Sentinel Lymph Node Mapping in Lung Cancer: A Pilot Study for the Detection of Micrometastases in Stage I Non-Small Cell Lung Cancer
Abstract
1. Introduction
- Inadequate lymph node dissection or incomplete removal of the lymph nodes draining the primary tumor [14].
- Inadequacy of definitive staging due to the sensitivity limits of the methods used to detect the presence of neoplastic cells in the lymph node tissue [14],
- Presence of “skip-metastases”: the lymphatic drainage of the primary tumor does not always follow the expected pattern, given the possibility that tumor cells exceed the regional lymph nodes and metastasize in distant stations.
2. Materials and Methods
2.1. Eligibility
2.2. Technique
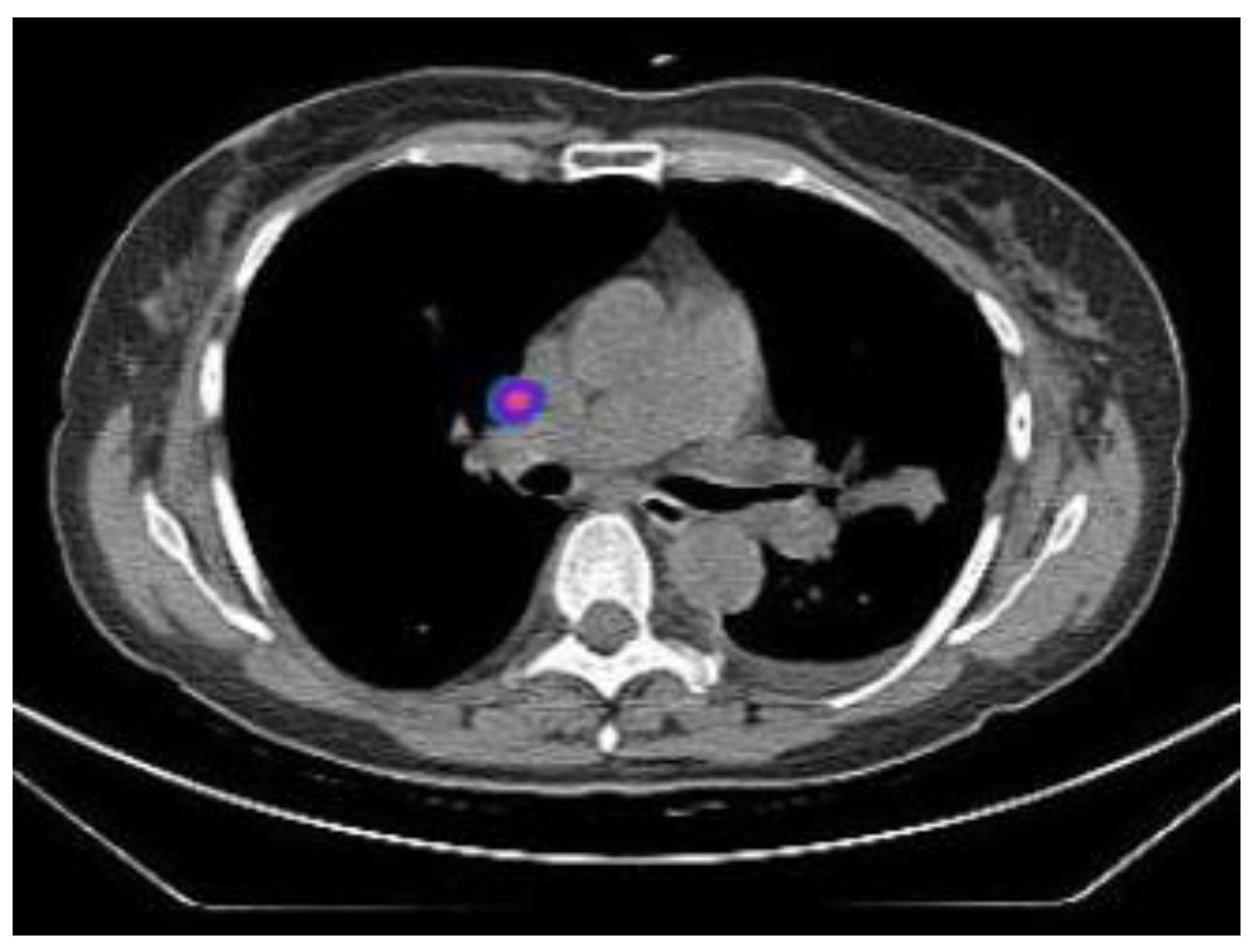
2.2.1. TIME 0: Lesion marking and SPECT Analysis
2.2.2. TIME 1 Surgery
2.2.3. TIME 2: Ex vivo Detection and OSNA Analysis
3. Results
4. Discussion
- The identification of micrometastases (“ultrastaging”);
- The identification of “skip metastases”;
- Choosing the extent of surgical resection.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Koning, H.J.; Van Der Aalst, C.M.; De Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Wu, F.Z.; Huang, Y.L.; Wu, Y.J.; Tang, E.K.; Wu, M.T.; Chen, C.S.; Lin, Y.P. Prognostic effect of implementation of the mass low-dose computed tomography lung cancer screening program: A hospital-based cohort study. Eur. J. Cancer Prev. 2020, 29, 445–451. [Google Scholar] [CrossRef]
- Wu, F.Z.; Huang, Y.L.; Wu, C.C.; Tang, E.K.; Chen, C.S.; Mar, G.Y.; Yen, Y.; Wu, M.T. Assessment of Selection Criteria for Low-Dose Lung Screening CT Among Asian Ethnic Groups in Taiwan: From Mass Screening to Specific Risk-Based Screening for Non-Smoker Lung Cancer. Clin. Lung Cancer 2016, 17, e45–e56. [Google Scholar] [CrossRef]
- Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al.; National Lung Screening Trial Research Team Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar]
- Goldstraw, P.; Crowley, J.; Chansky, K.; Giroux, D.J.; Groome, P.A.; Rami-Porta, R.; Postmus, P.E.; Rusch, V.; Sobin, L.; International Association for the Study of Lung Cancer International Staging Committee and Participating Institutions. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J. Thorac. Oncol. 2007, 2, 706–714. [Google Scholar] [CrossRef]
