Dual-Energy CT in Oncologic Imaging
Abstract
1. Introduction
Imaging Technique
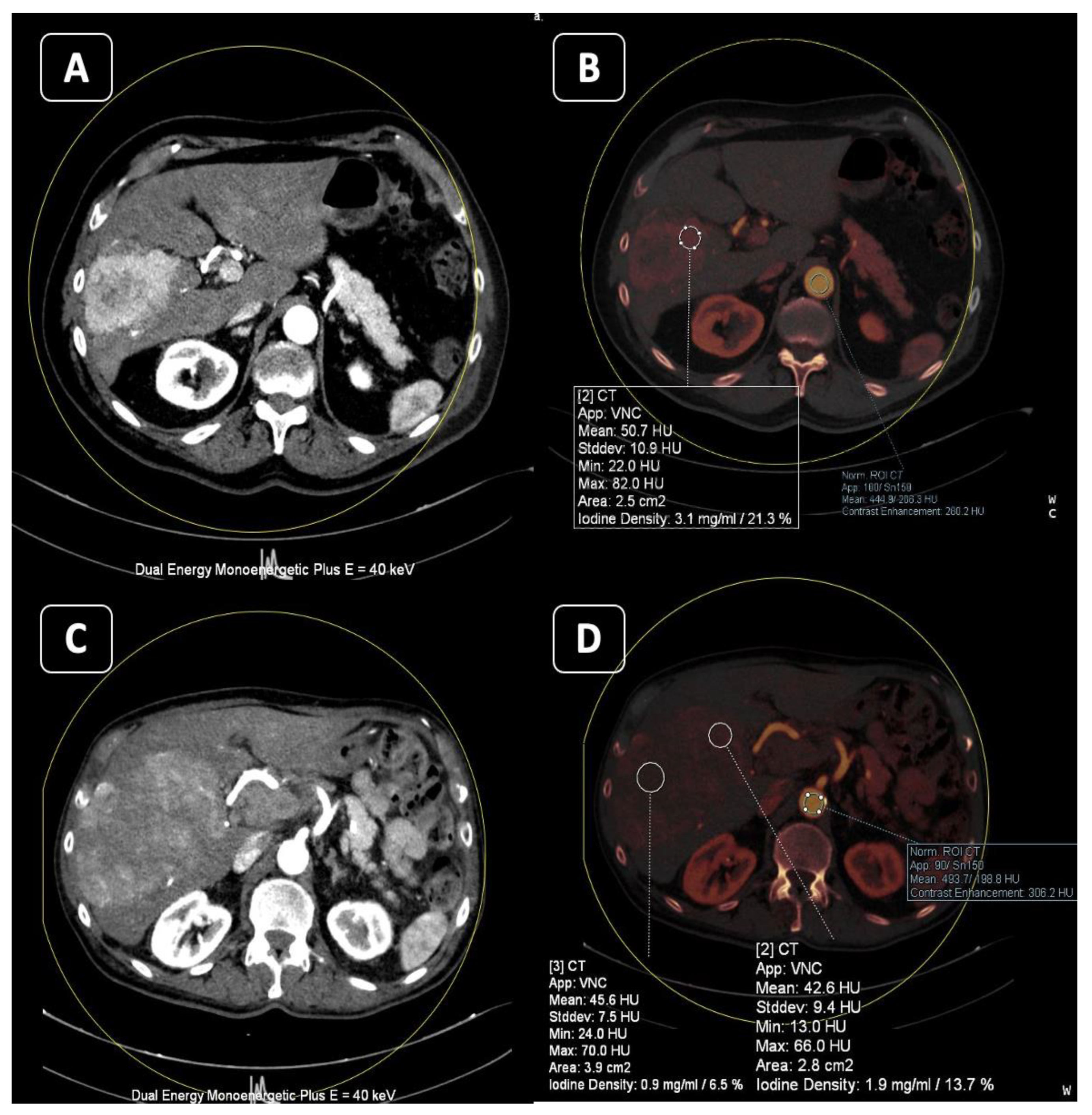
2. Virtual Non-Contrast (VNC)
Applications
3. Iodine MAP
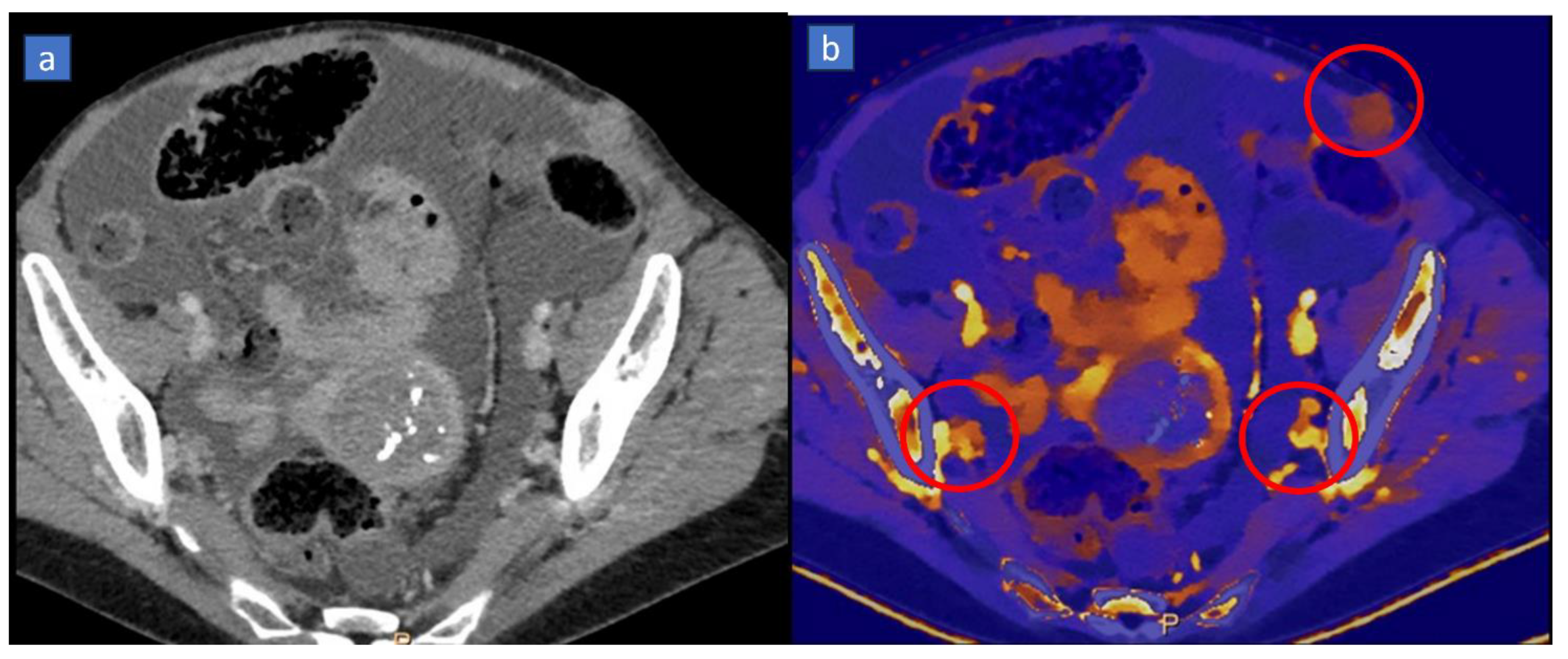
3.1. Applications
3.2. Response to Therapy
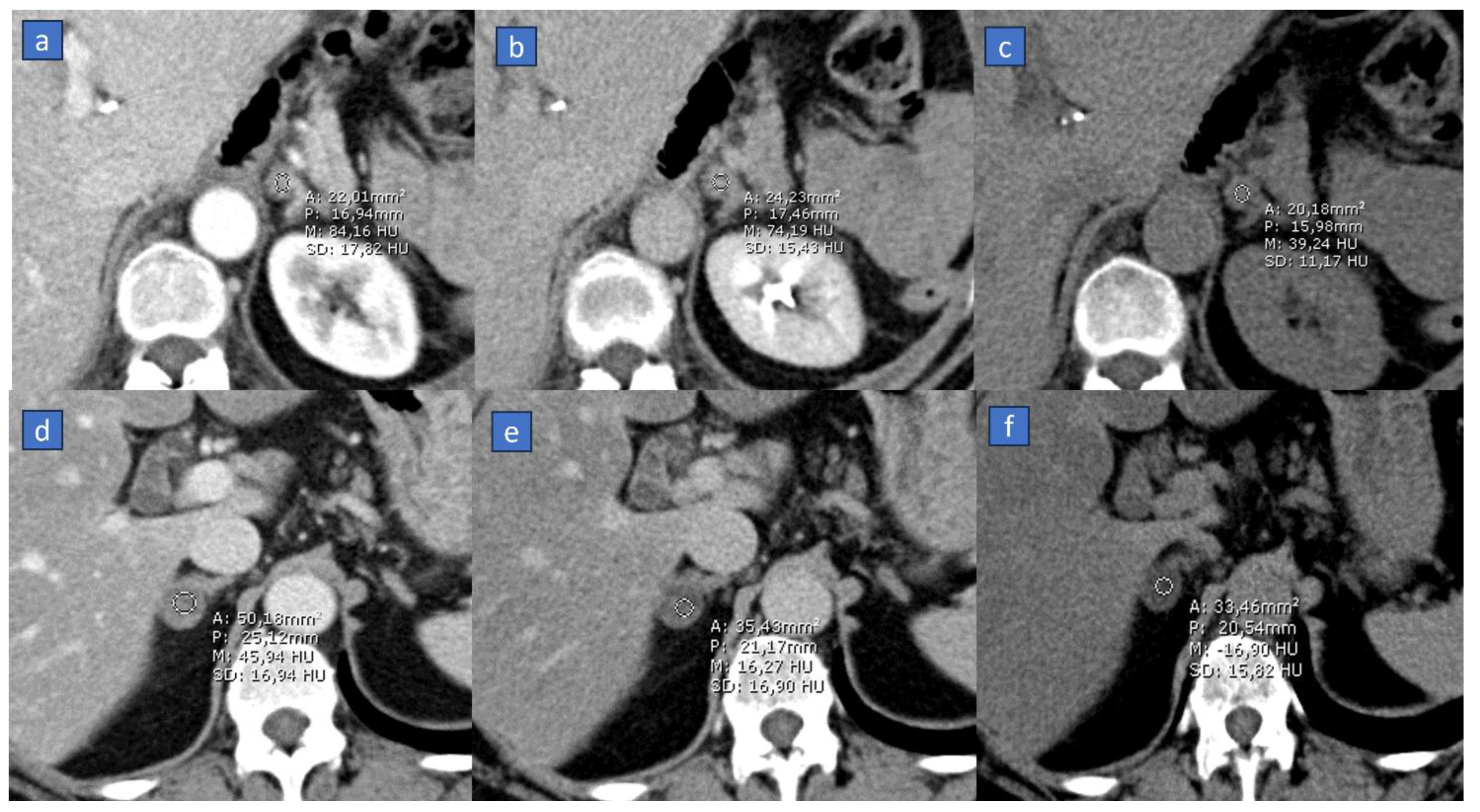
3.3. Organ Perfusion
4. Virtual Monoenergetic
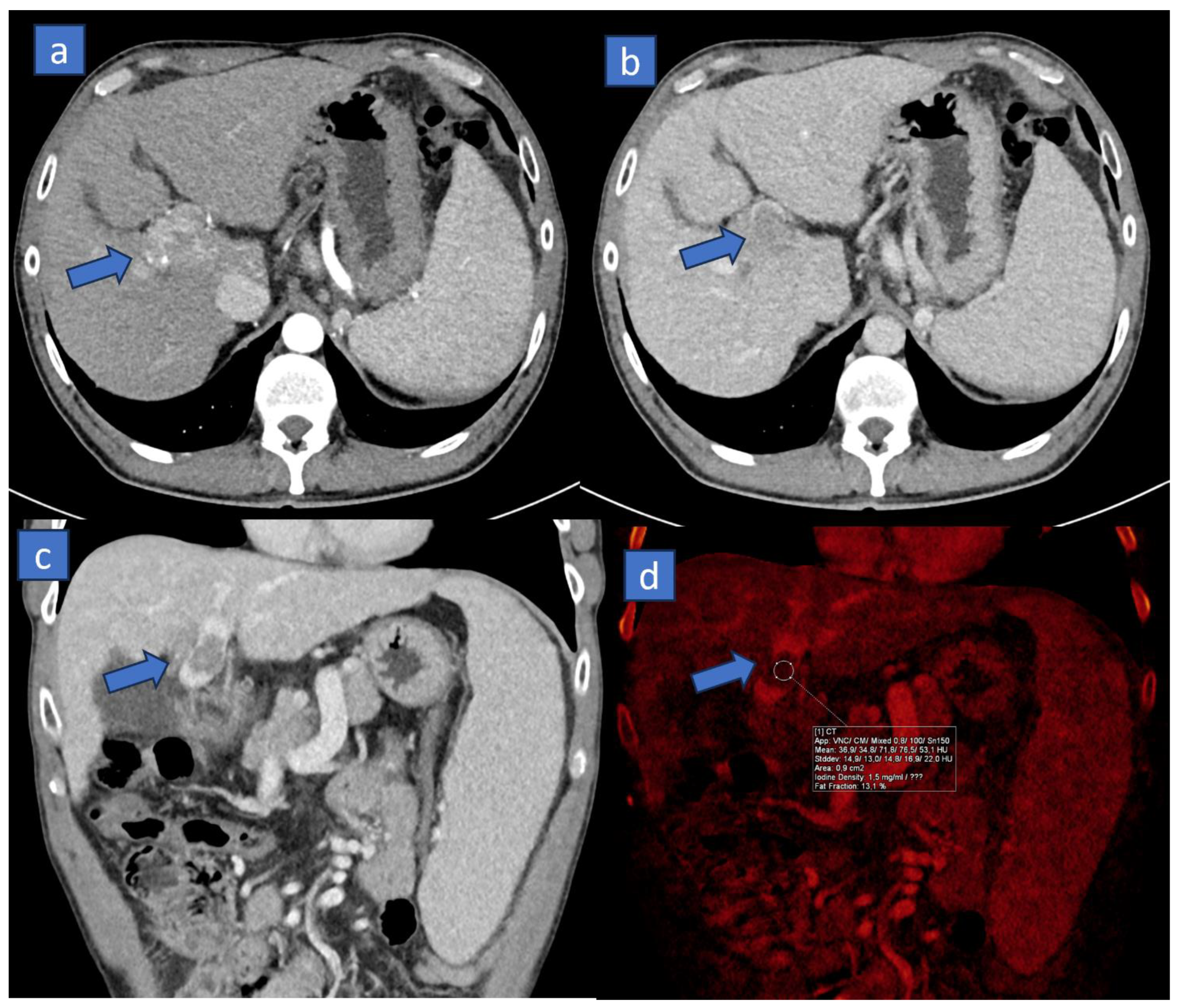
4.1. Better Conspicuity of Lesions
4.2. Less Contrast Material
4.3. Reduce Metal Artifacts
5. Bone Marrow Edema
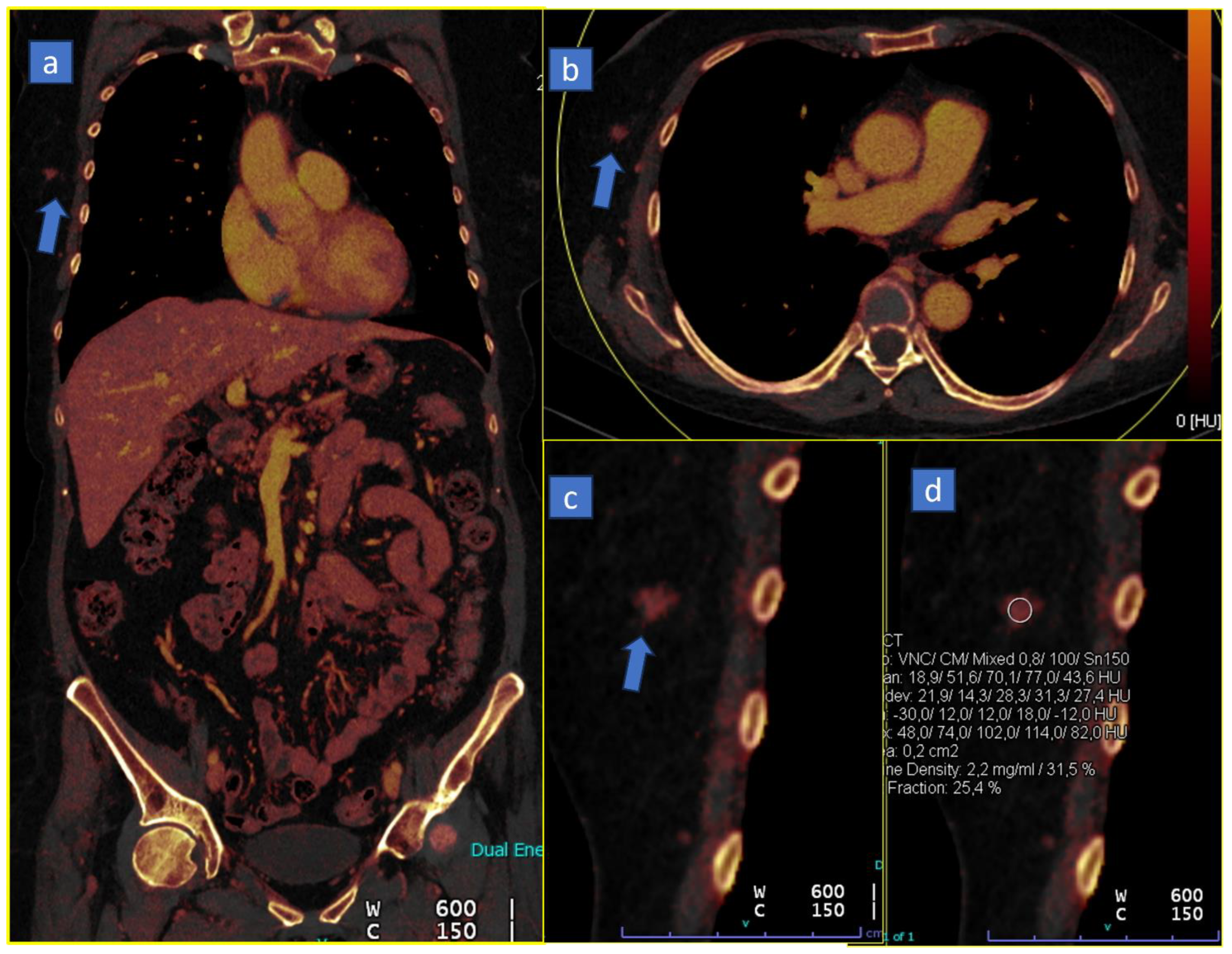
6. Lung Analysis
6.1. Pulmonary Thromboembolism
6.2. Lung Volumes and Perfusion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, A.J.N.; Wong, M.; Kutschera, P.; Lau, K.K. Dual-Energy CT in Musculoskeletal Trauma. Clin. Radiol. 2021, 76, 38–49. [Google Scholar] [CrossRef]
- Rajiah, P.; Sundaram, M.; Subhas, N. Dual-Energy CT in Musculoskeletal Imaging: What Is the Role Beyond Gout? Am. J. Roentgenol. 2019, 213, 493–505. [Google Scholar] [CrossRef]
- Cheraya, G.; Sharma, S.; Chhabra, A. Dual Energy CT in Musculoskeletal Applications beyond Crystal Imaging: Bone Marrow Maps and Metal Artifact Reduction. Skelet. Radiol. 2022, 51, 1521–1534. [Google Scholar] [CrossRef]
- Sandhu, R.; Aslan, M.; Obuchowski, N.; Primak, A.; Karim, W.; Subhas, N. Dual-Energy CT Arthrography: A Feasibility Study. Skelet. Radiol. 2021, 50, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Lutz, A.M. Using Dual-Energy CT for Painful Hip Arthroplasties. Radiology 2021, 300, 650–651. [Google Scholar] [CrossRef]
- Cicero, G.; Ascenti, G.; Albrecht, M.H.; Blandino, A.; Cavallaro, M.; D’Angelo, T.; Carerj, M.L.; Vogl, T.J.; Mazziotti, S. Extra-Abdominal Dual-Energy CT Applications: A Comprehensive Overview. Radiol. Medica 2020, 125, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Vlahos, I.; Jacobsen, M.C.; Godoy, M.C.; Stefanidis, K.; Layman, R.R. Dual-Energy CT in Pulmonary Vascular Disease. Br. J. Radiol. 2022, 95, 20210699. [Google Scholar] [CrossRef]
- Trabzonlu, T.A.; Mozaffary, A.; Kim, D.; Yaghmai, V. Dual-Energy CT Evaluation of Gastrointestinal Bleeding. Abdom. Radiol. 2020, 45, 1–14. [Google Scholar] [CrossRef]
- Tarkowski, P.; Czekajska-Chehab, E. Dual-Energy Heart CT: Beyond Better Angiography—Review. J. Clin. Med. 2021, 10, 5193. [Google Scholar] [CrossRef]
- Dell’Aversana, S.; Ascione, R.; De Giorgi, M.; De Lucia, D.R.; Cuocolo, R.; Boccalatte, M.; Sibilio, G.; Napolitano, G.; Muscogiuri, G.; Sironi, S.; et al. Dual-Energy CT of the Heart: A Review. J. Imaging 2022, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.D.; Pinho, D.F.; Kulkarni, N.M.; Hahn, P.F.; Guimaraes, A.R.; Sahani, D.V. Oncologic Applications of Dual-Energy CT in the Abdomen. RadioGraphics 2014, 34, 589–612. [Google Scholar] [CrossRef]
- Postma, A.A.; Das, M.; Stadler, A.A.R.; Wildberger, J.E. Dual-Energy CT: What the Neuroradiologist Should Know. Curr. Radiol. Rep. 2015, 3, 16. [Google Scholar] [CrossRef]
- Grajo, J.R.; Sahani, D.V. Dual-Energy CT of the Abdomen and Pelvis: Radiation Dose Considerations. J. Am. Coll. Radiol. 2018, 15, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Danad, I.; Fayad, Z.A.; Willemink, M.J.; Min, J.K. New Applications of Cardiac Computed Tomography. JACC Cardiovasc. Imaging 2015, 8, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, P.; Deviggiano, A.; Rodriguez-Granillo, G.A. Dual Energy Cardiac Computed Tomography. Minerva Cardiol. Angiol. 2017, 65, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yu, F.; Liu, M.; Yan, C. Clinical Value of Resting Cardiac Dual-Energy CT in Patients Suspected of Coronary Artery Disease. BMC Med. Imaging 2022, 22, 32. [Google Scholar] [CrossRef] [PubMed]
- Bodanapally, U.K.; Shanmuganathan, K.; Ramaswamy, M.; Tsymbalyuk, S.; Aarabi, B.; Parikh, G.Y.; Schwartzbauer, G.; Dreizin, D.; Simard, M.; Ptak, T.; et al. Iodine-Based Dual-Energy CT of Traumatic Hemorrhagic Contusions: Relationship to In-Hospital Mortality and Short-Term Outcome. Radiology 2019, 292, 730–738. [Google Scholar] [CrossRef]
- Nair, J.R.; Burrows, C.; Jerome, S.; Ribeiro, L.; Larrazabal, R.; Gupta, R.; Yu, E. Dual Energy CT: A Step Ahead in Brain and Spine Imaging. Br. J. Radiol. 2020, 93, 20190872. [Google Scholar] [CrossRef]
- Kazimierczak, W.; Kazimierczak, N.; Serafin, Z. Review of Clinical Applications of Dual-Energy CT in Patients after Endovascular Aortic Repair. J. Clin. Med. 2023, 12, 7766. [Google Scholar] [CrossRef] [PubMed]
