Qualitative and Quantitative Computed Tomography Analyses of Lung Adenocarcinoma for Predicting Spread Through Air Spaces
Abstract
1. Introduction
2. Materials and Methods
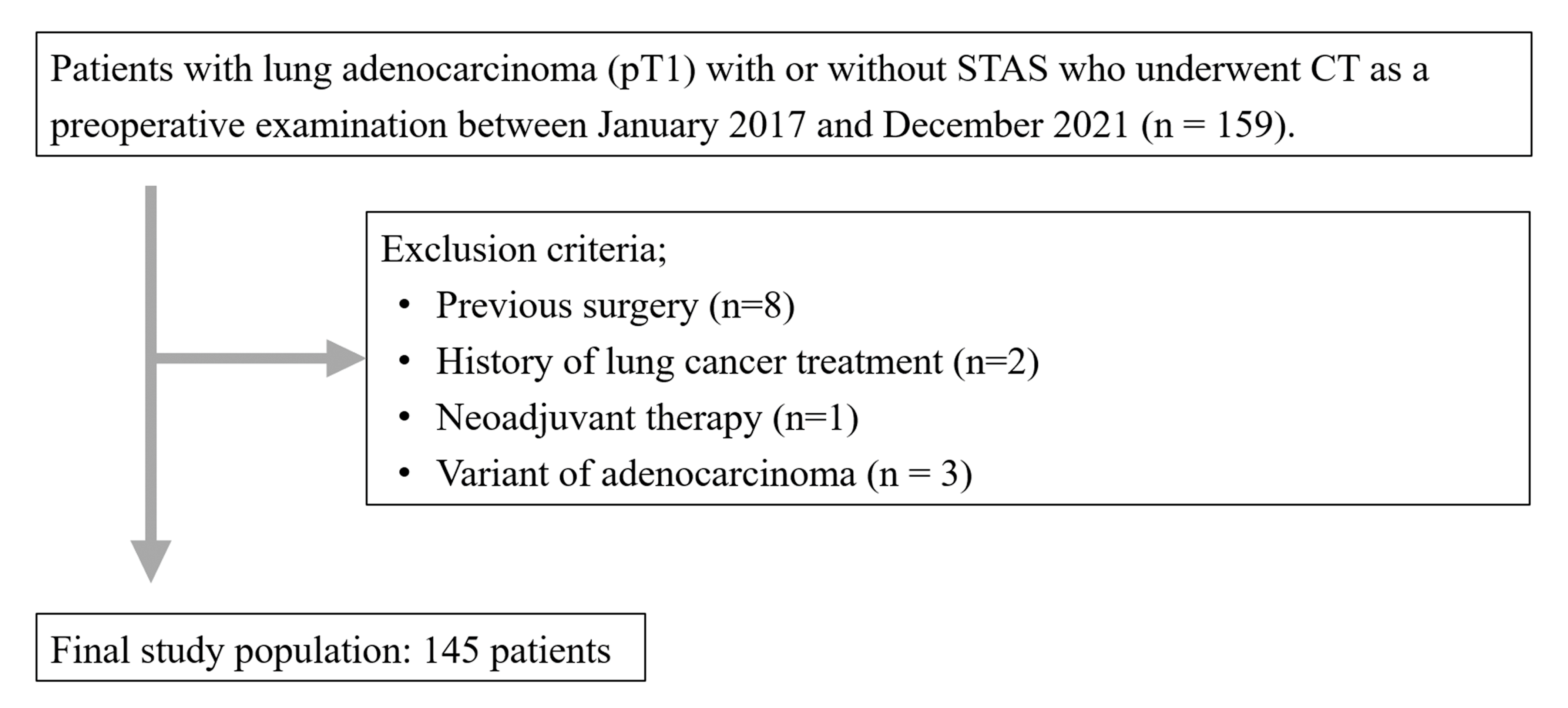
2.1. Patients
2.2. CT Examinations
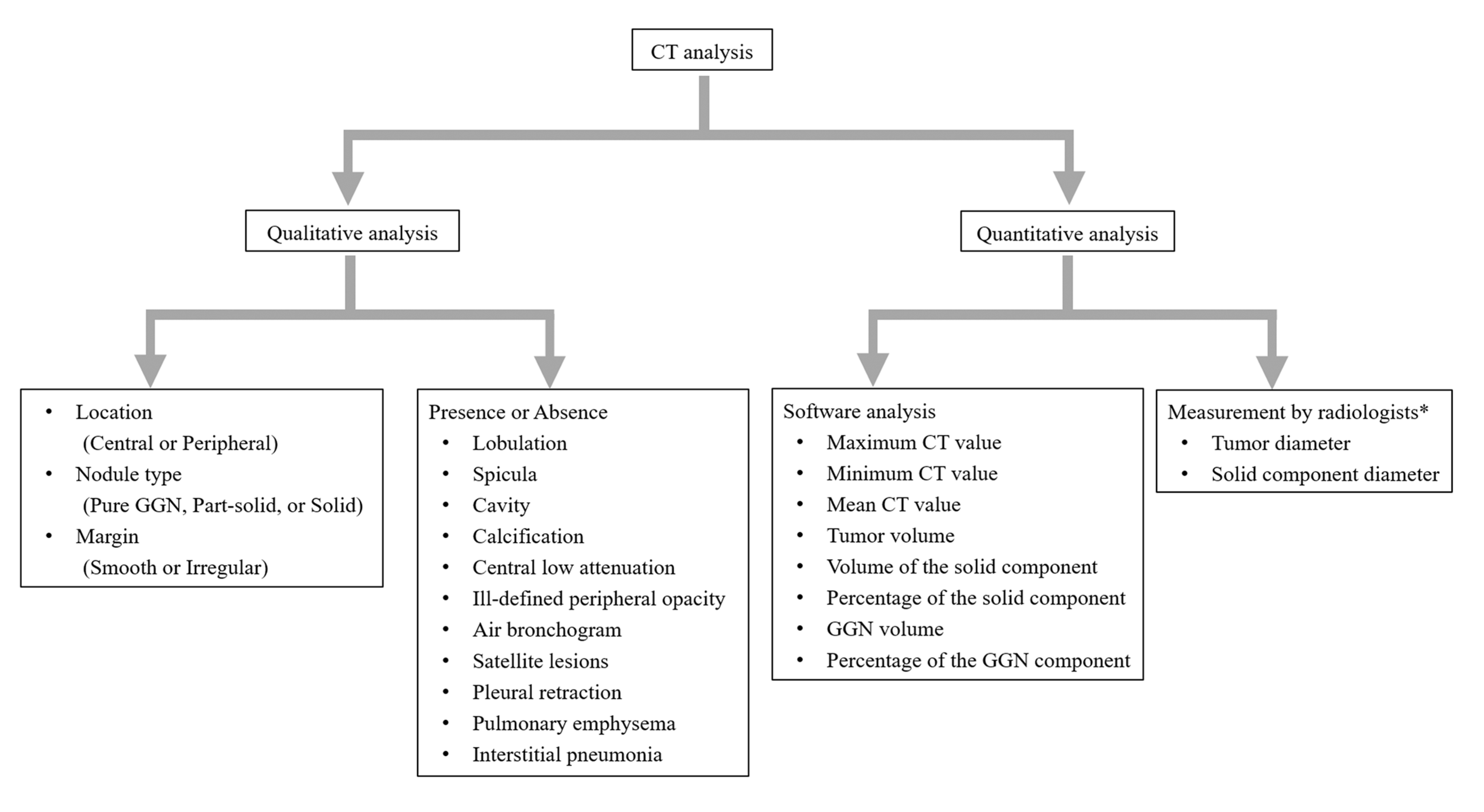
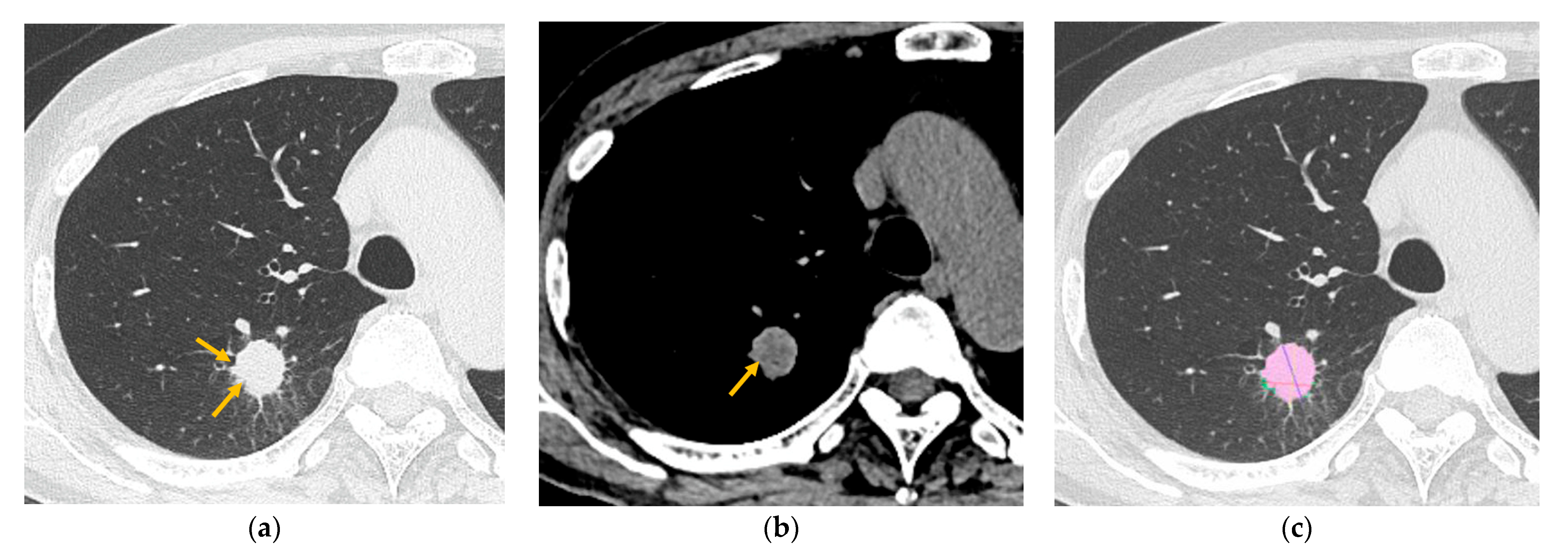
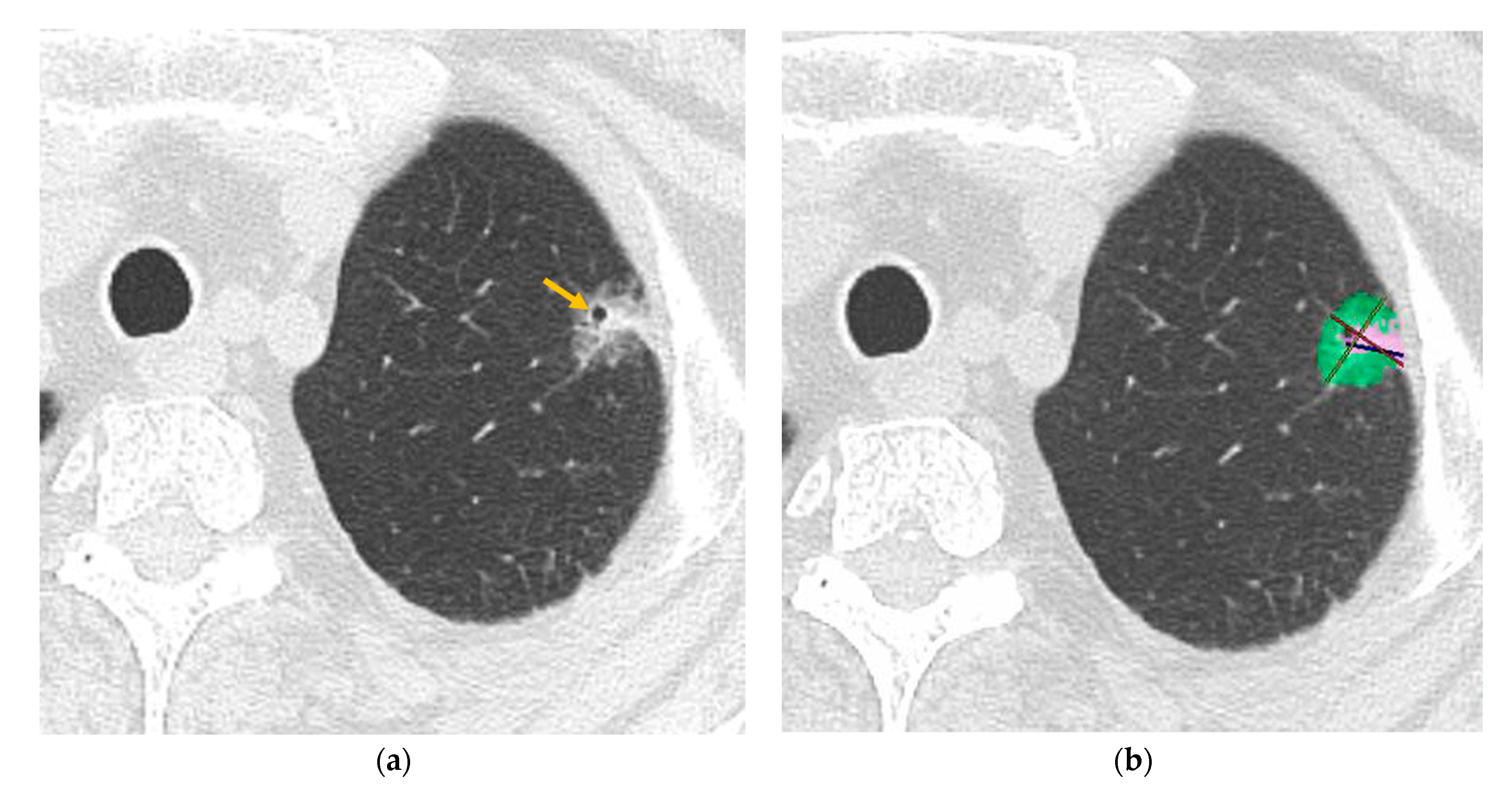
2.3. CT Analysis
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics and CT Analysis
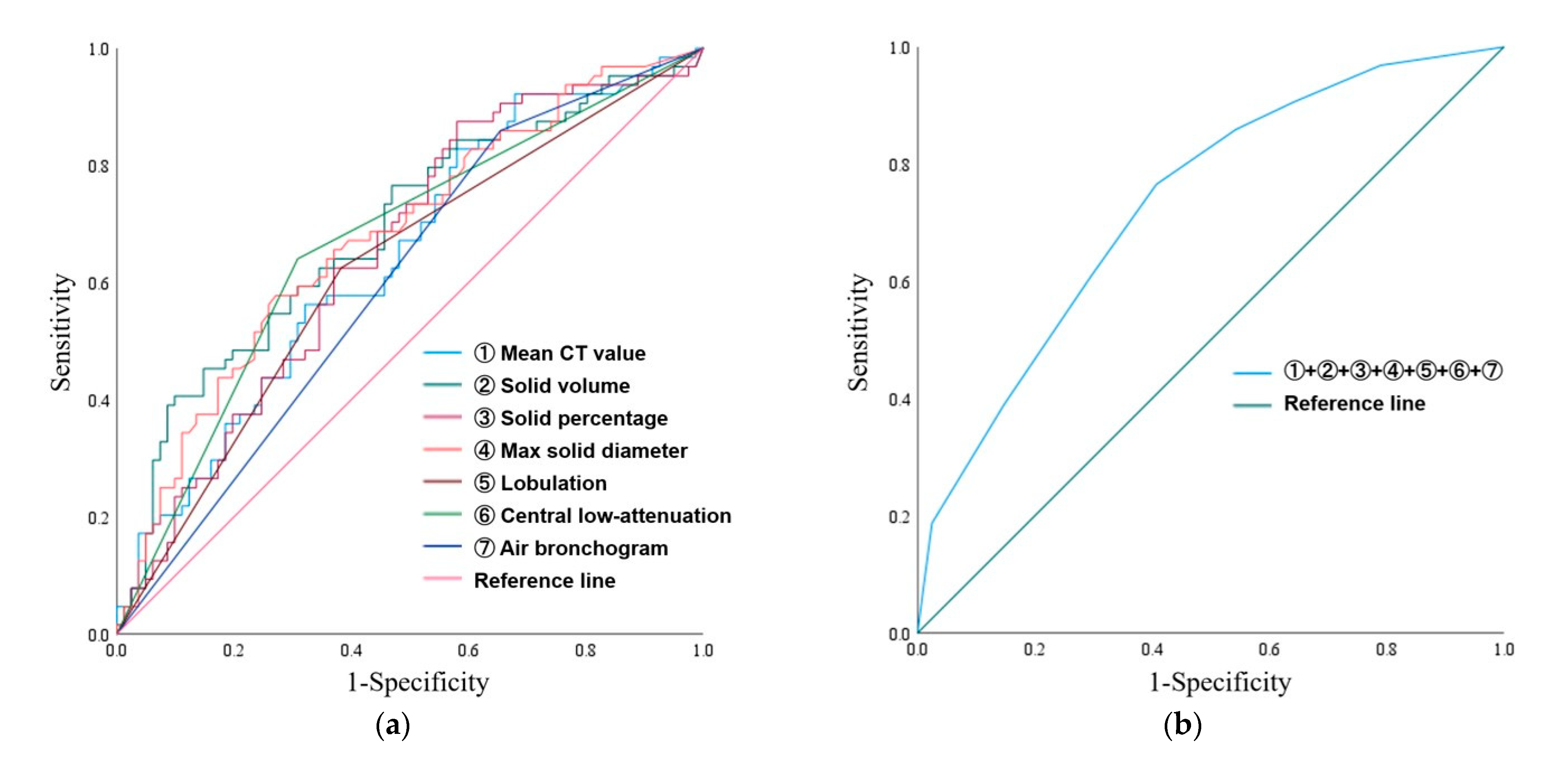
3.2. ROC Curve Analysis and Multiple Logistic Regression Analyses
3.3. Comparative Analysis Between the Cases With or Without Preoperative Biopsy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| STAS | Spread through air spaces |
| GGN | Ground-glass nodule |
| NSCLC | Non-small-cell lung cancer |
| TNM | Tumor-node-metastasis |
| AIS | Adenocarcinoma in situ |
| MIA | Minimally invasive adenocarcinoma |
| IAC | Invasive adenocarcinoma |
| 3D | Three-dimensional |
| AI | Artificial intelligence |
| WHO | World Health Organization |
| PACS | Picture archiving and communication system |
| WL | Window level |
| WW | Window width |
| HU | Hounsfield unit |
| BAL | Bronchoalveolar lavage |
| TBLB | Transbronchial lung biopsy |
| Max | Maximum |
| Min | Minimum |
| LN | Lymph node |
| ROC | Receiver operating characteristics |
| AUC | Area under the curve |
| CI | Confidence interval |
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Travis, W.D.; Asamura, H.; Bankier, A.A.; Beasley, M.B.; Detterbeck, F.; Flieder, D.B.; Goo, J.M.; MacMahon, H.; Naidich, D.; Nicholson, A.G.; et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J. Thorac. Oncol. 2016, 11, 1204–1223. [Google Scholar] [CrossRef]
- Kuriyama, K.; Tateishi, R.; Doi, O.; Kodama, K.; Tatsuta, M.; Matsuda, M.; Mitani, T.; Narumi, Y.; Fujita, M. CT-pathologic correlation in small peripheral lung cancers. AJR Am. J. Roentgenol. 1987, 149, 1139–1143. [Google Scholar] [CrossRef]
