Current Update on DWI-MRI and Its Radiomics in Liver Fibrosis—A Review of the Literature
Abstract
1. Introduction
2. Methods
3. Assessment Techniques for Liver Fibrosis
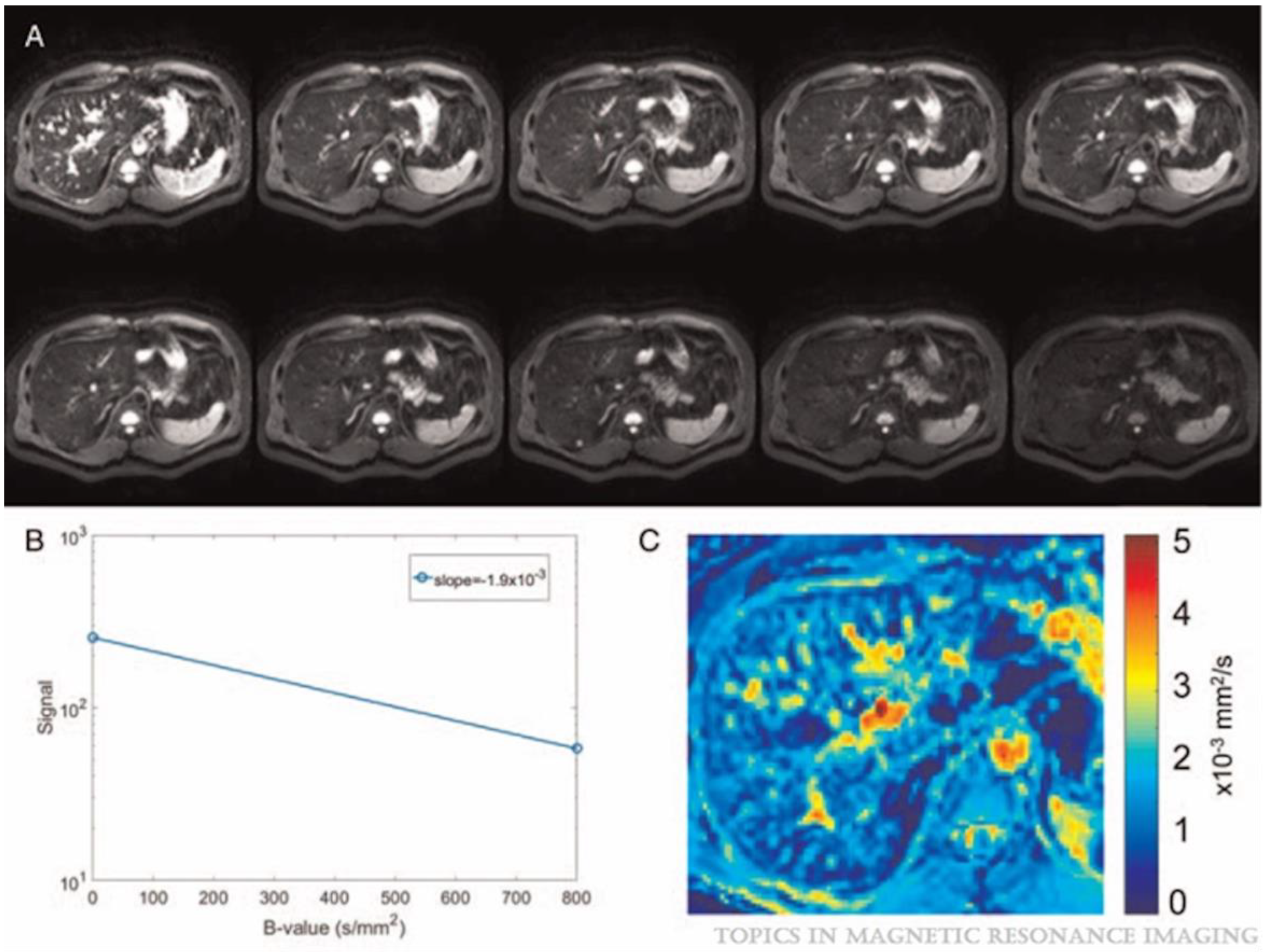
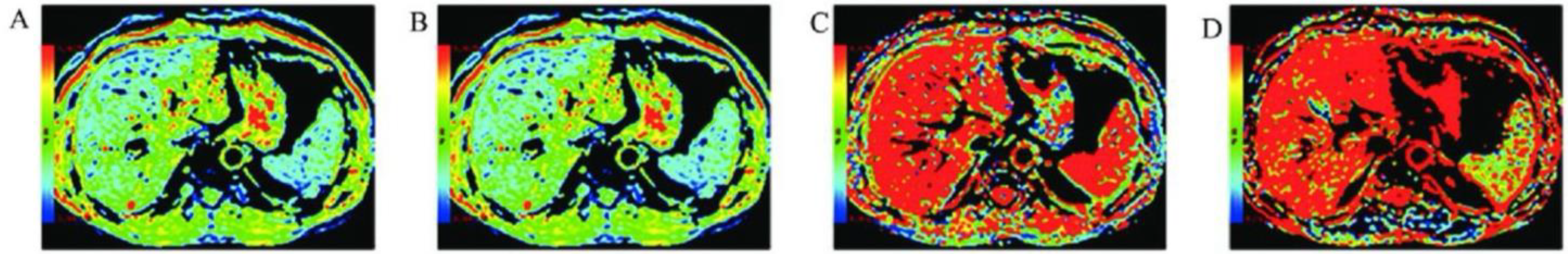
4. Basic Principles of DWI and Its Advancements
5. The Principal Changes in Hepatic Fibrosis Molecules Using DWI
6. Radiomics Analysis of DWI Images in Liver Fibrosis
7. Discussion
8. Future Research Directions for DWI in Liver Fibrosis Assessment
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parola, M.; Pinzani, M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol. Asp. Med. 2019, 65, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Berumen, J.; Baglieri, J.; Kisseleva, T.; Mekeel, K. Liver fibrosis: Pathophysiology and clinical implications. WIREs Mech. Dis. 2021, 13, e1499. [Google Scholar] [CrossRef] [PubMed]
- Goodman, Z.D. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J. Hepatol. 2007, 47, 598–607. [Google Scholar] [CrossRef]
- Khalifa, A.; Rockey, D.C. The utility of liver biopsy in 2020. Curr. Opin. Gastroenterol. 2020, 36, 184–191. [Google Scholar] [CrossRef]
- Maino, C.; Vernccio, F.; Cannella, R.; Cristoferi, L.; Franco, P.N.; Carbone, M.; Cortese, F.; Faletti, R.; De Bernardi, E.; Inchingolo, R. Non-invasive imaging biomarkers in chronic liver disease. Eur. J. Radiol. 2024, 181, 111749. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, J.; Gao, R.; Huang, Z.; Wu, M.; Song, B. Liver fibrosis staging with diffusion-weighted imaging: A systematic review and meta-analysis. Abdom. Radiol. 2017, 42, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Lincke, T.; Zech, C.J. Liver metastases: Detection and staging. Eur. J. Radiol. 2017, 97, 76–82. [Google Scholar] [CrossRef]
- Petitclerc, L.; Gilbert, G.; Nguyen, B.N.; Tang, A. Liver fibrosis quantification by magnetic resonance imaging. Top. Magn. Reson. Imaging 2017, 26, 229–241. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Z.-Y. Radiomics approaches in gastric cancer: A frontier in clinical decision making. Chin. Med. J. 2019, 132, 1983–1989. [Google Scholar]
- Liu, Z.; Wang, S.; Dong, D.; Wei, J.; Fang, C.; Zhou, X.; Sun, K.; Li, L.; Li, B.; Wang, M. The applications of radiomics in precision diagnosis and treatment of oncology: Opportunities and challenges. Theranostics 2019, 9, 1303. [Google Scholar]
- Zhang, Y.-P.; Zhang, X.-Y.; Cheng, Y.-T.; Li, B.; Teng, X.-Z.; Zhang, J.; Lam, S.; Zhou, T.; Ma, Z.-R.; Sheng, J.-B. Artificial intelligence-driven radiomics study in cancer: The role of feature engineering and modeling. Mil. Med. Res. 2023, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Neisius, U.; El-Rewaidy, H.; Kucukseymen, S.; Tsao, C.W.; Mancio, J.; Nakamori, S.; Manning, W.J.; Nezafat, R. Texture signatures of native myocardial T1 as novel imaging markers for identification of hypertrophic cardiomyopathy patients without scar. J. Magn. Reson. Imaging 2020, 52, 906–919. [Google Scholar] [CrossRef]