- De Cecco, C.N.; Darnell, A.; Rengo, M.; Muscogiuri, G.; Bellini, D.; Ayuso, C.; Laghi, A. Dual-Energy CT: Oncologic Applications. AJR Am. J. Roentgenol. 2012, 199, S98–S105. [Google Scholar] [CrossRef]
- Guerrini, S.; Bagnacci, G.; Perrella, A.; Meglio, N.D.; Sica, C.; Mazzei, M.A. Dual Energy CT in Oncology: Benefits for Both Patients and Radiologists From an Emerging Quantitative and Functional Diagnostic Technique. Semin. Ultrasound CT MR 2023, 44, 205–213. [Google Scholar] [CrossRef]
- Ersahin, D.; Rasla, J.; Singh, A. Dual Energy CT Applications in Oncological Imaging. Semin. Ultrasound CT MRI 2022, 43, 344–351. [Google Scholar] [CrossRef]
- Winkelmann, M.T.; Gassenmaier, S.; Walter, S.S.; Artzner, C.; Lades, F.; Faby, S.; Nikolaou, K.; Bongers, M.N. Differentiation of Adrenal Adenomas from Adrenal Metastases in Single-Phased Staging Dual-Energy CT and Radiomics. Diagn. Interv. Radiol. Ank. Turk. 2022, 28, 208–216. [Google Scholar] [CrossRef]
- Agostini, A.; Mari, A.; Lanza, C.; Schicchi, N.; Borgheresi, A.; Maggi, S.; Giovagnoni, A. Trends in Radiation Dose and Image Quality for Pediatric Patients with a Multidetector CT and a Third-Generation Dual-Source Dual-Energy CT. Radiol. Medica 2019, 124, 745–752. [Google Scholar] [CrossRef]
- McCoombe, K.; Dobeli, K.; Meikle, S.; Llewellyn, S.; Kench, P. Sensitivity of Virtual Non-Contrast Dual-Energy CT Urogram for Detection of Urinary Calculi: A Systematic Review and Meta-Analysis. Eur. Radiol. 2022, 32, 8588–8596. [Google Scholar] [CrossRef] [PubMed]
- Connolly, M.J.; McInnes, M.D.F.; El-Khodary, M.; McGrath, T.A.; Schieda, N. Diagnostic Accuracy of Virtual Non-Contrast Enhanced Dual-Energy CT for Diagnosis of Adrenal Adenoma: A Systematic Review and Meta-Analysis. Eur. Radiol. 2017, 27, 4324–4335. [Google Scholar] [CrossRef] [PubMed]
- Takane, Y.; Sato, K.; Kageyama, R.; Takano, H.; Kayano, S. Accuracy of Virtual Non-Contrast Images with Different Algorithms in Dual-Energy Computed Tomography. Radiol. Phys. Technol. 2022, 15, 234–244. [Google Scholar] [CrossRef]
- Si-Mohamed, S.; Dupuis, N.; Tatard-Leitman, V.; Rotzinger, D.; Boccalini, S.; Dion, M.; Vlassenbroek, A.; Coulon, P.; Yagil, Y.; Shapira, N.; et al. Virtual versus True Non-Contrast Dual-Energy CT Imaging for the Diagnosis of Aortic Intramural Hematoma. Eur. Radiol. 2019, 29, 6762–6771. [Google Scholar] [CrossRef]
- Zhang, P.P.; Choi, H.H.; Ohliger, M.A. Detection of Fatty Liver Using Virtual Non-Contrast Dual-Energy CT. Abdom. Radiol. N. Y. 2022, 47, 2046–2056. [Google Scholar] [CrossRef]
- Mingkwansook, V.; Puwametwongsa, K.; Watcharakorn, A.; Dechasasawat, T. Comparative Study of True and Virtual Non-Contrast Imaging Generated from Dual-Layer Spectral CT in Patients with Upper Aerodigestive Tract Cancer. Pol. J. Radiol. 2022, 87, e678–e687. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Cao, R.; Li, H.; Long, M.; Sun, P.; Zheng, Y.; Li, L.; Yin, J. Dual-Energy CT Iodine Map in Predicting the Efficacy of Neoadjuvant Chemotherapy for Hypopharyngeal Carcinoma: A Preliminary Study. Sci. Rep. 2022, 12, 21356. [Google Scholar] [CrossRef]
- Xu, X.-Q.; Zhou, Y.; Su, G.-Y.; Tao, X.-W.; Ge, Y.-Q.; Si, Y.; Shen, M.-P.; Wu, F.-Y. Iodine Maps from Dual-Energy CT to Predict Extrathyroidal Extension and Recurrence in Papillary Thyroid Cancer Based on a Radiomics Approach. AJNR Am. J. Neuroradiol. 2022, 43, 748–755. [Google Scholar] [CrossRef]
- Sun, H.; Hou, X.-Y.; Xue, H.-D.; Li, X.-G.; Jin, Z.-Y.; Qian, J.-M.; Yu, J.-C.; Zhu, H.-D. Dual-Source Dual-Energy CT Angiography with Virtual Non-Enhanced Images and Iodine Map for Active Gastrointestinal Bleeding: Image Quality, Radiation Dose and Diagnostic Performance. Eur. J. Radiol. 2015, 84, 884–891. [Google Scholar] [CrossRef]
- Li, J.-X.; Xie, F.-J.; Chen, C.-H.; Chen, K.-M.; Tsai, C.-J. Dual-Energy Computed Tomography for Evaluation of Breast Cancer Follow-Ups: Comparison of Virtual Monoenergetic Images and Iodine-Map. Diagnostics 2022, 12, 946. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Bernatz, S.; Althoff, F.C.; Koch, V.; Grünewald, L.D.; Scholtz, J.-E.; Walter, D.; Zeuzem, S.; Wild, P.J.; Vogl, T.J.; et al. Dual-Energy CT Based Material Decomposition to Differentiate Intrahepatic Cholangiocarcinoma from Hepatocellular Carcinoma. Eur. J. Radiol. 2022, 156, 110556. [Google Scholar] [CrossRef]