- Gierada, D.S.; Rydzak, C.E.; Zei, M.; Rhea, L. Improved Interobserver Agreement on Lung-RADS Classification of Solid Nodules Using Semiautomated CT Volumetry. Radiology 2020, 297, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Kadota, K.; Nitadori, J.I.; Sima, C.S.; Ujiie, H.; Rizk, N.P.; Jones, D.R.; Adusumilli, P.S.; Travis, W.D. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J. Thorac. Oncol. 2015, 10, 806–814. [Google Scholar] [CrossRef]
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J. Thorac. Oncol. 2022, 17, 362–387. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, T.J.; Chung, M.J.; Kim, T.S.; Lee, K.S.; Zo, J.I.; Shim, Y.M. Lung Adenocarcinoma: CT Features Associated with Spread through Air Spaces. Radiology 2018, 289, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Eisele, M.; Nishimura, K.K.; Aly, R.G.; Bertoglio, P.; Chou, T.Y.; Detterbeck, F.C.; Donnington, J.; Fang, W.; Joubert, P.; et al. The International Association for the Study of Lung Cancer (IASLC) Staging Project for Lung Cancer: Recommendation to Introduce Spread Through Air Spaces as a Histologic Descriptor in the Ninth Edition of the TNM Classification of Lung Cancer. Analysis of 4061 Pathologic Stage I NSCLC. J. Thorac. Oncol. 2024, 19, 1028–1051. [Google Scholar]
- Chen, Y.; Jiang, C.; Kang, W.; Gong, J.; Luo, D.; You, S.; Cheng, Z.; Luo, Y.; Wu, K. Development and validation of a CT-based nomogram to predict spread through air space (STAS) in peripheral stage IA lung adenocarcinoma. Jpn. J. Radiol. 2022, 40, 586–594. [Google Scholar] [CrossRef]
- de Margerie-Mellon, C.; Onken, A.; Heidinger, B.H.; VanderLaan, P.A.; Bankier, A.A. CT Manifestations of Tumor Spread Through Airspaces in Pulmonary Adenocarcinomas Presenting as Subsolid Nodules. J. Thorac. Imaging 2018, 33, 402–408. [Google Scholar] [CrossRef]
