Optimizing Imaging Parameters for Assessment of Hepatocellular Carcinoma Using Photon-Counting Detector Computed Tomography—Impact of Reconstruction Kernel and Slice Thickness
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Image Acquisition
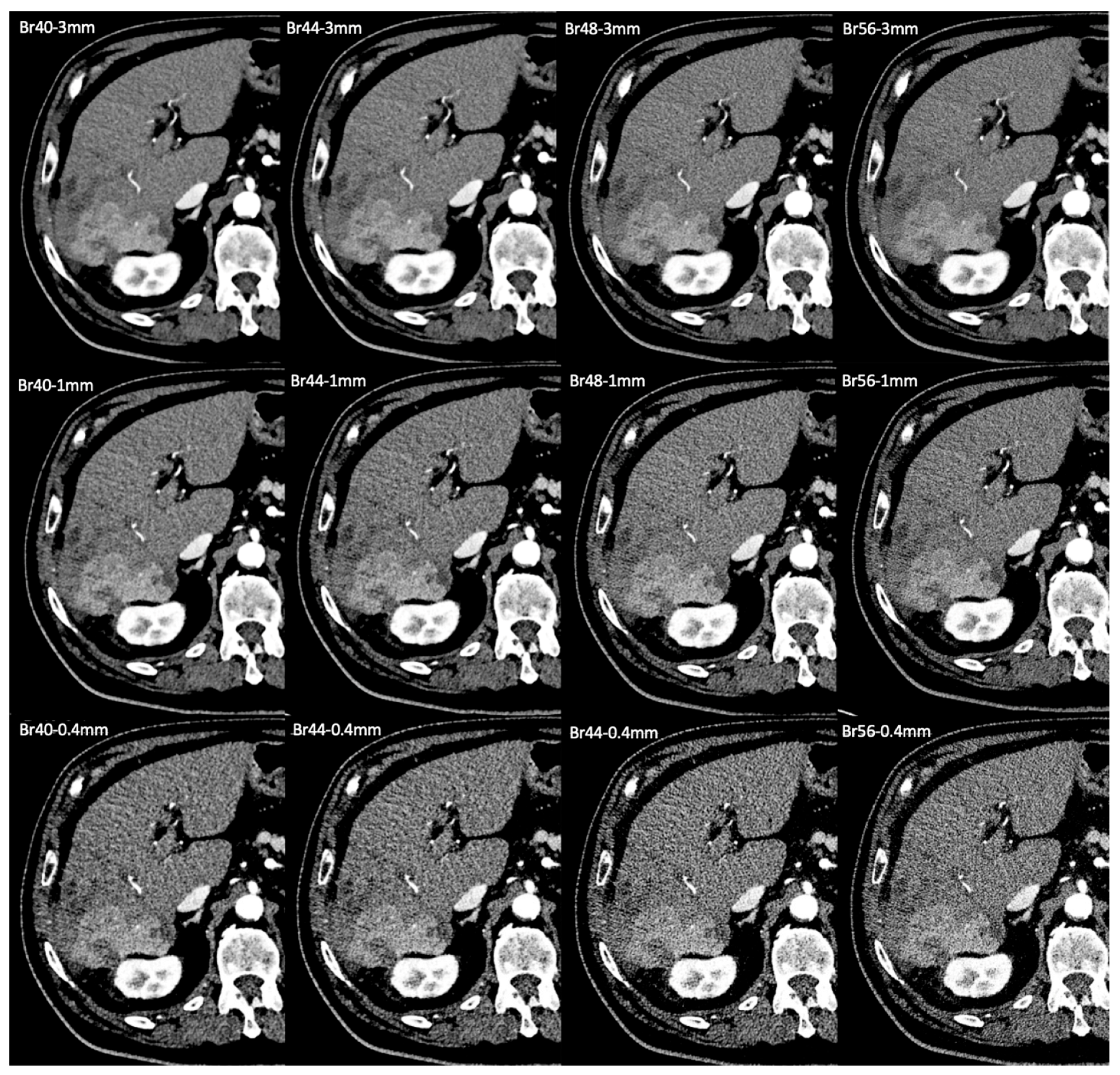
2.3. Image Reconstruction
2.4. Qualitative Image Analysis
2.5. Quantitative Analysis
2.6. Statistical Analysis
3. Results
3.1. Patients and Demographic Details
3.2. Objective Image Quality
3.3. Subjective Image Quality
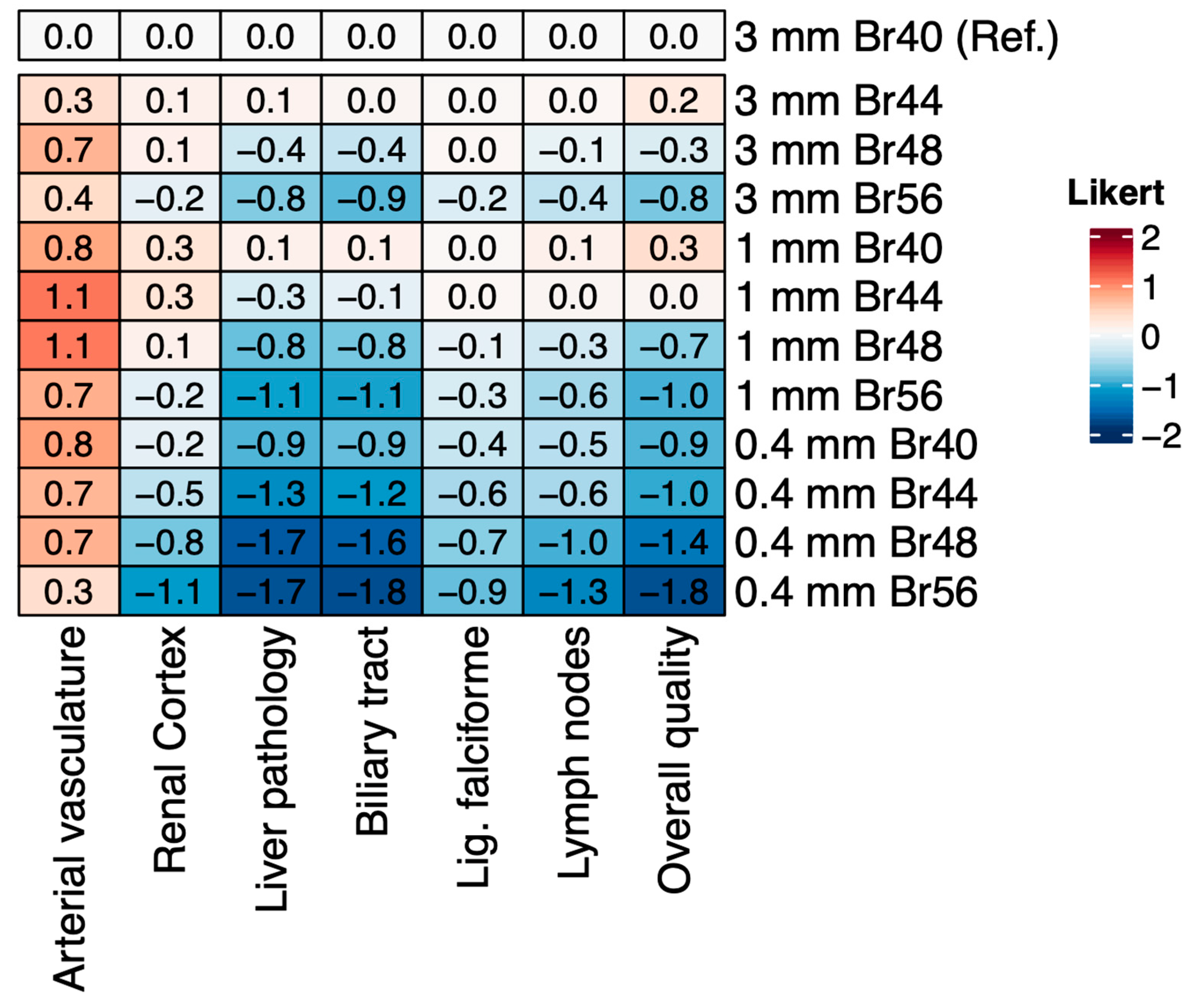

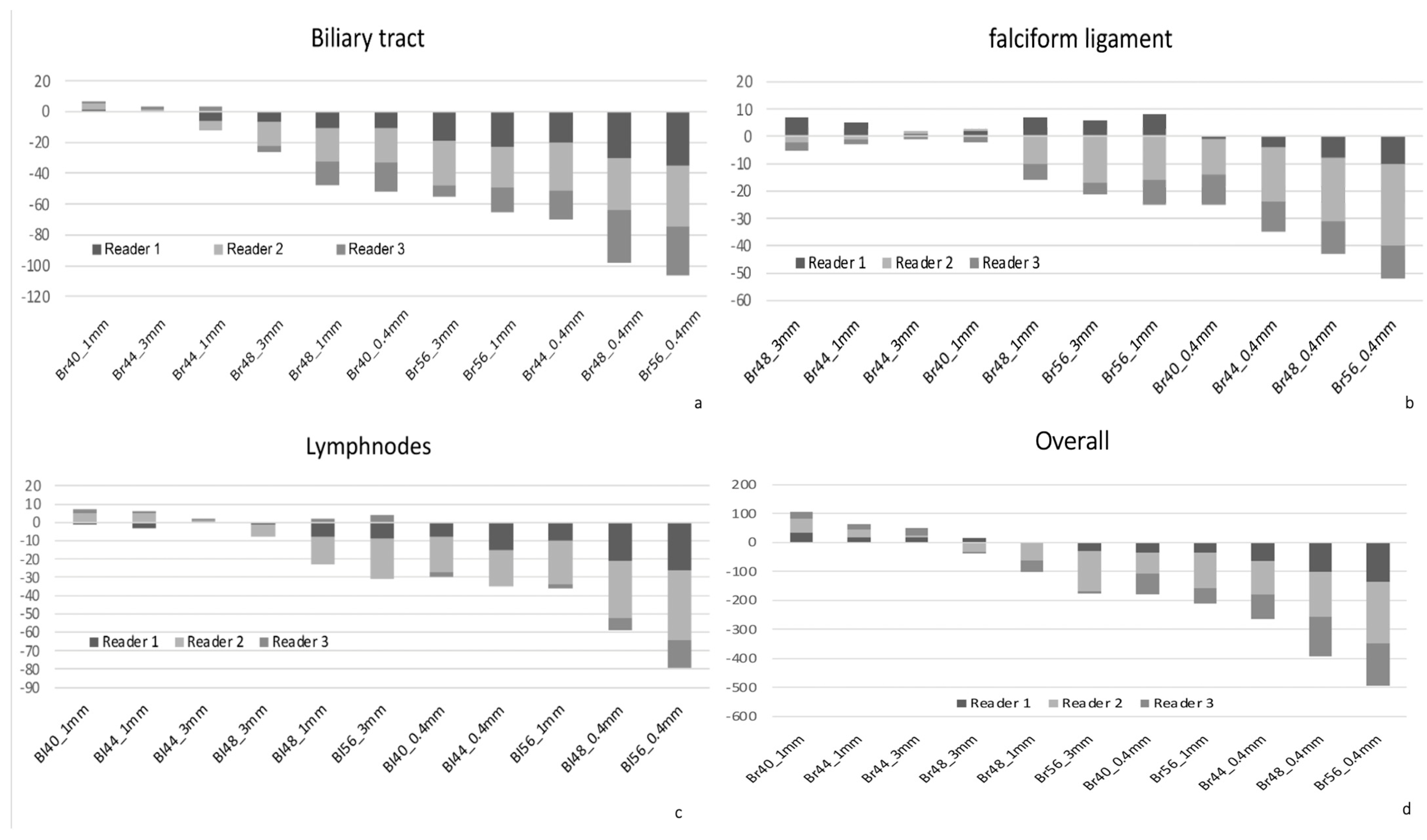
3.4. Assessment of Image Sharpness
3.5. Assessment of Intrahepatic Vasculature
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koons, E.; VanMeter, P.; Rajendran, K.; Yu, L.; McCollough, C.; Leng, S. Improved quantification of coronary artery luminal stenosis in the presence of heavy calcifications using photon-counting detector CT. Proc. SPIE Int. Soc. Opt. Eng. 2022, 12031, 120311A. [Google Scholar] [PubMed]
- Bette, S.J.; Braun, F.M.; Haerting, M.; Decker, J.A.; Luitjens, J.H.; Scheurig-Muenkler, C.; Kroencke, T.J.; Schwarz, F. Visualization of bone details in a novel photon-counting dual-source CT scanner-comparison with energy-integrating CT. Eur. Radiol. 2022, 32, 2930–2936. [Google Scholar] [CrossRef]
- Winkelmann, M.T.; Hagen, F.; Le-Yannou, L.; Weiss, J.; Riffel, P.; Gutjahr, R.; Faby, S.; Nikolaou, K.; Horger, M. Myeloma bone disease imaging on a 1st-generation clinical photon-counting detector CT vs. 2nd-generation dual-source dual-energy CT. Eur. Radiol. 2023, 33, 2415–2425. [Google Scholar] [CrossRef] [PubMed]
- Graafen, D.; Müller, L.; Halfmann, M.; Düber, C.; Hahn, F.; Yang, Y.; Emrich, T.; Kloeckner, R. Photon-counting detector CT improves quality of arterial phase abdominal scans: A head-to-head comparison with energy-integrating CT. Eur. J. Radiol. 2022, 156, 110514. [Google Scholar] [CrossRef] [PubMed]
