Longitudinal Overlap and Metabolite Analysis in Spectroscopic MRI-Guided Proton Beam Therapy in Pediatric High-Grade Glioma
Abstract
1. Introduction
2. Materials and Methods
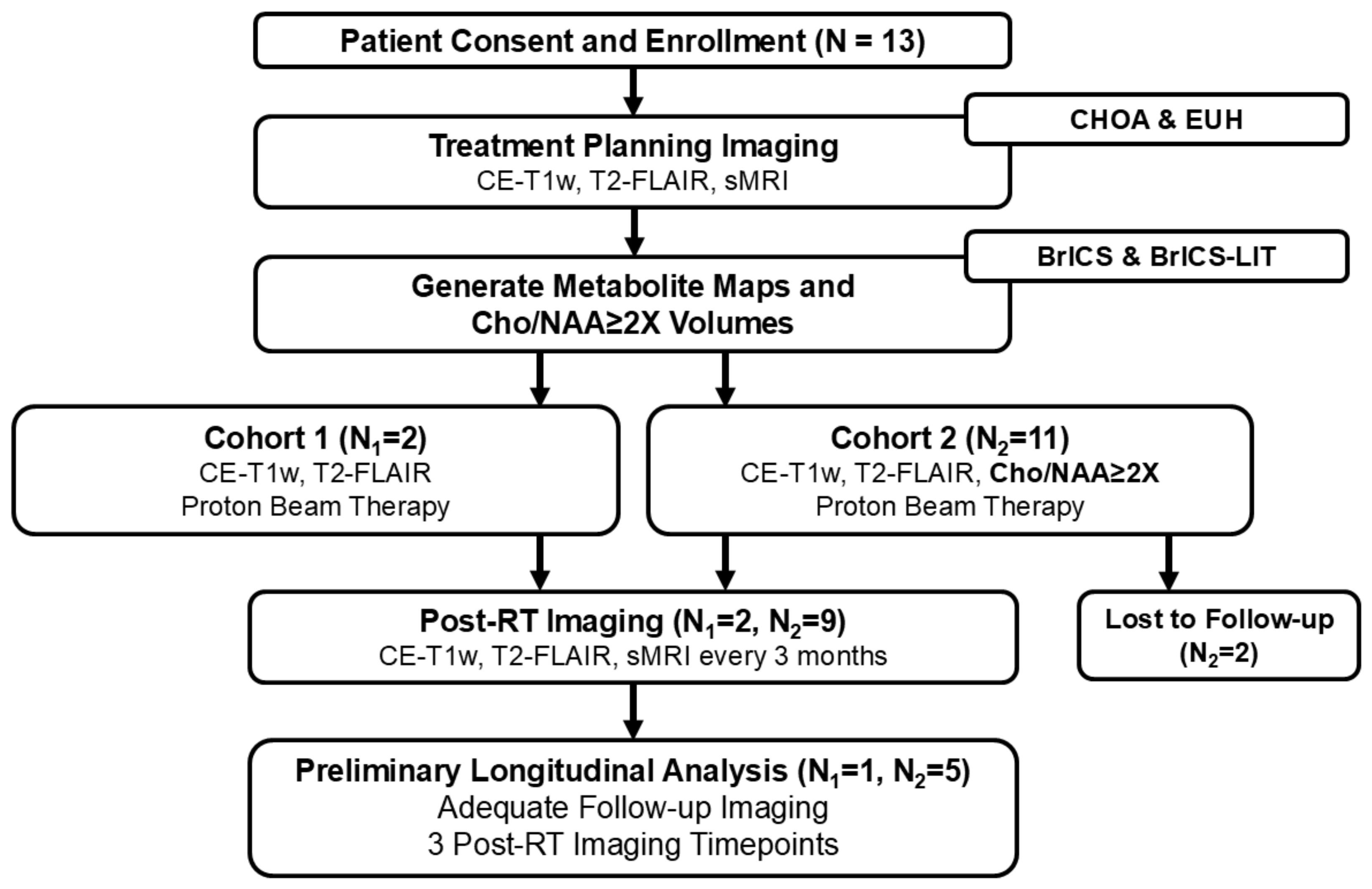
2.1. Study Design
2.2. Enrollment Eligibility Criteria
2.3. Imaging Parameters
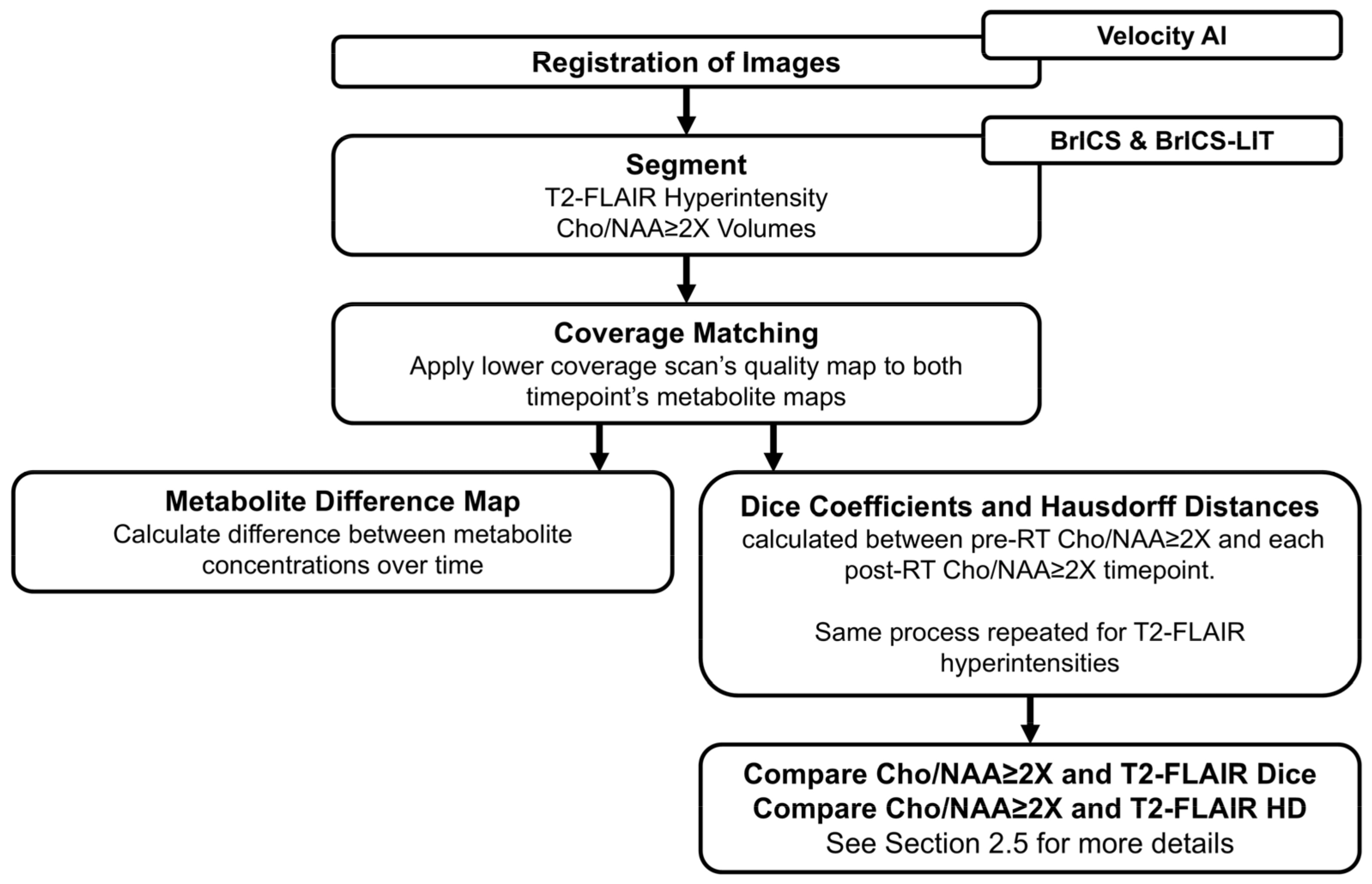
2.4. Longitudinal Overlap Analysis
2.5. Statistical Analysis
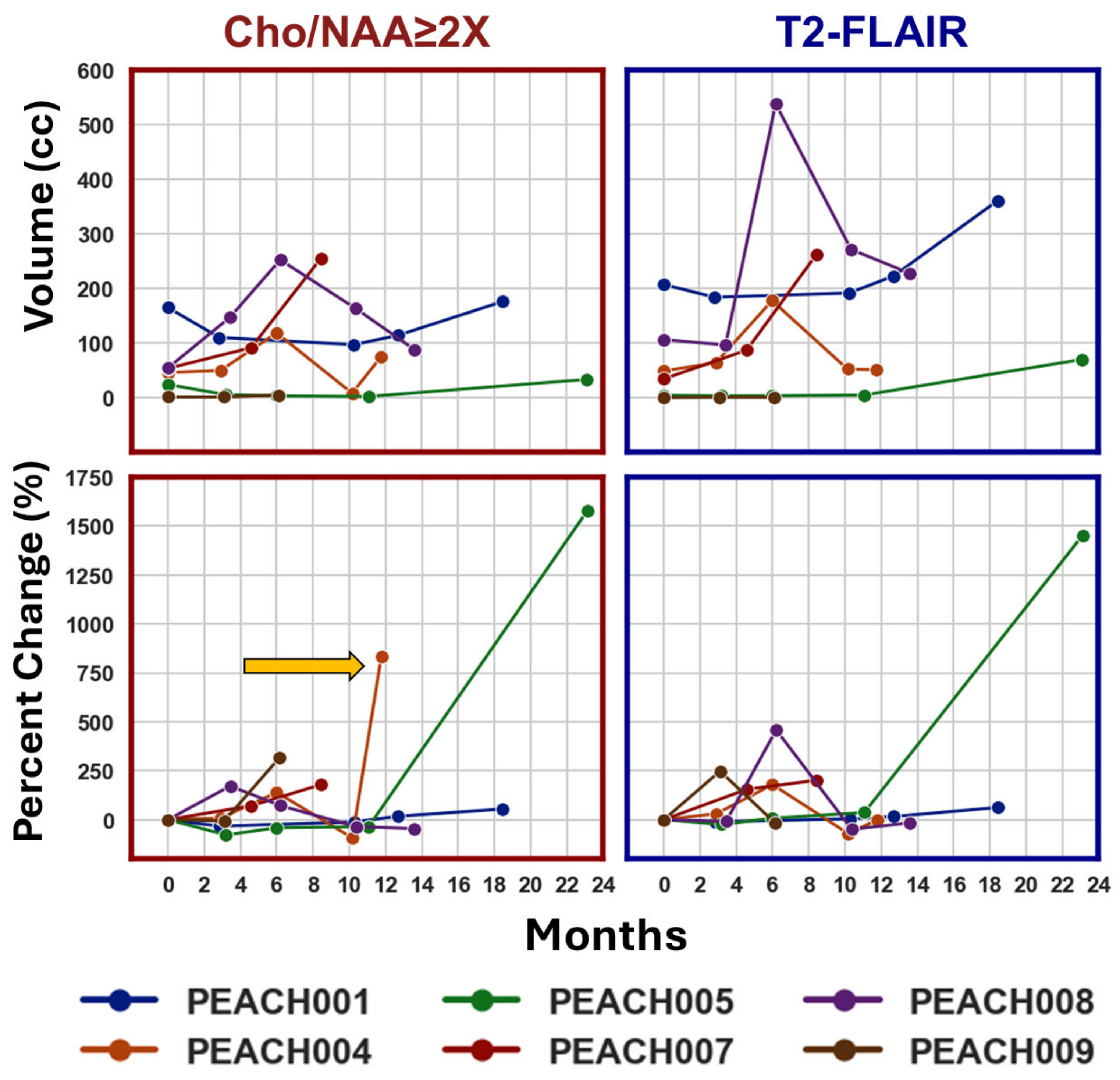
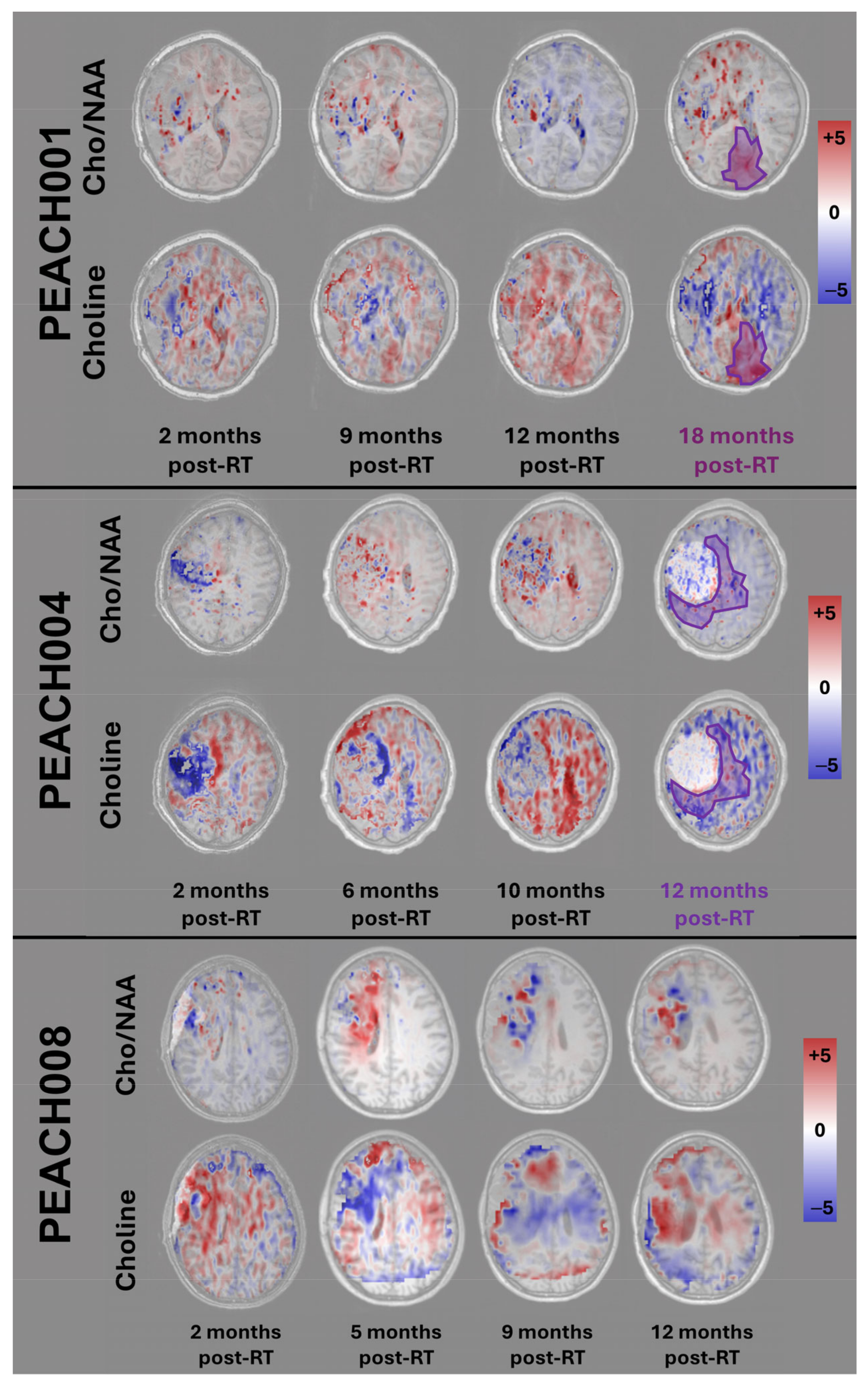
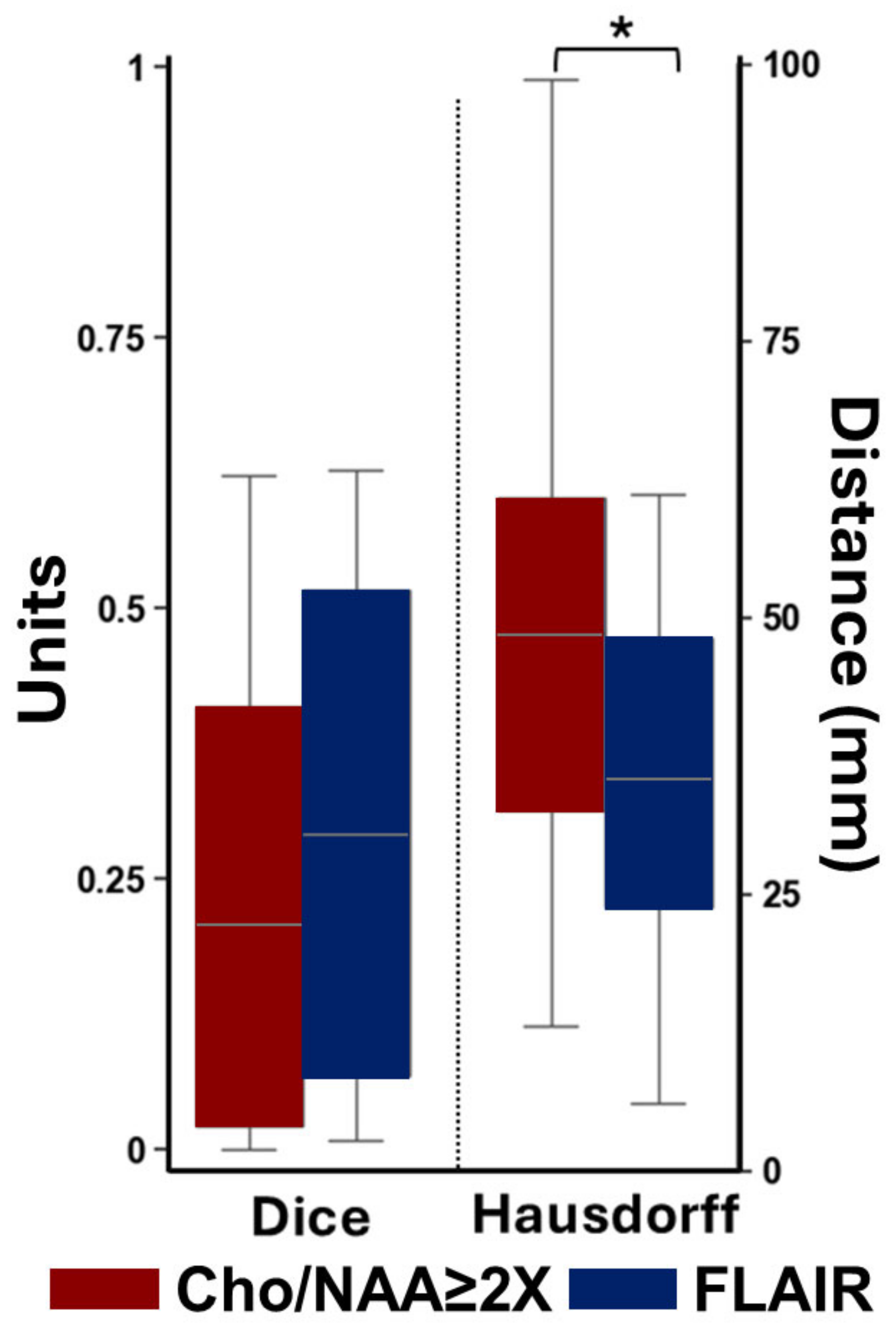
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| pHGG | Pediatric High-Grade Glioma |
| PBT | Proton Beam Therapy |
| sMRI | Spectroscopic Magnetic Resonance Imaging |
| EPSI | Echo-Planar Spectroscopic Imaging |
| Cho | Choline |
| NAA | N-acetyl Aspartate |
| BrICS | Brain Imaging Collaboration Suite |
| DSC | Dice Similarity Coefficient |
| HD | Hausdorff Distance |
References
- Ostrom, Q.T.; Gittleman, H.; Farah, P.; Ondracek, A.; Chen, Y.; Wolinsky, Y.; Stroup, N.E.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013, 15 (Suppl. S2), ii1–ii56. [Google Scholar] [CrossRef] [PubMed]
- Kline, C.; Felton, E.; Allen, I.E.; Tahir, P.; Mueller, S. Survival outcomes in pediatric recurrent high-grade glioma: Results of a 20-year systematic review and meta-analysis. J. Neurooncol. 2018, 137, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Luo, W.; Pehlivan, K.C.; Hoang, H.; Rajappa, P.; Cripe, T.P.; Cassady, K.A.; Lee, D.A.; Cairo, M.S. Pediatric versus adult high grade glioma: Immunotherapeutic and genomic considerations. Front. Immunol. 2022, 13, 1038096. [Google Scholar] [CrossRef] [PubMed]
- Chatwin, H.V.; Cruz, J.C.; Green, A.L. Pediatric high-grade glioma: Moving toward subtype-specific multimodal therapy. FEBS J. 2021, 288, 6127–6141. [Google Scholar] [CrossRef]
- Skowrońska-Gardas, A. A literature review of the recent radiotherapy clinical trials in pediatric brain tumors. Rev. Recent. Clin. Trials 2009, 4, 42–55. [Google Scholar] [CrossRef]