- Koezuka, S.; Mikami, T.; Tochigi, N.; Sano, A.; Azuma, Y.; Makino, T.; Otsuka, H.; Matsumoto, K.; Shiraga, N.; Iyoda, A. Toward improving prognosis prediction in patients undergoing small lung adenocarcinoma resection: Radiological and pathological assessment of diversity and intratumor heterogeneity. Lung Cancer 2019, 135, 40–46. [Google Scholar] [CrossRef]
- Qin, L.; Sun, Y.; Zhu, R.; Hu, B.; Wu, J. Clinicopathological and CT features of tumor spread through air space in invasive lung adenocarcinoma. Front. Oncol. 2022, 12, 959113. [Google Scholar] [CrossRef] [PubMed]
- Bankier, A.A.; MacMahon, H.; Colby, T.; Gevenois, P.A.; Goo, J.M.; Leung, A.N.C.; Lynch, D.A.; Schaefer-Prokop, C.M.; Tomiyama, N.; Travis, W.D.; et al. Fleischner Society: Glossary of Terms for Thoracic Imaging. Radiology 2024, 310, e232558. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.L.; Patz, E.F., Jr. CT of the lung: Patterns of calcification and other high-attenuation abnormalities. AJR Am. J. Roentgenol. 1994, 162, 1063–1066. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef]
- Dai, C.; Xie, H.; Su, H.; She, Y.; Zhu, E.; Fan, Z.; Zhou, F.; Ren, Y.; Xie, D.; Zheng, H.; et al. Tumor Spread through Air Spaces Affects the Recurrence and Overall Survival in Patients with Lung Adenocarcinoma >2 to 3 cm. J. Thorac. Oncol. 2017, 12, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, J.; Nakajima, T.; Suzuki, H.; Nagato, K.; Iwata, T.; Yoshida, S.; Fukuyo, M.; Ota, S.; Nakatani, Y.; Yoshino, I. Impact of free tumor clusters on prognosis after resection of pulmonary adenocarcinoma. J. Thorac. Cardiovasc. Surg. 2016, 152, 64–72.e1. [Google Scholar] [CrossRef]
