Endovascular Treatment of Extracranial Arteriovenous Malformations: A Retrospective Monocentric Case-Series Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Imaging Evaluation and Classification
2.3. Treatment Protocol and Embolization Strategy
- Arterial embolization was preferred in lesions with limited and accessible feeders.
- Venous embolization was favored when the dominant outflow vein (DOV) was accessible and formed the site of arteriovenous shunting.
- Combined arterial and venous embolization was used in complex lesions with multilevel shunting, high-flow recurrence, or partial response to single-route embolization.
2.4. Devices for AVM Embolization
2.4.1. Arterial Side
2.4.2. Venous Side
2.5. Variables and Outcome Measures
2.6. Statistical Analysis
3. Results
Subgroup Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schimmel, K.; Ali, M.K.; Tan, S.Y.; Teng, J.; Do, H.M.; Steinberg, G.K.; Stevenson, D.A.; Spiekerkoetter, E. Arteriovenous Malformations-Current Understanding of the Pathogenesis with Implications for Treatment. Int. J. Mol. Sci. 2021, 22, 9037. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, V.F.; Masthoff, M.; Vielsmeier, V.; Seebauer, C.T.; Cangir, Ö.; Meyer, L.; Mükke, A.; Lang, W.; Schmid, A.; Sporns, P.B.; et al. Clinical Outcome and Quality of Life of Multimodal Treatment of Extracranial Arteriovenous Malformations: The APOLLON Study Protocol. Cardiovasc. Interv. Radiol. 2023, 46, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Stapf, C.; Mohr, J.P.; Pile-Spellman, J.; Solomon, R.A.; Sacco, R.L.; Connolly, E.S., Jr. Epidemiology and natural history of arteriovenous malformations. Neurosurg. Focus 2001, 11, e1. