The Relationship between Liver Volume, Clinicopathological Characteristics and Survival in Patients Undergoing Resection with Curative Intent for Non-Metastatic Colonic Cancer
Abstract
1. Introduction
2. Methods
2.1. Patients
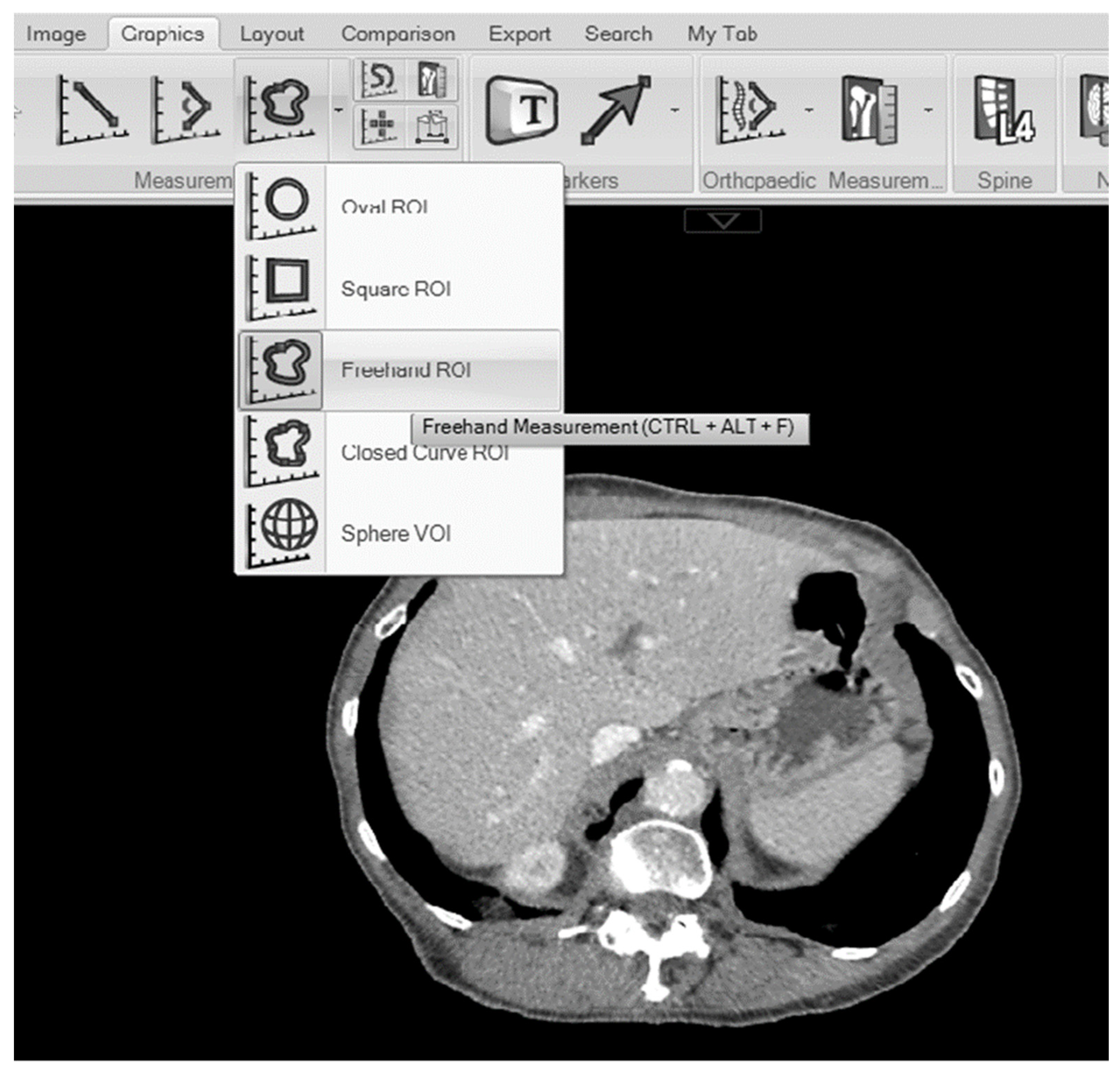
2.2. Measurements of the Maximal Liver Area and Total Liver Volume
2.3. Statistical Analysis
3. Results
3.1. Patient Inclusion
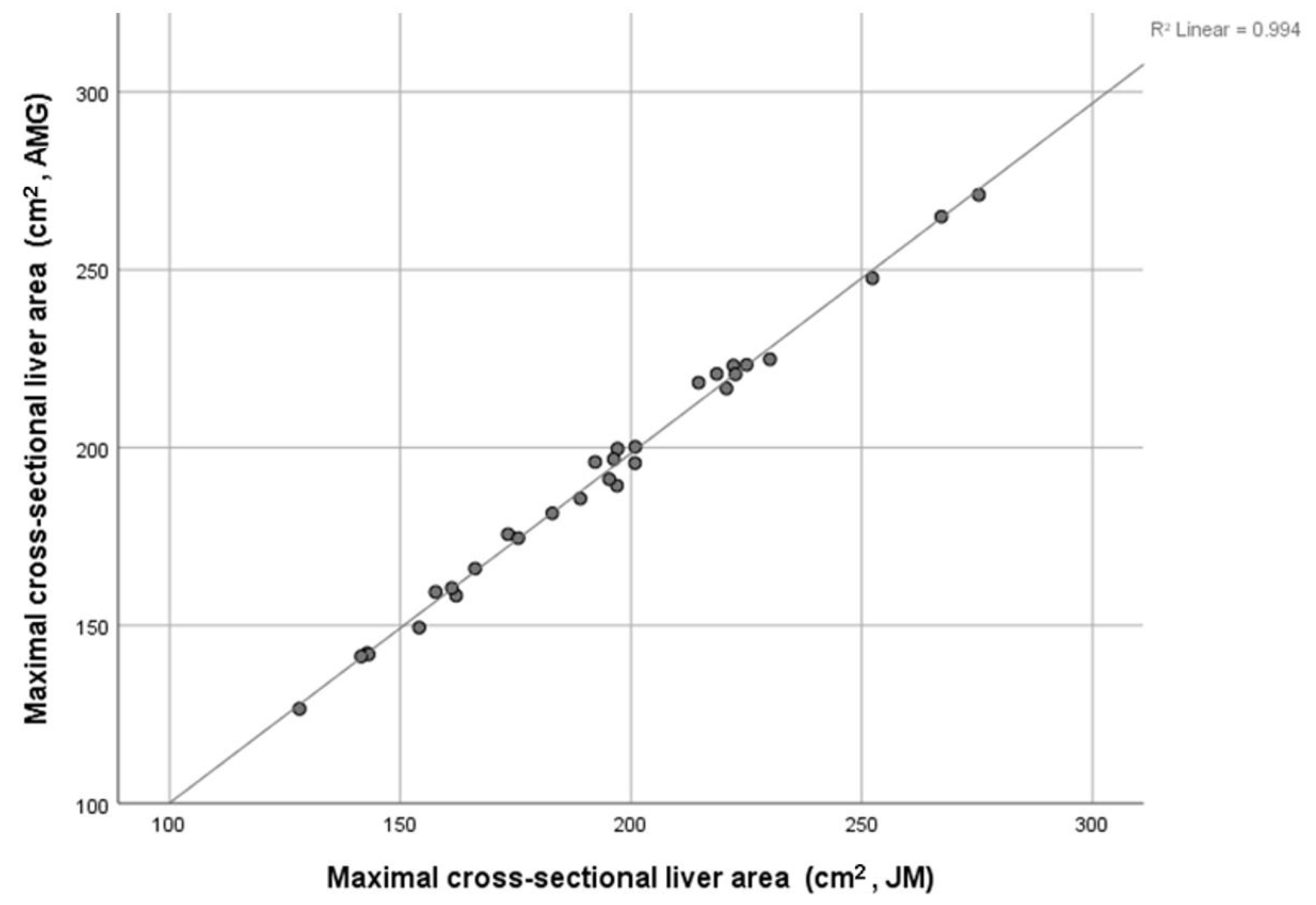
3.2. Relationships between Cross-Sectional Liver Area and Total Liver Volume
3.3. Relationship between Liver Mass Index (LI) and Age, Sex, BMI, BSA, ASA, T2DM, Liver Disease and OS
3.4. Relationship between LI (Lowest/Middle vs. Highest Tertiles) and Age, Sex, BMI, BSA, ASA, ASA and T2DM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suzuki, K.; Kohlbrenner, R.; Epstein, M.L.; Obajuluwa, A.M.; Xu, J.; Hori, M. Computer-aided measurement of liver volumes in CT by means of geodesic active contour segmentation coupled with level-set algorithms. Med. Phys. 2010, 37, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Gotra, A.; Sivakumaran, L.; Chartrand, G.; Vu, K.-N.; Vandenbroucke-Menu, F.; Kauffmann, C.; Kadoury, S.; Gallix, B.; de Guise, J.A.; Tang, A. Liver segmentation: Indications, techniques and future directions. Insights Imaging 2017, 8, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Vauthey, J.-N.; Abdalla, E.K.; Doherty, D.A.; Gertsch, P.; Fenstermacher, M.J.; Loyer, E.M.; Lerut, J.; Materne, R.; Wang, X.; Encarnacion, A.; et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transplant. 2002, 8, 233–240. [Google Scholar] [CrossRef]
- Harada, K.; Ishinuki, T.; Ohashi, Y.; Tanaka, T.; Chiba, A.; Numasawa, K.; Imai, T.; Hayasaka, S.; Tsugiki, T.; Miyanishi, K.; et al. Nature of the liver volume depending on the gender and age assessing volumetry from a reconstruction of the computed tomography. PLoS ONE 2021, 16, e0261094. [Google Scholar] [CrossRef]
- Martin, S.; Sorokin, E.P.; Thomas, E.L.; Sattar, N.; Cule, M.; Bell, J.D.; Yaghootkar, H. Estimating the Effect of Liver and Pancreas Volume and Fat Content on Risk of Diabetes: A Mendelian Randomization Study. Diabetes Care 2022, 45, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Chen, J.; Le, T.A.; Changchien, C.; Hamilton, G.; Middleton, M.S.; Loomba, R.; Sirlin, C.B. Cross-sectional and longitudinal evaluation of liver volume and total liver fat burden in adults with nonalcoholic steatohepatitis. Abdom. Imaging 2015, 40, 26–37. [Google Scholar] [CrossRef]