- Onozato, M.L.; Kovach, A.E.; Yeap, B.Y.; Morales-Oyarvide, V.; Klepeis, V.E.; Tammireddy, S.; Heist, R.S.; Mark, E.J.; Dias-Santagata, D.; Iafrate, A.J.; et al. Tumor islands in resected early-stage lung adenocarcinomas are associated with unique clinicopathologic and molecular characteristics and worse prognosis. Am. J. Surg. Pathol. 2013, 37, 287–294. [Google Scholar] [CrossRef]
- Shiono, S.; Yanagawa, N. Spread through air spaces is a predictive factor of recurrence and a prognostic factor in stage I lung adenocarcinoma. Interact. Cardiovasc. Thorac. Surg. 2016, 23, 567–572. [Google Scholar] [CrossRef]
- Warth, A.; Muley, T.; Kossakowski, C.A.; Goeppert, B.; Schirmacher, P.; Dienemann, H.; Weichert, W. Prognostic Impact of Intra-alveolar Tumor Spread in Pulmonary Adenocarcinoma. Am. J. Surg. Pathol. 2015, 39, 793–801. [Google Scholar] [CrossRef]
- Masai, K.; Sakurai, H.; Sukeda, A.; Suzuki, S.; Asakura, K.; Nakagawa, K.; Asamura, H.; Watanabe, S.I.; Motoi, N.; Hiraoka, N. Prognostic Impact of Margin Distance and Tumor Spread Through Air Spaces in Limited Resection for Primary Lung Cancer. J. Thorac. Oncol. 2017, 12, 1788–1797. [Google Scholar] [CrossRef]
- de Baère, T.; Palussière, J.; Aupérin, A.; Hakime, A.; Abdel-Rehim, M.; Kind, M.; Dromain, C.; Ravaud, A.; Tebboune, N.; Boige, V.; et al. Midterm local efficacy and survival after radiofrequency ablation of lung tumors with minimum follow-up of 1 year: Prospective evaluation. Radiology 2006, 240, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Loganadane, G.; Martinetti, F.; Mercier, O.; Krhili, S.; Riet, F.G.; Mbagui, R.; To, H.; Le Péchoux, C.; Levy, A. Stereotactic ablative radiotherapy for early stage non-small cell lung cancer: A critical literature review of predictive factors of relapse. Cancer Treat. Rev. 2016, 50, 240–246. [Google Scholar] [CrossRef]