- Symons, R.; Pourmorteza, A.; Sandfort, V.; Ahlman, M.A.; Cropper, T.; Mallek, M.; Kappler, S.; Ulzheimer, S.; Mahesh, M.; Jones, E.C.; et al. Feasibility of Dose-reduced Chest CT with Photon-counting Detectors: Initial Results in Humans. Radiology 2017, 285, 980–989. [Google Scholar] [CrossRef]
- Willemink, M.J.; Persson, M.; Pourmorteza, A.; Pelc, N.J.; Fleischmann, D. Photon-counting CT: Technical Principles and Clinical Prospects. Radiology 2018, 289, 293–312. [Google Scholar] [CrossRef]
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef]
- Flohr, T.; Petersilka, M.; Henning, A.; Ulzheimer, S.; Ferda, J.; Schmidt, B. Photon-counting CT review. Phys. Med. 2020, 79, 126–136. [Google Scholar] [CrossRef]
- Zhou, W.; Bartlett, D.J.; Diehn, F.E.; Glazebrook, K.N.; Kotsenas, A.L.; Carter, R.E.; Fletcher, J.G.; McCollough, C.H.; Leng, S. Reduction of Metal Artifacts and Improvement in Dose Efficiency Using Photon-Counting Detector Computed Tomography and Tin Filtration. Investig. Radiol. 2019, 54, 204–211. [Google Scholar] [CrossRef]
- Rajendran, K.; Petersilka, M.; Henning, A.; Shanblatt, E.R.; Schmidt, B.; Flohr, T.G.; Ferrero, A.; Baffour, F.; Diehn, F.E.; Yu, L.; et al. First Clinical Photon-counting Detector CT System: Technical Evaluation. Radiology 2022, 303, 130–138. [Google Scholar] [CrossRef]
- Gutjahr, R.; Halaweish, A.F.; Yu, Z.; Leng, S.; Yu, L.; Li, Z.; Jorgensen, S.M.; Ritman, E.L.; Kappler, S.; McCollough, C.H. Human Imaging With Photon Counting-Based Computed Tomography at Clinical Dose Levels: Contrast-to-Noise Ratio and Cadaver Studies. Investig. Radiol. 2016, 51, 421–429. [Google Scholar] [CrossRef]
