Quantitative Ultrasound Grayscale Analysis and Size of Benign and Malignant Solid Thyroid Nodules
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
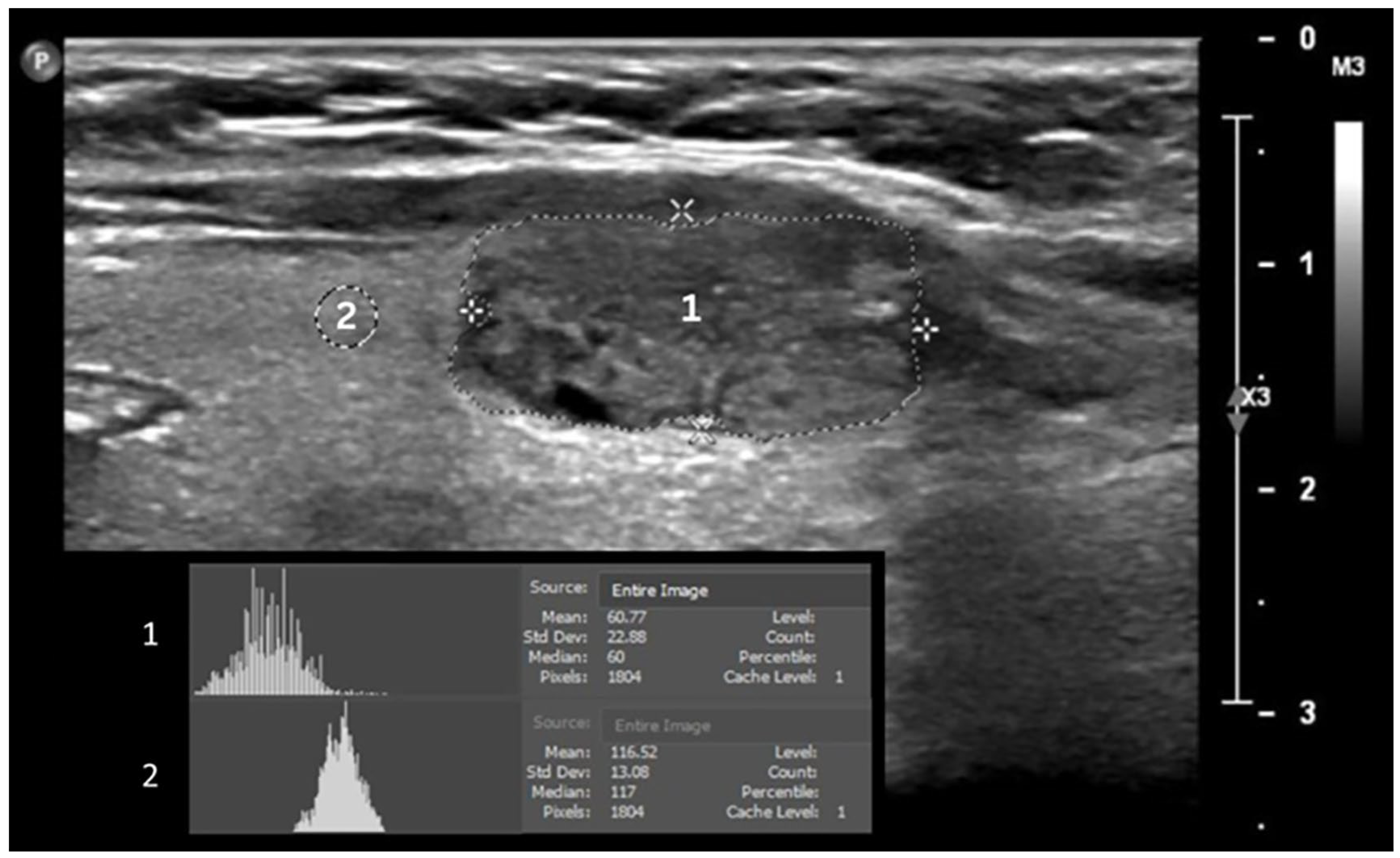
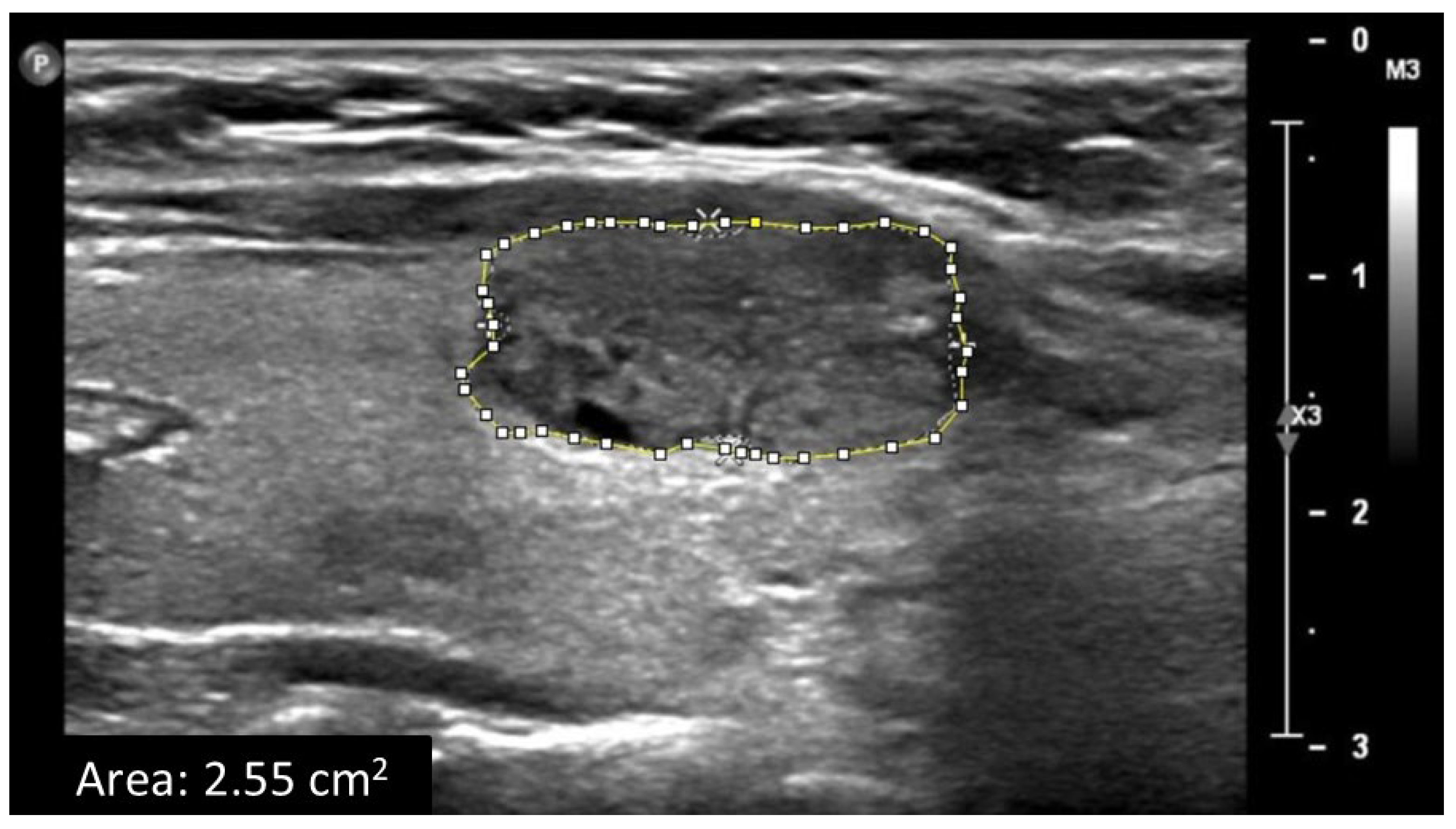
2.2. Data Acquisition and Ultrasound Image Analysis
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
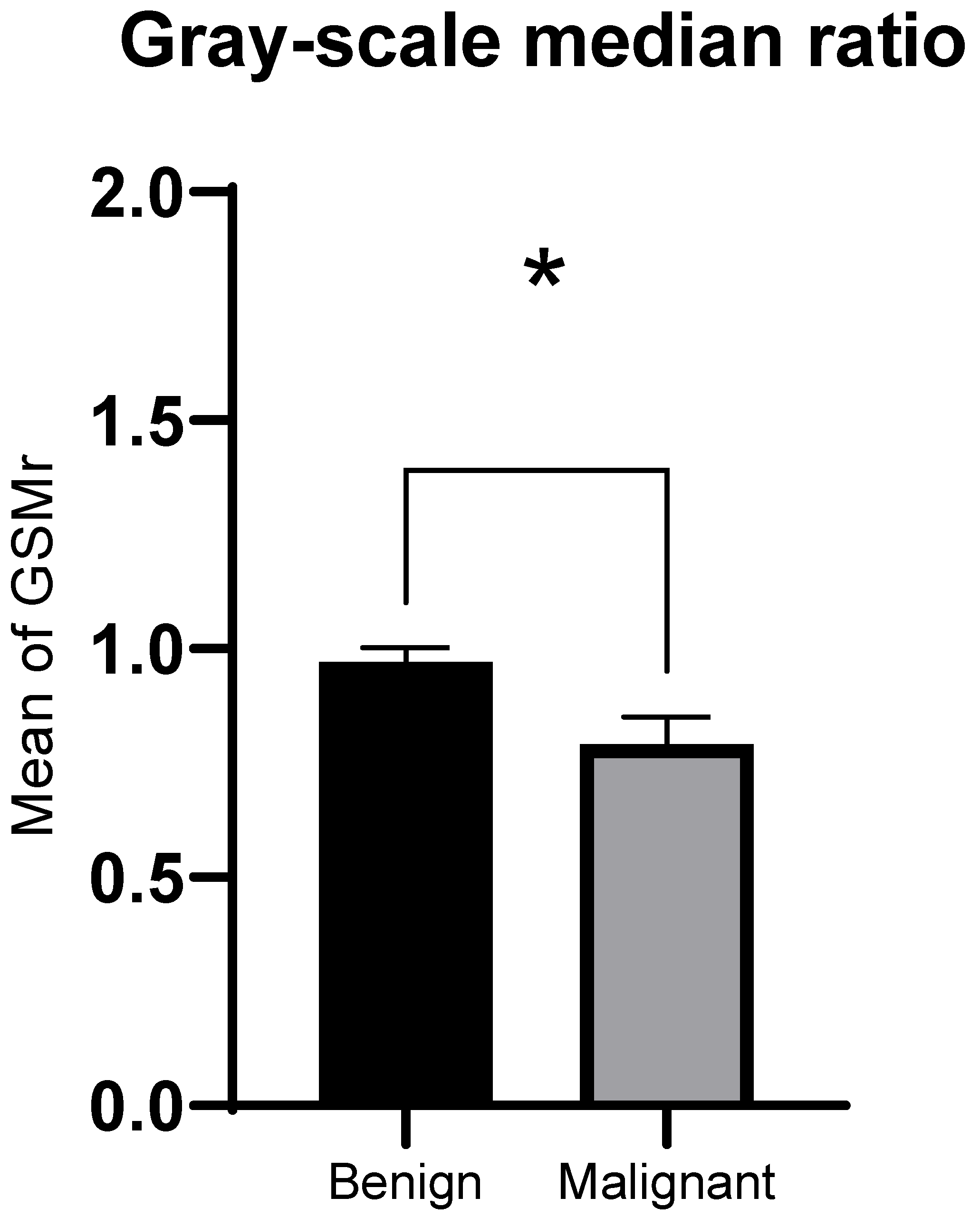
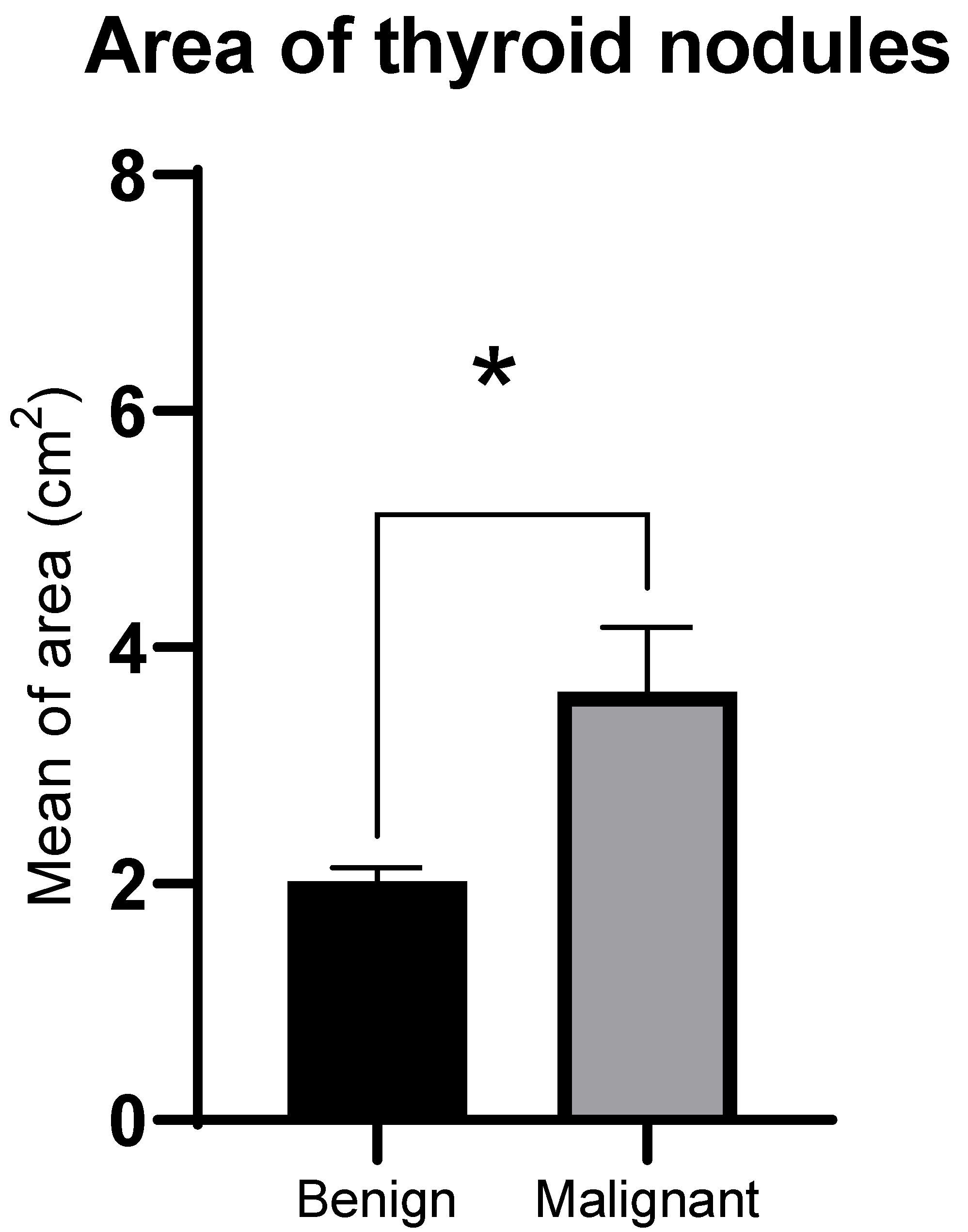
3.2. GSMr and Area of Benign and Malignant Solid Thyroid Nodules
3.3. Inter-Observer Agreement of GSMr and Area
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Castellana, M.; Grani, G.; Radzina, M.; Guerra, V.; Giovanella, L.; Deandrea, M.; Ngu, R.; Durante, C.; Trimboli, P. Performance of EU-TIRADS in malignancy risk stratification of thyroid nodules: A meta-analysis. Eur. J. Endocrinol. 2020, 183, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Grani, G.; Sponziello, M.; Filetti, S.; Durante, C. Thyroid nodules: Diagnosis and management. Nat. Rev. Endocrinol. 2024, 20, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Krzentowska, A.; Gołkowski, F.; Broniatowska, E.; Konturek, A.; Barczyński, M. Risk Factors for Malignancy of Thyroid Nodules in Patients Undergoing Thyroid Resection. J. Clin. Med. 2024, 13, 7559. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xiao, L.; He, M.; Feng, R.; Huang, X.; Su, Q.; Xue, X.; Hu, Z.; Zhang, Q.; Du, S.; et al. Prevalence of thyroid nodules and canceration risk assessment in TIRADS, and their relationships to obesity and dysglycemia. Front. Oncol. 2025, 15, 1658717. [Google Scholar] [CrossRef]
- David, E.; Grazhdani, H.; Tattaresu, G.; Pittari, A.; Foti, P.V.; Palmucci, S.; Spatola, C.; Lo Greco, M.C.; Inì, C.; Tiralongo, F.; et al. Thyroid Nodule Characterization: Overview and State of the Art of Diagnosis with Recent Developments, from Imaging to Molecular Diagnosis and Artificial Intelligence. Biomedicines 2024, 12, 1676. [Google Scholar] [CrossRef]