- Chea, P.; Mandell, J.C. Current applications and future directions of deep learning in musculoskeletal radiology. Skelet. Radiol. 2020, 49, 183–197. [Google Scholar] [CrossRef]
- Alyami, A.S. The role of radiomics in fibrosis Crohn’s disease: A review. Diagnostics 2023, 13, 1623. [Google Scholar] [CrossRef]
- Guan, Y.; Li, W.; Jiang, Z.; Chen, Y.; Liu, S.; He, J.; Zhou, Z.; Ge, Y. Whole-lesion apparent diffusion coefficient-based entropy-related parameters for characterizing cervical cancers: Initial findings. Acad. Radiol. 2016, 23, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Corino, V.D.; Montin, E.; Messina, A.; Casali, P.G.; Gronchi, A.; Marchianò, A.; Mainardi, L.T. Radiomic analysis of soft tissues sarcomas can distinguish intermediate from high-grade lesions. J. Magn. Reson. Imaging 2018, 47, 829–840. [Google Scholar] [CrossRef]
- Kocak, B.; Baessler, B.; Cuocolo, R.; Mercaldo, N.; dos Santos, D.P. Trends and statistics of artificial intelligence and radiomics research in Radiology, Nuclear Medicine, and Medical Imaging: Bibliometric analysis. Eur. Radiol. 2023, 33, 7542–7555. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, S.K.; Torbenson, M.S. Liver fibrosis quantification. Abdom. Radiol. 2022, 47, 1032–1052. [Google Scholar] [CrossRef]
- De, K. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef]
- Almpanis, Z.; Demonakou, M.; Tiniakos, D. Evaluation of liver fibrosis: “Something old, something new…”. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2016, 29, 445. [Google Scholar] [CrossRef] [PubMed]
- Nallagangula, K.S.; Nagaraj, S.K.; Venkataswamy, L.; Chandrappa, M. Liver fibrosis: A compilation on the biomarkers status and their significance during disease progression. Future Sci. OA 2018, 4, FSO250. [Google Scholar] [CrossRef] [PubMed]
- Gourtsoyianni, S.; Santinha, J.; Matos, C.; Papanikolaou, N. Diffusion-weighted imaging and texture analysis: Current role for diffuse liver disease. Abdom. Radiol. 2020, 45, 3523–3531. [Google Scholar] [CrossRef]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Grenier, P.; Cabanis, E.; Laval-Jeantet, M. MR imaging of intravoxel incoherent motions: Application to diffusion and perfusion in neurologic disorders. Radiology 1986, 161, 401–407. [Google Scholar] [CrossRef]
- Li, Y.T.; Cercueil, J.-P.; Yuan, J.; Chen, W.; Loffroy, R.; Wáng, Y.X.J. Liver intravoxel incoherent motion (IVIM) magnetic resonance imaging: A comprehensive review of published data on normal values and applications for fibrosis and tumor evaluation. Quant. Imaging Med. Surg. 2017, 7, 59. [Google Scholar] [CrossRef]
- Iima, M. Perfusion-driven intravoxel incoherent motion (IVIM) MRI in oncology: Applications, challenges, and future trends. Magn. Reson. Med. Sci. 2021, 20, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wei, Q.; Peng, H.; Zhang, W.; Tang, J.; Liu, T. Monoexponential and advanced diffusion-weighted imaging for hepatic fibrosis staging based on high inter-examiner reliability. Saudi Med. J. 2024, 45, 911. [Google Scholar] [CrossRef]