- Pourmorteza, A.; Symons, R.; Sandfort, V.; Mallek, M.; Fuld, M.K.; Henderson, G.; Jones, E.C.; Malayeri, A.A.; Folio, L.R.; Bluemke, D.A. Abdominal Imaging with Contrast-enhanced Photon-counting CT: First Human Experience. Radiology 2016, 279, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.; Bruesewitz, M.; Tao, S.; Rajendran, K.; Halaweish, A.F.; Campeau, N.G.; Fletcher, J.G.; McCollough, C.H. Photon-counting Detector CT: System Design and Clinical Applications of an Emerging Technology. Radiographics 2019, 39, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Mergen, V.; Sartoretti, T.; Baer-Beck, M.; Schmidt, B.; Petersilka, M.; Wildberger, J.E.; Euler, A.; Eberhard, M.; Alkadhi, H. Ultra-High-Resolution Coronary CT Angiography With Photon-Counting Detector CT: Feasibility and Image Characterization. Investig. Radiol. 2022, 57, 780–788. [Google Scholar] [CrossRef]
- Yu, Z.; Leng, S.; Jorgensen, S.M.; Li, Z.; Gutjahr, R.; Chen, B.; Halaweish, A.F.; Kappler, S.; Yu, L.; Ritman, E.L.; et al. Evaluation of conventional imaging performance in a research whole-body CT system with a photon-counting detector array. Phys. Med. Biol. 2016, 61, 1572–1595. [Google Scholar] [CrossRef]
- Milos, R.I.; Rohrich, S.; Prayer, F.; Strassl, A.; Beer, L.; Heidinger, B.H.; Weber, M.; Watzenboeck, M.L.; Kifjak, D.; Tamandl, D.; et al. Ultrahigh-Resolution Photon-Counting Detector CT of the Lungs: Association of Reconstruction Kernel and Slice Thickness With Image Quality. AJR Am. J. Roentgenol. 2023, 220, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, F.R.; Marin, D.; Lofino, L.; Abadia, A.; O’Donnell, T.; Dane, B. Protocol optimization for abdominal imaging using photon-counting CT: A consensus of two academic institutions. Abdom. Radiol. 2024, 49, 1762–1770. [Google Scholar] [CrossRef]