- Lewin, M.; Laurent-Bellue, A.; Desterke, C.; Radu, A.; Feghali, J.A.; Farah, J.; Agostini, H.; Nault, J.-C.; Vibert, E.; Guettier, C. Evaluation of Perfusion CT and Dual-Energy CT for Predicting Microvascular Invasion of Hepatocellular Carcinoma. Abdom. Radiol. N. Y. 2022, 47, 2115–2127. [Google Scholar] [CrossRef]
- Liang, H.; Du, S.; Yan, G.; Zhou, Y.; Yang, T.; Zhang, Z.; Luo, C.; Liao, H.; Li, Y. Dual-Energy CT of the Pancreas: Comparison between Virtual Non-Contrast Images and True Non-Contrast Images in the Detection of Pancreatic Lesion. Abdom. Radiol. N. Y. 2023, 48, 2596–2603. [Google Scholar] [CrossRef] [PubMed]
- Frellesen, C.; Fessler, F.; Hardie, A.D.; Wichmann, J.L.; De Cecco, C.N.; Schoepf, U.J.; Kerl, J.M.; Schulz, B.; Hammerstingl, R.; Vogl, T.J.; et al. Dual-Energy CT of the Pancreas: Improved Carcinoma-to-Pancreas Contrast with a Noise-Optimized Monoenergetic Reconstruction Algorithm. Eur. J. Radiol. 2015, 84, 2052–2058. [Google Scholar] [CrossRef] [PubMed]
- Elsherif, S.B.; Zheng, S.; Ganeshan, D.; Iyer, R.; Wei, W.; Bhosale, P.R. Does Dual-Energy CT Differentiate Benign and Malignant Ovarian Tumours? Clin. Radiol. 2020, 75, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Daoud, T.; Morani, A.C.; Waters, R.; Bhosale, P.; Virarkar, M.K. Diagnostic Approaches to Neuroendocrine Neoplasms of Unknown Primary Site. J. Comput. Assist. Tomogr. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Lenga, L.; Lange, M.; Arendt, C.T.; Yel, I.; Booz, C.; Durden, J.; Leithner, D.; Vogl, T.J.; Albrecht, M.H.; Martin, S.S. Can Dual-Energy CT-Based Virtual Monoenergetic Imaging Improve the Assessment of Hypodense Liver Metastases in Patients with Hepatic Steatosis? Acad. Radiol. 2021, 28, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ju, Y.; Wu, J.; Liu, A.; Chen, A.; Liu, J.; Liu, Y.; Li, J. Differentiation of Liver Abscess from Liver Metastasis Using Dual-Energy Spectral CT Quantitative Parameters. Eur. J. Radiol. 2019, 113, 204–208. [Google Scholar] [CrossRef]
- Lenga, L.; Czwikla, R.; Wichmann, J.L.; Leithner, D.; Albrecht, M.H.; Booz, C.; Arendt, C.T.; Yel, I.; D’Angelo, T.; Vogl, T.J.; et al. Dual-Energy CT in Patients with Colorectal Cancer: Improved Assessment of Hypoattenuating Liver Metastases Using Noise-Optimized Virtual Monoenergetic Imaging. Eur. J. Radiol. 2018, 106, 184–191. [Google Scholar] [CrossRef]
- Si, K.; Wu, H.; Yang, M.; Guo, Y.; Zhang, X.; Ding, C.; Xue, J.; Han, P.; Li, X. Utility of Dark-Blood Dual-Energy CT Images for Predicting Vascular Involvement and R0 Resection in Patients with Pancreatic Cancer. AJR Am. J. Roentgenol. 2023, 220, 838–848. [Google Scholar] [CrossRef]
- Albrecht, M.H.; Vogl, T.J.; Martin, S.S.; Nance, J.W.; Duguay, T.M.; Wichmann, J.L.; De Cecco, C.N.; Varga-Szemes, A.; van Assen, M.; Tesche, C.; et al. Review of Clinical Applications for Virtual Monoenergetic Dual-Energy CT. Radiology 2019, 293, 260–271. [Google Scholar] [CrossRef]
- Liang, H.; Zhou, Y.; Zheng, Q.; Yan, G.; Liao, H.; Du, S.; Zhang, X.; Lv, F.; Zhang, Z.; Li, Y.-M. Dual-Energy CT with Virtual Monoenergetic Images and Iodine Maps Improves Tumor Conspicuity in Patients with Pancreatic Ductal Adenocarcinoma. Insights Imaging 2022, 13, 153. [Google Scholar] [CrossRef]
- Foti, G.; Fighera, A.; Campacci, A.; Natali, S.; Guerriero, M.; Zorzi, C.; Carbognin, G. Diagnostic Performance of Dual-Energy CT for Detecting Painful Hip Prosthesis Loosening. Radiology 2021, 300, 641–649. [Google Scholar] [CrossRef]
- Darras, K.E.; Clark, S.J.; Kang, H.; Mohammed, M.F.; Barrett, S.; Chang, S.D.; Harris, A.C.; Nicolaou, S.; McLaughlin, P.D. Virtual Monoenergetic Reconstruction of Contrast-Enhanced CT Scans of the Abdomen and Pelvis at 40 keV Improves the Detection of Peritoneal Metastatic Deposits. Abdom. Radiol. N. Y. 2019, 44, 422–428. [Google Scholar] [CrossRef]
- Pourvaziri, A.; Parakh, A.; Cao, J.; Locascio, J.; Eisner, B.; Sahani, D.; Kambadakone, A. Comparison of Four Dual-Energy CT Scanner Technologies for Determining Renal Stone Composition: A Phantom Approach. Radiology 2022, 304, 580–589. [Google Scholar] [CrossRef] [PubMed]
- McCollough, C.H.; Rajiah, P.S. Milestones in CT: Past, Present, and Future. Radiology 2023, 309, e230803. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.P.; Antunes, C.; Curvo-Semedo, L. Pros and Cons of Dual-Energy CT Systems: “One Does Not Fit All”. Tomography 2023, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Obmann, M.M.; Punjabi, G.; Obmann, V.C.; Boll, D.T.; Heye, T.; Benz, M.R.; Yeh, B.M. Dual-Energy CT of Acute Bowel Ischemia. Abdom. Radiol. N. Y. 2022, 47, 1660–1683. [Google Scholar] [CrossRef] [PubMed]