- Chakrabarty, N.; Mahajan, A.; Basu, S.; D’Cruz, A.K. Comprehensive Review of the Imaging Recommendations for Diagnosis, Staging, and Management of Thyroid Carcinoma. J. Clin. Med. 2024, 13, 2904. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.W.; Basurrah, M.A.; Lee, J.; Hwang, S.H. Diagnostic Performance of Six Ultrasound Risk Stratification Systems for Thyroid Nodules: A Systematic Review and Network Meta-Analysis. Am. J. Roentgenol. 2023, 220, 791–804. [Google Scholar] [CrossRef]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Papotti, M.G.; Berruti, A. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef]
- Hoang, J.K.; Middleton, W.D.; Tessler, F.N. Update on ACR TI-RADS: Successes, Challenges, and Future Directions, From the AJR Special Series on Radiology Reporting and Data Systems. AJR. Am. J. Roentgenol. 2021, 216, 570–578. [Google Scholar] [CrossRef]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef]
- Ha, E.J.; Chung, S.R.; Na, D.G.; Ahn, H.S.; Chung, J.; Lee, J.Y.; Park, J.S.; Yoo, R.E.; Baek, J.H.; Baek, S.M.; et al. 2021 Korean Thyroid Imaging Reporting and Data System and Imaging-Based Management of Thyroid Nodules: Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J. Radiol. 2021, 22, 2094–2123. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Baek, J.H.; Chung, J.; Ha, E.J.; Kim, J.H.; Lee, Y.H.; Lim, H.K.; Moon, W.J.; Na, D.G.; Park, J.S.; et al. Ultrasonography Diagnosis and Imaging-Based Management of Thyroid Nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J. Radiol. 2016, 17, 370–395. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Na, D.G.; Joo, L.; Lee, J.Y.; Ha, E.J.; Kim, J.H.; Jung, S.L.; Baek, J.H. Standardized Imaging and Reporting for Thyroid Ultrasound: Korean Society of Thyroid Radiology Consensus Statement and Recommendation. Korean J. Radiol. 2023, 24, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid J. 2017, 6, 225–237. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, C.Y.; Hwang, I.; You, S.-H.; Park, S.-W.; Lee, B.; Yoon, R.G.; Yim, Y.; Kim, J.-H.; Na, D.G. Malignancy risk stratification of thyroid nodules according to echotexture and degree of hypoechogenicity: A retrospective multicenter validation study. Sci. Rep. 2022, 12, 16587. [Google Scholar] [CrossRef]
- Durante, C.; Hegedüs, L.; Czarniecka, A.; Paschke, R.; Russ, G.; Schmitt, F.; Soares, P.; Solymosi, T.; Papini, E. 2023 European Thyroid Association Clinical Practice Guidelines for thyroid nodule management. Eur. Thyroid J. 2023, 12, e230067. [Google Scholar] [CrossRef]