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Su, L.; Wang, D.; Fan, X. Overview of peripheral arteriovenous malformations: From diagnosis to treatment methods. J. Interv. Med. 2023, 6, 170–175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Osuga, K.; Yamamoto, K.; Higashihara, H.; Juri, H.; Yamamoto, K.; Higashiyama, A.; Matsutani, H.; Sugimoto, A.; Toda, S.; Fujitani, T. Endovascular and Percutaneous Embolotherapy for the Body and Extremity Arteriovenous Malformations. Interv. Radiol. 2023, 8, 36–48. [Google Scholar] [CrossRef]
- Limaye, N.; Boon, L.M.; Vikkula, M. From germline towards somatic mutations in the pathophysiology of vascular anomalies. Hum. Mol. Genet. 2009, 18, R65–R74. [Google Scholar] [CrossRef]
- Ardelean, D.S.; Letarte, M. Anti-angiogenic therapeutic strategies in hereditary hemorrhagic telangiectasia. Front. Genet. 2015, 6, 35. [Google Scholar] [CrossRef]
- Wassef, M.; Borsik, M.; Cerceau, P.; Faucon, B.; Laurian, C.; Le Clerc, N.; Lemarchand-Venencie, F.; Massoni, C.; Salvan, D.; Bisdorff-Bresson, A. Classification des tumeurs et malformations vasculaires. Apport de la classification ISSVA 2014/2018 [Classification of vascular tumours and vascular malformations. Contribution of the ISSVA 2014/2018 classification]. Ann. Pathol. 2021, 41, 58–70. [Google Scholar] [CrossRef]
- Sadick, M.; Müller-Wille, R.; Wildgruber, M.; Wohlgemuth, W.A. Vascular Anomalies (Part I): Classification and Diagnostics of Vascular Anomalies. Gefäßanomalien (Teil I): Klassifikation und Diagnostik von Gefäßanomalien. RoFo Fortschritte Geb. Rontgenstrahlen Nukl. 2018, 190, 825–835. [Google Scholar] [CrossRef]
