The Correlation between the Elastic Modulus of the Achilles Tendon Enthesis and Bone Microstructure in the Calcaneal Crescent
Abstract
1. Introduction
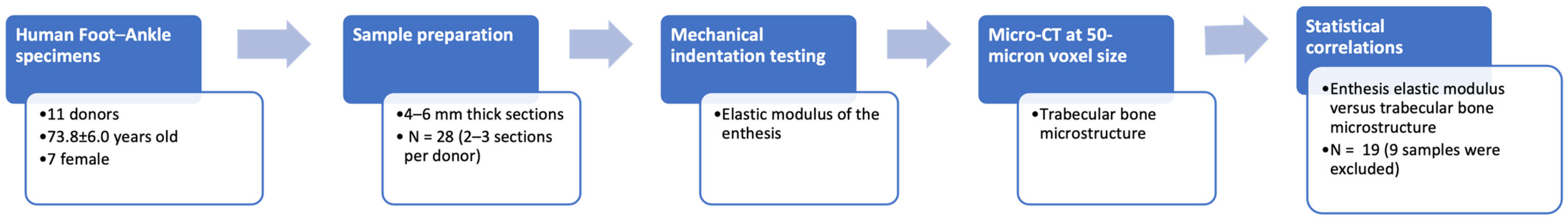
2. Materials and Methods
2.1. Sample Preparation
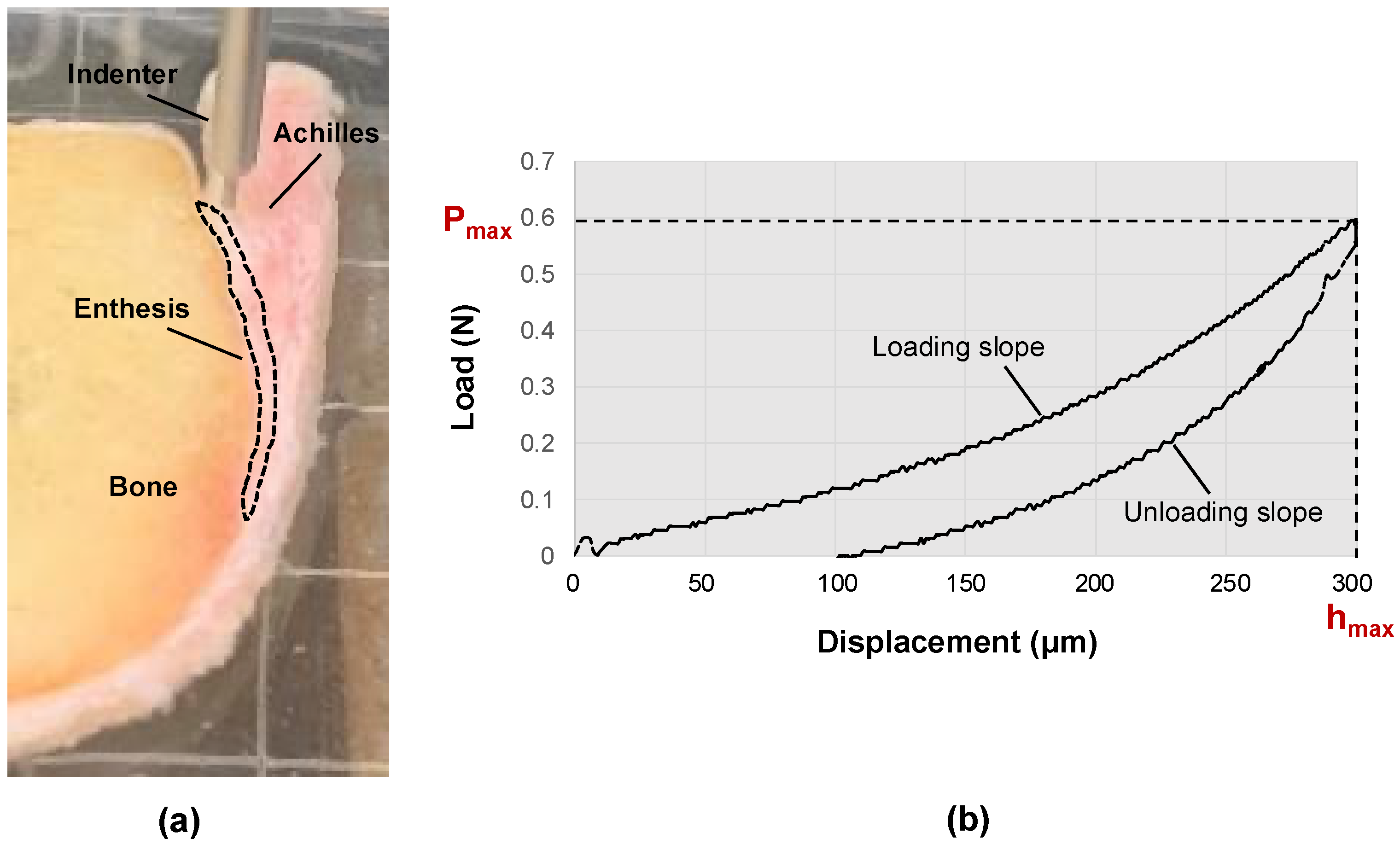
2.2. Mechanical Indentation Testing
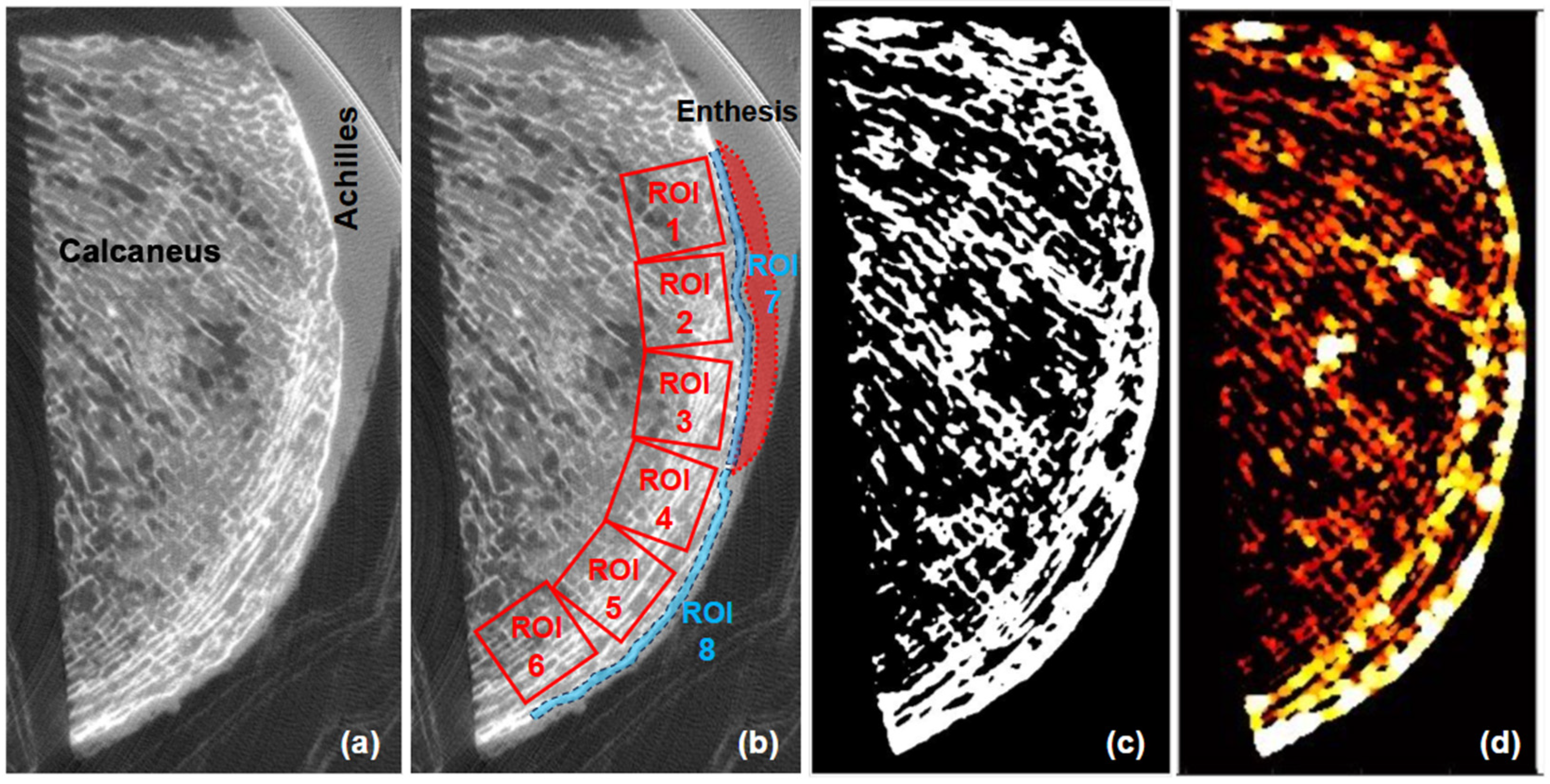
2.3. Micro-Computed Tomography (Micro-CT) and Trabecular Microstructure
2.4. Statistical Correlations
3. Results
3.1. Bone Microstructure and CT Values
3.2. Correlation between Achilles Elastic Modulus and Bone Microstructure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, M.; Ralphs, J.R. Fibrocartilage in tendons and ligaments--an adaptation to compressive load. J. Anat. 1998, 193 Pt 4, 481–494. [Google Scholar] [CrossRef]
- Benjamin, M.; McGonagle, D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J. Anat. 2001, 199 Pt 5, 503–526. [Google Scholar] [CrossRef]
- Rossetti, L.; Kuntz, L.A.; Kunold, E.; Schock, J.; Müller, K.W.; Grabmayr, H.; Stolberg-Stolberg, J.; Pfeiffer, F.; Sieber, S.A.; Burgkart, R.; et al. The microstructure and micromechanics of the tendon-bone insertion. Nat. Mater. 2017, 16, 664–670. [Google Scholar] [CrossRef]
- Hu, Y.; Birman, V.; Deymier-Black, A.; Schwartz, A.G.; Thomopoulos, S.; Genin, G.M. Stochastic interdigitation as a toughening mechanism at the interface between tendon and bone. Biophys. J. 2015, 108, 431–437. [Google Scholar] [CrossRef]
- Deymier, A.C.; An, Y.; Boyle, J.J.; Schwartz, A.G.; Birman, V.; Genin, G.M.; Thomopoulos, S.; Barber, A.H. Micro-mechanical properties of the tendon-to-bone attachment. Acta Biomater. 2017, 56, 25–35. [Google Scholar] [CrossRef]
- Moffat, K.L.; Sun, W.H.; Pena, P.E.; Chahine, N.O.; Doty, S.B.; Ateshian, G.A.; Hung, C.T.; Lu, H.H. Characterization of the structure-function relationship at the ligament-to-bone interface. Proc. Natl. Acad. Sci. USA 2008, 105, 7947–7952. [Google Scholar] [CrossRef]
