT-weighted MRI: Techniques, current neuro applications, and challenging issues. J. Magn. Reson. Imaging 2019, 50, 347–364. [Google Scholar] [CrossRef]
- Dou, W.; Lin, C.E.; Ding, H.; Shen, Y.; Dou, C.; Qian, L.; Wen, B.; Wu, B. Chemical exchange saturation transfer magnetic resonance imaging and its main and potential applications in pre-clinical and clinical studies. Quant. Imaging Med. Surg. 2019, 9, 1747–1766. [Google Scholar] [CrossRef]
- Deng, M.; Chen, S.Z.; Yuan, J.; Chan, Q.; Zhou, J.; Wang, Y.X. Chemical Exchange Saturation Transfer (CEST) MR Technique for Liver Imaging at 3.0 Tesla: An Evaluation of Different Offset Number and an After-Meal and Over-Night-Fast Comparison. Mol. Imaging Biol. 2016, 18, 274–282. [Google Scholar] [CrossRef]
- Chen, S.Z.; Yuan, J.; Deng, M.; Wei, J.; Zhou, J.; Wang, Y.X. Chemical exchange saturation transfer (CEST) MR technique for in-vivo liver imaging at 3.0 tesla. Eur. Radiol. 2016, 26, 1792–1800. [Google Scholar] [CrossRef][Green Version]
- Lindquist, D.M.; Fugate, E.M.; Wang, J.; Sharma, A.; Gandhi, C.R.; Dillman, J.R. MRI Measures of Murine Liver Fibrosis. J. Magn. Reson. Imaging 2021, 54, 739–749. [Google Scholar] [CrossRef]
- Gast, L.V.; Platt, T.; Nagel, A.M.; Gerhalter, T. Recent technical developments and clinical research applications of sodium ((23)Na) MRI. Prog. Nucl. Magn. Reson. Spectrosc. 2023, 138–139, 1–51. [Google Scholar] [CrossRef]
- Borthakur, A.; Shapiro, E.M.; Beers, J.; Kudchodkar, S.; Kneeland, J.B.; Reddy, R. Sensitivity of MRI to proteoglycan depletion in cartilage: Comparison of sodium and proton MRI. Osteoarthr. Cartil. 2000, 8, 288–293. [Google Scholar] [CrossRef]
- Haacke, E.M. Magnetic Resonance Imaging : Physical Principles and Sequence Design; Wiley: New York, NY, USA, 1999; p. xxvii. 914p. [Google Scholar]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Juluri, R.; Vuppalanchi, R.; Olson, J.; Unalp, A.; Van Natta, M.L.; Cummings, O.W.; Tonascia, J.; Chalasani, N. Generalizability of the nonalcoholic steatohepatitis Clinical Research Network histologic scoring system for nonalcoholic fatty liver disease. J. Clin. Gastroenterol. 2011, 45, 55–58. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Mark, S.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Grady, J.T.; Cyrus, J.W.; Sterling, R.K. Novel Noninvasive Tests for Liver Fibrosis: Moving Beyond Simple Tests in Metabolic Dysfunction Associated Steatotic Liver Disease. Clin. Gastroenterol. Hepatol. 2025. [Google Scholar] [CrossRef]
- NIH. Available online: https://imagej.nih.gov/ij/ (accessed on 16 January 2020).
- Bansal, N.; Germann, M.J.; Seshan, V.; Shires, G.T., 3rd; Malloy, C.R.; Sherry, A.D. Thulium 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrakis(methylene phosphonate) as a 23Na shift reagent for the in vivo rat liver. Biochemistry 1993, 32, 5638–5643. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Huttasch, M.; Roden, M.; Kahl, S. Obesity and MASLD: Is weight loss the (only) key to treat metabolic liver disease? Metabolism 2024, 157, 155937. [Google Scholar] [CrossRef]
- Ajmera, V.; Loomba, R. Imaging biomarkers of NAFLD, NASH, and fibrosis. Mol. Metab. 2021, 50, 101167. [Google Scholar] [CrossRef]
- Hagstrom, H.; Shang, Y.; Hegmar, H.; Nasr, P. Natural history and progression of metabolic dysfunction-associated steatotic liver disease. Lancet Gastroenterol. Hepatol. 2024, 9, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Birchall, J.R.; Horvat-Menih, I.; Kaggie, J.D.; Riemer, F.; Benjamin, A.J.V.; Graves, M.J.; Wilkinson, I.; Gallagher, F.A.; McLean, M.A. Quantitative (23)Na magnetic resonance imaging in the abdomen at 3 T. MAGMA 2024, 37, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Mikolasevic, I.; Domislovic, V.; Krznaric-Zrnic, I.; Krznaric, Z.; Virovic-Jukic, L.; Stojsavljevic, S.; Grgurevic, I.; Milic, S.; Vukoja, I.; Puz, P.; et al. The Accuracy of Serum Biomarkers in the Diagnosis of Steatosis, Fibrosis, and Inflammation in Patients with Nonalcoholic Fatty Liver Disease in Comparison to a Liver Biopsy. Medicina 2022, 58, 252. [Google Scholar] [CrossRef] [PubMed]