- Seo, N.; Chung, Y.E.; Park, Y.N.; Kim, E.; Hwang, J.; Kim, M.-J. Liver fibrosis: Stretched exponential model outperforms mono-exponential and bi-exponential models of diffusion-weighted MRI. Eur. Radiol. 2018, 28, 2812–2822. [Google Scholar] [CrossRef]
- Park, J.H.; Seo, N.; Chung, Y.E.; Kim, S.U.; Park, Y.N.; Choi, J.-Y.; Park, M.-S.; Kim, M.-J. Noninvasive evaluation of liver fibrosis: Comparison of the stretched exponential diffusion-weighted model to other diffusion-weighted MRI models and transient elastography. Eur. Radiol. 2021, 31, 4813–4823. [Google Scholar] [CrossRef]
- Jensen, J.H.; Helpern, J.A.; Ramani, A.; Lu, H.; Kaczynski, K. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med. 2005, 53, 1432–1440. [Google Scholar] [CrossRef]
- Lu, H.; Jensen, J.H.; Ramani, A.; Helpern, J.A. Three-dimensional characterization of non-gaussian water diffusion in humans using diffusion kurtosis imaging. NMR Biomed. 2006, 19, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lu, X.; Wang, X.; Shu, J. Diffusion tensor imaging quantifying the severity of chronic hepatitis in rats. BMC Med. Imaging 2020, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shenoy-Bhangle, A.; Baliyan, V.; Kordbacheh, H.; Guimaraes, A.R.; Kambadakone, A. Diffusion weighted magnetic resonance imaging of liver: Principles, clinical applications and recent updates. World J. Hepatol. 2017, 9, 1081. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.P.; Venkatesh, S.K. Imaging of hepatic fibrosis. Curr. Gastroenterol. Rep. 2018, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kromrey, M.-L.; Le Bihan, D.; Ichikawa, S.; Motosugi, U. Diffusion-weighted MRI-based virtual elastography for the assessment of liver fibrosis. Radiology 2020, 295, 127–135. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, X.; Yao, M.; Tan, W.; Zhan, S.; Liu, K.; Feng, Z.; Yan, H.; Dai, Y.; Yuan, J. Comparison and optimization of b value combinations for diffusion-weighted imaging in discriminating hepatic fibrosis. Abdom. Radiol. 2024, 49, 1113–1121. [Google Scholar] [CrossRef]
- Li, T.; Che-Nordin, N.; Wáng, Y.X.J.; Rong, P.-F.; Qiu, S.-W.; Zhang, S.-W.; Zhang, P.; Jiang, Y.-F.; Chevallier, O.; Zhao, F. Intravoxel incoherent motion derived liver perfusion/diffusion readouts can be reliable biomarker for the detection of viral hepatitis B induced liver fibrosis. Quant. Imaging Med. Surg. 2019, 9, 371. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Lee, J.M.; Lee, K.B.; Kim, D.; Kabasawa, H.; Han, J.K. Comparison of monoexponential, intravoxel incoherent motion diffusion-weighted imaging and diffusion kurtosis imaging for assessment of hepatic fibrosis. Acta Radiol. 2019, 60, 1593–1601. [Google Scholar] [CrossRef]