- Genin, G.M.; Kent, A.; Birman, V.; Wopenka, B.; Pasteris, J.D.; Marquez, P.J.; Thomopoulos, S. Functional grading of mineral and collagen in the attachment of tendon to bone. Biophys. J. 2009, 97, 976–985. [Google Scholar] [CrossRef]
- Sevick, J.L.; Abusara, Z.; Andrews, S.H.; Xu, M.; Khurshid, S.; Chatha, J.; Hart, D.A.; Shrive, N.G. Fibril deformation under load of the rabbit Achilles tendon and medial collateral ligament femoral entheses. J. Orthop. Res. 2018, 36, 2506–2515. [Google Scholar] [CrossRef]
- Milz, S.; Rufai, A.; Buettner, A.; Putz, R.; Ralphs, J.R.; Benjamin, M. Three-dimensional reconstructions of the Achilles tendon insertion in man. J. Anat. 2002, 200 Pt 2, 145–152. [Google Scholar] [CrossRef]
- Benjamin, M.; Toumi, H.; Ralphs, J.R.; Bydder, G.; Best, T.M.; Milz, S. Where tendons and ligaments meet bone: Attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J. Anat. 2006, 208, 471–490. [Google Scholar] [CrossRef]
- Freedman, B.R.; Gordon, J.A.; Soslowsky, L.J. The Achilles tendon: Fundamental properties and mechanisms governing healing. Muscles Ligaments Tendons J. 2014, 4, 245–255. [Google Scholar] [CrossRef]
- Cook, J.L.; Purdam, C.R. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br. J. Sports Med. 2009, 43, 409–416. [Google Scholar] [CrossRef]
- Sundararajan, P.P.; Wilde, T.S. Radiographic, clinical, and magnetic resonance imaging analysis of insertional Achilles tendinopathy. J. Foot Ankle Surg. 2014, 53, 147–151. [Google Scholar] [CrossRef]
- Lukas, C.; Ruffoni, D.; Lambers, F.M.; Schulte, F.A.; Kuhn, G.; Kollmannsberger, P.; Weinkamer, R.; Müller, R. Mineralization kinetics in murine trabecular bone quantified by time-lapsed in vivo micro-computed tomography. Bone 2013, 56, 55–60. [Google Scholar] [CrossRef]
- Galatz, L.M.; Ball, C.M.; Teefey, S.A.; Middleton, W.D.; Yamaguchi, K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J. Bone Jt. Surg. Am. 2004, 86, 219–224. [Google Scholar] [CrossRef]
