Feasibility of Low-Dose and Low-Contrast Media Volume Approach in Computed Tomography Cardiovascular Imaging Reconstructed with Model-Based Algorithm
Abstract
1. Introduction
2. Materials and Methods
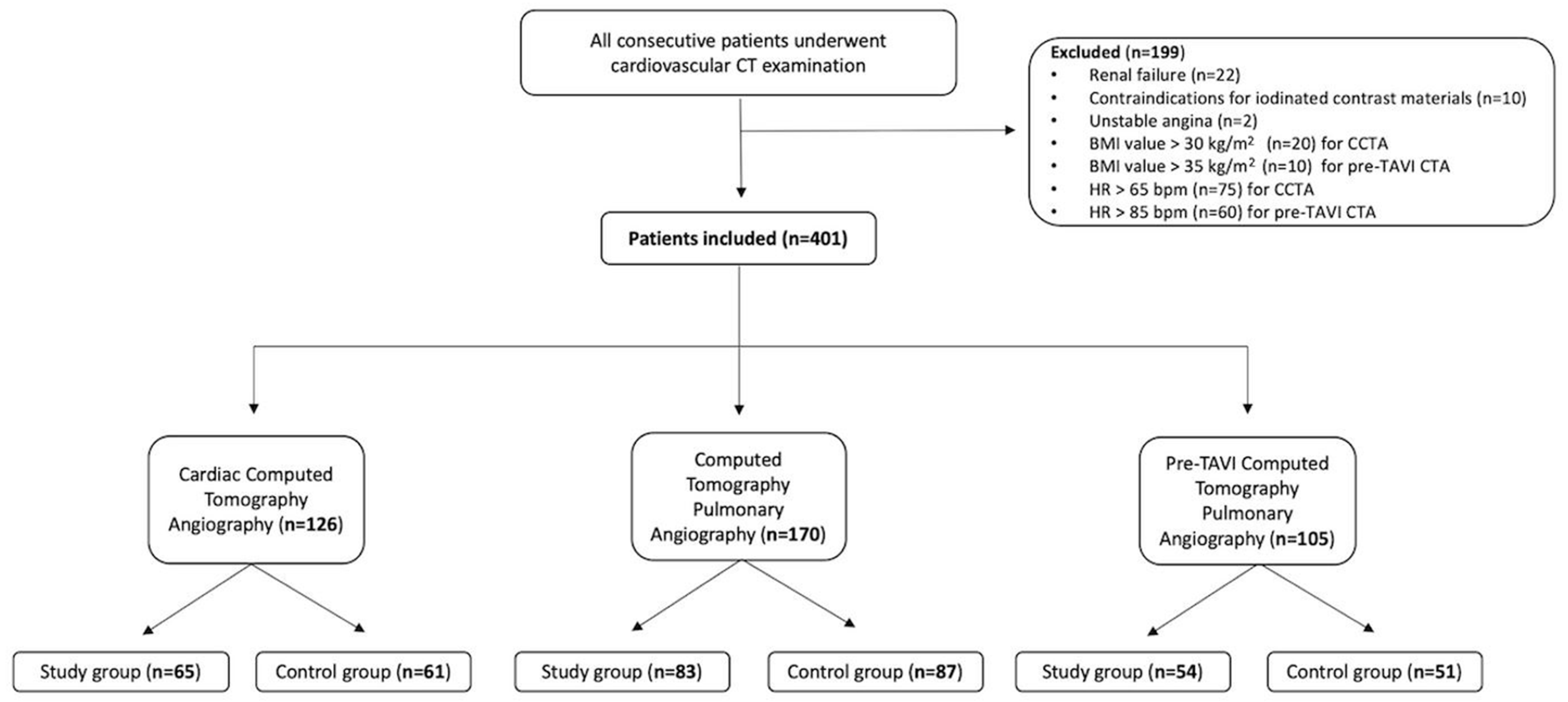
2.1. Study Population
2.2. CT Protocols
2.3. Reconstruction Algorithms
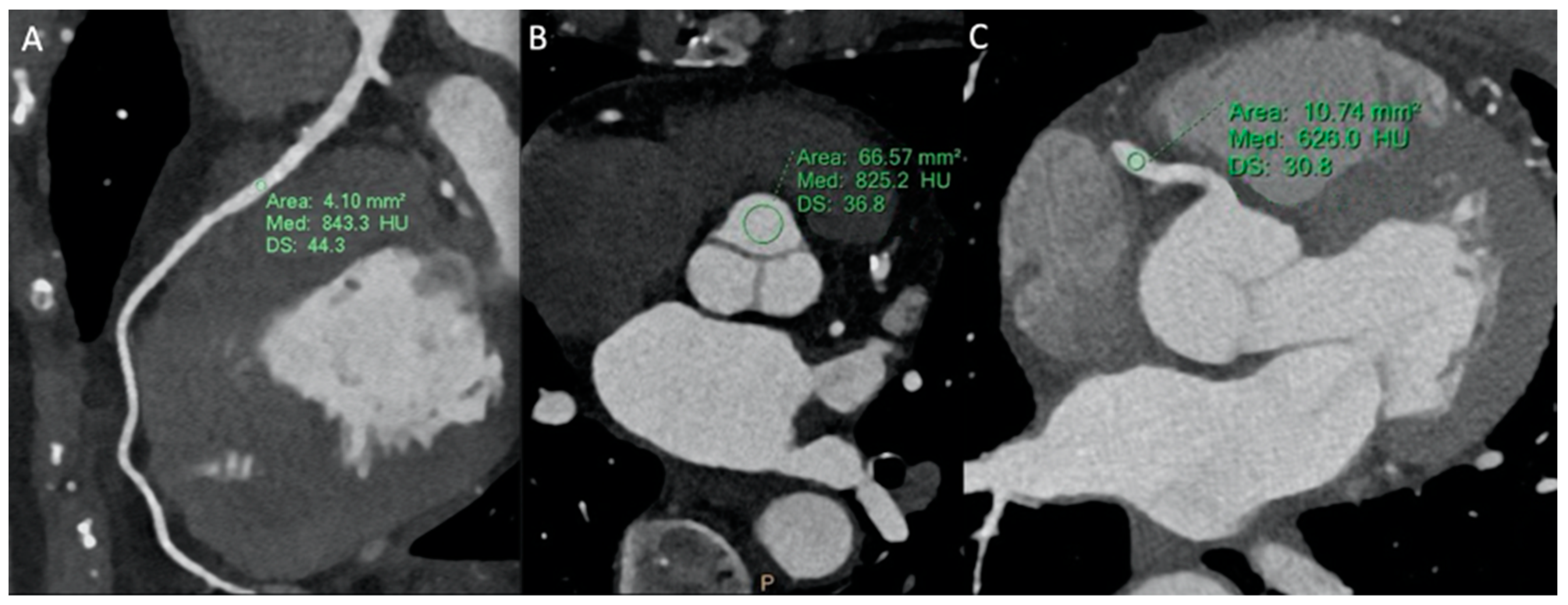
2.4. Image Analysis
2.5. Radiation Dose Quantification and Acquisition Time
2.6. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Image Analysis Results
3.2.1. CCTA
3.2.2. CTPA
3.2.3. Pre-TAVR CTA
3.3. Image Quality Results
3.4. Radiation Dose Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Moss, A.J.; Williams, M.C.; Newby, D.E.; Nicol, E.D. The Updated NICE Guidelines: Cardiac CT as the First-Line Test for Coronary Artery Disease. Curr. Cardiovasc. Imaging Rep. 2017, 10, 15. [Google Scholar] [CrossRef]
- Achenbach, S.; Delgado, V.; Hausleiter, J.; Schoenhagen, P.; Min, J.K.; Leipsic, J.A. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J. Cardiovasc. Comput. Tomogr. 2012, 6, 366–380. [Google Scholar] [CrossRef]
- Leipsic, J.; Gurvitch, R.; LaBounty, T.M.; Min, J.K.; Wood, D.; Johnson, M.; Ajlan, A.M.; Wijesinghe, N.; Webb, J.G. Multidetector Computed Tomography in Transcatheter Aortic Valve Implantation. JACC Cardiovasc. Imaging 2011, 4, 416–429. [Google Scholar] [CrossRef]