- Matsui, O.; Kobayashi, S.; Sanada, J.; Kouda, W.; Ryu, Y.; Kozaka, K.; Kitao, A.; Nakamura, K.; Gabata, T. Hepatocelluar nodules in liver cirrhosis: Hemodynamic evaluation (angiography-assisted CT) with special reference to multi-step hepatocarcinogenesis. Abdom. Imaging 2011, 36, 264–272. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Decker, J.A.; Bette, S.; Lubina, N.; Rippel, K.; Braun, F.; Risch, F.; Woznicki, P.; Wollny, C.; Scheurig-Muenkler, C.; Kroencke, T.J.; et al. Low-dose CT of the abdomen: Initial experience on a novel photon-counting detector CT and comparison with energy-integrating detector CT. Eur. J. Radiol. 2022, 148, 110181. [Google Scholar] [CrossRef]
- Higashigaito, K.; Euler, A.; Eberhard, M.; Flohr, T.G.; Schmidt, B.; Alkadhi, H. Contrast-Enhanced Abdominal CT with Clinical Photon-Counting Detector CT: Assessment of Image Quality and Comparison with Energy-Integrating Detector CT. Acad. Radiol. 2022, 29, 689–697. [Google Scholar] [CrossRef]
- Beer, L.; Toepker, M.; Ba-Ssalamah, A.; Schestak, C.; Dutschke, A.; Schindl, M.; Wressnegger, A.; Ringl, H.; Apfaltrer, P. Objective and subjective comparison of virtual monoenergetic vs. polychromatic images in patients with pancreatic ductal adenocarcinoma. Eur. Radiol. 2019, 29, 3617–3625. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, F.R.; Samei, E.; Marin, D. Exploiting the Potential of Photon-Counting CT in Abdominal Imaging. Investig. Radiol. 2023, 58, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Graafen, D.; Stoehr, F.; Halfmann, M.C.; Emrich, T.; Foerster, F.; Yang, Y.; Duber, C.; Muller, L.; Kloeckner, R. Quantum iterative reconstruction on a photon-counting detector CT improves the quality of hepatocellular carcinoma imaging. Cancer Imaging 2023, 23, 69. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, F.R.; Ashton, J.; Wildman-Tobriner, B.; Molvin, L.; Ramirez-Giraldo, J.C.; Samei, E.; Bashir, M.R.; Marin, D. Liver fat quantification in photon counting CT in head to head comparison with clinical MRI—First experience. Eur. J. Radiol. 2023, 161, 110734. [Google Scholar] [CrossRef]