- Broniscer, A.; Gajjar, A. Supratentorial High-Grade Astrocytoma and Diffuse Brainstem Glioma: Two Challenges for the Pediatric Oncologist. Oncologist 2004, 9, 197–206. [Google Scholar] [CrossRef]
- Mizumoto, M.; Fuji, H.; Miyachi, M.; Soejima, T.; Yamamoto, T.; Aibe, N.; Demizu, Y.; Iwata, H.; Hashimoto, T.; Motegi, A.; et al. Proton beam therapy for children and adolescents and young adults (AYAs): JASTRO and JSPHO Guidelines. Cancer Treat. Rev. 2021, 98, 102209. [Google Scholar] [CrossRef]
- Mohan, R.; Grosshans, D. Proton therapy—Present and future. Adv. Drug Deliv. Rev. 2017, 109, 26–44. [Google Scholar] [CrossRef]
- Odei, B.C.L.; Boothe, D.; Keole, S.R.; Vargas, C.E.; Foote, R.L.; Schild, S.E.; Ashman, J.B. A 20-Year Analysis of Clinical Trials Involving Proton Beam Therapy. Int. J. Part. Ther. 2017, 3, 398–406. [Google Scholar] [CrossRef]
- Vitti, E.T.; Parsons, J.L. The Radiobiological Effects of Proton Beam Therapy: Impact on DNA Damage and Repair. Cancers 2019, 11, 946. [Google Scholar] [CrossRef]
- Bekelman, J.E.; Lu, H.; Pugh, S.; Baker, K.; Berg, C.D.; de Gonzalez, A.B.; Braunstein, L.Z.; Bosch, W.; Chauhan, C.; Ellenberg, S.; et al. Pragmatic randomised clinical trial of proton versus photon therapy for patients with non-metastatic breast cancer: The Radiotherapy Comparative Effectiveness (RadComp) Consortium trial protocol. BMJ Open 2019, 9, e025556. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.C.; Habrand, J.L.; Hoppe, B.S.; Kayser, C.H.; Laack, N.N.; Langendijk, J.A.; MacDonald, S.M.; McGovern, S.L.; Pater, L.; Perentesis, J.P.; et al. Proton therapy for pediatric malignancies: Fact, figures and costs. A joint consensus statement from the pediatric subcommittee of PTCOG, PROS and EPTN. Radiother. Oncol. 2018, 128, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Jalali, R.; Goda, J.S. Proton beam therapy in pediatric brain tumor patients: Improved radiation delivery techniques improve neurocognitive outcomes. Neuro Oncol. 2019, 21, 830–831. [Google Scholar] [CrossRef] [PubMed]