- Holmes, D.R.; Mack, M.J.; Kaul, S.; Agnihotri, A.; Alexander, K.P.; Bailey, S.R.; Calhoon, J.H.; Carabello, B.A.; Desai, M.Y.; Edwards, F.H.; et al. 2012 ACCF/AATS/SCAI/STS Expert Consensus Document on Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2012, 59, 1200–1254. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- European Society of Radiology (ESR). White paper on radiation protection by the European Society of Radiology. Insights Imaging 2011, 2, 357–362. [Google Scholar] [CrossRef]
- Kalra, M.K.; Maher, M.M.; Sahani, D.V.; Blake, M.A.; Hahn, P.F.; Avinash, G.B.; Toth, T.L.; Halpern, E.; Saini, S. Low-Dose CT of the Abdomen: Evaluation of Image Improvement with Use of Noise Reduction Filters—Pilot Study. Radiology 2003, 228, 251–256. [Google Scholar] [CrossRef]
- Diel, J.; Perlmutter, S.; Venkataramanan, N.; Mueller, R.; Lane, M.J.; Katz, D.S. Unenhanced Helical CT Using Increased Pitch for Suspected Renal Colic: An Effective Technique for Radiation Dose Reduction? J. Comput. Assist. Tomogr. 2000, 24, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Sagara, Y.; Hara, A.K.; Pavlicek, W.; Silva, A.C.; Paden, R.G.; Wu, Q. Abdominal CT: Comparison of Low-Dose CT With Adaptive Statistical Iterative Reconstruction and Routine-Dose CT With Filtered Back Projection in 53 Patients. Am. J. Roentgenol. 2010, 195, 713–719. [Google Scholar] [CrossRef]