- Tang, J.Z.; Chua, J.M.E.; Woon, T.K.; Tan, B.S.; Kiong, K.L. Large thyroid nodules: Should size alone matter? Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 3139–3146. [Google Scholar] [CrossRef]
- Hammad, A.R.Y.; Noureldine, S.I.; Hu, T.; Ibrahim, Y.; Masoodi, H.M.; Kandil, E. A meta-analysis examining the independent association between thyroid nodule size and malignancy. Gland Surg. 2016, 5, 312. [Google Scholar] [CrossRef]
- Magister, M.J.; Chaikhoutdinov, I.; Schaefer, E.; Williams, N.; Saunders, B.; Goldenberg, D. Association of Thyroid Nodule Size and Bethesda Class With Rate of Malignant Disease. JAMA Otolaryngol. Neck Surg. 2015, 141, 1089–1095. [Google Scholar] [CrossRef]
- de Carlos, J.; Garcia, J.; Basterra, F.J.; Pineda, J.J.; Dolores Ollero, M.; Toni, M.; Munarriz, P.; Anda, E. Interobserver variability in thyroid ultrasound. Endocrine 2024, 85, 730–736. [Google Scholar] [CrossRef]
- Solymosi, T.; Hegedus, L.; Bonnema, S.J.; Frasoldati, A.; Jambor, L.; Karanyi, Z.; Kovacs, G.L.; Papini, E.; Rucz, K.; Russ, G.; et al. Considerable interobserver variation calls for unambiguous definitions of thyroid nodule ultrasound characteristics. Eur. Thyroid J. 2023, 12, e220134. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Qiu, Y.; Zhu, J.; Su, A.; Wu, W. Diagnostic performance of ultrasound risk stratification systems on thyroid nodules cytologically classified as indeterminate: A systematic review and meta-analysis. Ultrasonography 2023, 42, 518. [Google Scholar] [CrossRef] [PubMed]
- Harris-Love, M.O.; Seamon, B.A.; Teixeira, C.; Ismail, C. Ultrasound estimates of muscle quality in older adults: Reliability and comparison of Photoshop and ImageJ for the grayscale analysis of muscle echogenicity. PeerJ 2016, 2016, e1721. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, E.; Koshiyama, M.; Watanabe, Y.; Banba, A.; Yanagisawa, N.; Nakagawa, M.; Ono, A.; Seki, K.; Kambe, H.; Godo, T.; et al. A Histogram Analysis of the Pixel Grayscale (Luminous Intensity) of B-Mode Ultrasound Images of the Subcutaneous Layer Predicts the Grade of Leg Edema in Pregnant Women. Healthcare 2023, 11, 1328. [Google Scholar] [CrossRef]
