Optimal DaTQUANT Thresholds for Diagnostic Accuracy of Dementia with Lewy Bodies (DLB) and Parkinson’s Disease (PD)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
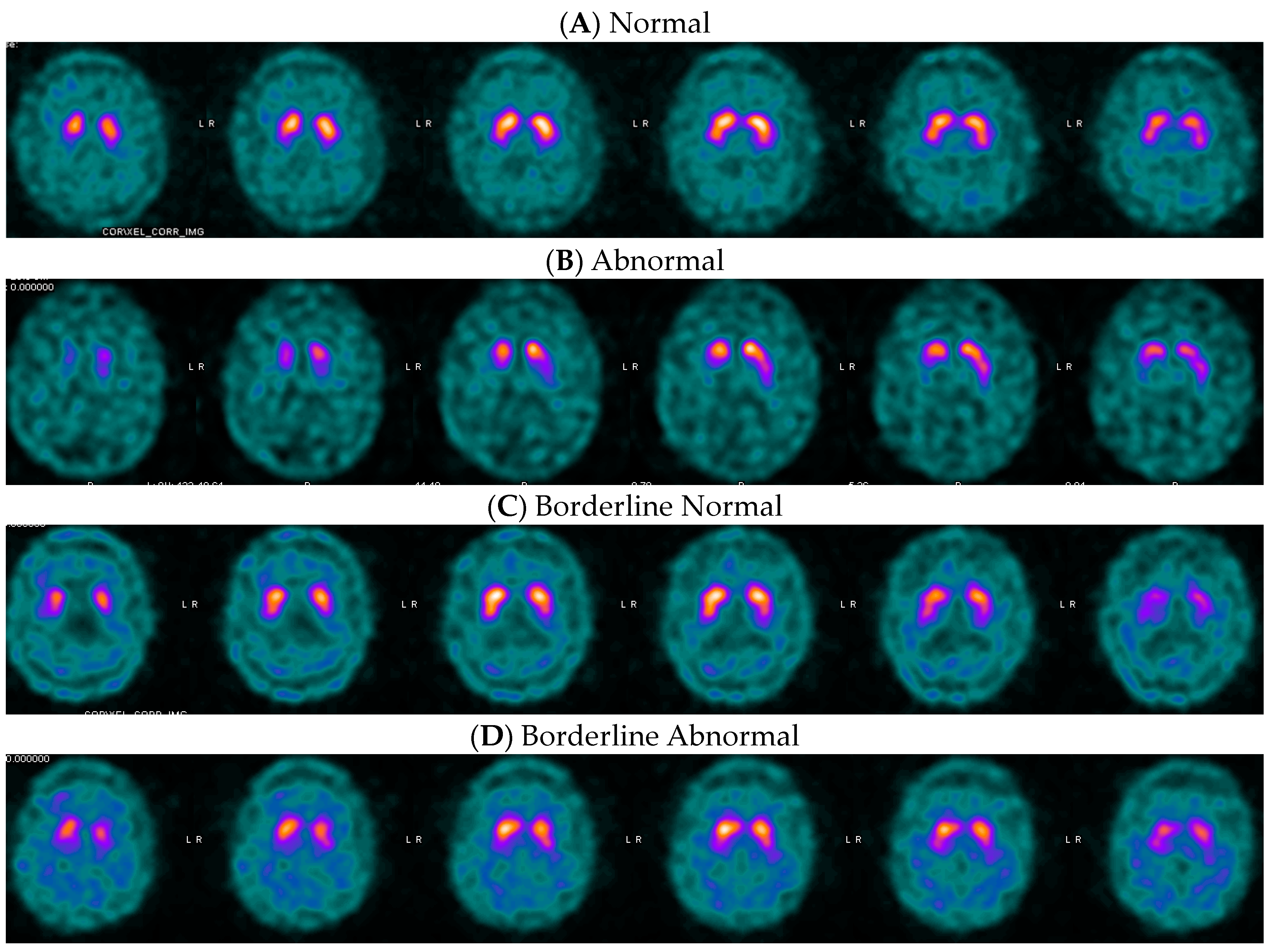
2.2. Quantification
2.3. Statistics
3. Results
3.1. Demographics
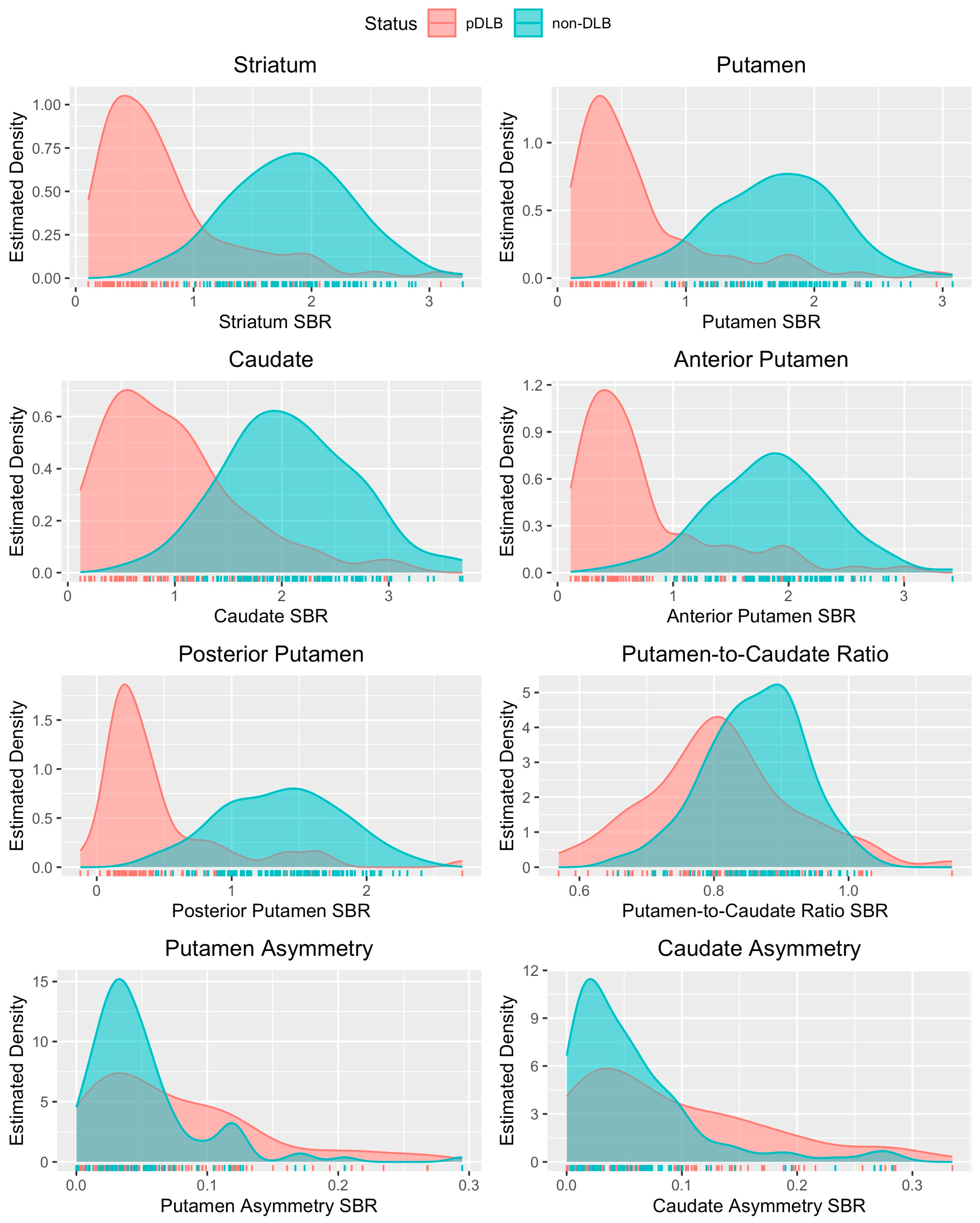
3.2. Putamen Distributions, Optimal Variables, and Thresholds for Dementia
3.3. Optimal Variable and Threshold for Movement Disorders
3.4. Comparison with Previously Published Thresholds
4. Discussion
4.1. Posterior Putamen as Optimal Single-Variable Model
4.2. Comparison with Previously Described Clinical DaTQUANT Thresholds
4.3. Clinical Implication of Differential Thresholds for DaT SPECT
4.4. Research Context, Future Perspectives, and Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bega, D.; Kuo, P.H.; Chalkidou, A.; Grzeda, M.T.; Macmillan, T.; Brand, C.; Sheikh, Z.H.; Antonini, A. Clinical utility of DaTscan in patients with suspected Parkinsonian syndrome: A systematic review and meta-analysis. npj Park. Dis. 2021, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Chouker, M.; Tatsch, K.; Linke, R.; Pogarell, O.; Hahn, K.; Schwarz, J. Striatal dopamine transporter binding in early to moderately advanced Parkinson’s disease: Monitoring of disease progression over 2 years. Nucl. Med. Commun. 2001, 22, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Colloby, S.J.; McParland, S.; O’Brien, J.T.; Attems, J. Neuropathological correlates of dopaminergic imaging in Alzheimer’s disease and Lewy body dementias. Brain 2012, 135, 2798–2808. [Google Scholar] [CrossRef]
- Perju-Dumbrava, L.D.; Kovacs, G.G.; Pirker, S.; Jellinger, K.; Hoffmann, M.; Asenbaum, S.; Pirker, W. Dopamine transporter imaging in autopsy-confirmed Parkinson’s disease and multiple system atrophy. Mov. Disord. 2012, 27, 65–71. [Google Scholar] [CrossRef]
- Seibyl, J.; Russell, D.; Jennings, D.; Marek, K. The molecular basis of dopaminergic brain imaging in Parkinson’s disease. Q. J. Nucl. Med. Mol. Imaging 2012, 56, 4–16. [Google Scholar]