- Nakaura, T.; Nakamura, S.; Maruyama, N.; Funama, Y.; Awai, K.; Harada, K.; Uemura, S.; Yamashita, Y. Low Contrast Agent and Radiation Dose Protocol for Hepatic Dynamic CT of Thin Adults at 256–Detector Row CT: Effect of Low Tube Voltage and Hybrid Iterative Reconstruction Algorithm on Image Quality. Radiology 2012, 264, 445–454. [Google Scholar] [CrossRef]
- Lee, S.; Shima, A.; Singh, S.; Kalra, M.K.; Kim, H.J.; Do, S. Co-registered image quality comparison in hybrid iterative reconstruction techniques: SAFIRE and SafeCT. In Medical Imaging 2013: Physics of Medical Imaging; SPIE: Cergy-Pontoise, France, 2013; pp. 931–936. [Google Scholar]
- Ippolito, D.; Maino, C.; Riva, L.; Pecorelli, A.; DE Vito, A.; Lombardi, S.; Ragusi, M.; Giandola, T.; Franzesi, C.T.; Sironi, S. Iterative model-based CT reconstruction algorithm: The background and added clinical value. J. Radiol. Rev. 2020, 7, 185–195. [Google Scholar] [CrossRef]
- Liu, L. Model-based Iterative Reconstruction: A Promising Algorithm for Today’s Computed Tomography Imaging. J. Med. Imaging Radiat. Sci. 2014, 45, 131–136. [Google Scholar] [CrossRef]
- Available online: https://www.esur.org/esur-guidelines-on-contrast-agents/ (accessed on 24 November 2023).