- Gondi, V.; Yock, T.I.; Mehta, M.P. Proton therapy for paediatric CNS tumours—improving treatment-related outcomes. Nat. Rev. Neurol. 2016, 12, 334–345. [Google Scholar] [CrossRef]
- Yahya, N.; Manan, H.A. Neurocognitive impairment following proton therapy for paediatric brain tumour: A systematic review of post-therapy assessments. Support. Care Cancer 2021, 29, 3035–3047. [Google Scholar] [CrossRef]
- Shahmohammadi Beni, M.; Krstic, D.; Nikezic, D.; Yu, K.N.; Zheng, D. A comparative study on dispersed doses during photon and proton radiation therapy in pediatric applications. PLoS ONE 2021, 16, e0248300. [Google Scholar] [CrossRef]
- Ramella, S.; D’Angelillo, R.M. Proton beam or photon beam radiotherapy in the treatment of non-small-cell lung cancer. Lancet Oncol. 2020, 21, 873–875. [Google Scholar] [CrossRef]
- Jumaniyazova, E.; Smyk, D.; Vishnyakova, P.; Fatkhudinov, T.; Gordon, K. Photon- and Proton-Mediated Biological Effects: What Has Been Learned? Life 2022, 13, 30. [Google Scholar] [CrossRef]
- Blanchard, P.; Gunn, G.B.; Lin, A.; Foote, R.L.; Lee, N.Y.; Frank, S.J. Proton Therapy for Head and Neck Cancers. Semin. Radiat. Oncol. 2018, 28, 53–63. [Google Scholar] [CrossRef]
- Doyen, J.; Falk, A.T.; Floquet, V.; Hérault, J.; Hannoun-Lévi, J.-M. Proton beams in cancer treatments: Clinical outcomes and dosimetric comparisons with photon therapy. Cancer Treat. Rev. 2016, 43, 104–112. [Google Scholar] [CrossRef]
- Ek, H.; Kristensen, I.F.; Stenberg, L.; Kinhult, S.; Benedek, H.; Ek, S.; Engelholm, S.A.; Engelholm, S.; Rosenschöld, P.M.A. Transitioning from conventional photon therapy to proton therapy for primary brain tumors. Acta Oncol. 2023, 62, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Baumann, B.C.; Mitra, N.; Harton, J.G.; Xiao, Y.; Wojcieszynski, A.P.; Gabriel, P.E.; Zhong, H.; Geng, H.; Doucette, A.; Wei, J.; et al. Comparative Effectiveness of Proton vs Photon Therapy as Part of Concurrent Chemoradiotherapy for Locally Advanced Cancer. JAMA Oncol. 2020, 6, 237–246. [Google Scholar] [CrossRef]
