Abstract
Background: We sought to determine whether pretreatment total masseter muscle volume (TMMV) measures can predict radiation-induced trismus (RIT) in patients with locally advanced nasopharyngeal carcinoma (LA-NPC) receiving concurrent chemoradiotherapy (C-CRT). Methods: We retrospectively reviewed the medical records of LA-NPC patients who received C-CRT and had pretreatment maximum mouth openings (MMO) greater than 35 mm. MMO of 35 mm or less after C-CRT were considered RIT. We employed receiver operating characteristic (ROC) curve analysis to explore the correlation between pre-treatment TMMV readings and RIT status. Results: Out of the 112 eligible patients, 22.0% of them received a diagnosis of RIT after C-CRT. The optimal TMMV cutoff that was significantly linked to post-C-CRT RIT rates was determined to be 35.0 cc [area under the curve: 79.5%; sensitivity: 75.0%; and specificity: 78.6%; Youden index: 0.536] in the ROC curve analysis. The incidence of RIT was significantly higher in patients with TMMV ≤ 5.0 cc than in those with TMMV > 35.0 cc [51.2% vs. 8.7%; Odds ratio: 6.79; p < 0.001]. A multivariate logistic regression analysis revealed that pre-C-CRT MMO ≤ 41.6 mm (p = 0.001), mean masticatory apparatus dose V56.5 ≥ 34% group (p = 0.002), and TMMV ≤ 35 cc were the independent predictors of significantly elevated rates of RIT. Conclusion: The presence of a smaller pretreatment TMMV is a reliable and independent novel biological marker that can confidently predict higher RIT rates in LA-NPC patients who receive C-CRT.
1. Introduction
Nasopharyngeal carcinoma (NPC), a malignant tumor arising from the epithelial cells of the nasopharynx, is typically managed using radiotherapy (RT) for early-stage disease and concurrent chemoradiotherapy (C-CRT) with intensity-modulated radiotherapy (IMRT) for locally advanced-stage disease [1,2,3]. However, the benefits of C-CRT, such as improved locoregional control and survival rates, often come at the cost of severe late side effects that can negatively impact patients’ quality of life [4]. Radiation-induced trismus (RIT) is a notable late side effect of RT or C-CRT, with an incidence ranging from 28% to 42% [5]. However, it is often less respected despite its substantial adverse consequences on the patient’s functional abilities and quality of life metrics.
Trismus, also known as lockjaw, is a condition that can be attributed to several factors, including but not limited to temporomandibular joint (TMJ) disorders, tumors, surgery, RT/C-CRT to the head and neck, and TMJ interventions for managing infections and connective tissue disorders [6]. The formation of RIT is attributed to RT-induced inflammation and fibrosis occurring in the intramuscular region and associated raphes of the masseter muscle, namely the strongest masticatory muscle [7,8]. Masha et al. [7] established that applying a maximum dose of RT > 44 Gy to the masseter muscle region was associated with RIT in a cohort of 35 patients with head and neck tumors (p = 0.003). In addition, recent studies have confirmed that the incidence rates of RIT may be affected by hypoxia and inflammation markers, such as pre-C-CRT hemoglobin-to-platelet ratios (HPR), neutrophil-to-lymphocyte ratios (NLR), and hemoglobin values [9,10,11].
Cancer cachexia is characterized by muscle wasting (sarcopenia) and weight loss, often accompanied by a reduction in fat mass. Systemic inflammation and cytokine storms are the main contributing factors to this clinical condition [12,13]. The evaluation of sarcopenia in head and neck cancer (HNC) through imaging-based studies includes the utilization of the skeletal muscle index. This index is determined by dividing the cross-sectional area of skeletal muscle at either the C3 or L3 level by the square of the individual’s height in meters [12,14]. Scholarly discourse has recently focused on the masseter muscle, an essential component of the masticatory apparatus, as an alternative indicator for evaluating sarcopenia. A reduction in the volume of the masseter muscle can result in decreased mastication capability, ultimately resulting in malnutrition [15]. Disusing the masseter muscle and other muscles involved in the mastication process may cause muscular atrophy and loss of volume, resulting in different degrees of trismus or heightened vulnerability to developing RIT.
A recently published article has reported a direct correlation between the pretreatment masseter muscle volume and the survival outcomes of patients with locally advanced NPC (LA-NPC) who received definitive C-CRT [16]. It is currently unknown whether the pre-C-CRT volume of the masseter muscle affects RIT rates, despite its known connection to disease prognosis and cachexia. Hence, our current research aimed to investigate the potential impact of pre-treatment total masseter muscle volume (TMMV) on the RIT rates in LA-NPC patients who have undergone C-CRT.