- Alkadhi, H.; Schindera, S.T. State of the art low-dose CT angiography of the body. Eur. J. Radiol. 2011, 80, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Leschka, S.; Stolzmann, P.; Schmid, F.T.; Scheffel, H.; Stinn, B.; Marincek, B.; Alkadhi, H.; Wildermuth, S. Low kilovoltage cardiac dual-source CT: Attenuation, noise, and radiation dose. EurRadiol 2008, 18, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Nakaura, T.; Awai, K.; Maruyama, N.; Takata, N.; Yoshinaka, I.; Harada, K.; Uemura, S.; Yamashita, Y. Abdominal Dynamic CT in Patients with Renal Dysfunction: Contrast Agent Dose Reduction with Low Tube Voltage and High Tube Current–Time Product Settings at 256–Detector Row CT. Radiology 2011, 261, 467–476. [Google Scholar] [CrossRef]
- Schindera, S.T.; Graca, P.B.; Patak, M.A.; Abderhalden, S.; von Allmen, G.R.; Vock, P.; Szucs-Farkas, Z. Thoracoabdominal-aortoiliac multidetector-row CT angiography at 80 and 100 kVp: Assessment of image quality and radiation dose. Invest. Radiol. 2009, 44, 650–655. [Google Scholar] [CrossRef]
- Tepel, M.; Aspelin, P.; Lameire, N. Contrast-Induced Nephropathy. Circulation 2006, 113, 1799–1806. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, J.; Oh, C.; Han, K.H.; Kim, T.H. The feasibility of sub-millisievert coronary CT angiography with low tube voltage, prospective ECG gating, and a knowledge-based iterative model reconstruction algorithm. Int. J. Cardiovasc. Imaging 2015, 31, 197–203. [Google Scholar] [CrossRef]
- Deak, P.D.; Smal, Y.; Kalender, W.A. Multisection CT protocols: Sex- and age-specific conversion factors used to determine effective dose from dose-length product. Radiology 2010, 257, 158–166. [Google Scholar] [CrossRef]
- Trattner, S.; Halliburton, S.; Thompson, C.M.; Xu, Y.; Chelliah, A.; Jambawalikar, S.R.; Peng, B.; Peters, M.R.; Jacobs, J.E.; Ghesani, M.; et al. Cardiac-Specific Conversion Factors to Estimate Radiation Effective Dose from Dose-Length Product in Computed Tomography. JACC Cardiovasc. Imaging 2018, 11, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Goetti, R.P.; Baumüller, S.; Feuchtner, G.; Stolzmann, P.; Karlo, C.; Alkadhi, H.; Leschka, S. High-pitch dual-source CT angiography of the thoracic and abdominal aorta: Is simultaneous coronary artery assessment possible? AJR Am. J. Roentgenol. 2010, 194, 938–944. [Google Scholar] [CrossRef]
- Komatsu, S.; Kamata, T.; Imai, A.; Ohara, T.; Takewa, M.; Ohe, R.; Miyaji, K.; Yoshida, J.; Kodama, K. Coronary computed tomography angiography using ultra-low-dose contrast media: Radiation dose and image quality. Int. J. Cardiovasc. Imaging 2013, 29, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.-X.; Wang, Y.-M.; Lu, J.-G.; Zhang, Y.; Wang, P.; Yang, C. Radiation and contrast agent doses reductions by using 80-kV tube voltage in coronary computed tomographic angiography: A comparative study. Eur. J. Radiol. 2014, 83, 309–314. [Google Scholar] [CrossRef]