| T1 Mapping | T2 Mapping | PDFF/T2* Mapping | Elastography | APT | Sodium | |
|---|---|---|---|---|---|---|
| Sequence | MOLLI (FFE) | TSE | mDIXON Quant | FFE | TSE | FFE |
| TR (ms) | 2.42 | 184 | 5.2 | 50 | 2500 | 50 |
| TE (ms) | 1.05 | 12 | 0.87 | 20 | 46 | 1.3 |
| Matrix | 480 × 480 | 256 × 256 | 240 × 240 | 432 × 432 | 224 × 224 | 68 × 67 |
| NSA | 1 | 1 | 1 | 1 | 1 | 256 |
| Breath-hold † | Y | N | Y | Y | Y | N |
| Sequence-Specific Parameters | Simulated heart rate of 60 beats/minute; 5(3)3 implementation | 8 echo times; respiratory triggered | 6 echoes | Driver frequency 60 Hz; driver amplitude was set to moderate | 16 echoes; ProSet fat suppression; 5 100-ms sinc-gauss RF pulses, 4.2 µT, at offsets of ±3.5 and 23.5 ppm relative to water | Flip angle: 90° |
| Indicator | Mean (SD) | Range |
|---|---|---|
| Inflammation Score | 1.3 (0.8) | 0–3 |
| Fibrosis Score | 1.3 (0.8) | 0–3 |
| NAS Score | 3.7 (1.4) | 2–6 |
| ALT (U/L) | 98 (99) | 7–381 |
| AST (U/L) | 53 (39) | 17–162 |
| Fib4 Score | 0.37 (0.14) | 0.20–0.69 |
| MRI Parameter | Healthy Controls | Patients | p Value * |
|---|---|---|---|
| T1 (ms) | 821 ± 87 (n = 18) | 983 ± 111 (n = 16) | <0.001 |
| T2 (ms) | 50 ± 8 (n = 18) | 53 ± 16 (n = 16) | 0.8 |
| PDFF (%) | 4.1 ± 1.3 (n = 18) | 15 ± 11 (n = 16) | <0.001 |
| Liver Stiffness (kPa) | 2.1 ± 0.4 (n = 17) | 2.8 ± 0.7 (n = 15) | 0.004 |
| APTw (%) | −5.3 ± 7.9 (n = 17) | −1.9 ± 6.4 (n = 16) | 0.2 |
| MTR+3.5 ppm (%) | 30.2 ± 4.8 (n = 17) | 24.3 ± 6.7 (n = 16) | 0.008 |
| MTR−3.5 ppm (%) | 34.7 ± 6.8 (n = 17) | 26.2 ± 7.6 (n = 16) | 0.002 |
| TSC (mM) | 33 ± 15 (n = 12) | 23 ± 5 (n = 12) | 0.046 |
| Inflammation | Fibrosis Score | NAS | ALT | AST | Fibrosis-4 Score | |
|---|---|---|---|---|---|---|
| Liver Stiffness | −0.03 (0.9) | 0.21 (0.4) | 0.33 (0.2) | 0.24 (0.4) | 0.04 (0.9) | −0.36 (0.2) |
| T1 | 0.62 (0.01) | 0.14 (0.6) | 0.53 (0.04) | 0.72 (0.002) | 0.45 (0.08) | −0.44 (0.09) |
| T2 | −0.27 (0.3) | −0.21 (0.4) | −0.80 (0.0002) | −0.62 (0.01) | −0.49 (0.05) | 0.28 (0.3) |
| PDFF | 0.40 (0.1) | 0.25 (0.3) | 0.75 (0.0008) | 0.69 (0.003) | 0.53 (0.03) | −0.16 (0.5) |
| APTw | −0.34 (0.2) | −0.02 (0.9) | −0.32 (0.2) | −0.19 (0.5) | −0.02 (0.9) | 0.26 (0.3) |
| MTR+3.5 ppm | −0.02 (0.9) | 0.12 (0.6) | 0.33 (0.2) | 0.18 (0.5) | 0.04 (0.9) | −0.04 (0.9) |
| MTR−3.5 ppm | 0.2 (0.5) | 0.06 (0.8) | 0.41 (0.1) | 0.24 (0.4) | 0.07 (0.8) | −0.11 (0.7) |
| TSC | 0.11 (0.7) | −0.24 (0.5) | −0.2 (0.5) | −0.39 (0.2) | −0.5 (0.1) | −0.69 (0.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindquist, D.M.; Manhard, M.K.; Levoy, J.; Dillman, J.R. Feasibility of Sodium and Amide Proton Transfer-Weighted Magnetic Resonance Imaging Methods in Mild Steatotic Liver Disease. Tomography 2025, 11, 89. https://doi.org/10.3390/tomography11080089
Lindquist DM, Manhard MK, Levoy J, Dillman JR. Feasibility of Sodium and Amide Proton Transfer-Weighted Magnetic Resonance Imaging Methods in Mild Steatotic Liver Disease. Tomography. 2025; 11(8):89. https://doi.org/10.3390/tomography11080089
Chicago/Turabian StyleLindquist, Diana M., Mary Kate Manhard, Joel Levoy, and Jonathan R. Dillman. 2025. "Feasibility of Sodium and Amide Proton Transfer-Weighted Magnetic Resonance Imaging Methods in Mild Steatotic Liver Disease" Tomography 11, no. 8: 89. https://doi.org/10.3390/tomography11080089
APA StyleLindquist, D. M., Manhard, M. K., Levoy, J., & Dillman, J. R. (2025). Feasibility of Sodium and Amide Proton Transfer-Weighted Magnetic Resonance Imaging Methods in Mild Steatotic Liver Disease. Tomography, 11(8), 89. https://doi.org/10.3390/tomography11080089