- Monroe, E.J. Brief Description of ISSVA Classification for Radiologists. Tech. Vasc. Interv. Radiol. 2019, 22, 100628. [Google Scholar] [CrossRef]
- Richter, G.T.; Friedman, A.B. Hemangiomas and vascular malformations: Current theory and management. Int. J. Pediatr. 2012, 2012, 645678. [Google Scholar] [CrossRef] [PubMed]
- Halut, M.; Dubois, J.; Giroux, M.-F.; Gilbert, P.; Amaral, R.H.D.; Nikolic, M.; Zhang, L.X.; Mahsouli, A.; Thérasse, É.; Soulez, G. Embolization of Extracranial Arteriovenous Malformations: Interventional Approaches according to the Yakes Classification System. RadioGraphics 2025, 45, e240219. [Google Scholar] [CrossRef]
- Iguchi, T.; Hiraki, T.; Matsui, Y.; Fujiwara, H.; Sakurai, J.; Baba, K.; Toyooka, S.; Gobara, H.; Kanazawa, S. Embolization using hydrogel-coated coils for pulmonary arteriovenous malformations. Diagn. Interv. Imaging 2020, 101, 129–135. [Google Scholar] [CrossRef]
- Fernández-Alvarez, V.; Suárez, C.; de Bree, R.; Nixon, I.J.; Mäkitie, A.A.; Rinaldo, A.; Downer, J.; Ferlito, A. Management of extracranial arteriovenous malformations of the head and neck. Auris Nasus Larynx 2020, 47, 181–190. [Google Scholar] [CrossRef]
- Case, D.; Folzenlogen, Z.; Rochon, P.; Kumpe, D.; Roark, C.; Seinfeld, J. Embolization of Head and Neck Vascular Malformations using Serial Arterial Embolization Followed by Dominant Arterial Embolization with Two Microcatheter Technique. J. Vasc. Interv. Neurol. 2018, 10, 47–51. [Google Scholar]