- Feuchtner, G.M.; Jodocy, D.; Klauser, A.; Haberfellner, B.; Aglan, I.; Spoeck, A.; Hiehs, S.; Soegner, P.; Jaschke, W. Radiation dose reduction by using 100-kV tube voltage in cardiac 64-slice computed tomography: A comparative study. Eur. J. Radiol. 2010, 75, e51–e56. [Google Scholar] [CrossRef]
- Viteri-Ramírez, G.; García-Lallana, A.; Simón-Yarza, I.; Broncano, J.; Ferreira, M.; Pueyo, J.; Villanueva, A.; Bastarrika, G. Low radiation and low-contrast dose pulmonary CT angiography: Comparison of 80 kVp/60 mL and 100 kVp/80 mL protocols. Clin. Radiol. 2012, 67, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, P.; Priya, S.; Eskandari, A.; Mullan, A.; Aggarwal, T.; Narayanasamy, S.; Parashar, K.; Bhat, A.P.; Sieren, J.C. Factors Affecting Radiation Dose in Computed Tomography Angiograms for Pulmonary Embolism: A Retrospective Cohort Study. J. Clin. Imaging Sci. 2020, 10, 74. [Google Scholar] [CrossRef]
- Montet, X.; Hachulla, A.-L.; Neroladaki, A.; Lador, F.; Rochat, T.; Botsikas, D.; Becker, C.D. Image quality of low mA CT pulmonary angiography reconstructed with model based iterative reconstruction versus standard CT pulmonary angiography reconstructed with filtered back projection: An equivalency trial. Eur. Radiol. 2015, 25, 1665–1671. [Google Scholar] [CrossRef]
- Sauter, A.; Koehler, T.; Brendel, B.; Aichele, J.; Neumann, J.; Noël, P.B.; Rummeny, E.J.; Muenzel, D. CT pulmonary angiography: Dose reduction via a next generation iterative reconstruction algorithm. Acta Radiol. 2019, 60, 478–487. [Google Scholar] [CrossRef]
- Franzesi, C.R.T.; Ippolito, D.; Riva, L.; Fior, D.; Cangiotti, C.; Sironi, S. Diagnostic value of iterative reconstruction algorithm in low kV CT angiography (CTA) with low contrast medium volume for transcatheter aortic valve implantation (TAVI) planning: Image quality and radiation dose exposure. Br. J. Radiol. 2018, 91, 20170802. [Google Scholar] [CrossRef]
- Onoda, H.; Ueno, H.; Hashimoto, M.; Kuwahara, H.; Sobajima, M.; Kinugawa, K. Clinical Advantages of Using Low Tube Voltage in Third-Generation 192-Slice Dual-Source Computed Tomographic Angiography Before Transcatheter Aortic Valve Implantation. Int. Heart J. 2019, 60, 1091–1097. [Google Scholar] [CrossRef]
- Gallo, G.S.; Gerasia, R.; Caruso, C.; Tafaro, C.; Iannazzo, E.; Cannataci, C.; Gentile, G.; Mamone, G.; Gandolfo, C.; Miraglia, R. Feasibility of combined ECG-Gated and Helical acquisition mode in a pre-TAVI computed tomography angiography protocol using a fixed low-volume contrast medium injection. Eur. J. Radiol. 2020, 131, 109239. [Google Scholar] [CrossRef] [PubMed]
- Löve, A.; Olsson, M.-L.; Siemund, R.; Stålhammar, F.; Björkman-Burtscher, I.M.; Söderberg, M. Six iterative reconstruction algorithms in brain CT: A phantom study on image quality at different radiation dose levels. BJR 2013, 86, 20130388. [Google Scholar] [CrossRef] [PubMed]