- He, J.; Liu, Y.; Zhang, X.; Li, B.; Yang, L.; Wang, H.; Wang, S.; Yu, J.; Wang, L. Comparison of proton therapy and photon therapy for early-stage non-small cell lung cancer: A meta-analysis. Biomark. Res. 2024, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Du, Y.; Zhang, Y.; Zhu, M.; Li, T.; Tian, W.; Wu, T.; Xiao, Z. Efficacy and Safety in Proton Therapy and Photon Therapy for Patients With Esophageal Cancer: A Meta-Analysis. JAMA Netw. Open 2023, 6, e2328136. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Bowen, S.R.; Thomas, H.M.; Sasidharan, B.K.; Rengan, R.; Zeng, J. Scanning Beam Proton Therapy versus Photon IMRT for Stage III Lung Cancer: Comparison of Dosimetry, Toxicity, and Outcomes. Adv. Radiat. Oncol. 2020, 5, 434–443. [Google Scholar] [CrossRef]
- Merchant, T.E.; Hoehn, M.E.; Khan, R.B.; Sabin, N.D.; Klimo, P.; A Boop, F.; Wu, S.; Li, Y.; A Burghen, E.; Jurbergs, N.; et al. Proton therapy and limited surgery for paediatric and adolescent patients with craniopharyngioma (RT2CR): A single-arm, phase 2 study. Lancet Oncol. 2023, 24, 523–534. [Google Scholar] [CrossRef]
- Warren, E.; Child, A.; Cirino, P.; Grosshans, D.; Mahajan, A.; Paulino, A.; Chintagumpala, M.; Okcu, F.; Ris, M.D.; Minard, C.; et al. QOL-42. Better social, cognitive, and academic outcomes among pediatric brain tumor survivors treated with proton versus photon radiation therapy. Neuro Oncol. 2018, 20 (Suppl. S2), i166. [Google Scholar] [CrossRef]
- Mohan, R. A Review of Proton Therapy—Current Status and Future Directions. Precis. Radiat. Oncol. 2022, 6, 164–176. [Google Scholar] [CrossRef]
- Lane, S.A.; Slater, J.M.; Yang, G.Y. Image-Guided Proton Therapy: A Comprehensive Review. Cancers 2023, 15, 2555. [Google Scholar] [CrossRef]
- Miklja, Z.; Pasternak, A.; Stallard, S.; Nicolaides, T.; Kline-Nunnally, C.; Cole, B.; Beroukhim, R.; Bandopadhayay, P.; Chi, S.; Ramkissoon, S.H.; et al. Molecular profiling and targeted therapy in pediatric gliomas: Review and consensus recommendations. Neuro Oncol. 2019, 21, 968–980. [Google Scholar] [CrossRef]