- Sabetai, M.M.; Tegos, T.J.; Nicolaides, A.N.; Dhanjil, S.; Pare, G.J.; Stevens, J.M. Reproducibility of computer-quantified carotid plaque echogenicity: Can we overcome the subjectivity? Stroke 2000, 31, 2189–2196. [Google Scholar] [CrossRef]
- Jug, R.; Foo, W.C.; Wildman-Tobriner, B.; Jiang, X. “Sara” Cytology-Radiology Correlation Series: Thyroid cytopathology. Cancer Cytopathol. 2025, 133, e70028. [Google Scholar] [CrossRef]
- Cibas, E.S.; Alexander, E.K.; Benson, C.B.; De Agustín, P.P.; Doherty, G.M.; Faquin, W.C.; Middleton, W.D.; Miller, T.; Raab, S.S.; White, M.L.; et al. Indications for thyroid FNA and pre-FNA requirements: A synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn. Cytopathol. 2008, 36, 390–399. [Google Scholar] [CrossRef]
- Moo-Young, T.A.; Traugott, A.L.; Moley, J.F. Sporadic and familial medullary thyroid carcinoma: State of the art. Surg. Clin. N. Am. 2009, 89, 1193–1204. [Google Scholar] [CrossRef]
- Moon, W.J.; So, L.J.; Jeong, H.L.; Dong, G.N.; Baek, J.H.; Young, H.L.; Kim, J.; Hyun, S.K.; Jun, S.B.; Dong, H.L. Benign and malignant thyroid nodules: US differentiation—Multicenter retrospective study. Radiology 2008, 247, 762–770. [Google Scholar] [CrossRef]
- Ha, E.J.; Na, D.G.; Baek, J.H.; Sung, J.Y.; Kim, J.-H.; Kang, S.Y. US Fine-Needle Aspiration Biopsy for Thyroid Malignancy: Diagnostic Performance of Seven Society Guidelines Applied to 2000 Thyroid Nodules. Radiology 2018, 287, 893–900. [Google Scholar] [CrossRef]
- Lee, J.Y.; Na, D.G.; Yoon, S.J.; Gwon, H.Y.; Paik, W.; Kim, T.; Kim, J.Y. Ultrasound malignancy risk stratification of thyroid nodules based on the degree of hypoechogenicity and echotexture. Eur. Radiol. 2020, 30, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Chen, C.N.; Chen, K.Y.; Ho, M.C.; Tai, H.C.; Wang, Y.H.; Chen, A.; Chang, K.J. Quantitative analysis of echogenicity for patients with thyroid nodules. Sci. Rep. 2016, 6, 35632. [Google Scholar] [CrossRef] [PubMed]
- Eng, O.S.; Potdevin, L.; Davidov, T.; Lu, S.-E.; Chen, C.; Trooskin, S.Z. Does nodule size predict compressive symptoms in patients with thyroid nodules? Gland Surg. 2014, 3, 23236. [Google Scholar] [CrossRef]