- Martini, N.; Bains, M.S.; Burt, M.E.; Zakowski, M.F.; McCormack, P.; Rusch, V.W.; Ginsberg, R.J. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J. Thorac. Cardiovasc. Surg. 1995, 109, 120–129. [Google Scholar] [CrossRef]
- Immerman, S.C.; Vanecko, R.M.; Fry, W.A.; Head, L.R.; Shields, T.W. Site of Recurrence in Patients with Stages I and II Carcinoma of the Lung Resected for Cure. Ann. Thorac. Surg. 1981, 32, 23–27. [Google Scholar] [CrossRef]
- Verhagen, A.F.; Bulten, J.; Shirango, H.; Thunnissen, F.B.; van der Drift, M.A.; van der Bruggen, W.; Tjan-Heijnen, V.C.; van Swieten, H.A. The clinical value of lymphatic micrometastases in patients with non-small cell lung cancer. J. Thorac. Oncol. 2010, 5, 1201–1205. [Google Scholar] [CrossRef]
- Passlick, B.; Kubuschock, B.; Sienel, W.; Thetter, O.; Pantel, K.; Izbicki, J. Mediastinal lymphadenectomy in non-small cell lung cancer: Effectiveness in patients with or without nodal micrometastases—Results of a preliminary study. Eur. J. Cardio-Thorac. Surg. 2002, 21, 520–526. [Google Scholar] [CrossRef]
- Marchevsky, A.M.; Qiao, J.-H.; Krajisnik, S.; Mirocha, J.M.; McKenna, R.J. The prognostic significance of intranodal isolated tumor cells and micrometastases in patients with non–small cell carcinoma of the lung. J. Thorac. Cardiovasc. Surg. 2003, 126, 551–557. [Google Scholar] [CrossRef]
- Osaki, T.; Oyama, T.; Gu, C.-D.; Yamashita, T.; So, T.; Takenoyama, M.; Sugio, K.; Yasumoto, K. Prognostic impact of micrometastatic tumor cells in the lymph nodes and bone marrow of patients with completely resected stage I non-small-cell lung cancer. J. Clin. Oncol. 2002, 20, 2930–2936. [Google Scholar] [CrossRef]
- Gwóźdź, P.; Pasieka-Lis, M.; Kołodziej, K.; Pankowski, J.; Banaś, R.; Wiłkojć, M.; Zieliński, M. Prognosis of Patients With Stages I and II Non-Small Cell Lung Cancer With Nodal Micrometastases. Ann. Thorac. Surg. 2018, 105, 1551–1557. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, L.; Xie, H.; She, Y.; Su, H.; Xie, D.; Zheng, H.; Zhang, L.; Jiang, G.; Wu, C.; et al. Lymph Node Micrometastasis Prognosticates Survival for Patients with Stage 1 Bronchogenic Adenocarcinoma. Ann. Surg. Oncol. 2018, 25, 3812–3819. [Google Scholar] [CrossRef]