- Cordova, J.S.; Shu, H.-K.G.; Liang, Z.; Gurbani, S.S.; Cooper, L.A.D.; Holder, C.A.; Olson, J.J.; Kairdolf, B.; Schreibmann, E.; Neill, S.G.; et al. Whole-brain spectroscopic MRI biomarkers identify infiltrating margins in glioblastoma patients. Neuro Oncol. 2016, 18, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Cordova, J.S.; Kandula, S.; Gurbani, S.; Zhong, J.; Tejani, M.; Kayode, O.; Patel, K.; Prabhu, R.; Schreibmann, E.; Crocker, I.; et al. Simulating the Effect of Spectroscopic MRI as a Metric for Radiation Therapy Planning in Patients with Glioblastoma. Tomography 2016, 2, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, K.; A Mellon, E.; Gurbani, S.S.; Weinberg, B.D.; Schreibmann, E.; A Sheriff, S.; Goryawala, M.; de le Fuente, M.; Eaton, B.R.; Zhong, J.; et al. A multi-institutional pilot clinical trial of spectroscopic MRI-guided radiation dose escalation for newly diagnosed glioblastoma. Neurooncol. Adv. 2022, 4, vdac006. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Huang, V.; Gurbani, S.S.; Ramesh, K.; Cordova, J.S.; Schreibmann, E.; Shu, H.-K.G.; Olson, J.; Han, H.; Giuffrida, A.; et al. 3D whole-brain metabolite imaging to improve characterization of low-to-intermediate grade gliomas. J. Neurooncol. 2021, 153, 303–311. [Google Scholar] [CrossRef]
- Rejimon, A.C.; Ramesh, K.K.; Trivedi, A.G.; Huang, V.; Schreibmann, E.; Weinberg, B.D.; Kleinberg, L.R.; Shu, H.-K.G.; Shim, H.; Olson, J.J. The Utility of Spectroscopic MRI in Stereotactic Biopsy and Radiotherapy Guidance in Newly Diagnosed Glioblastoma. Tomography 2024, 10, 428–443. [Google Scholar] [CrossRef]
- Maudsley, A.A.; Domenig, C.; Govind, V.; Darkazanli, A.; Studholme, C.; Arheart, K.; Bloomer, C. Mapping of brain metabolite distributions by volumetric proton MR spectroscopic imaging (MRSI). Magn. Reson. Med. An Off. J. Int. Soc. Magn. Reson. Med. 2009, 61, 548–559. [Google Scholar] [CrossRef]