- Zawada, E.; Serafin, Z.; Dybowska, D.; Halota, W.; Wypych, A.; Nadolska, K.; Rusak, G. Monoexponential and biexponential fitting of diffusional magnetic resonance imaging signal analysis for prediction of liver fibrosis severity. J. Comput. Assist. Tomogr. 2019, 43, 857–862. [Google Scholar] [CrossRef]
- Yang, L.; Rao, S.; Wang, W.; Chen, C.; Ding, Y.; Yang, C.; Grimm, R.; Yan, X.; Fu, C.; Zeng, M. Staging liver fibrosis with DWI: Is there an added value for diffusion kurtosis imaging? Eur. Radiol. 2018, 28, 3041–3049. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, Y.S.; Liu, Z.; Liu, H.F.; Zhai, Y.N.; Mao, X.R.; Lei, J.Q. Whole-liver apparent diffusion coefficient histogram analysis for the diagnosis and staging of liver fibrosis. J. Magn. Reson. Imaging 2020, 51, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, Y.-L.; Chen, T.-W.; Li, R.; Pu, Y.; Zhang, X.-M.; Li, H.-J.; Tang, S.; Cao, J.-M.; Yang, J.-Q. Liver lobe based intravoxel incoherent motion diffusion weighted imaging in hepatitis B related cirrhosis: Association with child-pugh class and esophageal and gastric fundic varices. Medicine 2020, 99, e18671. [Google Scholar] [CrossRef] [PubMed]
- Tosun, M.; Onal, T.; Uslu, H.; Alparslan, B.; Akhan, S.Ç. Intravoxel incoherent motion imaging for diagnosing and staging the liver fibrosis and inflammation. Abdom. Radiol. 2020, 45, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Wáng, Y.X.J.; Deng, M.; Li, Y.T.; Huang, H.; Leung, J.C.S.; Chen, W.; Lu, P.-X. A combined use of intravoxel incoherent motion MRI parameters can differentiate early-stage hepatitis-b fibrotic livers from healthy livers. SLAS Technol. Transl. Life Sci. Innov. 2018, 23, 259–268. [Google Scholar] [CrossRef]
- Ding, L.; Xiao, L.; Lin, X.; Xiong, C.; Lin, L.; Chen, S. Intravoxel Incoherent Motion (IVIM) Diffusion-Weighted Imaging (DWI) in Patients with Liver Dysfunction of Chronic Viral Hepatitis: Segmental Heterogeneity and Relationship with Child-Turcotte-Pugh Class at 3 Tesla. Gastroenterol. Res. Pract. 2018, 2018, 2983725. [Google Scholar] [CrossRef]
- Hu, F.; Yang, R.; Huang, Z.; Wang, M.; Zhang, H.; Yan, X.; Song, B. Liver fibrosis: In vivo evaluation using intravoxel incoherent motion-derived histogram metrics with histopathologic findings at 3.0 T. Abdom. Radiol. 2017, 42, 2855–2863. [Google Scholar] [CrossRef]
- Ren, H.; Xu, H.; Yang, D.; Tong, X.; Zhao, X.; Wang, Q.; Sun, Y.; Ou, X.; Jia, J.; You, H. Intravoxel incoherent motion assessment of liver fibrosis staging in MASLD. Abdom. Radiol. 2024, 49, 1411–1418. [Google Scholar] [CrossRef]
- Yoshimaru, D.; Miyati, T.; Suzuki, Y.; Hamada, Y.; Mogi, N.; Funaki, A.; Tabata, A.; Masunaga, A.; Shimada, M.; Tobari, M. Diffusion kurtosis imaging with the breath-hold technique for staging hepatic fibrosis: A preliminary study. Magn. Reson. Imaging 2018, 47, 33–38. [Google Scholar] [CrossRef]
- Fu, F.; Li, X.; Chen, C.; Bai, Y.; Liu, Q.; Shi, D.; Sang, J.; Wang, K.; Wang, M. Non-invasive assessment of hepatic fibrosis: Comparison of MR elastography to transient elastography and intravoxel incoherent motion diffusion-weighted MRI. Abdom. Radiol. 2020, 45, 73–82. [Google Scholar] [CrossRef]