- Tatsch, K. Imaging of the dopaminergic system in differential diagnosis of dementia. Eur. J. Nucl. Med. Mol. Imaging 2008, 35 (Suppl. S1), S51–S57. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, S.; Tinazzi, M.; Pasquin, I.; Nothdurfter, W.; Tomelleri, G.; Fincati, E.; Nordera, G.; Moretto, G.; Fiaschi, A.; Smania, N.; et al. Comparative analysis of visual and semi-quantitative assessment of striatal [123I]FP-CIT-SPET binding in Parkinson’s disease. Neurol. Sci. 2006, 27, 397–401. [Google Scholar] [CrossRef]
- Stokkel, M.P.; Dibbets-Schneider, P.; Koestering, E.; Dragoiescu, C.; Blokland, K.A. Reproducibility of a standardized quantitative analysis using fixed regions of interest to differentiate movement disorders on 123I-FP-CIT SPECT. J. Nucl. Med. Technol. 2007, 35, 21–26. [Google Scholar]
- Zubal, I.G.; Early, M.; Yuan, O.; Jennings, D.; Marek, K.; Seibyl, J.P. Optimized, automated striatal uptake analysis applied to SPECT brain scans of Parkinson’s disease patients. J. Nucl. Med. 2007, 48, 857–864. [Google Scholar] [CrossRef]
- Booij, J.; Dubroff, J.; Pryma, D.; Yu, J.; Agarwal, R.; Lakhani, P.; Kuo, P.H. Diagnostic Performance of the Visual Reading of (123)I-Ioflupane SPECT Images with or without Quantification in Patients with Movement Disorders or Dementia. J. Nucl. Med. 2017, 58, 1821–1826. [Google Scholar] [CrossRef]
- Kuo, P.H.; Avery, R.; Krupinski, E.; Lei, H.; Bauer, A.; Sherman, S.; McMillan, N.; Seibyl, J.; Zubal, G. Receiver-operating-characteristic analysis of an automated program for analyzing striatal uptake of 123I-ioflupane SPECT images: Calibration using visual reads. J. Nucl. Med. Technol. 2013, 41, 26–31. [Google Scholar] [CrossRef]
- Tinaz, S.; Chow, C.; Kuo, P.H.; Krupinski, E.A.; Blumenfeld, H.; Louis, E.D.; Zubal, G. Semiquantitative Analysis of Dopamine Transporter Scans in Patients with Parkinson Disease. Clin. Nucl. Med. 2018, 43, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Marcus, C.; Sheikhbahaei, S.; Solnes, L.B.; Leal, J.P.; Du, Y.; Rowe, S.P.; Higuchi, T.; Buck, A.K.; Lapa, C.; et al. Visual and Semiquantitative Accuracy in Clinical Baseline 123I-Ioflupane SPECT/CT Imaging. Clin. Nucl. Med. 2019, 44, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, R.M.; Frey, K.A.; Hunt, C.H.; Mercier, G.A., Jr.; Solnes, L.B.; Colletti, P.M.; Lu, Y.; Savir-Baruch, B.; Williams, H.T. ACR-ACNM