- Dabus, G.; Linfante, I.; Benenati, J.; Perlyn, C.A.; Martínez-Galdámez, M. Interventional management of high-flow craniofacial vascular malformations: A database analysis and review of the literature. J. Neurointerv. Surg. 2017, 9, 92–96. [Google Scholar] [CrossRef]
- Spreafico, R.; Sordo, L.; Bellotto, R.; Schipano, M.; Rescaldani, A.; Parmigiani, F. Arterio-venous malformation of the mandible. Case report and review of literature. Malformazione arterovenosa della mandibola. Caso clinico e revisione della letteratura. Acta Otorhinolaryngol. Ital. Organo Uff. Della Soc. Ital. Otorinolaringol. Chir. Cervico-Facciale 2016, 36, 333–336. [Google Scholar] [CrossRef]
- Siekmann, R. Basics and Principles in the Application of Onyx LD Liquid Embolic System in the Endovascular Treatment of Cerebral Arteriovenous Malformations. Interv. Neuroradiol. J. Peritherapeutic Neuroradiol. Surg. Proced. Relat. Neurosci. 2005, 11 (Suppl. S1), 131–140. [Google Scholar] [CrossRef]
- Vaidya, S.; Tozer, K.R.; Chen, J. An overview of embolic agents. Semin. Interv. Radiol. 2008, 25, 204–215. [Google Scholar] [CrossRef]
- Miyayama, S.; Yamashiro, M.; Ikeda, R.; Matsumoto, J.; Ogawa, N.; Sakuagawa, N. Fibrin Glue in Interventional Radiology: How to Use It. Interv. Radiol. 2021, 6, 122–129. [Google Scholar] [CrossRef]
- Çay, F.; Altunbulak, A.Y.; Özbay, Y.; Eldem, G.; Çil, B.E.; Vargel, İ.; Kutluk, M.T.; Yalçın, B.; Peynircioğlu, B. Clinical results of polidocanol sclerotherapy in venous malformation treatment: Patient-perceived improvement and satisfaction. Phlebology 2023, 38, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Filippiadis, D.K.; Pereira, P.L.; Hausegger, K.A.; Binkert, C.A. CIRSE Classification System for Complications’ Reporting: A Project Evaluation Process. Cardiovasc. Intervent. Radiol. 2024, 47, 1160–1162. [Google Scholar] [CrossRef]