- Xie, S.; Li, Q.; Cheng, Y.; Zhou, L.; Xia, S.; Li, J.; Shen, W. Differentiating mild and substantial hepatic fibrosis from healthy controls: A comparison of diffusion kurtosis imaging and conventional diffusion-weighted imaging. Acta Radiol. 2020, 61, 1012–1020. [Google Scholar] [CrossRef]
- Ren, H.; Liu, Y.; Lu, J.; An, W.; Wang, W.; Yan, T.; Li, Y.; Dong, J.; Cai, J. Evaluating the clinical value of MRI multi-model diffusion-weighted imaging on liver fibrosis in chronic hepatitis B patients. Abdom. Radiol. 2021, 46, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-L.; Li, J.; Zhang, P.-F.; Fan, F.-X.; Zou, J.; Yang, P.; Wang, P.-F.; Wang, S.-Y.; Zhang, J. Staging liver fibrosis with various diffusion-weighted magnetic resonance imaging models. World J. Gastroenterol. 2024, 30, 1164. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Che-Nordin, N.; Wang, L.-F.; Xiao, B.-H.; Chevallier, O.; Yun, Y.-X.; Guo, S.-W.; Wáng, Y.X.J. High performance of intravoxel incoherent motion diffusion MRI in detecting viral hepatitis-b induced liver fibrosis. Ann. Transl. Med. 2019, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Sollini, M.; Antunovic, L.; Chiti, A.; Kirienko, M. Towards clinical application of image mining: A systematic review on artificial intelligence and radiomics. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2656–2672. [Google Scholar] [CrossRef]
- Zheng, W.; Guo, W.; Xiong, M.; Chen, X.; Gao, L.; Song, Y.; Cao, D. Clinic-radiological features and radiomics signatures based on Gd-BOPTA-enhanced MRI for predicting advanced liver fibrosis. Eur. Radiol. 2023, 33, 633–644. [Google Scholar] [CrossRef]
- Qiu, Q.-T.; Zhang, J.; Duan, J.-H.; Wu, S.-Z.; Ding, J.-L.; Yin, Y. Development and validation of radiomics model built by incorporating machine learning for identifying liver fibrosis and early-stage cirrhosis. Chin. Med. J. 2020, 133, 2653–2659. [Google Scholar] [CrossRef]
- Xiao, L.; Zhao, H.; Liu, S.; Dong, W.; Gao, Y.; Wang, L.; Huang, B.; Li, Z. Staging liver fibrosis: Comparison of radiomics model and fusion model based on multiparametric MRI in patients with chronic liver disease. Abdom. Radiol. 2024, 49, 1165–1174. [Google Scholar] [CrossRef]
- Gotta, J.; Gruenewald, L.D.; Reschke, P.; Booz, C.; Mahmoudi, S.; Stieltjes, B.; Choi, M.H.; D’Angelo, T.; Bernatz, S.; Vogl, T.J. Noninvasive Grading of Liver Fibrosis Based on Texture Analysis From MRI-Derived Radiomics. NMR Biomed. 2025, 38, e5301. [Google Scholar] [CrossRef]
| Fibrosis Stages | METAVIR System | Ishak System | Kleiner System |
|---|---|---|---|
| Stage: 0 | Absence of fibrosis | No evidence of fibrosis | No fibrosis observed |
| Stage: 1 | Fibrosis limited to portal areas without evidence of bridging septa | Mild fibrous expansion in some portal areas, accompanied by or without short septa | 1: Fibrosis in periportal or presinusoidal regions |