- Gurbani, S.; Weinberg, B.; Cooper, L.; Mellon, E.; Schreibmann, E.; Sheriff, S.; Maudsley, A.; Goryawala, M.; Shu, H.-K.; Shim, H. The Brain Imaging Collaboration Suite (BrICS): A Cloud Platform for Integrating Whole-Brain Spectroscopic MRI into the Radiation Therapy Planning Workflow. Tomography 2019, 5, 184–191. [Google Scholar] [CrossRef]
- Ramesh, K.; Gurbani, S.S.; Mellon, E.A.; Huang, V.; Goryawala, M.; Barker, P.B.; Kleinberg, L.; Shu, H.-K.G.; Shim, H.; Weinberg, B.D. The Longitudinal Imaging Tracker (BrICS-LIT):A Cloud Platform for Monitoring Treatment Response in Glioblastoma Patients. Tomography 2020, 6, 93–100. [Google Scholar] [CrossRef]
- Sabati, M.; Sheriff, S.; Gu, M.; Wei, J.; Zhu, H.; Barker, P.B.; Spielman, D.M.; Alger, J.R.; Maudsley, A.A. Multivendor implementation and comparison of volumetric whole-brain echo-planar MR spectroscopic imaging. Magn. Reson. Med. 2015, 74, 1209–1220. [Google Scholar] [CrossRef]
- Maudsley, A.; Darkazanli, A.; Alger, J.R.; Hall, L.O.; Schuff, N.; Studholme, C.; Yu, Y.; Ebel, A.; Frew, A.; Goldgof, D.; et al. Comprehensive processing, display and analysis for in vivo MR spectroscopic imaging. NMR Biomed. 2006, 19, 492–503. [Google Scholar] [CrossRef]
- Gurbani, S.; Schreibmann, E.; Sheriff, S.; Cooper, L.; Shu, H.; Holder, C.; Maudsley, A.; Shim, H. A Software Platform for Collaborative Radiation Therapy Planning Using Spectroscopic MRI. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, E667. [Google Scholar] [CrossRef]
- Trivedi, A.G.; Kim, S.H.; Ramesh, K.K.; Giuffrida, A.S.; Weinberg, B.D.; Mellon, E.A.; Kleinberg, L.R.; Barker, P.B.; Han, H.; Shu, H.-K.G.; et al. Applying a Radiation Therapy Volume Analysis Pipeline to Determine the Utility of Spectroscopic MRI-Guided Adaptive Radiation Therapy for Glioblastoma. Tomography 2023, 9, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Champ, C.; Siglin, J.; Mishra, M.V.; Shen, X.; Werner-Wasik, M.; Andrews, D.W.; Mayekar, S.U.; Liu, H.; Shi, W. Evaluating changes in radiation treatment volumes from post-operative to same-day planning MRI in High-grade gliomas. Radiat. Oncol. 