- Spetzler, R.F.; Martin, N.A. A proposed grading system for arteriovenous malformations. J. Neurosurg. 2008, 108, 186–193. [Google Scholar] [CrossRef]
- Mulliken, J.B.; Zetter, B.R.; Folkman, J. In vitro characteristics of endothelium from hemangiomas and vascular malformations. Surgery 1982, 92, 348–353. [Google Scholar]
- Kohout, M.P.; Hansen, M.; Pribaz, J.J.; Mulliken, J.B. Arteriovenous malformations of the head and neck: Natural history and management. Plast. Reconstr. Surg. 1998, 102, 643–654. [Google Scholar] [CrossRef]
- Schmidt, V.F.; Masthoff, M.; Czihal, M.; Cucuruz, B.; Häberle, B.; Brill, R.; Wohlgemuth, W.A.; Wildgruber, M. Imaging of peripheral vascular malformations—Current concepts and future perspectives. Mol. Cell. Pediatr. 2021, 8, 19. [Google Scholar] [CrossRef]
- Vaišnytė, B.; Vajauskas, D.; Palionis, D.; Misonis, N.; Kurminas, M.; Nevidomskytė, D.; Matačiūnas, M.; Gutauskas, M.; Laucevičius, A. Diagnostic methods, treatment modalities, and follow-up of extracranial arteriovenous malformations. Medicina 2012, 48, 58. [Google Scholar] [CrossRef]
- Liu, A.S.; Mulliken, J.B.; Zurakowski, D.; Fishman, S.J.; Greene, A.K. Extracranial arteriovenous malformations: Natural progression and recurrence after treatment. Plast. Reconstr. Surg. 2010, 125, 1185–1194. [Google Scholar] [CrossRef]
- Jia, H.; Chen, Y.; Yang, X.; Lee, Y.; Zou, Y.; Zhou, J.; Jin, Y.; Hua, C.; Lin, X. Treatment of Challenging Extracranial Arteriovenous Malformations: A Single-Center Experience and Literature Review. Ann. Plast. Surg. 2023, 90 (Suppl. S2), S177–S182. [Google Scholar] [CrossRef]