- Kobayashi, T.; Tanaka, N.; Matsumoto, T.; Ueda, K.; Hoshii, Y.; Kunihiro, Y.; Tanaka, T.; Hayashi, M.; Matsunaga, N. HRCT findings of small cell lung cancer measuring 30 mm or less located in the peripheral lung. Jpn. J. Radiol. 2015, 33, 67–75. [Google Scholar] [CrossRef]
- Oshiro, Y.; Kusumoto, M.; Matsuno, Y.; Asamura, H.; Tsuchiya, R.; Terasaki, H.; Takei, H.; Maeshima, A.; Murayama, S.; Moriyama, N. CT findings of surgically resected large cell neuroendocrine carcinoma of the lung in 38 patients. AJR Am. J. Roentgenol. 2004, 182, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, W.; Yang, M.; Du, H.; Zhou, F.; Song, N.; Wan, Z.; Zhu, J.; Li, W. Air bronchogram on chest CT in radiological pure-solid appearance lung cancer: Correlation analysis with genetic pathological features and survival outcomes. Eur. J. Radiol. 2023, 169, 111194. [Google Scholar] [CrossRef]
- Toyokawa, G.; Takada, K.; Okamoto, T.; Kawanami, S.; Kozuma, Y.; Matsubara, T.; Haratake, N.; Takamori, S.; Akamine, T.; Katsura, M.; et al. Relevance Between Programmed Death Ligand 1 and Radiologic Invasiveness in Pathologic Stage I Lung Adenocarcinoma. Ann. Thorac. Surg. 2017, 103, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Akira, M.; Atagi, S.; Kawahara, M.; Iuchi, K.; Johkoh, T. High-resolution CT findings of diffuse bronchioloalveolar carcinoma in 38 patients. AJR Am. J. Roentgenol. 1999, 173, 1623–1629. [Google Scholar] [CrossRef]
- Gaeta, M.; Blandino, A.; Pergolizzi, S.; Mazziotti, S.; Caruso, R.; Barone, M.; Cascinu, S. Patterns of recurrence of bronchioloalveolar cell carcinoma after surgical resection: A radiological, histological, and immunohistochemical study. Lung Cancer 2003, 42, 319–326. [Google Scholar] [CrossRef]