- Sugi, K.; Nawata, K.; Fujita, N.; Ueda, K.; Tanaka, T.; Matsuoka, T.; Kaneda, Y.; Esato, K. Systematic lymph node dissection for clinically diagnosed peripheral non-small-cell lung cancer less than 2 cm in diameter. World J. Surg. 1998, 22, 290–295. [Google Scholar] [CrossRef]
- Pirker, R. Adjuvant chemotherapy in patients with completely resected non-small cell lung cancer. Transl. Lung Cancer Res. 2014, 3, 305–310. [Google Scholar] [CrossRef]
- Mountain, C.F. Revisions in the International System for Staging Lung Cancer. Chest 1997, 111, 1710–1717. [Google Scholar] [CrossRef]
- Manfredini, B.; Zirafa, C.C.; Filosso, P.L.; Stefani, A.; Romano, G.; Davini, F.; Melfi, F. The Role of Lymphadenectomy in Early-Stage NSCLC. Cancers 2023, 15, 3735. [Google Scholar] [CrossRef]
- Luo, J.; Yang, S.; Dong, S. Selective Mediastinal Lymphadenectomy or Complete Mediastinal Lymphadenectomy for Clinical Stage I Non-Small Cell Lung Cancer: A Meta-Analysis. Adv. Ther. 2021, 38, 5671–5683. [Google Scholar] [CrossRef]
- Liptay, M.J. Sentinel Node Mapping in Lung Cancer: The Holy Grail? Ann. Thorac. Surg. 2008, 85, S778–S779. [Google Scholar] [CrossRef]
- Little, A.G.; DeHoyos, A.; Kirgan, D.M.; Arcomano, T.R.; Murray, K.D. Intraoperative lymphatic mapping for non–small cell lung cancer: The sentinel node technique. J. Thorac. Cardiovasc. Surg. 1999, 117, 220–224. [Google Scholar] [CrossRef]
- Melfi, F.M.; Chella, A.; Menconi, G.F.; Givigliano, F.; Boni, G.; Mariani, G.; Sbragia, P.; Angeletti, C.A. Intraoperative radioguided sentinel lymph node biopsy in non-small cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2003, 23, 214–220. [Google Scholar] [CrossRef]
- Nomori, H.; Watanabe, K.; Ohtsuka, T.; Naruke, T.; Suemasu, K. In vivo identification of sentinel lymph nodes for clinical stage I non-small cell lung cancer for abbreviation of mediastinal lymph node dissection. Lung Cancer 2004, 46, 49–55. [Google Scholar] [CrossRef]
- Rovera, G.; de Koster, E.J.; Rufini, V.; Zollino, M.; Zagaria, L.; Giammarile, F.; Vidal-Sicart, S.; Olmos, R.V.; Collarino, A. 99mTc-Tilmanocept performance for sentinel node mapping in breast cancer, melanoma, and head and neck cancer: A systematic review and meta-analysis from a European expert panel. Eur. J. Nucl. Med. 2023, 50, 3375–3389. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, X.; Jiang, L.; Chen, X.; Bao, X.; Chen, X. The diagnostic value of one step nucleic acid amplification (OSNA) in differentiating lymph node metastasis of tumors: A systematic review and meta-analysis. Int. J. Surg. 2018, 56, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.M.; Hoh, C.K.; Vera, D.R.; Darrah, D.D.; Schulteis, G. Lymphoseek: A molecular radiopharmaceutical for sentinel node detection. Ann. Surg. Oncol. 2003, 10, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, M.; Nakabayashi, K.; Yoshidome, K.; Kaneko, T.; Iwase, T.; Akiyama, F.; Kato, Y.; Tsuda, H.; Ueda, S.; Sato, K.; et al. One-step nucleic acid amplification for intraoperative detection of lymph node metastasis in breast cancer patients. Clin. Cancer Res. 2007, 13, 4807–4816. [Google Scholar] [CrossRef]
- Gęca, K.; Rawicz-Pruszyński, K.; Mielko, J.; Mlak, R.; Sędłak, K.; Polkowski, W.P. Rapid Detection of Free Cancer Cells in Intraoperative Peritoneal Lavage Using One-Step Nucleic Acid Amplification (OSNA) in Gastric Cancer Patients. Cells 2020, 9, 2168. [Google Scholar] [CrossRef]
- Little, A.G.; Rusch, V.W.; Bonner, J.A.; Gaspar, L.E.; Green, M.R.; Webb, W.R.; Stewart, A.K. Patterns of surgical care of lung cancer patients. Ann. Thorac. Surg. 2005, 80, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Alex, J.; Krag, D. Gamma-probe guided localization of lymph nodes. Surg. Oncol. 1993, 2, 137–143. [Google Scholar] [CrossRef]
- Nomori, H.; Horio, H.; Naruke, T.; Orikasa, H.; Yamazaki, K.; Suemasu, K. Use of technetium-99m tin colloid for sentinel lymph node identification in non–small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2002, 124, 486–492. [Google Scholar] [CrossRef]
- Melfi, F.M.; Davini, F.; Boni, G.; Mussi, A. Sentinel lymph node in lung cancer surgery. Thorac. Surg. Clin. 2012, 22, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Melfi, F.M.; Lucchi, M.; Davini, F.; Viti, A.; Fontanini, G.; Boldrini, L.; Boni, G.; Mussi, A. Intraoperative sentinel lymph node mapping in stage I non-small cell lung cancer: Detection of micrometastases by polymerase chain reaction. Eur. J. Cardio-Thorac. Surg. 2008, 34, 181–186. [Google Scholar] [CrossRef]
- Alongi, P.; Garau, L.M.; Albalá González, M.D.; Zucchetta, P.; Manca, G.; Margolin, G.; Vidal-Sicart, S. Sentinel node identification with [99mTc]-tilmanocept SPECT/CT: A pictorial essay of clinical applications. Clin. Transl. Imaging 2020, 8, 279–288. [Google Scholar] [CrossRef]