- Di Girolamo, D.; Tajbakhsh, S. Pathological features of tissues and cell populations during cancer cachexia. Cell Regen. 2022, 11, 15. [Google Scholar] [CrossRef]
- Lieffers, J.R.; Mourtzakis, M.; Hall, K.D.; McCargar, L.J.; Prado, C.M.M.; Baracos, V.E. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: Contributions of organ and tumor mass to whole-body energy demands. Am. J. Clin. Nutr. 2009, 89, 1173–1179. [Google Scholar] [CrossRef]
- Dello, S.A.; Lodewick, T.M.; van Dam, R.M.; Reisinger, K.W.; van den Broek, M.A.; von Meyenfeldt, M.F.; Bemelmans, M.H.; Olde Damink, S.W.; Dejong, C.H. Sarcopenia negatively affects preoperative total functional liver volume in patients undergoing liver resection. HPB 2013, 15, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Lodewick, T.M.; Roeth, A.A.; Olde Damink, S.W.; Alizai, P.H.; van Dam, R.M.; Gassler, N.; Schneider, M.; Dello, S.A.; Schmeding, M.; Dejong, C.H.; et al. Sarcopenia, obesity and sarcopenic obesity: Effects on liver function and volume in patients scheduled for major liver resection. J. Cachexia Sarcopenia Muscle 2015, 6, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Rubbia-Brandt, L.; Audard, V.; Sartoretti, P.; Roth, A.D.; Brezault, C.; Le Charpentier, M.; Dousset, B.; Morel, P.; Soubrane, O.; Chaussade, S.; et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann. Oncol. 2004, 15, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Bilchik, A.J.; Poston, G.; Curley, S.A.; Strasberg, S.; Saltz, L.; Adam, R.; Nordlinger, B.; Rougier, P.; Rosen, L.S. Neoadjuvant chemotherapy for metastatic colon cancer: A cautionary note. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 9073–9078. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fearon, K.C.; Glass, D.J.; Guttridge, D.C. Cancer Cachexia: Mediators, Signaling, and Metabolic Pathways. Cell Metab. 2012, 16, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Rohm, M.; Zeigerer, A.; Machado, J.; Herzig, S. Energy metabolism in cachexia. EMBO Rep. 2019, 20, e47258. [Google Scholar] [CrossRef] [PubMed]