- De Vito, A.; Maino, C.; Lombardi, S.; Ragusi, M.; Franzesi, C.T.; Ippolito, D.; Sironi, S. Model-based reconstruction algorithm in the detection of acute trauma-related lesions in brain CT examinations. Neuroradiol. J. 2021, 34, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, S.; Riva, L.; Patassini, M.; Remida, P.; Capraro, C.; Canonico, F.; Franzesi, C.T.; Ippolito, D. “Hyperdense artery sign” in early ischemic stroke: Diagnostic value of model-based reconstruction approach in comparison with standard hybrid iterative reconstruction algorithm. Neuroradiology 2018, 60, 1273–1280. [Google Scholar] [CrossRef]
- Hata, A.; Yanagawa, M.; Yoshida, Y.; Miyata, T.; Tsubamoto, M.; Honda, O.; Tomiyama, N. Combination of Deep Learning-Based Denoising and Iterative Reconstruction for Ultra-Low-Dose CT of the Chest: Image Quality and Lung-RADS Evaluation. AJR Am. J. Roentgenol. 2020, 215, 1321–1328. [Google Scholar] [CrossRef]
- Shuman, W.P.; Chan, K.T.; Busey, J.M.; Mitsumori, L.M.; Choi, E.; Koprowicz, K.M.; Kanal, K.M. Standard and reduced radiation dose liver CT images: Adaptive statistical iterative reconstruction versus model-based iterative reconstruction-comparison of findings and image quality. Radiology 2014, 273, 793–800. [Google Scholar] [CrossRef]
- Chang, W.; Lee, J.M.; Lee, K.; Yoon, J.H.; Yu, M.H.; Han, J.K.; Choi, B.I. Assessment of a model-based, iterative reconstruction algorithm (MBIR) regarding image quality and dose reduction in liver computed tomography. Investig. Radiol. 2013, 48, 598–606. [Google Scholar] [CrossRef]
- Sun, J.; Yang, L.; Zhou, Z.; Zhang, D.; Han, W.; Zhang, Q.; Peng, Y. Performance evaluation of two iterative reconstruction algorithms, MBIR and ASIR, in low radiation dose and low contrast dose abdominal CT in children. Radiol. Med. 2020, 125, 918–925. [Google Scholar] [CrossRef]
- Aschoff, A.J.; Catalano, C.; A Kirchin, M.; Krix, M.; Albrecht, T. Low radiation dose in computed tomography: The role of iodine. Br. J. Radiol. 2017, 90, 20170079. [Google Scholar] [CrossRef]
- Davenport, M.S.; Perazella, M.A.; Yee, J.; Dillman, J.R.; Fine, D.; McDonald, R.J.; Rodby, R.A.; Wang, C.L.; Weinreb, J.C. Use of Intravenous Iodinated Contrast Media in Patients with Kidney Disease: Consensus Statements from the American College of Radiology and the National Kidney Foundation. Radiology 2020, 294, 660–668. [Google Scholar] [CrossRef]
- Warty, V.S.; Calvo, W.J.; Berceli, S.A.; Pham, S.M.; Durham, S.J.; Tanksale, S.K.; Klein, E.C.; Herman, I.M.; Borovetz, H.S. Hemodynamics alter arterial low-density lipoprotein metabolism. J. Vasc. Surg. 1989, 10, 392–399. [Google Scholar] [CrossRef][Green Version]
- Károlyi, M.; Szilveszter, B.; Kolossváry, M.; Takx, R.A.; Celeng, C.; Bartykowszki, A.; Jermendy, L.; Panajotu, A.; Karády, J.; Raaijmakers, R.; et al. Iterative model reconstruction reduces calcified plaque volume in coronary CT angiography. Eur. J. Radiol. 2017, 87, 83–89. [Google Scholar] [CrossRef]
- Tsukada, J.; Yamada, M.; Yamada, Y.; Yamazaki, S.; Imanishi, N.; Tamura, K.; Hashimoto, M.; Nakatsuka, S.; Jinzaki, M. Comparison of the diagnostic accuracy of FBP, ASiR, and MBIR reconstruction during CT angiography in the evaluation of a vessel phantom with calcified stenosis in a distal superficial femoral artery in a cadaver extremity. Medicine 2016, 95, e4127. [Google Scholar] [CrossRef] [PubMed]