- Dillinger, D.; Overhoff, D.; Booz, C.; Kaatsch, H.L.; Piechotka, J.; Hagen, A.; Froelich, M.F.; Vogl, T.J.; Waldeck, S. Impact of CT Photon-Counting Virtual Monoenergetic Imaging on Visualization of Abdominal Arterial Vessels. Diagnostics 2023, 13, 938. [Google Scholar] [CrossRef]

| Scan Parameters | |
|---|---|
| Collimation | 144 × 0.4 mm |
| Rotation time | 0.5 s |
| Pitch factor | 0.80 |
| Tube potential | 120 kVp |
| CARE keV IQ Level | 170 |
| Reconstruction Parameters | |
| Slice thickness | 3 mm/1 mm/0.4 mm |
| Reconstruction Increment | 2 mm/0.8 mm/0.3 mm |
| Matrix Size | 512 × 512 |
| Kernel | Br40/Br44/Br48/Br56 |
| Quantum iterative reconstruction | Level 3 |
| Window | User specific |
| CNR | Noise | ||||
|---|---|---|---|---|---|
| Reconstruction | HCC | Aorta | CHA | Kidney | |
| Br40–3 mm | 4.5 ± 2.0 | 39.1 ± 13.0 | 37.9 ± 11.9 | 15.1 ± 5.2 | 12.4 ± 1.6 |
| Br44–3 mm | 3.5 ± 1.5 | 30.5 ± 9.6 ** | 29.8 ± 9.1 * | 11.8 ± 4.0 * | 15.8 ± 1.9 |
| Br48–3 mm | 2.7 ± 1.2 *** | 24.2 ± 7.6 *** | 23.0 ± 7.3 *** | 9.2 ± 3.2 *** | 19.8 ± 2.3 *** |
| Br56–3 mm | 2.0 ± 0.8 *** | 17.9 ± 5.1 *** | 16.8 ± 4.9 *** | 6.6 ± 2.3 *** | 26.8 ± 2.8 ** |
| Br40–1 mm | 3.0 ± 1.2 ** | 26.5 ± 9.2 *** | 27.8 ± 9.7 *** | 10.4 ± 3.7 *** | 18.4 ± 2.7 *** |
| Br44–1 mm | 2.4 ± 0.9 *** | 20.4 ± 6.6 *** | 21.4 ± 7.1 *** | 8.0 ± 2.7 *** | 23.7 ± 3.1 *** |
| Br48–1 mm | 1.8 ± 0.7 *** | 15.9 ± 5.2 *** | 16.2 ± 5.5 *** | 6.1 ± 2.2 *** | 30.2 ± 4.2 *** |
| Br56–1 mm | 1.3 ± 0.5 *** | 11.4 ± 3.3 *** | 11.5 ± 3.6 *** | 4.3 ± 1.5 *** | 42.1 ± 4.8 *** |
| Br40–0.4 mm | 2.0 ± 0.7 *** | 17.6 ± 6.0 *** | 18.5 ± 6.6 *** | 6.9 ± 2.3 *** | 27.7 ± 3.8 *** |
| Br44–0.4 mm | 1.5 ± 0.6 *** | 13.3 ± 4.5 *** | 14.0 ± 5.0 *** | 5.2 ± 1.8 *** | 36.6 ± 6.8 *** |
| Br48–0.4 mm | 1.2 ± 0.5 *** | 10.5 ± 3.4 *** | 10.7 ± 3.7 *** | 4.1 ± 1.4 *** | 45.6 ± 5.5 *** |