- Dello, S.A.W.G.; van Dam, R.M.; Slangen, J.J.G.; van de Poll, M.C.G.; Bemelmans, M.H.A.; Greve, J.W.W.M.; Beets-Tan, R.G.H.; Wigmore, S.J.; Dejong, C.H.C. Liver volumetry plug and play: Do it yourself with ImageJ. World J. Surg. 2007, 31, 2215–2221. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.C.; Tan, C.H.; Cai, J.; Zheng, J.; Kow, A.W. CT volumetry of the liver: Where does it stand in clinical practice? Clin. Radiol. 2014, 69, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Schiano, T.D.; Bodian, C.; Schwartz, M.E.; Glajchen, N.; Min, A.D. Accuracy and significance of computed tomographic scan assessment of hepatic volume in patients undergoing liver transplantation. Transplantation 2000, 69, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Abbass, T.; Dolan, R.D.; McMillan, D.C. Computed tomography-derived body composition analysis in patients with advanced cancer: Clinical utility and future research. Curr. Opin. Support. Palliat. Care 2020, 14, 309–315. [Google Scholar] [CrossRef]
- Mosteller, R.D. Simplified calculation of body-surface area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar]
- Dripps, R.D.; Lamont, A.; Eckenhoff, J.E. The Role of Anesthesia in Surgical Mortality. JAMA 1961, 178, 261–266. [Google Scholar] [CrossRef]
- Hori, M.; Suzuki, K.; Epstein, M.L.; Baron, R.L. Computed tomography liver volumetry using 3-dimensional image data in living donor liver transplantation: Effects of the slice thickness on the volume calculation. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2011, 17, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Epstein, M.L.; Kohlbrenner, R.; Garg, S.; Hori, M.; Oto, A.; Baron, R.L. Quantitative radiology: Automated CT liver volumetry compared with interactive volumetry and manual volumetry. AJR Am. J. Roentgenol. 2011, 197, W706–W712. [Google Scholar] [CrossRef] [PubMed]
- Lodewick, T.M.; Arnoldussen, C.W.; Lahaye, M.J.; van Mierlo, K.M.; Neumann, U.P.; Beets-Tan, R.G.; Dejong, C.H.; van Dam, R.M. Fast and accurate liver volumetry prior to hepatectomy. HPB 2016, 18, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Seppelt, D.; Kromrey, M.L.; Ittermann, T.; Kolb, C.; Haubold, A.; Kampfrath, N.; Fedders, D.; Heiss, P.; Hoberück, S.; Hoffmann, R.T.; et al. Reliability and accuracy of straightforward measurements for liver volume determination in ultrasound and computed tomography compared to real volumetry. Sci. Rep. 2022, 12, 12465. [Google Scholar] [CrossRef] [PubMed]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef]