- Baxa, J.; Vondráková, A.; Matoušková, T.; Růžičková, O.; Schmidt, B.; Flohr, T.; Sedlmair, M.; Ferda, J. Dual-Phase Dual-Energy CT in Patients with Lung Cancer: Assessment of the Additional Value of Iodine Quantification in Lymph Node Therapy Response. Eur. Radiol. 2014, 24, 1981–1988. [Google Scholar] [CrossRef]
- Mohammadinejad, P.; Kwapisz, L.; Fidler, J.L.; Sheedy, S.P.; Heiken, J.P.; Khandelwal, A.; Wells, M.L.; Froemming, A.T.; Hansel, S.L.; Lee, Y.S.; et al. The Utility of a Dual-Phase, Dual-Energy CT Protocol in Patients Presenting with Overt Gastrointestinal Bleeding. Acta Radiol. Open 2021, 10, 20584601211030658. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Higuchi, S.; Kimura, H.; Gabata, T. Liver Metastases: Correlation between Imaging Features and Pathomolecular Environments. RadioGraphics 2022, 42, 1994–2013. [Google Scholar] [CrossRef]
- Silva, A.C.; Evans, J.M.; McCullough, A.E.; Jatoi, M.A.; Vargas, H.E.; Hara, A.K. MR Imaging of Hypervascular Liver Masses: A Review of Current Techniques. Radiographics 2009, 29, 385–402. [Google Scholar] [CrossRef] [PubMed]
- Mastrodicasa, D.; Willemink, M.J.; Madhuripan, N.; Chima, R.S.; Ho, A.A.; Ding, Y.; Marin, D.; Patel, B.N. Diagnostic Performance of Single-Phase Dual-Energy CT to Differentiate Vascular and Nonvascular Incidental Renal Lesions on Portal Venous Phase: Comparison with CT. Eur. Radiol. 2021, 31, 9600–9611. [Google Scholar] [CrossRef]
- Hering, D.A.; Kröger, K.; Bauer, R.W.; Eich, H.T.; Haverkamp, U. Comparison of Virtual Non-Contrast Dual-Energy CT and a True Non-Contrast CT for Contouring in Radiotherapy of 3D Printed Lung Tumour Models in Motion: A Phantom Study. Br. J. Radiol. 2020, 93, 20200152. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Lennartz, S.; Pisuchpen, N.; Mroueh, N.; Kongboonvijit, S.; Parakh, A.; Sahani, D.V.; Kambadakone, A. Renal Lesion Characterization by Dual-Layer Dual-Energy CT: Comparison of Virtual and True Unenhanced Images. AJR Am. J. Roentgenol. 2022, 219, 614–623. [Google Scholar] [CrossRef]
- Çamlıdağ, İ.; Nural, M.S.; Danacı, M.; Özden, E. Usefulness of Rapid kV-Switching Dual Energy CT in Renal Tumor Characterization. Abdom. Radiol. N. Y. 2019, 44, 1841–1849. [Google Scholar] [CrossRef]
- Foti, G.; Malleo, G.; Faccioli, N.; Guerriero, A.; Furlani, L.; Carbognin, G. Characterization of Adrenal Lesions Using MDCT Wash-out Parameters: Diagnostic Accuracy of Several Combinations of Intermediate and Delayed Phases. Radiol. Medica 2018, 123, 833–840. [Google Scholar] [CrossRef]
- Nagayama, Y.; Inoue, T.; Oda, S.; Tanoue, S.; Nakaura, T.; Ikeda, O.; Yamashita, Y. Adrenal Adenomas versus Metastases: Diagnostic Performance of Dual-Energy Spectral CT Virtual Noncontrast Imaging and Iodine Maps. Radiology 2020, 296, 324–332. [Google Scholar] [CrossRef]
- Badawy, M.; Gaballah, A.H.; Ganeshan, D.; Abdelalziz, A.; Remer, E.M.; Alsabbagh, M.; Westphalen, A.; Siddiqui, M.A.; Taffel, M.T.; Itani, M.; et al. Adrenal Hemorrhage and Hemorrhagic Masses; Diagnostic Workup and Imaging Findings. Br. J. Radiol. 2021, 94, 20210753. [Google Scholar] [CrossRef]
- Nelles, C.; Laukamp, K.R.; Große Hokamp, N.; Zaeske, C.; Celik, E.; Schoenfeld, M.H.; Borggrefe, J.; Kabbasch, C.; Schlamann, M.; Lennartz, S.; et al. Virtual Non-Contrast Reconstructions Improve Differentiation between Vascular Enhancement and Calcifications in Stereotactic Planning CT Scans of Cystic Intracranial Tumors. Eur. J. Radiol. 2022, 157, 110583. [Google Scholar] [CrossRef] [PubMed]
- Lehti, L.; Söderberg, M.; Höglund, P.; Wassélius, J. Comparing Arterial- and Venous-Phase Acquisition for Optimization of Virtual Noncontrast Images From Dual-Energy Computed Tomography Angiography. J. Comput. Assist. Tomogr. 2019, 43, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Lehti, L.; Söderberg, M.; Höglund, P.; Nyman, U.; Gottsäter, A.; Wassélius, J. Reliability of Virtual Non-Contrast Computed Tomography Angiography: Comparing It with the Real Deal. Acta Radiol. Open 2018, 7, 205846011879011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, X.; Xue, H.; Jin, Z.; Sun, H.; Chen, Y.; He, Y. Determinants of Detection of Stones and Calcifications in the Hepatobiliary System on Virtual Nonenhanced Dual-Energy CT. Chin. Med. Sci. J. 2016, 31, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, J.M.; Baek, J.H.; Han, J.K.; Choi, B.I. Initial Assessment of Dual-Energy CT in Patients with Gallstones or Bile Duct Stones: Can Virtual Nonenhanced Images Replace True Nonenhanced Images? AJR Am. J. Roentgenol. 2012, 198, 817–824. [Google Scholar] [CrossRef]