| 1A: Zone 3 has mild perisinusoidal fibrosis | |||
| 1B: Zone 3 has moderate perisinusoidal fibrosis | |||
| 1C: Fibrosis in periportal or portal areas | |||
| Stage: 2 | Portal fibrosis with minimal septa | Fibrous expansion in most portal areas, with or without short septa | Combined perisinusoidal and periportal/portal fibrosis |
| Stage: 3 | Significant septal formation without cirrhosis | Fibrous expansion in majority portal regions, with occasional bridging | Presence of bridging fibrosis |
| Stage: 4 | Development of cirrhosis | Extensive bridging fibrosis, including portal-to-central bridging and portal-to-portal | Cirrhosis |
| Stage: 5 | Prominent bridging fibrosis with early nodule formation | ||
| Stage: 6 | Established cirrhosis |
| Technique | Advantages | Disadvantages |
|---|---|---|
| Conventional DWI |
|
|
| IVIM-DWI |
|
|
| DKI |
|
|
| DTI |
|
|
| Ref. | Target and Study Type | Aim | Results |
|---|---|---|---|
| Kromrey et al. [35] | N = 74 Retrospective | To assess the effectiveness of DW-MRI-based elastography for staging liver fibrosis | There was a strong agreement between the µDiff and µMRE values (mean difference of −0.02 kPa ± 0.88; p < 0.001). In 55% of patients, DWI-based fibrosis staging matched magnetic MRE staging, with a one-stage difference in 35% of cases. |
| Park et al. [29] | N = 87 Retrospective | To assess the diagnostic effectiveness of the stretched exponential model of DWI and to examine the impact of confounding factors on the staging of liver fibrosis | The DDC showed no correlation with steatosis (p = 0.619) but demonstrated a significant correlation with inflammation (p = 0.001) and fibrosis (p < 0.001). The DDC exhibited superior performance for liver fibrosis with an area under the curve of 0.717 (95% CI: 0.653–0.765) compared to transient elastography, which had a performance of 0.681 (95% CI: 0.623–0.733). |
| Jiaoyan Wang et al. [36] | 110 subjects (21 healthy liver and 81 patients with liver fibrosis) Prospective | To identify the optimal high and low b-values of DWI for assessing hepatic fibrosis | The analysis revealed significant correlations between all tested b-value-derived ADC measurements and MRE values (all p < 0.05). Particularly strong associations were observed for three specific b-value ranges: 0–800 s/mm2, 200–1000 s/mm2, and 200–1200 s/mm2. The ADC with b-values of 200–800 s/mm2 and 200–1000 s/mm2 had area under the receiver operating characteristic (AUROC) values greater than 0.750 for detecting hepatic fibrosis in the F1 and F2–4 groups, F1–2 and F3–4 groups, and F1–3 and F4 groups, respectively. The b-value combination of 200–800 s/mm2 demonstrated superior diagnostic accuracy for fibrosis staging compared to the 200–1000 s/mm2. |