- Tateishi, U.; Müller, N.L.; Johkoh, T.; Maeshima, A.; Asamura, H.; Satake, M.; Kusumoto, M.; Arai, Y. Mucin-producing adenocarcinoma of the lung: Thin-section computed tomography findings in 48 patients and their effect on prognosis. J. Comput. Assist. Tomogr. 2005, 29, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Chung, J.H.; Cho, S.; Han, Y.B.; Park, Y.M.; Kim, H.J.; Song, M.J.; Kwon, B.S.; Lim, S.Y.; Lee, Y.J.; et al. Impact of Preoperative Diagnostic Biopsy Procedure on Spread Through Airspaces and Related Outcomes in Resected Stage I Non-Small Cell Lung Cancer. Chest 2022, 162, 1199–1212. [Google Scholar] [CrossRef] [PubMed]
| All Patients (n = 145) | Positive for STAS (n = 64) | Negative for STAS (n = 81) | p Value | |
|---|---|---|---|---|
| Age | 71.4 ± 9.2 | 71.3 ± 8.7 | 71.5 ± 9.6 | 0.658 |
| Sex, n (%) | 0.848 | |||
| Male | 78 (53.7) | 35 (54.7) | 43 (53.1) | |
| Female | 67 (46.2) | 29 (45.3) | 38 (46.9) | |
| LN metastasis, n (%) | 11 (7.6) | 9 (14.1) | 2 (2.5) | 0.011 |
| Location, n (%) | 0.615 | |||
| Central | 40 (27.6) | 19 (29.7) | 21 (25.9) | |
| Peripheral | 105 (72.4) | 45 (70.3) | 60 (74.1) | |
| Preoperative biopsy, n (%) | 70 (48.3) | 37/70 (52.9) | 33/70 (47.1) | 0.041 |
| Positive for carcinoma | 29/70 (41.4) | 17/70 (24.3) | 12/70 (17.1) | 0.472 |
| Negative for carcinoma | 41/70 (58.6) | 20/70 (28.6) | 21/70 (30.0) | |
| Qualitative analysis | ||||
| Nodule type, n (%) | 0.351 | |||
| Pure GGN | 9 (6.2) | 2 (3.1) | 7 (8.6) | |
| Part-solid nodule | 100 (69.0) | 44 (68.8) | 56 (69.1) | |
| Solid nodule | 36 (24.8) | 18 (28.1) | 18 (22.2) | |
| Margin, n (%) | 0.647 | |||
| Smooth | 39 (26.9) | 16 (25.0) | 23 (28.4) | |
| Irregular | 106 (73.1) | 48 (75.0) | 58 (71.6) | |
| Lobulation, n (%) | 71 (49.0) | 40 (62.5) | 31 (38.3) | 0.004 |
| Spicula, n (%) | 69 (47.6) | 35 (54.7) | 34 (42.0) | 0.128 |
| Cavity, n (%) | 9 (6.2) | 4 (6.3) | 5 (6.2) | 1.000 |
| Calcification, n (%) | 7 (4.8) | 5 (7.8) | 2 (2.5) | 0.241 |