- Schulte, F.A.; Ruffoni, D.; Lambers, F.M.; Christen, D.; Webster, D.J.; Kuhn, G.; Müller, R. Local mechanical stimuli regulate bone formation and resorption in mice at the tissue level. PLoS ONE 2013, 8, e62172. [Google Scholar] [CrossRef]
- Christen, P.; Ito, K.; Ellouz, R.; Boutroy, S.; Sornay-Rendu, E.; Chapurlat, R.D.; van Rietbergen, B. Bone remodelling in humans is load-driven but not lazy. Nat. Commun. 2014, 5, 4855. [Google Scholar] [CrossRef]
- Cheong, V.S.; Roberts, B.C.; Kadirkamanathan, V.; Dall’Ara, E. Bone remodelling in the mouse tibia is spatio-temporally modulated by oestrogen deficiency and external mechanical loading: A combined in vivo/in silico study. Acta Biomater. 2020, 116, 302–317. [Google Scholar] [CrossRef]
- Tits, A.; Ruffoni, D. Joining soft tissues to bone: Insights from modeling and simulations. Bone Rep. 2020, 14, 100742. [Google Scholar] [CrossRef]
- Mullender, M.G.; Huiskes, R. Proposal for the regulatory mechanism of Wolff’s law. J. Orthop. Res. 1995, 13, 503–512. [Google Scholar] [CrossRef]
- Ruff, C.; Holt, B.; Trinkaus, E. Who’s afraid of the big bad Wolff?: “Wolff’s law” and bone functional adaptation. Am. J. Phys. Anthropol. 2006, 129, 484–498. [Google Scholar] [CrossRef]
- Tits, A.; Plougonven, E.; Blouin, S.; Hartmann, M.A.; Kaux, J.F.; Drion, P.; Fernandez, J.; van Lenthe, G.H.; Ruffoni, D. Local anisotropy in mineralized fibrocartilage and subchondral bone beneath the tendon-bone interface. Sci. Rep. 2021, 11, 16534. [Google Scholar] [CrossRef]
- Ficek, K.; Filipek, J.; Ficek, J.; Muzalewska, M.; Humpa, F. Calcaneal CT is a useful tool for identifying Achilles tendon disorders: A pilot study. J. Orthop. Surg. Res. 2017, 12, 139. [Google Scholar] [CrossRef]
- Finkenstaedt, T.; Siriwanarangsun, P.; Statum, S.; Biswas, R.; Anderson, K.E.; Bae, W.C.; Chung, C.B. The Calcaneal Crescent in Patients With and Without Plantar Fasciitis: An Ankle MRI Study. AJR Am. J. Roentgenol. 2018, 211, 1075–1082. [Google Scholar] [CrossRef]
- Frost, H.M. Wolff’s Law and bone’s structural adaptations to mechanical usage: An overview for clinicians. Angle Orthod. 1994, 64, 175–188. [Google Scholar]