| Br56–0.4 mm | 0.9 ± 0.3 *** | 7.6 ± 2.2 *** | 7.6 ± 2.5 *** | 2.9 ± 1.0 *** | 63.8 ± 7.3 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szelenyi, A.; Stelzer, P.; Wassipaul, C.; Kittinger, J.; Strassl, A.; Schmidbauer, V.; Watzenböck, M.L.; Lindenlaub, F.; Arnoldner, M.; Weber, M.; et al. Optimizing Imaging Parameters for Assessment of Hepatocellular Carcinoma Using Photon-Counting Detector Computed Tomography—Impact of Reconstruction Kernel and Slice Thickness. Tomography 2025, 11, 77. https://doi.org/10.3390/tomography11070077
Szelenyi A, Stelzer P, Wassipaul C, Kittinger J, Strassl A, Schmidbauer V, Watzenböck ML, Lindenlaub F, Arnoldner M, Weber M, et al. Optimizing Imaging Parameters for Assessment of Hepatocellular Carcinoma Using Photon-Counting Detector Computed Tomography—Impact of Reconstruction Kernel and Slice Thickness. Tomography. 2025; 11(7):77. https://doi.org/10.3390/tomography11070077
Chicago/Turabian StyleSzelenyi, Anna, Philipp Stelzer, Christian Wassipaul, Jakob Kittinger, Andreas Strassl, Victor Schmidbauer, Martin Luther Watzenböck, Florian Lindenlaub, Michael Arnoldner, Michael Weber, and et al. 2025. "Optimizing Imaging Parameters for Assessment of Hepatocellular Carcinoma Using Photon-Counting Detector Computed Tomography—Impact of Reconstruction Kernel and Slice Thickness" Tomography 11, no. 7: 77. https://doi.org/10.3390/tomography11070077
APA StyleSzelenyi, A., Stelzer, P., Wassipaul, C., Kittinger, J., Strassl, A., Schmidbauer, V., Watzenböck, M. L., Lindenlaub, F., Arnoldner, M., Weber, M., Pinter, M., Milos, R.-I., & Tamandl, D. (2025). Optimizing Imaging Parameters for Assessment of Hepatocellular Carcinoma Using Photon-Counting Detector Computed Tomography—Impact of Reconstruction Kernel and Slice Thickness. Tomography, 11(7), 77. https://doi.org/10.3390/tomography11070077