- Hatem, M.A.; Farheen, S. Thyroid nodule size as an indicator for surgery. Int. Surg. J. 2018, 5, 2401. [Google Scholar] [CrossRef]
- Cotter, A.; Jinih, M. Thyroid nodule size and risk of malignancy: A systematic review. Discov. Oncol. 2025, 16, 1188. [Google Scholar] [CrossRef]
- Cavallo, A.; Johnson, D.N.; White, M.G.; Siddiqui, S.; Antic, T.; Mathew, M.; Grogan, R.H.; Angelos, P.; Kaplan, E.L.; Cipriani, N.A. Thyroid Nodule Size at Ultrasound as a Predictor of Malignancy and Final Pathologic Size. Thyroid 2017, 27, 641–650. [Google Scholar] [CrossRef]
- Raj, M.D.; Grodski, S.; Woodruff, S.; Yeung, M.; Paul, E.; Serpell, J.W. Diagnostic lobectomy is not routinely required to exclude malignancy in thyroid nodules greater than four centimetres. ANZ J. Surg. 2012, 82, 73–77. [Google Scholar] [CrossRef]
- Meko, J.B.; Norton, J.A. Large cystic/solid thyroid nodules: A potential false-negative fine-needle aspiration. Surgery 1995, 118, 996–1004. [Google Scholar] [CrossRef]
- Ucler, R.; Usluogullari, C.A.; Tam, A.A.; Ozdemir, D.; Balkan, F.; Yalcin, S.; Kiyak, G.; Ersoy, P.E.; Guler, G.; Ersoy, R.; et al. The diagnostic accuracy of ultrasound-guided fine-needle aspiration biopsy for thyroid nodules three centimeters or larger in size. Diagn. Cytopathol. 2015, 43, 622–628. [Google Scholar] [CrossRef]
- Kamran, S.C.; Marqusee, E.; Kim, M.I.; Frates, M.C.; Ritner, J.; Peters, H.; Benson, C.B.; Doubilet, P.M.; Cibas, E.S.; Barletta, J.; et al. Thyroid nodule size and prediction of cancer. J. Clin. Endocrinol. Metab. 2013, 98, 564–570. [Google Scholar] [CrossRef]
- Al-Hakami, H.A.; Alqahtani, R.; Alahmadi, A.; Almutairi, D.; Algarni, M.; Alandejani, T. Thyroid Nodule Size and Prediction of Cancer: A Study at Tertiary Care Hospital in Saudi Arabia. Cureus 2020, 12, e7478. [Google Scholar] [CrossRef]
- Shayganfar, A.; Hashemi, P.; Esfahani, M.M.; Ghanei, A.M.; Moghadam, N.A.; Ebrahimian, S. Prediction of thyroid nodule malignancy using thyroid imaging reporting and data system (TIRADS) and nodule size. Clin. Imaging 2020, 60, 222–227. [Google Scholar] [CrossRef]