2012, 7, 220. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yao, C.; Chen, H.; Zhuang, D.; Tang, W.; Ren, G.; Wang, Y.; Wu, J.; Huang, F.; Zhou, L. The relationship between Cho/NAA and glioma metabolism: Implementation for margin delineation of cerebral gliomas. Acta Neurochir. 2012, 154, 1361–1370; discussion 1370. [Google Scholar] [CrossRef]
- Chuang, M.T.; Liu, Y.-S.; Tsai, Y.-S.; Chen, Y.-C.; Wang, C.-K.; Hendrikse, J. Differentiating Radiation-Induced Necrosis from Recurrent Brain Tumor Using MR Perfusion and Spectroscopy: A Meta-Analysis. PLoS ONE 2016, 11, e0141438. [Google Scholar] [CrossRef]
- Prager, A.J.; Martinez, N.; Beal, K.; Omuro, A.; Zhang, Z.; Young, R. Diffusion and perfusion MRI to differentiate treatment-related changes including pseudoprogression from recurrent tumors in high-grade gliomas with histopathologic evidence. AJNR Am. J. Neuroradiol. 2015, 36, 877–885. [Google Scholar] [CrossRef]
- Alexiou, G.A.; Zikou, A.; Tsiouris, S.; Goussia, A.; Kosta, P.; Papadopoulos, A.; Voulgaris, S.; Tsekeris, P.; Kyritsis, A.P.; Fotopoulos, A.D.; et al. Comparison of diffusion tensor, dynamic susceptibility contrast MRI and (99m)Tc-Tetrofosmin brain SPECT for the detection of recurrent high-grade glioma. Magn. Reson. Imaging 2014, 32, 854–859. [Google Scholar] [CrossRef]
- Huang, J.; Wang, A.-M.; Shetty, A.; Maitz, A.H.; Yan, D.; Doyle, D.; Richey, K.; Park, S.; Pieper, D.R.; Chen, P.Y.; et al. Differentiation between intra-axial metastatic tumor progression and radiation injury following fractionated radiation therapy or stereotactic radiosurgery using MR spectroscopy, perfusion MR imaging or volume progression modeling. Magn. Reson. Imaging 2011, 29, 993–1001. [Google Scholar] [CrossRef]