- Mileto, A.; Guimaraes, L.S.; McCollough, C.H.; Fletcher, J.G.; Yu, L. State of the Art in Abdominal CT: The Limits of Iterative Reconstruction Algorithms. Radiology 2019, 293, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Barras, H.; Dunet, V.; Hachulla, A.-L.; Grimm, J.; Beigelman-Aubry, C. Influence of model based iterative reconstruction algorithm on image quality of multiplanar reformations in reduced dose chest CT. Acta Radiol. Open 2016, 5, 205846011666229. [Google Scholar] [CrossRef]


| CT Scan Parameters | CCTA | CTPA | Pre-TAVR CTA | |||
|---|---|---|---|---|---|---|
| Study Group | Control Group | Study Group | Control Group | Study Group | Control Group | |
| Tube voltage (kV) | 80 | 100 | 80 | 100 | 80 | 100 |
| Tube current (mAs) | Automated | Automated | Automated | Automated | Automated | Automated |
| Gantry rotation time (s) | 0.27 | 0.27 | 0.27 | 0.27 | 0.33 | 0.33 |
| Slices | 256 | 256 | 256 | 256 | 256 | 256 |
| Matrix | 512 × 512 | 512 × 512 | 512 × 512 | 512 × 512 | 512 × 512 | 512 × 512 |
| Pitch | Prospective gating | Prospective gating | 0.17 | 0.17 | 0.30 | 0.30 |
| FOV (mm) | 250 | 250 | 350 | 350 | 350 | 350 |
| Thickness/increment (mm) | 0.67/0.34 | 1/1 | 0.8/0.4 | 1/0.5 | 0.8/0.4 | 1.0/1.0 |
| CM | Iobiditrol 350 | Iobiditrol 350 | Iobiditrol 350 | Iobiditrol 350 | Iobiditrol 350 | Iobiditrol 350 |
| CM volume (mL)/flow rate (mL/s) | 60/4.5 | 60/4.5 | 50/3.5 | 50/3.5 | 60/3.5 | 60/3.5 |
| Saline volume (mL)/flow rate (mL/s) | 40/4.5 | 40/4.5 | 50/3.5 | 50/3.5 | 50/3.5 | 50/3.5 |
| Reconstruction algorithm | MBIR | HIR | MBIR | HIR | MBIR | HIR |
| N = 401 | CCTA | CTPA | Pre-TAVR CTA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Study Group (n = 65) | Control Group (n = 61) | p-Value | Study Group (n = 83) | Control Group (n = 87) | p-Value | Study Group (n = 54) | Control Group (n = 51) | p-Value | |
| M/F (n) | 35/30 | 33/28 | >0.05 | 42/41 | 42/45 | >0.05 | 30/24 | 23/28 | >0.05 |
| Age (yo, mean ± SD) | 67.4 ± 12.5 | 62.86 ± 10.46 | >0.05 | 64.45 ±13.44 | 65.24 ± 12.47 | >0.05 | 72.55 ± 13.44 | 73.88 ± 13.15 | >0.05 |
| BMI (kg/m2, mean ± SD) | 26.3 ± 3.87 | 26.8 ± 4.95, | >0.05 | 23.7 ± 2.1 | 22.9 ± 1.5 | >0.05 | 24.1 ± 2.6 | 25.66 ± 4.33 | >0.05 |
| Heart rate (bpm, mean ± SD) | 62.7 ± 5.29 | 61.5 ± 8.2 | >0.05 | - | - | 75.3 ± 7.66 (n = 4 β-blocked) | 76.2 ± 9.1 (n = 3 β-blocked) | >0.05 | |
| Arterial Level | Study Group HU | Control Group HU | p-Value | Study Group SNR | Control Group SNR | p-Value | Study Group CNR | Control Group CNR | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| CCTA | LV | 591.97 ± 121.8 | 519.76 ± 117.52 | 0.001 | 15.77 ± 3.54 | 11.14 ± 5.33 | <0.001 | 19.61 ± 6.57 | 14.65 ± 8.44 | <0.001 |
| Aorta | 624.58 ± 117.8 | 489.33 ± 101.45 | <0.0001 | 16.73 ± 4.11 | 9.85 ± 7.15 | <0.0001 | 21.11 ± 6.9 | 13.77 ± 8.39 | <0.0001 | |
| LAD-prox | 619.77 ± 99.31 | 475.17 ± 105.21 | <0.0001 | 13.45 ± 6.71 | 7.79 ± 5.93 | <0.0001 | 20.28 ± 9.6 | 12.87 ± 10.4 | <0.0001 | |