- Aleixo, G.F.P.; Shachar, S.S.; Nyrop, K.A.; Muss, H.B.; Malpica, L.; Williams, G.R. Myosteatosis and prognosis in cancer: Systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2020, 145, 102839. [Google Scholar] [CrossRef]
- Cakir, H.; Heus, C.; van der Ploeg, T.J.; Houdijk, A.P. Visceral obesity determined by CT scan and outcomes after colorectal surgery; a systematic review and meta-analysis. Int. J. Color. Dis. 2015, 30, 875–882. [Google Scholar] [CrossRef]
- Lee, C.M.; Kang, J. Prognostic impact of myosteatosis in patients with colorectal cancer: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2020, 11, 1270–1282. [Google Scholar] [CrossRef]
- Trejo-Avila, M.; Bozada-Gutiérrez, K.; Valenzuela-Salazar, C.; Herrera-Esquivel, J.; Moreno-Portillo, M. Sarcopenia predicts worse postoperative outcomes and decreased survival rates in patients with colorectal cancer: A systematic review and meta-analysis. Int. J. Color. Dis. 2021, 36, 1077–1096. [Google Scholar] [CrossRef]
- Erickson, B.J.; Korfiatis, P.; Kline, T.L.; Akkus, Z.; Philbrick, K.; Weston, A.D. Deep Learning in Radiology: Does One Size Fit All? J. Am. Coll. Radiol. 2018, 15, 521–526. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Xiao, J.; Caan, B.J.; Weltzien, E.; Cespedes Feliciano, E.M.; Kroenke, C.H.; Meyerhardt, J.A.; Baracos, V.E.; Kwan, M.L.; Castillo, A.L.; Prado, C.M. Associations of pre-existing co-morbidities with skeletal muscle mass and radiodensity in patients with non-metastatic colorectal cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Doyle, S.L.; Bennett, A.M.; Donohoe, C.L.; Mongan, A.M.; Howard, J.M.; Lithander, F.E.; Pidgeon, G.P.; Reynolds, J.V.; Lysaght, J. Establishing computed tomography-defined visceral fat area thresholds for use in obesity-related cancer research. Nutr. Res. 2013, 33, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Friesen, D.E.; Baracos, V.E.; Tuszynski, J.A. Modeling the energetic cost of cancer as a result of altered energy metabolism: Implications for cachexia. Theor. Biol. Med. Model. 2015, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Purkiss, S.F.; Williams, N.S. Growth rate and percentage hepatic replacement of colorectal liver metastases. Br. J. Surg. 1993, 80, 1036–1038. [Google Scholar] [CrossRef] [PubMed]