- Leong, S.P.; Kim, J.; Ross, M.; Faries, M.; Scoggins, C.R.; Rich Metz, W.L.; Cope, F.O.; Orahood, R.C. A phase 2 study of 99mTc-tilmanocept in the detection of sentinel lymph nodes in melanoma and breast cancer. Ann. Surg. Oncol. 2011, 18, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Unkart, J.T.; Proudfoot, J.; Wallace, A.M. Outcomes of “oneday” vs “two-day” injection protocols using Tc-99m tilmanocept for sentinel lymph node biopsy in breast cancer. Breast J. 2018, 24, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, A.; Resto, V.A. Oral cavity squamous cell carcinoma and the clinically N0 Neck: The past, present, and future of sentinel lymph node biopsy. Curr. Oncol. Rep. 2010, 12, 129–135. [Google Scholar] [CrossRef][Green Version]
- Azad, A.K.; Rajaram, M.V.S.; Metz, W.L.; Cope, F.O.; Blue, M.S.; Vera, D.R.; Schlesinger, L.S. γ-tilmanocept, a new radiopharmaceutical tracer for cancer sentinel lymph nodes, binds to the mannose receptor (CD206). J. Immunol. 2015, 195, 2019–2029. [Google Scholar] [CrossRef] [PubMed]
- Riquet, M.; Assouad, J.; Bagan, P.; Foucault, C.; Barthes, F.L.P.; Dujon, A.; Danel, C. Skip mediastinal lymph node metastasis and lung cancer: A particular n2 subgroup with a better prognosis. Ann. Thorac. Surg. 2005, 79, 225–233. [Google Scholar] [CrossRef]
- Stasiak, F.; Seitlinger, J.; Streit, A.; Wollbrett, C.; Piccoli, J.; Siat, J.; Gauchotte, G.; Renaud, S. Sentinel Lymph Node in Non-Small Cell Lung Cancer: Assessment of Feasibility and Safety by Near-Infrared Fluorescence Imaging and Clinical Consequences. J. Pers. Med. 2022, 13, 90. [Google Scholar] [CrossRef]
- Croner, R.S.; Geppert, C.-I.; Bader, F.G.; Nitsche, U.; Späth, C.; Rosenberg, R.; Zettl, A.; Matias-Guiu, X.; Tarragona, J.; Güller, U.; et al. Molecular staging of lymph node-negative colon carcinomas by one-step nucleic acid amplification (OSNA) results in upstaging of a quarter of patients in a prospective, European, multicentre study. Br. J. Cancer 2014, 110, 2544–2550. [Google Scholar] [CrossRef]