Practice Parameter for the Performance of Dopamine Transporter (DaT) Single Photon Emission Computed Tomography (SPECT) Imaging for Movement Disorders. Clin. Nucl. Med. 2017, 42, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Neill, M.; Fisher, J.M.; Brand, C.; Lei, H.; Sherman, S.J.; Chou, Y.H.; Kuo, P.H. Practical Application of DaTQUANT with Optimal Threshold for Diagnostic Accuracy of Dopamine Transporter SPECT. Tomography 2021, 7, 980–989. [Google Scholar] [CrossRef]
- AlMahadin, G.; Lotfi, A.; Zysk, E.; Siena, F.L.; Carthy, M.M.; Breedon, P. Parkinson’s disease: Current assessment methods and wearable devices for evaluation of movement disorder motor symptoms—A patient and healthcare professional perspective. BMC Neurol. 2020, 20, 419. [Google Scholar] [CrossRef]
- Lanfranchi, F.; Arnaldi, D.; Miceli, A.; Mattioli, P.; D’Amico, F.; Raffa, S.; Donegani, M.I.; Chiola, S.; Massa, F.; Pardini, M.; et al. Different z-score cut-offs for striatal binding ratio (SBR) of DaT SPECT are needed to support the diagnosis of Parkinson’s Disease (PD) and dementia with Lewy bodies (DLB). Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1090–1102. [Google Scholar] [CrossRef]
- Jennings, D.; Siderowf, A.; Stern, M.; Seibyl, J.; Eberly, S.; Oakes, D.; Marek, K. Imaging prodromal Parkinson disease: The Parkinson Associated Risk Syndrome Study. Neurology 2014, 83, 1739–1746. [Google Scholar] [CrossRef]
- Brogley, J.E. DaTQUANT: The Future of Diagnosing Parkinson Disease. J. Nucl. Med. Technol. 2019, 47, 21–26. [Google Scholar] [CrossRef]
- Miyamoto, T.; Miyamoto, M.; Numahata, K.; Onoue, H.; Akaiwa, Y.; Sairenchi, T. Reduced dopamine transporter binding predicts early transition to Lewy body disease in Japanese patients with idiopathic rapid eye movement sleep behavior disorder. J. Neurol. Sci. 2020, 414, 116821. [Google Scholar] [CrossRef]