- Faughnan, M.E.; Mager, J.J.; Hetts, S.W.; Palda, V.A.; Lang-Robertson, K.; Buscarini, E.; Deslandres, E.; Kasthuri, R.S.; Lausman, A.; Poetker, D.; et al. Second International Guidelines for the Diagnosis and Management of Hereditary Hemorrhagic Telangiectasia. Ann. Intern. Med. 2020, 173, 989–1001. [Google Scholar] [CrossRef]
- Do, Y.S.; Yakes, W.F.; Shin, S.W.; Lee, B.B.; Kim, D.I.; Liu, W.C.; Shin, B.S.; Kim, D.K.; Choo, S.W.; Choo, I.W. Ethanol embolization of arteriovenous malformations: Interim results. Radiology 2005, 235, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Milic, A.; Chan, R.P.; Cohen, J.H.; Faughnan, M.E. Reperfusion of pulmonary arteriovenous malformations after embolotherapy. J. Vasc. Interv. Radiol. 2005, 16, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Venot, Q.; Blanc, T.; Rabia, S.H.; Berteloot, L.; Ladraa, S.; Duong, J.P.; Blanc, E.; Johnson, S.C.; Hoguin, C.; Boccara, O.; et al. Targeted therapy in patients with PIK3CA-related overgrowth syndrome. Nature 2018, 558, 540–546. [Google Scholar] [CrossRef]
- Adams, D.M.; Trenor, C.C., 3rd; Hammill, A.M.; Vinks, A.A.; Patel, M.N.; Chaudry, G.; Wentzel, M.S.; Mobberley-Schuman, P.S.; Campbell, L.M.; Brookbank, C.; et al. Efficacy and Safety of Sirolimus in the Treatment of Complicated Vascular Anomalies. Pediatrics 2016, 137, e20153257. [Google Scholar] [CrossRef]
- Nicholson, C.L.; Flanagan, S.; Murati, M.; Boull, C.; McGough, E.; Ameduri, R.; Weigel, B.; Maguiness, S. Successful management of an arteriovenous malformation with trametinib in a patient with capillary-malformation arteriovenous malformation syndrome and cardiac compromise. Pediatr. Dermatol. 2022, 39, 316–319. [Google Scholar] [CrossRef]