- Anyene, I.; Caan, B.; Williams, G.R.; Popuri, K.; Lenchik, L.; Giri, S.; Chow, V.; Beg, M.F.; Cespedes Feliciano, E.M. Body composition from single versus multi-slice abdominal computed tomography: Concordance and associations with colorectal cancer survival. J. Cachexia Sarcopenia Muscle 2022, 13, 2974–2984. [Google Scholar] [CrossRef]
| n = (%) | |
|---|---|
| Age | |
| <65 | 98 (27%) |
| 65–74 | 128 (36%) |
| >74 | 133 (37%) |
| Sex | |
| Female | 177 (49%) |
| Male | 182 (51%) |
| BMI (kg/m2) | |
| <18.5 | 13 (4%) |
| 18.5–24.99 | 112 (31%) |
| 25–29.99 | 113 (31%) |
| ≥30 | 121 (34%) |
| BSA (m2) | |
| <1.51 | 119 (33%) |
| 1.51–1.94 | 120 (33.5%) |
| >1.94 | 120 (33.5%) |
| ASA | |
| 1 | 53 (15%) |
| 2 | 165 (46%) |
| ≥3 | 141 (39%) |
| T2DM | |
| No | 289 (81%) |
| Yes | 69 (19%) |
| Liver Disease | |
| No | 341 (96%) |
| Yes | 15 (4%) |
| Median maximal cross-sectional liver area (cm2) | 178.7 (163.7–198.4) |
| Median LI (cm2/m2) | 66.8 (62.0–71.6) |
| Median total liver volume (cm3) | 1509.13 (857.8–3337.1) |
| 3-year survival | |
| Yes | 305 (80%) |
| No | 75 (20%) |
| LI < 61.9 cm2/m2 (n = 119) | LI 61.9–71.6 cm2/m2 (n = 120) | LI > 71.6 cm2/m2 (n = 120) | p Value 1 | |
|---|---|---|---|---|
| Age | <0.001 | |||
| <65 | 25 (21%) | 30 (25%) | 43 (36%) | |
| 65–74 | 36 (30%) | 44 (37%) | 48 (40%) | |
| >74 | 58 (49%) | 46 (38%) | 29 (24%) | |
| Sex | 0.106 | |||
| Female | 61 (51%) | 67 (56%) | 71 (59%) | |
| Male | 58 (49%) | 53 (44%) | 49 (41%) | |
| BMI (kg/m2) | <0.001 | |||
| <18.5 | 8 (7%) | 3 (3%) | 2 (2%) | |
| 18.5–24.99 | 59 (49%) | 40 (33%) | 13 (11%) | |
| 25–29.99 | 31 (26%) | 43 (36%) | 39 (32%) | |
| ≥30 | 21 (18%) | 34 (28%) | 66 (55%) | |
| BSA (m2) | <0.001 | |||
| <1.51 | 56 (47%) | 38 (32%) | 25 (21%) | |
| 1.51–1.94 | 35 (29%) | 46 (38%) | 39 (32%) | |
| >1.94 | 28 (24%) | 36 (30%) | 56 (47%) | |
| ASA | 0.053 | |||
| 1 | 24 (20%) | 17 (14%) | 12 (10%) | |
| 2 | 50 (42%) | 61 (51%) | 54 (45%) | |
| ≥3 | 45 (38%) | 42 (35%) | 54 (45%) | |
| T2DM | <0.001 | |||
| No | 110 (92%) | 98 (82%) | 81 (67%) | |
| Yes | 9 (8%) | 21 (18%) | 39 (33%) | |
| Liver Disease | 0.347 | |||
| No | 114 (97%) | 114 (97%) | 113 (94%) | |