- Ozel, A.; Cakmakcı, E.; Camurcuoglu, E. Enhancing the Detection of Malignancy in Thyroid Nodules by Assessing Longitudinal Diameter-to-Anteroposterior Diameter Ratio. Ultrasound Q. 2025, 41, e00714. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Huang, L.-F.; Han, Y.-J.; Cen, X.-X.; Tao, Z.-X. Histopathological Analysis of Thyroid Nodules with Taller-Than-Wide Shape in Adults. Int. J. Gen. Med. 2024, 17, 5123–5131. [Google Scholar] [CrossRef] [PubMed]
- Prete, A.; Borges de Souza, P.; Censi, S.; Muzza, M.; Nucci, N.; Sponziello, M. Update on Fundamental Mechanisms of Thyroid Cancer. Front. Endocrinol. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Fagin, J.A.; Krishnamoorthy, G.P.; Landa, I. Pathogenesis of cancers derived from thyroid follicular cells. Nat. Rev. Cancer 2023, 23, 631–650. [Google Scholar] [CrossRef] [PubMed]
- Anil, G.; Hegde, A.; Chong, F.H.V. Thyroid nodules: Risk stratification for malignancy with ultrasound and guided biopsy. Cancer Imaging 2011, 11, 209. [Google Scholar] [CrossRef]
- Angell, T.E.; Vyas, C.M.; Medici, M.; Wang, Z.; Barletta, J.A.; Benson, C.B.; Cibas, E.S.; Cho, N.L.; Doherty, G.M.; Doubilet, P.M.; et al. Differential Growth Rates of Benign vs. Malignant Thyroid Nodules. J. Clin. Endocrinol. Metab. 2017, 102, 4642–4647. [Google Scholar] [CrossRef]
- Nasr, H.; Farghaly, H.; Alqarni, A.; Al-Salem, S.; Sayed, M. Characteristics of malignant thyroid lesions on [18F] fluorodeoxyglucose (FDG)-Positron emission tomography (PET)/Computed tomography (CT). Eur. J. Radiol. Open 2021, 8, 100373. [Google Scholar] [CrossRef]
- Kurti, M.; Sabeti, S.; Robinson, K.A.; Scalise, L.; Larson, N.B.; Fatemi, M.; Alizad, A. Quantitative Biomarkers Derived from a Novel Contrast-Free Ultrasound High-Definition Microvessel Imaging for Distinguishing Thyroid Nodules. Cancers 2023, 15, 1888. [Google Scholar] [CrossRef]
- Boers, T.; Braak, S.J.; Rikken, N.E.T.; Versluis, M.; Manohar, S. Ultrasound imaging in thyroid nodule diagnosis, therapy, and follow-up: Current status and future trends. J. Clin. Ultrasound 2023, 51, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Moraes, P.H.D.M.; Sigrist, R.; Takahashi, M.S.; Schelini, M.; Chammas, M.C. Ultrasound elastography in the evaluation of thyroid nodules: Evolution of a promising diagnostic tool for predicting the risk of malignancy. Radiol. Bras. 2019, 52, 247. [Google Scholar] [CrossRef] [PubMed]