| Huang et al. [53] | Hepatitis-b induced liver fibrosis group (n = 12, F1–2 = 7, and F3–4 = 5) and non-fibrosis group (n = 30) Prospective | To assess the diagnostic effectiveness of the modified IVIM technique in detecting early-stage liver fibrosis | To differentiate between liver fibrosis patients and healthy volunteers, a threshold b-value of 60 s/mm2 was preferred above 200 s/mm2. When a threshold b-value of 60 s/mm2 was used, a PF (f) less than 6.49% effectively distinguished healthy livers from all fibrotic livers with 100% sensitivity and specificity. For the patient measurements, there was a strong correlation between PF and Dfast, with a Pearson correlation coefficient of r = 0.865 (p < 0.001). However, no significant correlation was observed between the slow diffusion component (Dslow) and the fast diffusion compartment (Dfast or PF). For Dslow, Dfast, and PF, the intraclass correlation coefficient (ICC) of intra-reader agreement was 0.909, 0.949, and 0.925, respectively. |
| Wáng et al. [44] | N = 49 subjects (33 hepatitis-b induced liver fibrosis and 16 healthy participants | To investigate a combined use of intravoxe IVIM parameters for liver fibrosis assessment | Compared to healthy volunteers, liver fibrosis had lower values in terms of PF, Dslow, and Dfast. Among these parameters, PF demonstrated the highest diagnostic value, followed by Dslow. The regression and classification tree analysis indicated that a combination of Dfast (Dfast < 13.36 × 10−3 mm2/s), Dslow (Dslow < 1.152 × 10−3 mm2/s), and PF (PF < 12.55%), effectively distinguished healthy individuals from all fibrotic livers (F1–4), achieving AUC of 0.986 in logistic regression. The study concluded that a combination of Dfast, Dslow and PF shows the potential of IVIM to detect early-stage liver fibrosis. |
| Lesheng Huang et al. [27] | N = 145 subjects (48 healthy volunteers, 59 early liver fibrosis patients, and 38 advanced liver fibrosis patients) Prospective | To assess the diagnostic effectiveness of metrics obtained from DWI, IVIM, and DKI for the purpose of staging liver fibrosis, emphasizing robust inter-examiner reliability as a guiding principle | In all study groups, the inter-examiner reliability of the parameters DDC, D, and particularly D* was low was found. Parameters from DTI, DKI, and DWI displayed good to excellent reliability; nonetheless, the majority of DKI, DTI, and DKI parameters did not exhibit notable variances among the cohorts under investigation The study findings reported that the specificity and sensitivity of the models distinguishing healthy volunteers from early liver fibrosis and those differentiating early liver fibrosis from advanced liver fibrosis were poor. |
| Li Yang [40] | N = 81 patients chronic liver disease Prospective | Compared the efficacy of KDI to conventional DWI among these patients | The kurtosis model does not provide any additional benefit over the conventional monoexponentially model. |
| Yoshimaru et al. [48] | N = 67 Prospective | To assess the potential of DKI analysis using the breath-hold technique for evaluating liver fibrosis | The DKI cutoff values for assessing F0, ≥F1, ≥F2, and F4 were 0.923, 0.955, and 1.11, respectively. ADC values did not demonstrate a correlation with the severity of liver fibrosis staging |