| LCx-prox | 626.30 ± 82.54 | 434.89 ± 94.3 | <0.0001 | 13.84 ± 7.24 | 7.55 ± 6.24 | <0.0001 | 21.05 ± 7.61 | 10.85 ± 8.52 | <0.0001 | |
| RCA-prox | 618.53 ± 109.3 | 445.7 ± 78.56 | <0.0001 | 14.68 ± 7.25 | 8.22 ± 6.13 | <0.0001 | 22.36 ± 8.8 | 11.42 ± 9.19 | <0.0001 | |
| CTPA | MPA | 697.91 ± 10.76 | 334.87 ± 23.3 | <0.0001 | 62.14 ± 17.3 | 20.47 ± 13.5 | <0.0001 | 60.7 ± 19.45 | 10.77 ± 5.76 | <0.0001 |
| LPA | 644.61 ± 11.88 | 302.89 ± 19.45 | <0.0001 | 59.88 ± 18.2 | 19.5 ± 9.87 | <0.0001 | 57.43 ± 15.2 | 13.98 ± 6.32 | <0.0001 | |
| RPA | 651.43 ± 12.17 | 318.31 ± 26.84 | <0.0001 | 55.16 ± 16.1 | 22.54 ± 10.7 | <0.0001 | 54.6 ± 13.33 | 14.6 ± 8.69 | <0.0001 | |
| Pre-TAVR CTA | Aortic arch | 533.60 ± 79.92 | 379.66 ± 23.28 | <0.0001 | 24.46 ± 7.59 | 16.23 ± 6.54 | <0.0001 | 27.29 ± 8.70 | 13.77 ± 4.82 | <0.0001 |
| Aorta at renal a. | 523.9 ± 82.7 | 345.7 ± 31.55 | <0.0001 | 21.30 ± 5.88 | 13.69 ± 4.23 | <0.0001 | 26.70 ± 7.96 | 15.02 ± 7.21 | <0.0001 |
| CCTA | CTPA | Pre-TAVR CTA | ||||
|---|---|---|---|---|---|---|
| Data/Protocol | Study Group | Control Group | Study Group | Control Group | Study Group | Control Group |
| CDTIvol (mGy) | 4.32 ± 1.46 | 10.33 ± 1.75 | 5.92 ± 1.09 | 9.82 ± 3.67 | 8.59 ± 3.28 | 27.33 ± 5.89 |
| DLP (mGy∙cm) | 63.90 ± 32.51 | 147.9 ± 33.41 | 211.82 ± 31.95 | 355.56 ± 3.51 | 588.15 ± 223.87 | 1600.1 ± 339.2 |
| ED (mSv) | 1.66 ± 0.85 | 3.75 ± 1.26 | 3.09 ± 0.46 | 5.19 ± 1.79 | 10.00 ± 3.81 | 23.36 ± 4.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ippolito, D.; Porta, M.; Maino, C.; Riva, L.; Ragusi, M.; Giandola, T.; Franco, P.N.; Cangiotti, C.; Gandola, D.; De Vito, A.; et al. Feasibility of Low-Dose and Low-Contrast Media Volume Approach in Computed Tomography Cardiovascular Imaging Reconstructed with Model-Based Algorithm. Tomography 2024, 10, 286-298. https://doi.org/10.3390/tomography10020023
Ippolito D, Porta M, Maino C, Riva L, Ragusi M, Giandola T, Franco PN, Cangiotti C, Gandola D, De Vito A, et al. Feasibility of Low-Dose and Low-Contrast Media Volume Approach in Computed Tomography Cardiovascular Imaging Reconstructed with Model-Based Algorithm. Tomography. 2024; 10(2):286-298. https://doi.org/10.3390/tomography10020023
Chicago/Turabian StyleIppolito, Davide, Marco Porta, Cesare Maino, Luca Riva, Maria Ragusi, Teresa Giandola, Paolo Niccolò Franco, Cecilia Cangiotti, Davide Gandola, Andrea De Vito, and et al. 2024. "Feasibility of Low-Dose and Low-Contrast Media Volume Approach in Computed Tomography Cardiovascular Imaging Reconstructed with Model-Based Algorithm" Tomography 10, no. 2: 286-298. https://doi.org/10.3390/tomography10020023
APA StyleIppolito, D., Porta, M., Maino, C., Riva, L., Ragusi, M., Giandola, T., Franco, P. N., Cangiotti, C., Gandola, D., De Vito, A., Talei Franzesi, C., & Corso, R. (2024). Feasibility of Low-Dose and Low-Contrast Media Volume Approach in Computed Tomography Cardiovascular Imaging Reconstructed with Model-Based Algorithm. Tomography, 10(2), 286-298. https://doi.org/10.3390/tomography10020023