- Pencharz, D.R.; Hanlon, P.; Chakravartty, R.; Navalkissoor, S.; Quigley, A.M.; Wagner, T.; Wagner, T. Automated quantification with BRASS reduces equivocal reporting of DaTSCAN (123I-FP-CIT) SPECT studies. Nucl. Med. Rev. Cent. East. Eur. 2014, 17, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Steinkrüger, H.; Lange, C.; Apostolova, I.; Amthauer, H.; Lehnert, W.; Klutmann, S.; Buchert, R. Impact of the size of the normal database on the performance of the specific binding ratio in dopamine transporter SPECT. EJNMMI Phys. 2020, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, D.M.; Arkhipenko, A.; Galasko, D.; Goldman, J.G.; Sheikh, Z.H.; Petrides, G.; Toledo, J.B.; Galvin, J.E. Practical use of DAT SPECT imaging in diagnosing dementia with Lewy bodies: A US perspective of current guidelines and future directions. Front. Neurol. 2024, 15, 1395413. [Google Scholar] [CrossRef] [PubMed]
- GE HealthCare. DATSCAN (Ioflupane I 123 Injection) [Prescribing Information]. U.S. Food and Drug Administration Website. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/022454s010lbl.pdf (accessed on 11 June 2024).
- Marshall, V.L.; Reininger, C.B.; Marquardt, M.; Patterson, J.; Hadley, D.M.; Oertel, W.H.; Benamer, H.T.; Kemp, P.; Burn, D.; Tolosa, E.; et al. Parkinson’s disease is overdiagnosed clinically at baseline in diagnostically uncertain cases: A 3-year European multicenter study with repeat [123I]FP-CIT SPECT. Mov. Disord. 2009, 24, 500–508. [Google Scholar] [CrossRef]
- Kupsch, A.R.; Bajaj, N.; Weiland, F.; Tartaglione, A.; Klutmann, S.; Buitendyk, M.; Sherwin, P.; Tate, A.; Grachev, I.D. Impact of DaTscan SPECT imaging on clinical management, diagnosis, confidence of diagnosis, quality of life, health resource use and safety in patients with clinically uncertain parkinsonian syndromes: A prospective 1-year follow-up of an open-label controlled study. J. Neurol. Neurosurg. Psychiatry 2012, 83, 620–628. [Google Scholar] [CrossRef]
- McKeith, I.; O’Brien, J.; Walker, Z.; Tatsch, K.; Booij, J.; Darcourt, J.; Padovani, A.; Giubbini, R.; Bonuccelli, U.; Volterrani, D.; et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: A phase III, multicentre study. Lancet Neurol. 2007, 6, 305–313. [Google Scholar] [CrossRef]
- R Core Team. R: A language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2021. Available online: https://www.R-project.org/ (accessed on 30 September 2024).