- Matsusue, E.; Fink, J.R.; Rockhill, J.K.; Ogawa, T.; Maravilla, K.R. Distinction between glioma progression and post-radiation change by combined physiologic MR imaging. Neuroradiology 2010, 52, 297–306. [Google Scholar] [CrossRef]
- Barajas, R.F.; Chang, J.; Sneed, P.; Segal, M.; McDermott, M.; Cha, S. Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am. J. Neuroradiol. 2009, 30, 367–372. [Google Scholar] [CrossRef]
- Farche, M.K.; O Fachinetti, N.; da Silva, L.R.; A Matos, L.; Appenzeller, S.; Cendes, F.; Reis, F. Revisiting the use of proton magnetic resonance spectroscopy in distinguishing between primary and secondary malignant tumors of the central nervous system. Neuroradiol. J. 2022, 35, 619–626. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rejimon, A.C.; Trivedi, A.G.; Huang, V.; Ramesh, K.K.; Esiashvilli, N.; Schreibmann, E.; Shim, H.; Reddy, K.; Eaton, B.R. Longitudinal Overlap and Metabolite Analysis in Spectroscopic MRI-Guided Proton Beam Therapy in Pediatric High-Grade Glioma. Tomography 2025, 11, 71. https://doi.org/10.3390/tomography11060071
Rejimon AC, Trivedi AG, Huang V, Ramesh KK, Esiashvilli N, Schreibmann E, Shim H, Reddy K, Eaton BR. Longitudinal Overlap and Metabolite Analysis in Spectroscopic MRI-Guided Proton Beam Therapy in Pediatric High-Grade Glioma. Tomography. 2025; 11(6):71. https://doi.org/10.3390/tomography11060071
Chicago/Turabian StyleRejimon, Abinand C., Anuradha G. Trivedi, Vicki Huang, Karthik K. Ramesh, Natia Esiashvilli, Eduard Schreibmann, Hyunsuk Shim, Kartik Reddy, and Bree R. Eaton. 2025. "Longitudinal Overlap and Metabolite Analysis in Spectroscopic MRI-Guided Proton Beam Therapy in Pediatric High-Grade Glioma" Tomography 11, no. 6: 71. https://doi.org/10.3390/tomography11060071
APA StyleRejimon, A. C., Trivedi, A. G., Huang, V., Ramesh, K. K., Esiashvilli, N., Schreibmann, E., Shim, H., Reddy, K., & Eaton, B. R. (2025). Longitudinal Overlap and Metabolite Analysis in Spectroscopic MRI-Guided Proton Beam Therapy in Pediatric High-Grade Glioma. Tomography, 11(6), 71. https://doi.org/10.3390/tomography11060071