- Baig, F.N.; Liu, S.Y.W.; Lam, H.C.; Yip, S.P.; Law, H.K.W.; Ying, M. Shear Wave Elastography Combining with Conventional Grey Scale Ultrasound Improves the Diagnostic Accuracy in Differentiating Benign and Malignant Thyroid Nodules. Appl. Sci. 2017, 7, 1103. [Google Scholar] [CrossRef]
- Qiu, Y.; Xing, Z.; Yang, Q.; Luo, Y.; Ma, B. Diagnostic performance of shear wave elastography in thyroid nodules with indeterminate cytology: A systematic review and meta-analysis. Heliyon 2023, 9, e20654. [Google Scholar] [CrossRef] [PubMed]
- Radzina, M.; Ratniece, M.; Putrins, D.S.; Saule, L.; Cantisani, V. Performance of Contrast-Enhanced Ultrasound in Thyroid Nodules: Review of Current State and Future Perspectives. Cancers 2021, 13, 5469. [Google Scholar] [CrossRef]
- Mi, J.; Wang, R.; Feng, Q.; Han, L.; Zhuang, Y.; Chen, K.; Chen, Z.; Hua, Z.; Luo, Y.; Lin, J. Three-dimensional visualization of thyroid ultrasound images based on multi-scale features fusion and hierarchical attention. Biomed. Eng. OnLine 2024, 23, 31. [Google Scholar] [CrossRef]
- Andermann, P.; Schlögl, S.; Mäder, U.; Luster, M.; Lassmann, M.; Reiners, C. Intra- and interobserver variability of thyroid volume measurements in healthy adults by 2D versus 3D ultrasound. Nuklearmedizin 2007, 46, 1–7. [Google Scholar]
- Licht, K.; Darr, A.; Opfermann, T.; Winkens, T.; Freesmeyer, M. 3D ultrasonography is as accurate as low-dose CT in thyroid volumetry. Nuklearmedizin 2014, 53, 99–104. [Google Scholar] [CrossRef]
- Sparchez, Z.; Mocan, T.; Craciun, R.; Sparchez, M.; Nolsøe, C. Contrast enhancement for ultrasound-guided interventions: When to use it and what to expect? Ultrasonography 2021, 41, 263. [Google Scholar] [CrossRef]
- Boers, T.; Braak, S.J.; Versluis, M.; Manohar, S. Matrix 3D ultrasound-assisted thyroid nodule volume estimation and radiofrequency ablation: A phantom study. Eur. Radiol. Exp. 2021, 5, 31. [Google Scholar] [CrossRef]
- Guiot, J.; Vaidyanathan, A.; Deprez, L.; Zerka, F.; Danthine, D.; Frix, A.N.; Lambin, P.; Bottari, F.; Tsoutzidis, N.; Miraglio, B.; et al. A review in radiomics: Making personalized medicine a reality via routine imaging. Med. Res. Rev. 2022, 42, 426–440. [Google Scholar] [CrossRef]