| Yes | 4 (3%) | 4 (3%) | 7 (6%) | |
| 3-year survival | 0.350 | |||
| Yes | 102 (79%) | 95 (79%) | 108 (83%) | |
| No | 28 (21%) | 25 (21%) | 22 (17%) |
| OR (Univariate) | p-Value | OR (Multivariate) | p-Value | |
|---|---|---|---|---|
| Age (<65/65–74/>74 years) | 0.60 (0.45–0.79) | <0.001 | 0.53 (0.38–0.74) | <0.001 |
| Sex (Female/Male) | 1.67 (1.08–2.61) | 0.023 | 2.10 (1.14–3.82) | 0.017 |
| BMI (<18.5/18.5–24.9/25–30/>30 kg/m2) | 2.69 (2.00–3.61) | <0.001 | 3.04 (1.99–4.65) | <0.001 |
| BSA (<1.51/1.51–1.94/>1.94 m2) | 1.81 (1.37–2.41) | <0.001 | - | 0.058 |
| ASA (1/2/≥3) | 1.40 (1.01–1.94) | 0.043 | - | 0.058 |
| T2DM (No/Yes) | 3.34 (1.94–5.73) | <0.001 | 2.48 (1.33–4.62) | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGovern, J.; Mackay, C.; Freireich, R.; Golder, A.M.; Dolan, R.D.; Horgan, P.G.; Holroyd, D.; Jamieson, N.B.; McMillan, D.C. The Relationship between Liver Volume, Clinicopathological Characteristics and Survival in Patients Undergoing Resection with Curative Intent for Non-Metastatic Colonic Cancer. Tomography 2024, 10, 349-359. https://doi.org/10.3390/tomography10030027
McGovern J, Mackay C, Freireich R, Golder AM, Dolan RD, Horgan PG, Holroyd D, Jamieson NB, McMillan DC. The Relationship between Liver Volume, Clinicopathological Characteristics and Survival in Patients Undergoing Resection with Curative Intent for Non-Metastatic Colonic Cancer. Tomography. 2024; 10(3):349-359. https://doi.org/10.3390/tomography10030027
Chicago/Turabian StyleMcGovern, Josh, Charles Mackay, Rhiannon Freireich, Allan M. Golder, Ross D. Dolan, Paul G. Horgan, David Holroyd, Nigel B. Jamieson, and Donald C. McMillan. 2024. "The Relationship between Liver Volume, Clinicopathological Characteristics and Survival in Patients Undergoing Resection with Curative Intent for Non-Metastatic Colonic Cancer" Tomography 10, no. 3: 349-359. https://doi.org/10.3390/tomography10030027
APA StyleMcGovern, J., Mackay, C., Freireich, R., Golder, A. M., Dolan, R. D., Horgan, P. G., Holroyd, D., Jamieson, N. B., & McMillan, D. C. (2024). The Relationship between Liver Volume, Clinicopathological Characteristics and Survival in Patients Undergoing Resection with Curative Intent for Non-Metastatic Colonic Cancer. Tomography, 10(3), 349-359. https://doi.org/10.3390/tomography10030027