- Jiang, W.; Varma, S.; Simon, R. Calculating confidence intervals for prediction error in microarray classification using resampling. Stat. Appl. Genet. Mol. Biol. 2008, 7, 8. [Google Scholar] [CrossRef]
- Walker, Z.; Jaros, E.; Walker, R.W.; Lee, L.; Costa, D.C.; Livingston, G.; Ince, P.G.; Perry, R.; McKeith, I.; Katona, C.L. Dementia with Lewy bodies: A comparison of clinical diagnosis, FP-CIT single photon emission computed tomography imaging and autopsy. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1176–1181. [Google Scholar] [CrossRef]
- Pagano, G.; Niccolini, F.; Politis, M. Imaging in Parkinson’s disease. Clin. Med. 2016, 16, 371–375. [Google Scholar] [CrossRef]
- Thomas, A.J.; Attems, J.; Colloby, S.J.; O’Brien, J.T.; McKeith, I.; Walker, R.; Lee, L.; Burn, D.; Lett, D.J.; Walker, Z. Autopsy validation of 123I-FP-CIT dopaminergic neuroimaging for the diagnosis of DLB. Neurology 2017, 88, 276–283. [Google Scholar] [CrossRef]
- Surmeier, D.J. Determinants of dopaminergic neuron loss in Parkinson’s disease. FEBS J. 2018, 285, 3657–3668. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G.; Giannoni, S.; Bellini, G.; Siciliano, G.; Ceravolo, R. Dopamine Transporter Imaging, Current Status of a Potential Biomarker: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 11234. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, F.; Kane, J.P.M.; Ibañez, A.; Lewis, S.J.G.; Camicioli, R.; Wang, H.; Yu, Y.; Zhang, J.; Ji, Y.; Borda, M.G.; et al. Dementia with Lewy bodies research consortia: A global perspective from the ISTAART Lewy Body Dementias Professional Interest Area working group. Alzheimer’s Dement. 2021, 13, e12235. [Google Scholar] [CrossRef] [PubMed]
- Siderowf, A.; Concha-Marambio, L.; Lafontant, D.E.; Farris, C.M.; Ma, Y.; Urenia, P.A.; Nguyen, H.; Alcalay, R.N.; Chahine, L.M.; Foroud, T.; et al. Assessment of heterogeneity among participants in the Parkinson’s Progression Markers Initiative cohort using α-synuclein seed amplification: A cross-sectional study. Lancet Neurol. 2023, 22, 407–417. [Google Scholar] [CrossRef]
- Simuni, T.; Chahine, L.M.; Poston, K.; Brumm, M.; Buracchio, T.; Campbell, M.; Chowdhury, S.; Coffey, C.; Concha-Marambio, L.; Dam, T.; et al. A biological definition of neuronal α-synuclein disease: Towards an integrated staging system for research. Lancet Neurol. 2024, 23, 178–190. [Google Scholar] [CrossRef]

| Dementia | Movement Disorder | Total | |
|---|---|---|---|
| Total | 185 | 118 | 303 |
| Sex | |||
| Male | 102 (55%) | 67 (57%) | 169 (56%) |
| Female | 83 (45%) | 51 (43%) | 134 (44%) |
| Age * | 73.78 (±7.2) | 66.34 (±10.99) | 70.88 (±9.57) |
| NSDD ** | 73 (39%) | 78 (66%) | 151 (49%) |
| Single-Variable Model Posterior Putamen | Example of Best-Performing Multi-Variable Model | ||
|---|---|---|---|
| SBR | Post Put + PCR | ||
| Accuracy | 0.90 [0.85, 0.94] | 0.89 [0.84, 0.94] | |
| Sensitivity | 0.81 | 0.80 | |
| Specificity | 0.96 | 0.96 | |
| Threshold | 0.65 | Not applicable | |