| Central low attenuation, n (%) | 66 (45.5) | 41 (64.1) | 25 (30.9) | <0.001 |
| Peripheral opacity, n (%) | 0.327 | |||
| Well-defined | 91 (62.8) | 43 (67.2) | 48(59.3) | |
| Ill-defined | 54(37.2) | 21(32.8) | 33(40.7) | |
| Air bronchogram, n (%) | 37 (25.5) | 9 (14.1) | 28 (34.6) | 0.005 |
| Satellite lesions, n (%) | 5 (3.4) | 4 (6.3) | 1 (1.2) | 0.170 |
| Pleural retraction, n (%) | 66 (45.5) | 33 (51.6) | 33 (40.7) | 0.194 |
| Pulmonary emphysema, n (%) | 34 (23.4) | 18 (28.1) | 16 (19.8) | 0.237 |
| Interstitial pneumonia, n (%) | 16 (11.0) | 5 (7.8) | 11 (13.6) | 0.271 |
| Quantitative analysis | ||||
| Max CT value (HU) | 200.1 ± 207.2 | 216.2 ± 205.3 | 187.5 ± 209.0 | 0.168 |
| Min CT value (HU) | −872.2 ± 198.1 | −856.8 ± 227.4 | −884.3 ± 172.1 | 0.684 |
| Mean CT value (HU) | −370.0 ± 251.6 | −300.1 ± 246.9 | −425.2 ± 242.8 | 0.003 |
| Tumor volume (mm3) | 3953.4 ± 5606.8 | 4349.4 ± 5382.7 | 3640.5 ± 5791.7 | 0.055 |
| Solid volume (mm3) | 1588.6 ± 2236.0 | 2187.2 ± 2506.2 | 1115.7 ± 1881.5 | <0.001 |
| Solid percentage (%) | 48.6 ± 33.2 | 57.8 ± 31.5 | 41.3 ± 32.9 | 0.002 |
| Max tumor diameter (mm) | 22.6 ± 9.9 | 23.7 ± 9.0 | 21.7 ± 10.5 | 0.063 |
| Max solid diameter (mm) | 15.0 ± 9.8 | 18.1 ± 9.8 | 12.5 ± 9.1 | <0.001 |
| AUC (95% CI) | Optimal Cutoff | Sensitivity | Specificity | |
|---|---|---|---|---|
| Quantitative analysis | ||||
| Mean CT value (HU) | 0.643 (0.553–0.733) | −527.9 | 0.828 | 0.420 |
| Solid volume (mm3) | 0.687 (0.599–0.775) | 2055.2 | 0.406 | 0.901 |
| Solid percentage (%) | 0.651 (0.561–0.740) | 24.2 | 0.875 | 0.420 |
| Max solid diameter (mm) | 0.678 (0.591–0.766) | 16.3 | 0.578 | 0.728 |
| Qualitative analysis | ||||
| Lobulation | 0.621 (0.529–0.713) | – | – | – |
| Central low attenuation | 0.666 (0.576–0.756) | – | – | – |
| Air bronchogram | 0.603 (0.511–0.694) | – | – | – |