- Jerban, S.; Afsahi, A.M.; Ma, Y.; Moazamian, D.; Statum, S.; Lombardi, A.F.; Kakos, L.; Dorthe, E.; Dlima, D.; Du, J.; et al. Correlations between elastic modulus and ultrashort echo time (UTE) adiabatic T1ρ relaxation time (UTE-Adiab-T1ρ) in Achilles tendons and entheses. J. Biomech. 2023, 160, 111825. [Google Scholar] [CrossRef]
- Hayes, W.C.; Keer, L.M.; Herrmann, G.; Mockros, L.F. A mathematical analysis for indentation tests of articular cartilage. J. Biomech. 1972, 5, 541–551. [Google Scholar] [CrossRef]
- Wren, T.A.; Yerby, S.A.; Beaupré, G.S.; Carter, D.R. Influence of bone mineral density, age, and strain rate on the failure mode of human Achilles tendons. Clin. Biomech. 2001, 16, 529–534. [Google Scholar] [CrossRef]
- Hart, N.H.; Nimphius, S.; Rantalainen, T.; Ireland, A.; Siafarikas, A.; Newton, R.U. Mechanical basis of bone strength: Influence of bone material, bone structure and muscle action. J. Musculoskelet. Neuronal Interact. 2017, 17, 114–139. [Google Scholar]
- McGonagle, D.; Wakefield, R.J.; Tan, A.L.; D’Agostino, M.A.; Toumi, H.; Hayashi, K.; Emery, P.; Benjamin, M. Distinct topography of erosion and new bone formation in achilles tendon enthesitis: Implications for understanding the link between inflammation and bone formation in spondylarthritis. Arthritis Rheum. 2008, 58, 2694–2699. [Google Scholar] [CrossRef]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R. Alterations in periarticular bone and cross talk between subchondral bone and articular cartilage in osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2012, 4, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Pušnik, L.; Lechner, L.; Serša, I.; Cvetko, E.; Haas, P.; Jengojan, S.A.; Snoj, Ž. 3D fascicular reconstruction of median and ulnar nerve: Initial experience and comparison between high-resolution ultrasound and MR microscopy. Eur. Radiol. Exp. 2024, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, T.; Verstraete, M.; De Roo, K.; De Waele, W.; Bellemans, J.; Victor, J. Digital image correlation as a tool for three-dimensional strain analysis in human tendon tissue. J. Exp. Orthop. 2014, 1, 7. [Google Scholar] [CrossRef]