- Karnezis, T.T.; Davidson, T.M. Treatment of hereditary hemorrhagic telangiectasia with submucosal and topical bevacizumab therapy. Laryngoscope 2012, 122, 495–497. [Google Scholar] [CrossRef]
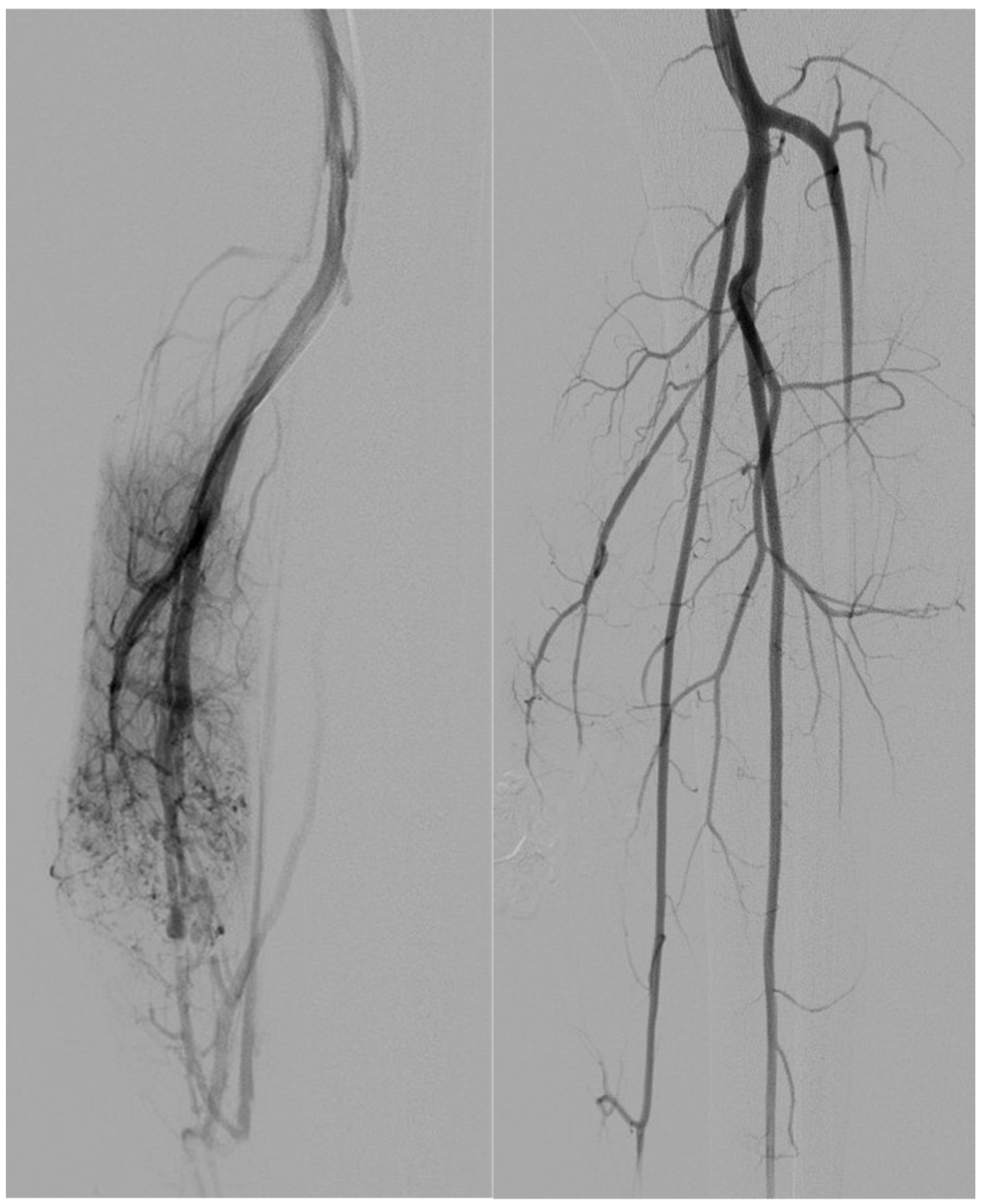
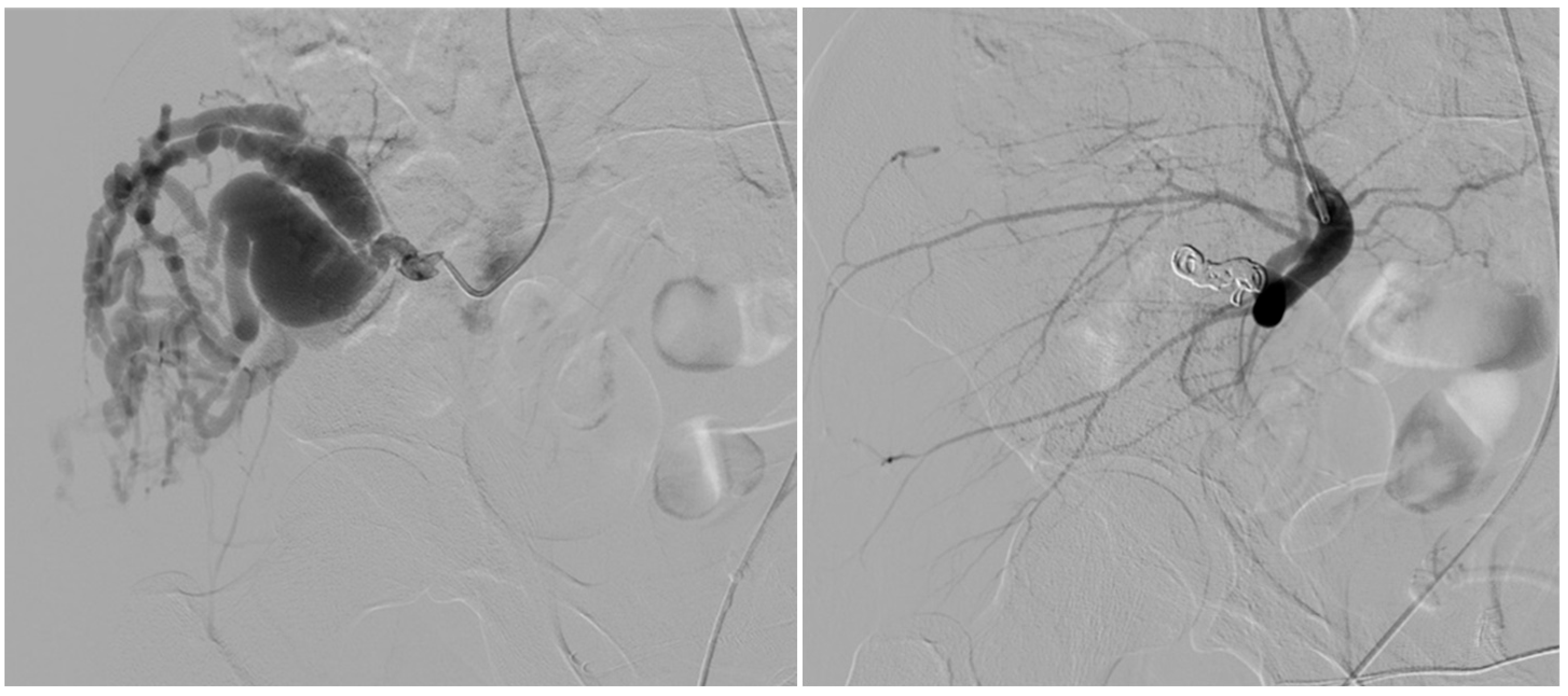
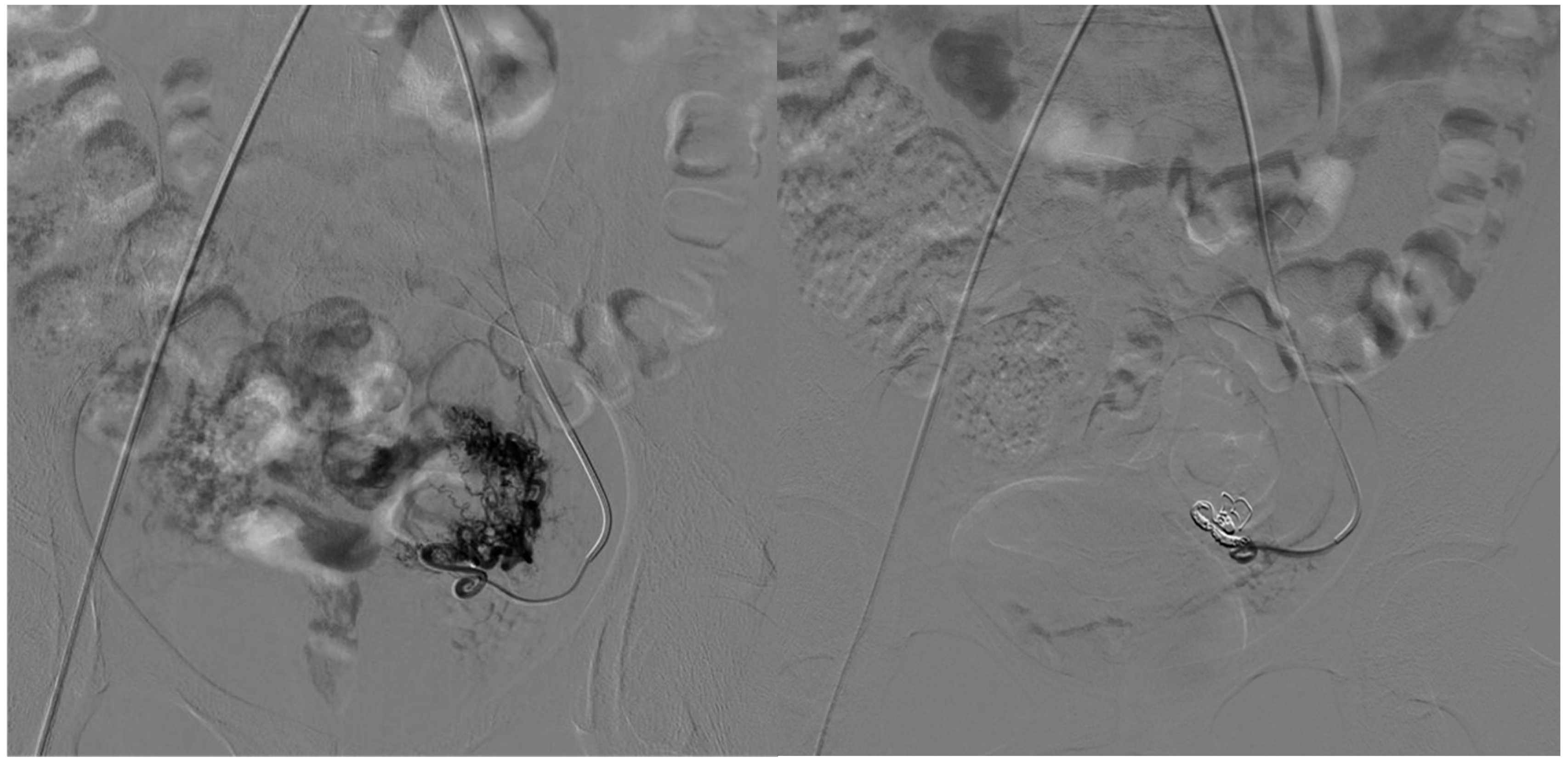
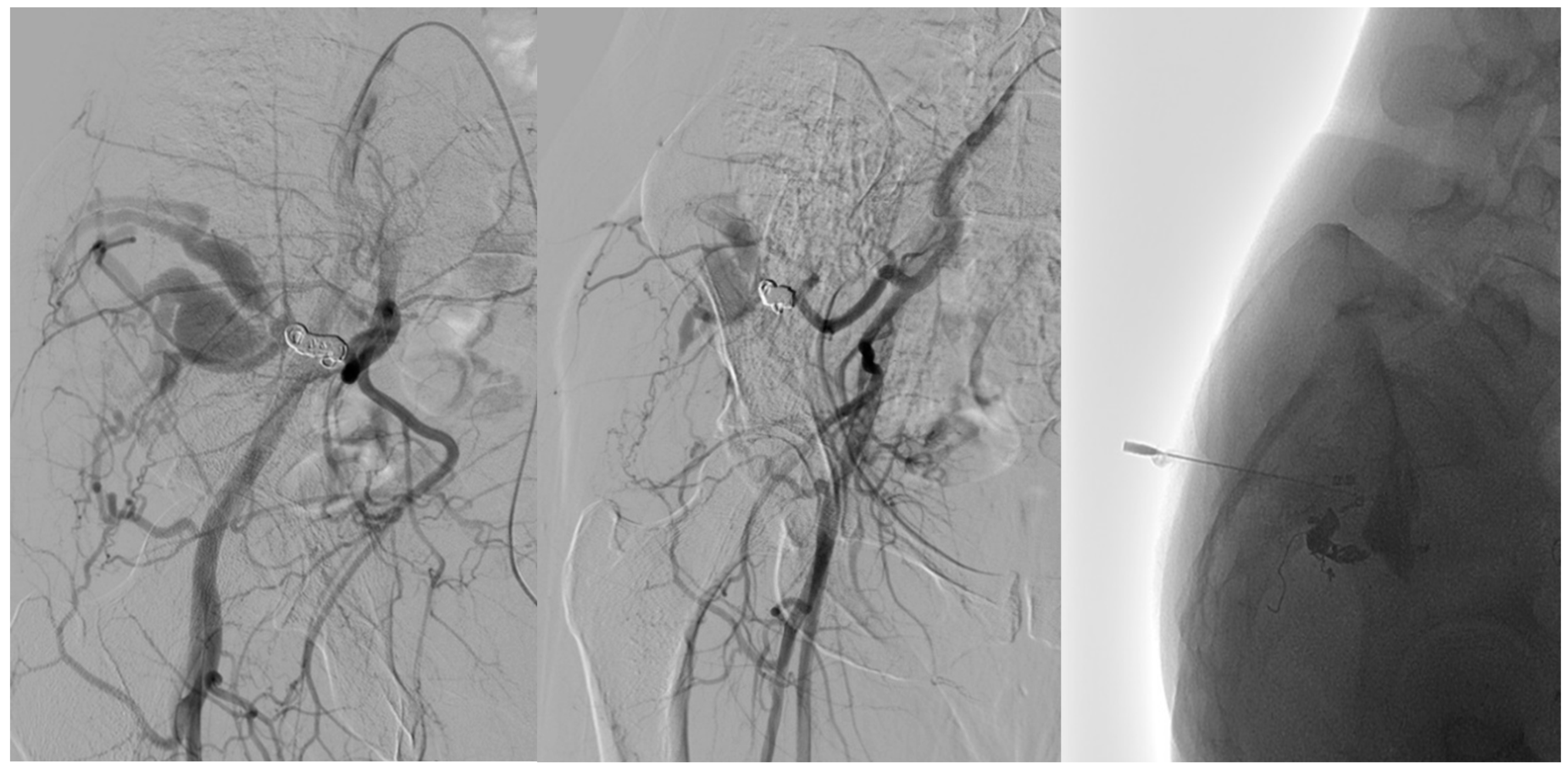
| Variable | Type I (n = 9) | Type IV (n = 5) | p-Value |
|---|---|---|---|
| Sex (male) | 3/9 (33.3%) | 2/5 (40%) | 1.00 |
| Age (years, mean ± SD) | 34.14 ± 22.66 | 27.75 ± 22.44 | 0.66 |
| Location | |||
| Lower limb | 5 (55.6%) | 5 (100%) | 0.22 |
| Neck | 2 (22.2%) | 0 | 0.50 |
| Pelvic | 1 (11.1%) | 0 | 1.00 |
| Upper limb | 1 (11.1%) | 0 | 1.00 |
| Embolic Material for the Arterial Side | |||
| Coils | 3 (33.3%) | 1 (20%) | - |
| Coils + PVA | 4 (44.4%) | 0 | - |
| Coils + EVOH | 0 | 1 (20%) | - |
| EVOH | 1 (11.1%) | 1 (20%) | - |
| PVA | 0 | 1 (20%) | - |
| EVOH + PVA | 1 (11.1%) | 0 | - |
| Treatment Approach | |||
| One-stage | 5 (55.6%) | 3 (60%) | 1.00 |
| Two-stages | 4 (44.4%) | 2 (40%) | 1.00 |
| Outcomes | |||
| Recurrence | 2 (22.2%) | 0 | 0.50 |
| Complications | 1 (11.1%) | 0 | 1.00 |
| Variable | Single Attempt (n = 8) | Two or More Attempts (n = 6) |
|---|---|---|
| AVM Type | ||
| Type I | 5 | 4 |
| Type IV | 3 | 2 |
| Location | ||
| Lower limb | 5 | 5 |
| Neck | 1 | 1 |
| Upper limb | 1 | 0 |
| Pelvis | 1 | 0 |
| Access Site | ||
| Arterial | 5 | 4 |
| Arterial + Venous direct access | 3 | 2 |