| z-score | Striatum + Caudate + PCR | ||
| Accuracy | 0.89 [0.84, 0.94] | 0.89 [0.83, 0.94] | |
| Sensitivity | 0.80 | 0.75 | |
| Specificity | 0.96 | 0.97 | |
| Threshold | −2.36 | Not applicable | |
| % Dev | Post Put + PCR | ||
| Accuracy | 0.90 [0.85, 0.94] | 0.89 [0.84, 0.94] | |
| Sensitivity | 0.81 | 0.80 | |
| Specificity | 0.96 | 0.96 | |
| Threshold | −0.54 | Not applicable |
| Single-Variable Model: Posterior Putamen | Example of Best Performing Multi-Variable Model | ||
|---|---|---|---|
| SBR | Striatum + Caudate | ||
| Acc | 0.82 [0.75, 0.89] | 0.83 [0.75, 0.90] | |
| Sens | 0.77 | 0.78 | |
| Spec | 0.93 | 0.93 | |
| Threshold | 0.92 | Not applicable | |
| z-score | Striatum + Post Put + Caud Asy | ||
| Acc | 0.83 [0.76, 0.90] | 0.84 [0.75, 0.92] | |
| Sens | 0.78 | 0.80 | |
| Spec | 0.93 | 0.93 | |
| Threshold | −1.53 | Not applicable | |
| % Dev | Striatum + Post Put + Caud Asy | ||
| Acc | 0.82 [0.75, 0.89] | 0.84 [0.74, 0.92] | |
| Sens | 0.78 | 0.80 | |
| Spec | 0.90 | 0.93 | |
| Threshold | −0.33 | Not applicable |
| Neill et al. 2021 [15] | Lanfranchi et al. 2023 [17] a | ||||
|---|---|---|---|---|---|
| Post Put—Movement Disorders | Post Put—Dementia | Post Put—Movement Disorders | Put—Dementia | ||
| SBR | Acc | 0.82 | 0.82 | - | - |
| Sens | 0.78 | 0.89 | - | - | |
| Spec | 0.90 | 0.78 | - | - | |
| Threshold | 1.0 | 1.0 | - | - | |
| z-score | Acc | 0.81 | 0.87 | 0.82 | 0.81 |
| Sens | 0.74 | 0.85 | 0.78 | 0.88 | |
| Spec | 0.92 | 0.88 | 0.90 | 0.77 | |
| Threshold | −1.8 | −1.8 | −1.27 | −0.96 ** | |
| % Dev | Acc | 0.82 | 0.86 | - | - |
| Sens | 0.78 | 0.89 | - | - | |
| Spec | 0.90 | 0.84 | - | - | |
| Threshold | −0.34 | −0.34 | - | - | |
| Posterior Putamen | ||
|---|---|---|
| Dementia | Movement Disorders | |
| SBR Threshold | 0.65 | 0.92 |
| z-score Threshold | −2.36 | −1.53 |
| Percent Dev Threshold | −0.54 | −0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, P.H.; Cella, P.; Chou, Y.-H.; Arkhipenko, A.; Fisher, J.M. Optimal DaTQUANT Thresholds for Diagnostic Accuracy of Dementia with Lewy Bodies (DLB) and Parkinson’s Disease (PD). Tomography 2024, 10, 1608-1621. https://doi.org/10.3390/tomography10100119
Kuo PH, Cella P, Chou Y-H, Arkhipenko A, Fisher JM. Optimal DaTQUANT Thresholds for Diagnostic Accuracy of Dementia with Lewy Bodies (DLB) and Parkinson’s Disease (PD). Tomography. 2024; 10(10):1608-1621. https://doi.org/10.3390/tomography10100119
Chicago/Turabian StyleKuo, Phillip H., Patrick Cella, Ying-Hui Chou, Alexander Arkhipenko, and Julia M. Fisher. 2024. "Optimal DaTQUANT Thresholds for Diagnostic Accuracy of Dementia with Lewy Bodies (DLB) and Parkinson’s Disease (PD)" Tomography 10, no. 10: 1608-1621. https://doi.org/10.3390/tomography10100119
APA StyleKuo, P. H., Cella, P., Chou, Y.-H., Arkhipenko, A., & Fisher, J. M. (2024). Optimal DaTQUANT Thresholds for Diagnostic Accuracy of Dementia with Lewy Bodies (DLB) and Parkinson’s Disease (PD). Tomography, 10(10), 1608-1621. https://doi.org/10.3390/tomography10100119