| Biopsy (n = 70) | Non-Biopsy (n = 75) | p Value | |
|---|---|---|---|
| Nodule type, n (%) | 0.029 | ||
| Pure GGN | 3 (4.3) | 6 (8.0) | |
| Part-solid nodule | 43 (61.4) | 57 (76.0) | |
| Solid nodule | 24 (34.0) † | 12 (16.0) ‡ | |
| Tumor volume (mm3) | 4663.6 ± 6228.2 | 3290.6 ± 4907.2 | 0.009 |
| Solid volume (mm3) | 1942.8 ± 2214.2 | 1258.1 ± 2220.1 | <0.001 |
| Solid percentage (%) | 59.2 ± 33.0 | 38.7 ± 30.4 | <0.001 |
| Max tumor diameter (mm) | 24.7 ± 9.9 | 20.7 ± 9.5 | 0.003 |
| Max solid diameter (mm) | 17.5 ± 9.9 | 12.6 ± 9.2 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kameda, F.; Kunihiro, Y.; Tanabe, M.; Nakashima, M.; Kobayashi, T.; Tanaka, T.; Hoshii, Y.; Ito, K. Qualitative and Quantitative Computed Tomography Analyses of Lung Adenocarcinoma for Predicting Spread Through Air Spaces. Tomography 2025, 11, 76. https://doi.org/10.3390/tomography11070076
Kameda F, Kunihiro Y, Tanabe M, Nakashima M, Kobayashi T, Tanaka T, Hoshii Y, Ito K. Qualitative and Quantitative Computed Tomography Analyses of Lung Adenocarcinoma for Predicting Spread Through Air Spaces. Tomography. 2025; 11(7):76. https://doi.org/10.3390/tomography11070076
Chicago/Turabian StyleKameda, Fumi, Yoshie Kunihiro, Masahiro Tanabe, Masatoshi Nakashima, Taiga Kobayashi, Toshiki Tanaka, Yoshinobu Hoshii, and Katsuyoshi Ito. 2025. "Qualitative and Quantitative Computed Tomography Analyses of Lung Adenocarcinoma for Predicting Spread Through Air Spaces" Tomography 11, no. 7: 76. https://doi.org/10.3390/tomography11070076
APA StyleKameda, F., Kunihiro, Y., Tanabe, M., Nakashima, M., Kobayashi, T., Tanaka, T., Hoshii, Y., & Ito, K. (2025). Qualitative and Quantitative Computed Tomography Analyses of Lung Adenocarcinoma for Predicting Spread Through Air Spaces. Tomography, 11(7), 76. https://doi.org/10.3390/tomography11070076