- Slama, J.; Dundr, P.; Dusek, L.; Cibula, D. High false negative rate of frozen section examination of sentinel lymph nodes in patients with cervical cancer. Gynecol. Oncol. 2013, 129, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Hyslop, T.; Waldman, S.A. Molecular staging of node negative patients with colorectal cancer. J. Cancer 2013, 4, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Miyashiro, I.; Hiratsuka, M.; Sasako, M.; Sano, T.; Mizusawa, J.; Nakamura, K.; Nashimoto, A.; Tsuburaya, A.; Fukushima, N.; The Gastric Cancer Surgical Study Group (GCSSG) in the Japan Clinical Oncology Group (JCOG). High false-negative proportion of intraoperative histological examination as a serious problem for clinical application of sentinel node biopsy for early gastric cancer: Final results of the Japan Clinical Oncology Group multicenter trial JCOG0302. Gastric Cancer 2014, 17, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Hayama, M.; Chida, M.; Karube, Y.; Tamura, M.; Kobayashi, S.; Oyaizu, T.; Honma, K. One-step nucleic acid amplification for detection of lymph node metastasis in lung cancer. Ann. Thorac. Cardiovasc. Surg. 2014, 20, 181–184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vogelaar, F.J.; Reimers, M.S.; van der Linden, R.L.A.; van der Linden, J.C.; Smit, V.T.H.B.M.; Lips, D.J.; van de Velde, C.J.H.; Bosscha, K. The diagnostic value of one-step nucleic acid amplification (osna) for sentinel lymph nodes in colon cancer patients. Ann. Surg. Oncol. 2014, 21, 3924–3930. [Google Scholar] [CrossRef] [PubMed]
- Vodicka, J.; Mukensnabl, P.; Vejvodova, S.; Spidlen, V.; Kulda, V.; Topolcan, O.; Pesta, M. A more sensitive detection of micrometastases of NSCLC in lymph nodes using the one-step nucleic acid amplification (OSNA) method. J. Surg. Oncol. 2018, 117, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Masai, K.; Nakagawa, K.; Yoshida, A.; Sakurai, H.; Watanabe, S.-I.; Asamura, H.; Tsuta, K. Cytokeratin 19 expression in primary thoracic tumors and lymph node metastases. Lung Cancer 2014, 86, 318–323. [Google Scholar] [CrossRef]
- Inoue, M.; Hiyama, K.; Nakabayashi, K.; Morii, E.; Minami, M.; Sawabata, N.; Shintani, Y.; Nakagiri, T.; Susaki, Y.; Maeda, J.; et al. An accurate and rapid detection of lymph node metastasis in non-small cell lung cancer patients based on one-step nucleic acid amplification assay. Lung Cancer 2012, 78, 212–218. [Google Scholar] [CrossRef]