- Lee, M.H.; Park, H.J.; Kim, J.N.; Kim, M.S.; Hong, S.W.; Park, J.H.; Kang, C.H. Virtual Non-Contrast Images from Dual-Energy CT Angiography of the Abdominal Aorta and Femoral Arteries: Comparison with True Non-Contrast CT Images. Br. J. Radiol. 2022, 95, 20220378. [Google Scholar] [CrossRef]
- Graser, A.; Johnson, T.R.C.; Hecht, E.M.; Becker, C.R.; Leidecker, C.; Staehler, M.; Stief, C.G.; Hildebrandt, H.; Godoy, M.C.B.; Finn, M.E.; et al. Dual-Energy CT in Patients Suspected of Having Renal Masses: Can Virtual Nonenhanced Images Replace True Nonenhanced Images? Radiology 2009, 252, 433–440. [Google Scholar] [CrossRef]
- Pulickal, G.G.; Singh, D.; Lohan, R.; Chawla, A. Dual-Source Dual-Energy CT in Submandibular Sialolithiasis: Reliability and Radiation Burden. AJR Am. J. Roentgenol. 2019, 213, 1291–1296. [Google Scholar] [CrossRef]
- Meyer, M.; Nelson, R.C.; Vernuccio, F.; González, F.; Farjat, A.E.; Patel, B.N.; Samei, E.; Henzler, T.; Schoenberg, S.O.; Marin, D. Virtual Unenhanced Images at Dual-Energy CT: Influence on Renal Lesion Characterization. Radiology 2019, 291, 381–390. [Google Scholar] [CrossRef]
- Coursey, C.A.; Nelson, R.C.; Boll, D.T.; Paulson, E.K.; Ho, L.M.; Neville, A.M.; Marin, D.; Gupta, R.T.; Schindera, S.T. Dual-Energy Multidetector CT: How Does It Work, What Can It Tell Us, and When Can We Use It in Abdominopelvic Imaging? RadioGraphics 2010, 30, 1037–1055. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Lee, J.M.; Kim, J.H.; Lee, K.-B.; Kim, H.; Hong, S.K.; Yi, N.-J.; Lee, K.-W.; Suh, K.-S. Hepatic Fibrosis Grading with Extracellular Volume Fraction from Iodine Mapping in Spectral Liver CT. Eur. J. Radiol. 2021, 137, 109604. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fan, Y.; You, H.; Li, C.; Luo, M.; Zhou, J.; Li, A.; Zhang, L.; Yu, X.; Deng, W.; et al. Dual-Energy CT Deep Learning Radiomics to Predict Macrotrabecular-Massive Hepatocellular Carcinoma. Radiology 2023, 308, e230255. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.M.; Kim, K.W.; Klotz, E.; Kim, S.H.; Lee, J.Y.; Han, J.K.; Choi, B.I. Dual-Energy Computed Tomography to Assess Tumor Response to Hepatic Radiofrequency Ablation: Potential Diagnostic Value of Virtual Noncontrast Images and Iodine Maps. Investig. Radiol. 2011, 46, 77–84. [Google Scholar] [CrossRef]
- Lee, J.-A.; Jeong, W.K.; Kim, Y.; Song, S.-Y.; Kim, J.; Heo, J.N.; Park, C.K. Dual-Energy CT to Detect Recurrent HCC after TACE: Initial Experience of Color-Coded Iodine CT Imaging. Eur. J. Radiol. 2013, 82, 569–576. [Google Scholar] [CrossRef]
- Gupta, S.; Wagner-Bartak, N.; Jensen, C.T.; Hui, A.; Wei, W.; Lertdilok, P.; Qayyum, A.; Tamm, E.P. Dual-Energy CT of Pancreatic Adenocarcinoma: Reproducibility of Primary Tumor Measurements and Assessment of Tumor Conspicuity and Margin Sharpness. Abdom. Radiol. 2016, 41, 1317–1324. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, Y.; Hu, X.; Shi, S.; Zhang, N.; Wang, L.; Deng, W.; Feng, S.-T.; Peng, Z.; Luo, Y. Use of Dual-Layer Spectral Detector Computed Tomography in the Diagnosis of Pancreatic Neuroendocrine Neoplasms. Eur. J. Radiol. 2023, 159, 110660. [Google Scholar] [CrossRef] [PubMed]
- Lennartz, S.; Mager, A.; Große Hokamp, N.; Schäfer, S.; Zopfs, D.; Maintz, D.; Reinhardt, H.C.; Thomas, R.K.; Caldeira, L.; Persigehl, T. Texture Analysis of Iodine Maps and Conventional Images for K-Nearest Neighbor Classification of Benign and Metastatic Lung Nodules. Cancer Imaging 2021, 21, 17. [Google Scholar] [CrossRef]
- Özdeniz, İ.; İdilman, İ.S.; Köklü, S.; Hamaloğlu, E.; Özmen, M.; Akata, D.; Karçaaltıncaba, M. Dual-Energy CT Characteristics of Colon and Rectal Cancer Allows Differentiation from Stool by Dual-Source CT. Diagn. Interv. Radiol. Ank. Turk. 2017, 23, 251–256. [Google Scholar] [CrossRef]
- May, M.S.; Wiesmueller, M.; Heiss, R.; Brand, M.; Bruegel, J.; Uder, M.; Wuest, W. Comparison of Dual- and Single-Source Dual-Energy CT in Head and Neck Imaging. Eur. Radiol. 2019, 29, 4207–4214. [Google Scholar] [CrossRef]
- Rizzo, S.; Radice, D.; Femia, M.; De Marco, P.; Origgi, D.; Preda, L.; Barberis, M.; Vigorito, R.; Mauri, G.; Mauro, A.; et al. Metastatic and Non-Metastatic Lymph Nodes: Quantification and Different Distribution of Iodine Uptake Assessed by Dual-Energy CT. Eur. Radiol. 2018, 28, 760–769. [Google Scholar] [CrossRef]