- Sorrenti, S.; Dolcetti, V.; Radzina, M.; Bellini, M.I.; Frezza, F.; Munir, K.; Grani, G.; Durante, C.; D’Andrea, V.; David, E.; et al. Artificial Intelligence for Thyroid Nodule Characterization: Where Are We Standing? Cancers 2022, 14, 3357. [Google Scholar] [CrossRef]

| Variable | Patient (n = 107) | Benign (n = 96) | Malignant (n = 20) |
|---|---|---|---|
| Gender | |||
| Female, n (%) | 94 (87.9) | 79 | 15 |
| Male, n (%) | 13 (12.1) | 8 | 5 |
| Hypertension, n (%) | 19 (17.8) | 17 | 2 |
| Diabetes Mellitus, n (%) | 24 (22.4) | 22 | 2 |
| Nodules evaluated, n | 116 | 96 | 20 |
| Nodule GSM, median | 84.5 | 86.5 | 80 |
| Thyroid tissue GSM, median | 90 | 86.5 | 101.5 |
| Nodule area (median, cm2) | 1.8 | 1.7 | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultan, S.R.; Albin Hajji, F.; Alhazmi, A.; Alamri, S.; Alsulami, A.; Albukhari, A.; Filimban, A.; Almutairi, B.; Albngali, A.; Kaifi, R.; et al. Quantitative Ultrasound Grayscale Analysis and Size of Benign and Malignant Solid Thyroid Nodules. Tomography 2025, 11, 133. https://doi.org/10.3390/tomography11120133
Sultan SR, Albin Hajji F, Alhazmi A, Alamri S, Alsulami A, Albukhari A, Filimban A, Almutairi B, Albngali A, Kaifi R, et al. Quantitative Ultrasound Grayscale Analysis and Size of Benign and Malignant Solid Thyroid Nodules. Tomography. 2025; 11(12):133. https://doi.org/10.3390/tomography11120133
Chicago/Turabian StyleSultan, Salahaden R., Faisal Albin Hajji, Abdulrahman Alhazmi, Shahad Alamri, Abrar Alsulami, Ahmed Albukhari, Asseel Filimban, Bander Almutairi, Ahmad Albngali, Reham Kaifi, and et al. 2025. "Quantitative Ultrasound Grayscale Analysis and Size of Benign and Malignant Solid Thyroid Nodules" Tomography 11, no. 12: 133. https://doi.org/10.3390/tomography11120133
APA StyleSultan, S. R., Albin Hajji, F., Alhazmi, A., Alamri, S., Alsulami, A., Albukhari, A., Filimban, A., Almutairi, B., Albngali, A., Kaifi, R., Khayat, M., Alkharaiji, M., Khalil, M., & Alfatni, A. (2025). Quantitative Ultrasound Grayscale Analysis and Size of Benign and Malignant Solid Thyroid Nodules. Tomography, 11(12), 133. https://doi.org/10.3390/tomography11120133