| ROI | BV/TV (%) | Tb.Th (μm) | Bone Radiodensity (HU) | Tb.Sp (μm) |
|---|---|---|---|---|
| 1 | 46.6 ± 6.4 | 601 ± 198 | 362 ± 217 | 666 ± 76 |
| 2 | 48.4 ± 6.1 | 617 ± 98 | 397 ± 238 | 646 ± 76 |
| 3 | 46.2 ± 3.9 | 588 ± 101 | 415 ± 247 | 674 ± 83 |
| 4 | 47.1 ± 6.2 | 574 ± 90 | 403 ± 242 | 683 ± 170 |
| 5 | 42.5 ± 10.4 | 608 ± 106 | 366 ± 227 | 788 ± 281 |
| 6 | 43.1 ± 13.5 | 704 ± 165 | 360 ± 227 | 833 ± 252 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doi, K.; Moazamian, D.; Namiranian, B.; Statum, S.; Afsahi, A.M.; Yamamoto, T.; Cheng, K.Y.; Chung, C.B.; Jerban, S. The Correlation between the Elastic Modulus of the Achilles Tendon Enthesis and Bone Microstructure in the Calcaneal Crescent. Tomography 2024, 10, 1665-1675. https://doi.org/10.3390/tomography10100122
Doi K, Moazamian D, Namiranian B, Statum S, Afsahi AM, Yamamoto T, Cheng KY, Chung CB, Jerban S. The Correlation between the Elastic Modulus of the Achilles Tendon Enthesis and Bone Microstructure in the Calcaneal Crescent. Tomography. 2024; 10(10):1665-1675. https://doi.org/10.3390/tomography10100122
Chicago/Turabian StyleDoi, Kenichiro, Dina Moazamian, Behnam Namiranian, Sheronda Statum, Amir Masoud Afsahi, Takuaki Yamamoto, Karen Y. Cheng, Christine B. Chung, and Saeed Jerban. 2024. "The Correlation between the Elastic Modulus of the Achilles Tendon Enthesis and Bone Microstructure in the Calcaneal Crescent" Tomography 10, no. 10: 1665-1675. https://doi.org/10.3390/tomography10100122
APA StyleDoi, K., Moazamian, D., Namiranian, B., Statum, S., Afsahi, A. M., Yamamoto, T., Cheng, K. Y., Chung, C. B., & Jerban, S. (2024). The Correlation between the Elastic Modulus of the Achilles Tendon Enthesis and Bone Microstructure in the Calcaneal Crescent. Tomography, 10(10), 1665-1675. https://doi.org/10.3390/tomography10100122