- Nakagawa, K.; Asamura, H.; Tsuta, K.; Nagai, K.; Yamada, E.; Ishii, G.; Mitsudomi, T.; Ito, A.; Higashiyama, M.; Tomita, Y.; et al. The novel one-step nucleic acid amplification (OSNA) assay for the diagnosis of lymph node metastasis in patients with non-small cell lung cancer (NSCLC): Results of a multicenter prospective study. Lung Cancer 2016, 97, 1–7. [Google Scholar] [CrossRef]
- Oezkan, F.; Khan, A.M.; Hager, T.; Freitag, L.; Christoph, D.; Darwiche, K. OSNA: A Fast Molecular Test Based on CK19 mRNA Concentration for Assessment of EBUS-TBNA Samples in Lung Cancer Patients. Clin. Lung Cancer 2016, 17, 198–204. [Google Scholar] [CrossRef]
- Escalante Pérez, M.; Hermida Romero, M.T.; Otero Alén, B.; Álvarez Martínez, M.; Fernández Prado, R.; de la Torre Bravos, M.; Concha López, Á. Detection of lymph node metastasis in lung cancer patients using a one-step nucleic acid amplification assay: A single-centre prospective study. J. Transl. Med. 2019, 17, 233. [Google Scholar] [CrossRef] [PubMed]
- Ose, N.; Takeuchi, Y.; Sakamaki, Y.; Kadota, Y.; Urasaki, K.; Tsuji, H.; Kawahara, K.; Noguchi, M.; Shintani, Y. Detection of lymph node metastasis in non-small cell lung cancer using the new system of one-step nucleic acid amplification assay. PLoS ONE 2022, 17, e0265603. [Google Scholar] [CrossRef]
- Namba, K.; Suzawa, K.; Shien, K.; Miura, A.; Takahashi, Y.; Miyauchi, S.; Araki, K.; Nakata, K.; Tomida, S.; Tanaka, S.; et al. One-step nucleic acid amplification for intraoperative diagnosis of lymph node metastasis in lung cancer patients: A single-center prospective study. Sci. Rep. 2022, 12, 7297. [Google Scholar] [CrossRef]
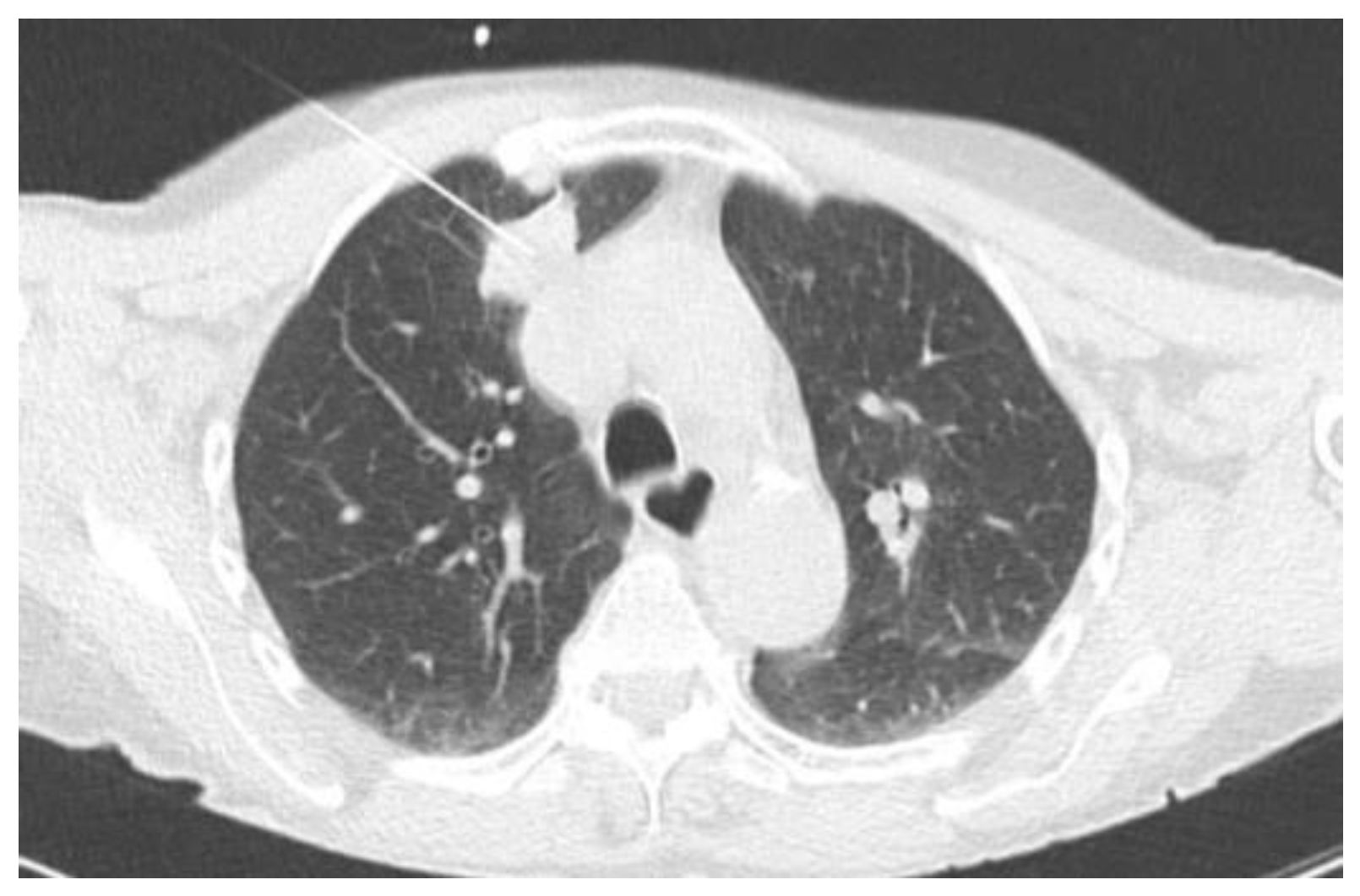

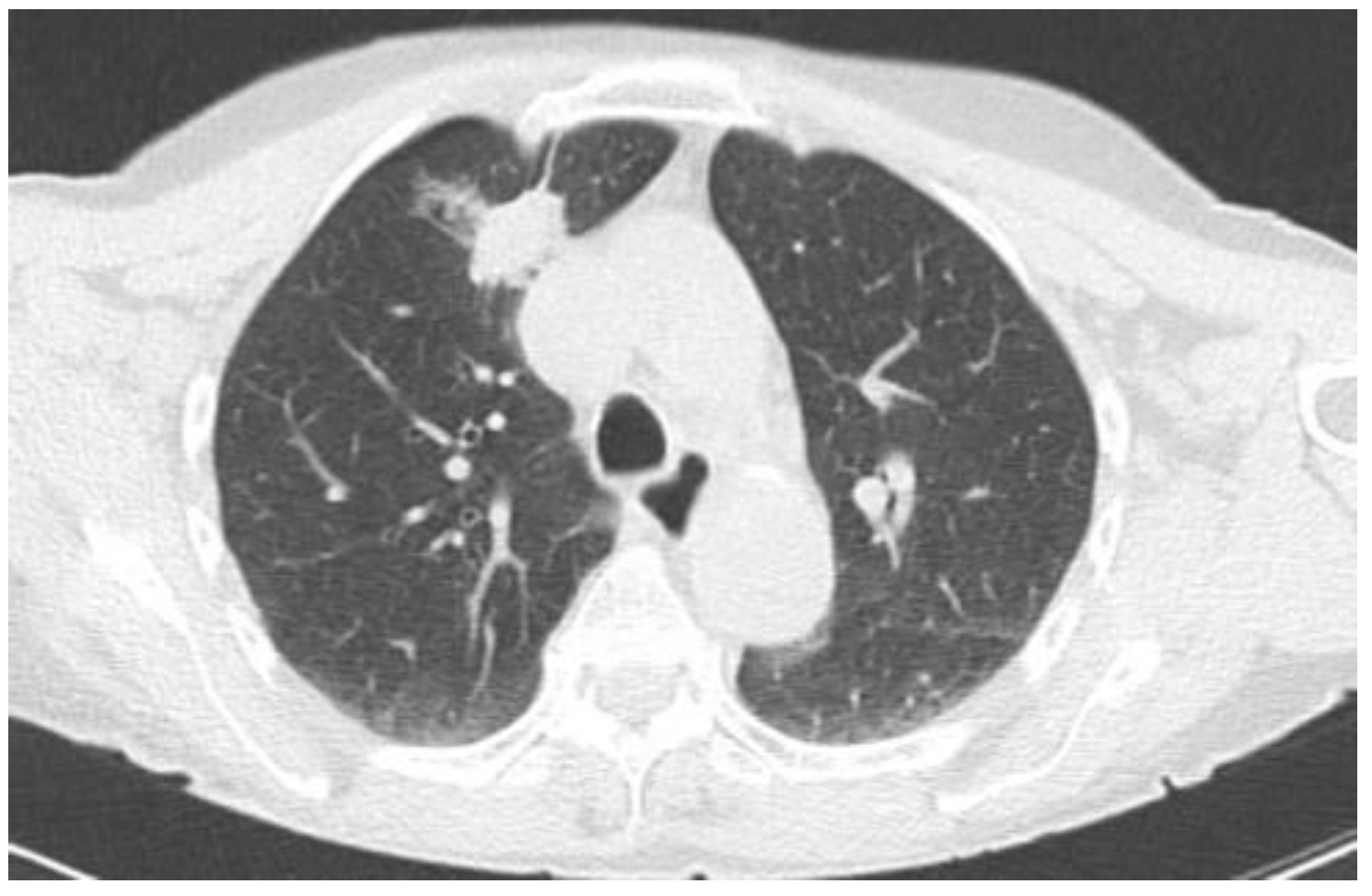
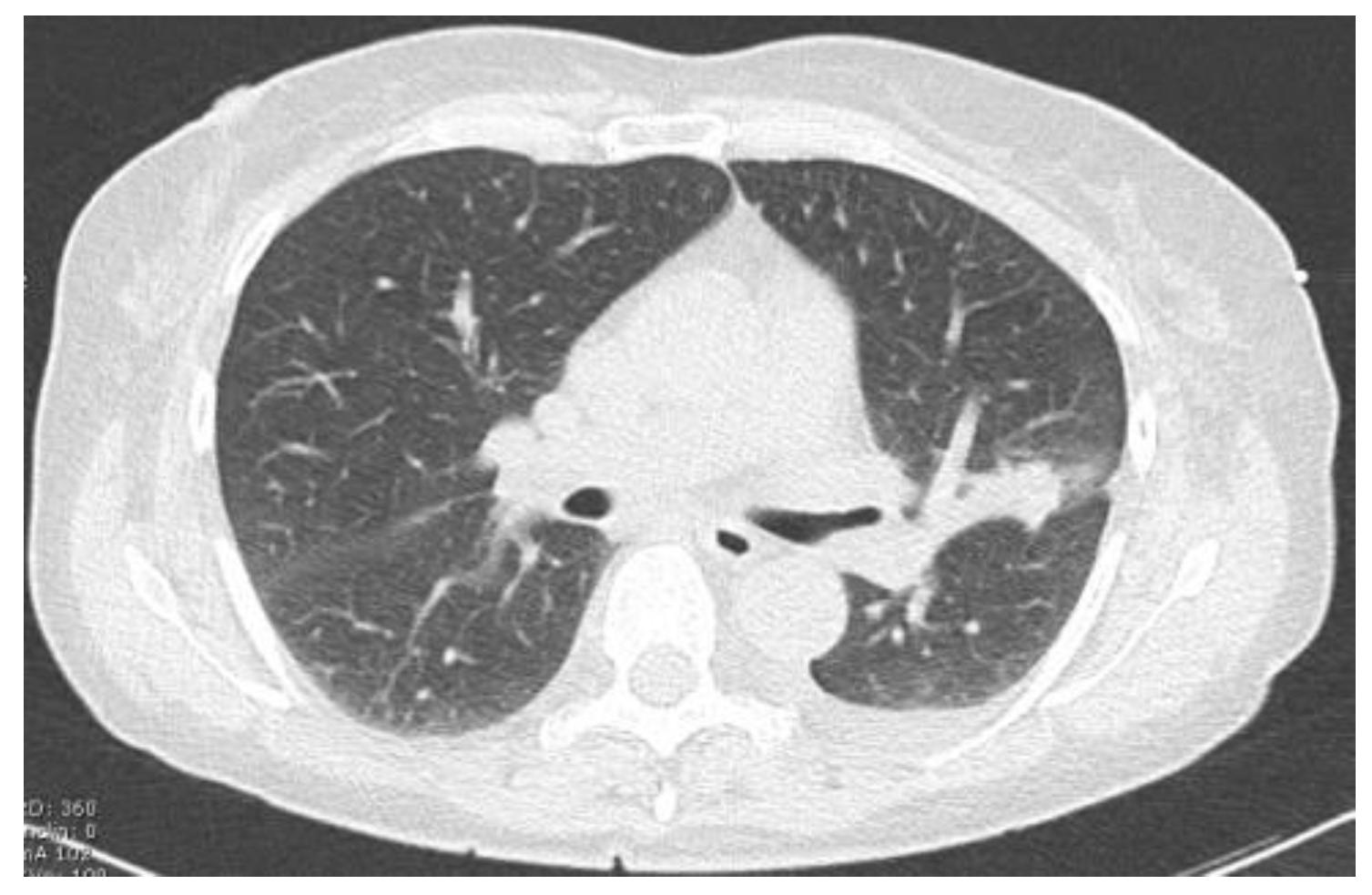
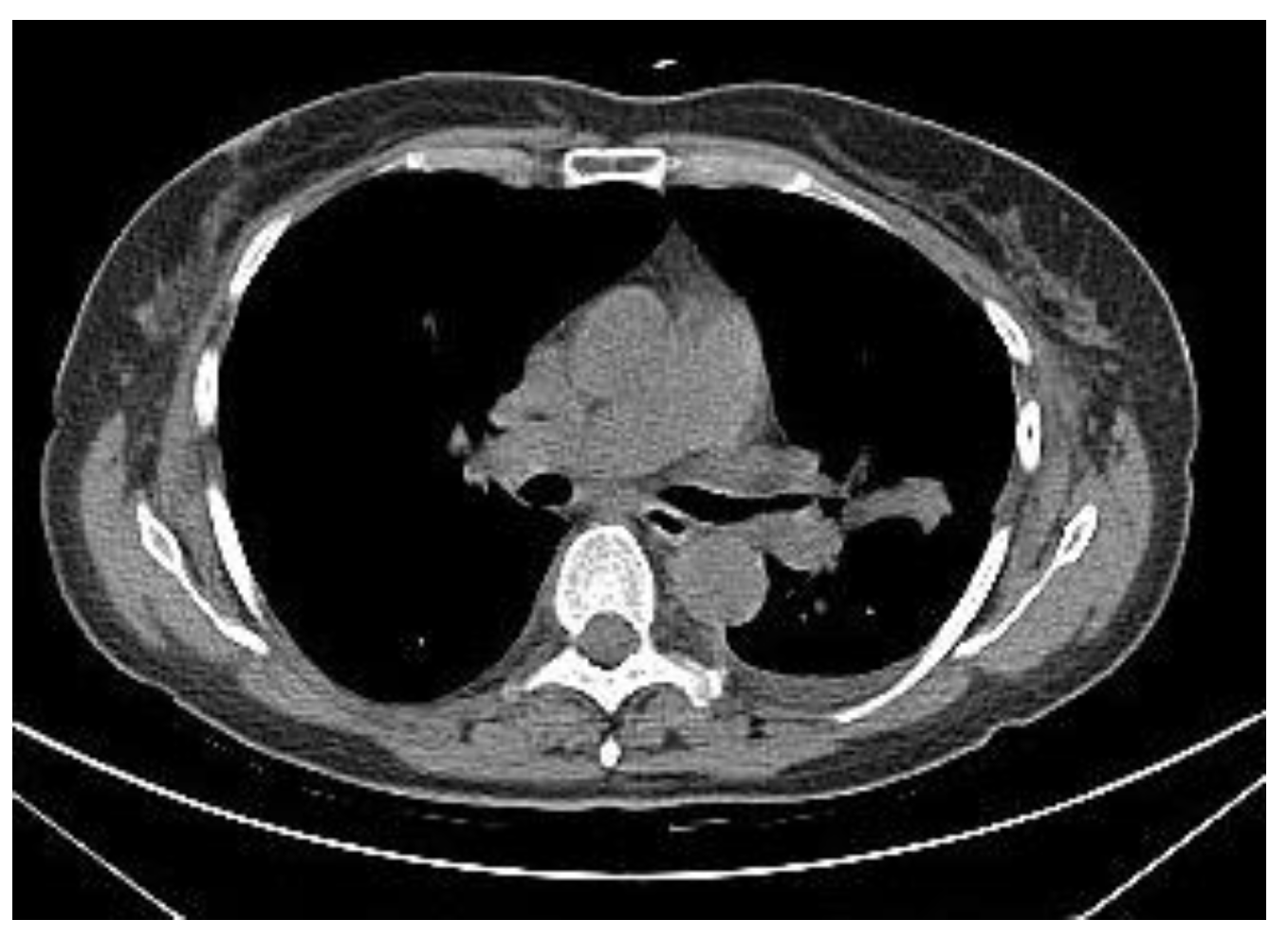
| Pt 1 | Pt 2 | Pt 3 | Pt 4 | Pt 5 | Pt 6 | Pt 7 | Pt 8 | |
|---|---|---|---|---|---|---|---|---|
| Sex | F | M | M | F | M | F | M | M |
| Age | 61 | 66 | 65 | 73 | 70 | 74 | 71 | 48 |
| Smoker/previous smoker | No | Yes | Yes | No | No | Yes | Yes | Yes |
| Charlson index | 5 | 4 | 2 | 4 | 2 | 2 | 5 | 2 |
| Site of lung cancer | LUL | RLL | RUL | LLL | RUL | RUL | LUL | RUL |
| Clinical stage | IB | IB | IB | IA | IA | IB | IB | IB |
| Histotype | ADC | ADC | ADC | ADC | ADC | ADC | SCC | ADC |
| Postoperative length of stay | 4 | 6 | 4 | 3 | 3 | 4 | 4 | 3 |
| Postoperative complications | No | No | No | No | No | No | No | No |