- Fervers, P.; Celik, E.; Bratke, G.; Maintz, D.; Baues, C.; Ruffing, S.; Pollman-Schweckhorst, P.; Kottlors, J.; Lennartz, S.; Große Hokamp, N. Radiotherapy Response Assessment of Multiple Myeloma: A Dual-Energy CT Approach With Virtual Non-Calcium Images. Front. Oncol. 2021, 11, 734819. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Tachibana, Y.; Kishimoto, R.; Omatsu, T.; Hotta, E.; Tanimoto, K.; Wakatsuki, M.; Obata, T.; Tsuji, H. Dual-Energy Computed Tomography-Based Iodine Concentration Estimation for Evaluating Choroidal Malignant Melanoma Response to Treatment: Optimization and Primary Validation. Diagnostics 2022, 12, 2692. [Google Scholar] [CrossRef] [PubMed]
- Hellbach, K.; Sterzik, A.; Sommer, W.; Karpitschka, M.; Hummel, N.; Casuscelli, J.; Ingrisch, M.; Schlemmer, M.; Graser, A.; Staehler, M. Dual Energy CT Allows for Improved Characterization of Response to Antiangiogenic Treatment in Patients with Metastatic Renal Cell Cancer. Eur. Radiol. 2017, 27, 2532–2537. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.N.; De Cecco, C.N.; Caruso, D.; Tesche, C.; Spandorfer, A.; Varga-Szemes, A.; Schoepf, U.J. Myocardial Perfusion Imaging with Dual Energy CT. Eur. J. Radiol. 2016, 85, 1914–1921. [Google Scholar] [CrossRef] [PubMed]
- Foti, G.; Silva, R.; Faccioli, N.; Fighera, A.; Menghini, R.; Campagnola, A.; Carbognin, G. Identification of Pulmonary Embolism: Diagnostic Accuracy of Venous-Phase Dual-Energy CT in Comparison to Pulmonary Arteries CT Angiography. Eur. Radiol. 2021, 31, 1923–1931. [Google Scholar] [CrossRef]
- Parakh, A.; Lennartz, S.; An, C.; Rajiah, P.; Yeh, B.M.; Simeone, F.J.; Sahani, D.V.; Kambadakone, A.R. Dual-Energy CT Images: Pearls and Pitfalls. Radiographics 2021, 41, 98–119. [Google Scholar] [CrossRef]
- Nagayama, Y.; Iyama, A.; Oda, S.; Taguchi, N.; Nakaura, T.; Utsunomiya, D.; Kikuchi, Y.; Yamashita, Y. Dual-Layer Dual-Energy Computed Tomography for the Assessment of Hypovascular Hepatic Metastases: Impact of Closing k-Edge on Image Quality and Lesion Detectability. Eur. Radiol. 2019, 29, 2837–2847. [Google Scholar] [CrossRef]
- De Cecco, C.N.; Caruso, D.; Schoepf, U.J.; De Santis, D.; Muscogiuri, G.; Albrecht, M.H.; Meinel, F.G.; Wichmann, J.L.; Burchett, P.F.; Varga-Szemes, A.; et al. A Noise-Optimized Virtual Monoenergetic Reconstruction Algorithm Improves the Diagnostic Accuracy of Late Hepatic Arterial Phase Dual-Energy CT for the Detection of Hypervascular Liver Lesions. Eur. Radiol. 2018, 28, 3393–3404. [Google Scholar] [CrossRef]
- Bhosale, P.; Le, O.; Balachandran, A.; Fox, P.; Paulson, E.; Tamm, E. Quantitative and Qualitative Comparison of Single-Source Dual-Energy Computed Tomography and 120-kVp Computed Tomography for the Assessment of Pancreatic Ductal Adenocarcinoma. J. Comput. Assist. Tomogr. 2015, 39, 907–913. [Google Scholar] [CrossRef]
- Marin, D.; Nelson, R.C.; Barnhart, H.; Schindera, S.T.; Ho, L.M.; Jaffe, T.A.; Yoshizumi, T.T.; Youngblood, R.; Samei, E. Detection of Pancreatic Tumors, Image Quality, and Radiation Dose during the Pancreatic Parenchymal Phase: Effect of a Low-Tube-Voltage, High-Tube-Current CT Technique-Preliminary Results. Radiology 2010, 256, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.Z.; Wu, Z.Y.; Tao, R.; Guo, Y.; Li, J.Y.; Zhang, J.; Chen, K.M. Dual Energy Spectral CT Imaging of Insulinoma—Value in Preoperative Diagnosis Compared with Conventional Multi-Detector CT. Eur. J. Radiol. 2012, 81, 2487–2494. [Google Scholar] [CrossRef]
- Benveniste, A.P.; de Castro Faria, S.; Broering, G.; Ganeshan, D.M.; Tamm, E.P.; Iyer, R.B.; Bhosale, P. Potential Application of Dual-Energy CT in Gynecologic Cancer: Initial Experience. AJR Am. J. Roentgenol. 2017, 208, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.N.; Farjat, A.; Schabel, C.; Duvnjak, P.; Mileto, A.; Ramirez-Giraldo, J.C.; Marin, D. Energy-Specific Optimization of Attenuation Thresholds for Low-Energy Virtual Monoenergetic Images in Renal Lesion Evaluation. AJR Am. J. Roentgenol. 2018, 210, W205–W217. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, H.; Yan, J.; Wang, B.; Du, L.; Pan, Z.; Yan, F. Decreased Stage Migration Rate of Early Gastric Cancer with a New Reconstruction Algorithm Using Dual-Energy CT Images: A Preliminary Study. Eur. Radiol. 2017, 27, 671–680. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Y.; Luo, D.; Yang, L.; Hu, L.; Zhao, X.; Wang, Y.; Liu, W. Diagnostic Value of Single-Source Dual-Energy Spectral Computed Tomography in Differentiating Parotid Gland Tumors: Initial Results. Quant. Imaging Med. Surg. 2018, 8, 588–596. [Google Scholar] [CrossRef]