| Embolic Material | ||
| EVOH | 1 | 1 |
| Coils + PVA | 2 | 2 |
| Coils | 2 | 1 |
| Coils + Lauromacrogol | 1 | 1 |
| PVA + Lauromacrogol | 1 | 0 |
| Recurrence | 1/8 (12.5%) | 1/6 (16.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarti, G.; Barbato, G.; Tiralongo, F.; Santini, G.; Arienzo, F.; Nilo, D.; Tortora, F.; Reginelli, A.; Comune, R.; Borrelli, M.; et al. Endovascular Treatment of Extracranial Arteriovenous Malformations: A Retrospective Monocentric Case-Series Study. Tomography 2025, 11, 75. https://doi.org/10.3390/tomography11070075
Sarti G, Barbato G, Tiralongo F, Santini G, Arienzo F, Nilo D, Tortora F, Reginelli A, Comune R, Borrelli M, et al. Endovascular Treatment of Extracranial Arteriovenous Malformations: A Retrospective Monocentric Case-Series Study. Tomography. 2025; 11(7):75. https://doi.org/10.3390/tomography11070075
Chicago/Turabian StyleSarti, Giuseppe, Giovanni Barbato, Francesco Tiralongo, Gianpaolo Santini, Francesco Arienzo, Davide Nilo, Fabio Tortora, Alfonso Reginelli, Rosita Comune, Maria Borrelli, and et al. 2025. "Endovascular Treatment of Extracranial Arteriovenous Malformations: A Retrospective Monocentric Case-Series Study" Tomography 11, no. 7: 75. https://doi.org/10.3390/tomography11070075
APA StyleSarti, G., Barbato, G., Tiralongo, F., Santini, G., Arienzo, F., Nilo, D., Tortora, F., Reginelli, A., Comune, R., Borrelli, M., Tamburrini, S., Basile, A., & Scaglione, M. (2025). Endovascular Treatment of Extracranial Arteriovenous Malformations: A Retrospective Monocentric Case-Series Study. Tomography, 11(7), 75. https://doi.org/10.3390/tomography11070075