| Shuangshuang Xie et al. [50] | N = 45 patients (n = 25 mild (S1) or n = 20 substantial (S2)) and 27 healthy controls Prospective | To assess the effectiveness of DKI in distinguishing between healthy controls and patients with S1 and S2 fibrosis, and to compare its diagnostic accuracy to traditional DWI | Strong agreement was found in the inter-observer reproducibility of the ADC, MK, and MD measures (ICC = 0.912, 0.908, and 0.894, respectively). Both ADC (p = 0.013) and MD (p < 0.001) values showed a decreasing trend with increasing fibrotic stage, showing notable distinctions between healthy participants and individuals with S1 and S2 fibrosis. Furthermore, there were statistically significant differences in MD values between S0 and S1 (p = 0.028) as well as S0 and S2 (p = 0.005), with no notable difference observed between S1 and S2 (p = 0.452). There was a negative association between the stages of fibrosis and the ADC as well as MD values (rs = 0.668, −0.341; p < 0.01), while MK levels did not show a significant connection with fibrosis stages (rs = 0.180; p = 0.13). |
| Ref. | Study Design | Target | Aim | Findings |
|---|---|---|---|---|
| Qiu et al. [56] | Retrospective | 369 participants with liver fibrosis (n = 108) and early-stage cirrhosis (n = 116) and control (n = 145) | To develop a radiomics model to detect early-stage cirrhosis and liver fibrosis | Radiomics analysis of DW images can accurately identify early-stage cirrhosis and liver fibrosis, with AUC values ranging from 0.944 to 0.973 |
| Gotta et al. [58] | Prospective | 79 participants (no liver fibrosis n = 31 participants and 48 with histologically proven fibrosis. | To develop a reliable grading system for liver fibrosis that does not require invasive procedures including ADC | The combination of ADC with radiomics did not reliably improve the predictive accuracy for grading fibrosis. Although there was a tendency for an increase in AUC for fibrosis grade 3 with the inclusion of ADC, the variations observed across all grades were not statistically significant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alyami, A.S. Current Update on DWI-MRI and Its Radiomics in Liver Fibrosis—A Review of the Literature. Tomography 2025, 11, 63. https://doi.org/10.3390/tomography11060063
Alyami AS. Current Update on DWI-MRI and Its Radiomics in Liver Fibrosis—A Review of the Literature. Tomography. 2025; 11(6):63. https://doi.org/10.3390/tomography11060063
Chicago/Turabian StyleAlyami, Ali S. 2025. "Current Update on DWI-MRI and Its Radiomics in Liver Fibrosis—A Review of the Literature" Tomography 11, no. 6: 63. https://doi.org/10.3390/tomography11060063
APA StyleAlyami, A. S. (2025). Current Update on DWI-MRI and Its Radiomics in Liver Fibrosis—A Review of the Literature. Tomography, 11(6), 63. https://doi.org/10.3390/tomography11060063