- Johansen, C.B.; Martinsen, A.C.T.; Enden, T.R.; Svanteson, M. The Potential of Iodinated Contrast Reduction in Dual-Energy CT Thoracic Angiography; an Evaluation of Image Quality. Radiography 2022, 28, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Shuman, W.P.; O’Malley, R.B.; Busey, J.M.; Ramos, M.M.; Koprowicz, K.M. Prospective Comparison of Dual-Energy CT Aortography Using 70% Reduced Iodine Dose versus Single-Energy CT Aortography Using Standard Iodine Dose in the Same Patient. Abdom. Radiol. N. Y. 2017, 42, 759–765. [Google Scholar] [CrossRef]
- Nicolaou, S.; Liang, T.; Murphy, D.T.; Korzan, J.R.; Ouellette, H.; Munk, P. Dual-Energy CT: A Promising New Technique for Assessment of the Musculoskeletal System. AJR Am. J. Roentgenol. 2012, 199, S78–S86. [Google Scholar] [CrossRef]
- Mallinson, P.I.; Coupal, T.M.; McLaughlin, P.D.; Nicolaou, S.; Munk, P.L.; Ouellette, H.A. Dual-Energy CT for the Musculoskeletal System. Radiology 2016, 281, 690–707. [Google Scholar] [CrossRef]
- Chawla, A.; Srinivasan, S.; Lim, T.-C.; Pulickal, G.G.; Shenoy, J.; Peh, W.C.G. Dual-Energy CT Applications in Salivary Gland Lesions. Br. J. Radiol. 2017, 90, 20160859. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, H.; Takayanagi, T.; Ishikawa, T.; Katada, Y.; Fukui, R.; Yamamoto, Y.; Suzuki, S. New Fast kVp Switching Dual-Energy CT: Reduced Severity of Beam Hardening Artifacts and Improved Image Quality in Reduced-Iodine Virtual Monochromatic Imaging. Acad. Radiol. 2020, 27, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Gentili, F.; Guerrini, S.; Mazzei, F.G.; Monteleone, I.; Di Meglio, N.; Sansotta, L.; Perrella, A.; Puglisi, S.; De Filippo, M.; Gennaro, P.; et al. Dual Energy CT in Gland Tumors: A Comprehensive Narrative Review and Differential Diagnosis. Gland Surg. 2020, 9, 2269–2282. [Google Scholar] [CrossRef] [PubMed]
- Laukamp, K.R.; Zopfs, D.; Wagner, A.; Lennartz, S.; Pennig, L.; Borggrefe, J.; Ramaiya, N.; Große Hokamp, N. CT Artifacts from Port Systems: Virtual Monoenergetic Reconstructions from Spectral-Detector CT Reduce Artifacts and Improve Depiction of Surrounding Tissue. Eur. J. Radiol. 2019, 121, 108733. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Yang, M.; Jia, Y.; Zhang, L.; Sun, X.; Zhang, Y.; Nie, Z.; Wu, H.; Zhang, X.; Lei, Z.; et al. A Novel Subtraction Method to Reduce Metal Artifacts of Cerebral Aneurysm Embolism Coils. Clin. Neuroradiol. 2022, 32, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, M.P.; Mekhail, A.; Benzel, E.C. Management of Metastatic Tumors of the Spine: Strategies and Operative Indications. Neurosurg. Focus 2001, 11, e2. [Google Scholar] [CrossRef] [PubMed]
- Pache, G.; Krauss, B.; Strohm, P.; Saueressig, U.; Blanke, P.; Bulla, S.; Schäfer, O.; Helwig, P.; Kotter, E.; Langer, M.; et al. Dual-Energy CT Virtual Noncalcium Technique: Detecting Posttraumatic Bone Marrow Lesions—Feasibility Study. Radiology 2010, 256, 617–624. [Google Scholar] [CrossRef]
- Foti, G.; Guerriero, M.; Faccioli, N.; Fighera, A.; Romano, L.; Zorzi, C.; Carbognin, G. Identification of Bone Marrow Edema around the Ankle Joint in Non-Traumatic Patients: Diagnostic Accuracy of Dual-Energy Computed Tomography. Clin. Imaging 2021, 69, 341–348. [Google Scholar] [CrossRef]
- Foti, G.; Faccioli, N.; Silva, R.; Oliboni, E.; Zorzi, C.; Carbognin, G. Bone Marrow Edema around the Hip in Non-Traumatic Pain: Dual-Energy CT vs MRI. Eur. Radiol. 2020, 30, 4098–4106. [Google Scholar] [CrossRef]
- Foti, G.; Catania, M.; Caia, S.; Romano, L.; Beltramello, A.; Zorzi, C.; Carbognin, G. Identification of Bone Marrow Edema of the Ankle: Diagnostic Accuracy of Dual-Energy CT in Comparison with MRI. Radiol. Medica 2019, 124, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Foti, G.; Mantovani, W.; Faccioli, N.; Crivellari, G.; Romano, L.; Zorzi, C.; Carbognin, G. Identification of Bone Marrow Edema of the Knee: Diagnostic Accuracy of Dual-Energy CT in Comparison with MRI. Radiol. Medica 2021, 126, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Frellesen, C.; Azadegan, M.; Martin, S.S.; Otani, K.; DʼAngelo, T.; Booz, C.; Eichler, K.; Panahi, B.; Kaup, M.; Bauer, R.W.; et al. Dual-Energy Computed Tomography-Based Display of Bone Marrow Edema in Incidental Vertebral Compression Fractures: Diagnostic Accuracy and Characterization in Oncological Patients Undergoing Routine Staging Computed Tomography. Investig. Radiol. 2018, 53, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Suh, C.H.; Yun, S.J.; Jin, W.; Lee, S.H.; Park, S.Y.; Ryu, C.-W. Diagnostic Performance of Dual-Energy CT for the Detection of Bone Marrow Oedema: A Systematic Review and Meta-Analysis. Eur. Radiol. 2018, 28, 4182–4194. [Google Scholar] [CrossRef] [PubMed]