| Sentinel lymph node detection | + | + | + | + | + | + | + | + |
| Site of Lung Cancer | Histotype | SPECT-Positive SLN Station | Intraoperative Positive SLN Station | |
|---|---|---|---|---|
| Patient 1 | LUL | ADC | 10 | 10 |
| Patient 2 | RLL | ADC | * | 9 |
| Patient 3 | RUL | ADC | 11 | 11 |
| Patient 4 | LLL | ADC | # | 11 |
| Patient 5 | RUL | ADC | # | 4 |
| Patient 6 | RUL | ADC | 10 | 10 |
| Patient 7 | LUL | SCC | * | 5 |
| Patient 8 | RUL | ADC | 10 | 10 |
| Surgical Procedure | Histotype | Number of Positive LN Stations | Rate of Positive LN Stations | Level of Positive LN Station (s) | |
|---|---|---|---|---|---|
| Patient 1 | LUL | Adenocarcinoma | 4 | 80% | 5, 6, 7, 10 |
| Patient 2 | RLL | Adenocarcinoma | 4 | 80% | 4, 7, 9, 11 |
| Patient 3 | RUL | Adenocarcinoma | 1 | 14% | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, G.; Zirafa, C.C.; Calabrò, F.; Alì, G.; Manca, G.; De Liperi, A.; Proietti, A.; Manfredini, B.; Di Stefano, I.; Marciano, A.; et al. Sentinel Lymph Node Mapping in Lung Cancer: A Pilot Study for the Detection of Micrometastases in Stage I Non-Small Cell Lung Cancer. Tomography 2024, 10, 761-772. https://doi.org/10.3390/tomography10050058
Romano G, Zirafa CC, Calabrò F, Alì G, Manca G, De Liperi A, Proietti A, Manfredini B, Di Stefano I, Marciano A, et al. Sentinel Lymph Node Mapping in Lung Cancer: A Pilot Study for the Detection of Micrometastases in Stage I Non-Small Cell Lung Cancer. Tomography. 2024; 10(5):761-772. https://doi.org/10.3390/tomography10050058
Chicago/Turabian StyleRomano, Gaetano, Carmelina Cristina Zirafa, Fabrizia Calabrò, Greta Alì, Gianpiero Manca, Annalisa De Liperi, Agnese Proietti, Beatrice Manfredini, Iosè Di Stefano, Andrea Marciano, and et al. 2024. "Sentinel Lymph Node Mapping in Lung Cancer: A Pilot Study for the Detection of Micrometastases in Stage I Non-Small Cell Lung Cancer" Tomography 10, no. 5: 761-772. https://doi.org/10.3390/tomography10050058
APA StyleRomano, G., Zirafa, C. C., Calabrò, F., Alì, G., Manca, G., De Liperi, A., Proietti, A., Manfredini, B., Di Stefano, I., Marciano, A., Davini, F., Volterrani, D., & Melfi, F. (2024). Sentinel Lymph Node Mapping in Lung Cancer: A Pilot Study for the Detection of Micrometastases in Stage I Non-Small Cell Lung Cancer. Tomography, 10(5), 761-772. https://doi.org/10.3390/tomography10050058