- Gosangi, B.; Mandell, J.C.; Weaver, M.J.; Uyeda, J.W.; Smith, S.E.; Sodickson, A.D.; Khurana, B. Bone Marrow Edema at Dual-Energy CT: A Game Changer in the Emergency Department. RadioGraphics 2020, 40, 859–874. [Google Scholar] [CrossRef] [PubMed]
- Abdullayev, N.; Große Hokamp, N.; Lennartz, S.; Holz, J.A.; Romman, Z.; Pahn, G.; Neuhaus, V.; Maintz, D.; Krug, B.; Borggrefe, J. Improvements of Diagnostic Accuracy and Visualization of Vertebral Metastasis Using Multi-Level Virtual Non-Calcium Reconstructions from Dual-Layer Spectral Detector Computed Tomography. Eur. Radiol. 2019, 29, 5941–5949. [Google Scholar] [CrossRef]
- Kosmala, A.; Weng, A.M.; Krauss, B.; Knop, S.; Bley, T.A.; Petritsch, B. Dual-Energy CT of the Bone Marrow in Multiple Myeloma: Diagnostic Accuracy for Quantitative Differentiation of Infiltration Patterns. Eur. Radiol. 2018, 28, 5083–5090. [Google Scholar] [CrossRef]
- Gruenewald, L.D.; Koch, V.; Martin, S.S.; Yel, I.; Mahmoudi, S.; Bernatz, S.; Eichler, K.; Gruber-Rouh, T.; Pinto Dos Santos, D.; D’Angelo, T.; et al. Dual-Energy CT-Based Opportunistic Volumetric Bone Mineral Density Assessment of the Distal Radius. Radiology 2023, 308, e223150. [Google Scholar] [CrossRef]
- Heinrich, A.; Schenkl, S.; Buckreus, D.; Güttler, F.V.; Teichgräber, U.K.-M. CT-Based Thermometry with Virtual Monoenergetic Images by Dual-Energy of Fat, Muscle and Bone Using FBP, Iterative and Deep Learning-Based Reconstruction. Eur. Radiol. 2022, 32, 424–431. [Google Scholar] [CrossRef]
- Patel, A.A.; Sutphin, P.D.; Xi, Y.; Abbara, S.; Kalva, S.P. Arterial Phase CTA Replacement by a Virtual Arterial Phase Reconstruction from a Venous Phase CTA: Preliminary Results Using Detector-Based Spectral CT. Cardiovasc. Interv. Radiol. 2019, 42, 250–259. [Google Scholar] [CrossRef]
- Choe, J.; Lee, S.M.; Chae, E.J.; Lee, S.M.; Kim, Y.-H.; Kim, N.; Seo, J.B. Evaluation of Postoperative Lung Volume and Perfusion Changes by Dual-Energy Computed Tomography in Patients with Lung Cancer. Eur. J. Radiol. 2017, 90, 166–173. [Google Scholar] [CrossRef]
- Suh, Y.J.; Lee, C.Y.; Lee, S.; Kim, H.E.; Kim, Y.J. Patterns of Postoperative Changes in Lung Volume and Perfusion Assessed by Dual-Energy CT: Comparison of Lobectomy and Limited Resection. AJR Am. J. Roentgenol. 2023, 220, 660–671. [Google Scholar] [CrossRef]
- Chae, E.J.; Kim, N.; Seo, J.B.; Park, J.-Y.; Song, J.-W.; Lee, H.J.; Hwang, H.J.; Lim, C.; Chang, Y.J.; Kim, Y.H. Prediction of Postoperative Lung Function in Patients Undergoing Lung Resection: Dual-Energy Perfusion Computed Tomography versus Perfusion Scintigraphy. Investig. Radiol. 2013, 48, 622–627. [Google Scholar] [CrossRef]
- Bahig, H.; Campeau, M.-P.; Lapointe, A.; Bedwani, S.; Roberge, D.; de Guise, J.; Blais, D.; Vu, T.; Lambert, L.; Chartrand-Lefebvre, C.; et al. Phase 1-2 Study of Dual-Energy Computed Tomography for Assessment of Pulmonary Function in Radiation Therapy Planning. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 334–343. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foti, G.; Ascenti, G.; Agostini, A.; Longo, C.; Lombardo, F.; Inno, A.; Modena, A.; Gori, S. Dual-Energy CT in Oncologic Imaging. Tomography 2024, 10, 299-319. https://doi.org/10.3390/tomography10030024
Foti G, Ascenti G, Agostini A, Longo C, Lombardo F, Inno A, Modena A, Gori S. Dual-Energy CT in Oncologic Imaging. Tomography. 2024; 10(3):299-319. https://doi.org/10.3390/tomography10030024
Chicago/Turabian StyleFoti, Giovanni, Giorgio Ascenti, Andrea Agostini, Chiara Longo, Fabio Lombardo, Alessandro Inno, Alessandra Modena, and Stefania Gori. 2024. "Dual-Energy CT in Oncologic Imaging" Tomography 10, no. 3: 299-319. https://doi.org/10.3390/tomography10030024
APA StyleFoti, G., Ascenti, G., Agostini, A., Longo, C., Lombardo, F., Inno, A., Modena, A., & Gori, S. (2024). Dual-Energy CT in Oncologic Imaging. Tomography, 10(3), 299-319. https://doi.org/10.3390/tomography10030024