2. Patients and Methods
2.1. Ethical Approval
The retrospective study protocol adhered to the official guidelines and amendments of the Declaration of Helsinki with utmost accuracy. Baskent University Faculty of Medicine’s Ethics and Scientific Committee granted approval before the collection of patient data, ensuring complete adherence to ethical standards (project No: KA23/196).
2.2. Study Population
A retrospective analysis was conducted on patients diagnosed with LA-NPC who received C-CRT and were evaluated at the Departments of Radiation Oncology, Radiology, and Dentistry at Baskent University Faculty of Medicine between January 2010 and January 2023. Patients were required to be at least 18 years old, have been diagnosed with nasopharyngeal squamous cell cancer through histopathological analysis, undergone a neck MRI evaluation before treatment, have been classified as LA-NPC (T1-2N1-3M0 or T3-4N0-3M0) according to the eighth edition of the AJCC staging framework, and did not have a diagnosis of temporomandibular disorder (TMD) or trismus before C-CRT, based on the current diagnostic criteria for TMD (Diagnostic criteria (DC)/TMD), to be eligible for the study [17,18].
The exclusion criteria included immunosuppressive drug usage within 30 days before C-CRT, blood transfusions within 90 days, evidence of dehydration, evidence of acute and chronic infectious diseases, being treated for a local recurrence, a prior history of surgery and/or RT to the head and neck region, and a follow-up duration of less than 6 months. Patients who had missing central incisors in their upper or lower jaws, had previously undergone surgery for TMJ issues, TMJ ankylosis, head or neck trauma, muscle-related pain, or myofascial pain syndrome, and those with primary tumors or lymph node invasion in their masticatory muscles were also excluded from the study.
2.3. Treatment Protocol
Our institutional gold standard treatment approach for LA-NPC is simultaneous integrated boost intensity-modulated radiotherapy (SIB-IMRT). The target volumes for RT were determined by using pretreatment co-registered computed tomography (CT), 18-fluorodeoxyglucose-positron emission tomography (PET)-CT, and/or magnetic resonance imaging (MRI) scans of both the involved nasopharyngeal primary and the whole neck. The target volumes and corresponding RT doses were as previously described [19]. To provide a brief overview, the doses administered to the planning target volumes (PTVs) were as follows: 70.0 Gy for high-risk-, 59.5 Gy for intermediate-risk-, and 54.0 Gy for low-risk PTVs, delivered using single daily fractions over a period of 33 days [19]. The treatment plan mandated the administration of 1 to 3 cycles of concurrent chemotherapy, comprising cisplatin and 5-fluorouracil. All patients were recommended to undergo adjuvant treatment, which involved two cycles of the same chemotherapy regimen. Necessary supportive care measures, such as analgesic and antiemetic medications, were administered whenever required.
2.4. Baseline Total Masseter Muscle Volume Measurements
The masseter muscle is a quadrangular muscle that consists of two divisions: a superficial division and a deep division. Its superficial division originates from a thick aponeurosis located on the temporal process of the zygomatic bone and the anterior two-thirds of the inferior border of the zygomatic arch. The superficial division fibers attach to the lower part of the mandibular ramus and the mandibular angle (masseteric tubercle) by going downwards and backward over the deep portion. The whole zygomatic arch serves as the origin for the deep division of the masseter muscle. The fibers originate superiorly to the masseter muscle and traverse inferiorly along the mandibular ramus. The evaluation of masseter muscles was performed using pre-contrast T2-weighted MRI images without fat saturation, using a 20-channel head and neck coil from either the MAGNETOM Skyra 3T or MAGNETOM Avanto Fit 1.5T, manufactured by Siemens Healthcare in Erlangen, Germany. The parameters used for pre-contrast T2-weighted images without fat saturation were as follows: for the 3T MR service, the TR was 7060 ms, TE was 79 ms, slice thickness was 3 mm, matrix was 224 × 320, field of view (FOV) was 220 × 220 mm, number of excitations was 1, and flip angle was 180; for the 1.5T MR service, the TR was 5550 ms, TE was 102 ms, slice thickness was 3 mm, matrix was 217 × 320, FOV was 230 × 230 mm, number of excitations was 2, and flip angle was 150.
The masseter muscle, which arises from the zygomatic arch and inserts into the mandibular angle, is the largest of all the muscles involved in mastication. MRI and planning CT scans obtained for SIB-IMRT planning were co-registered for the masseter muscle delineation and TMMV (total volume of right and left masseter muscles) calculation processes. A skilled radiologist (UAP) who specializes in head and neck imaging performed all radiologic evaluations, measurements, and analyses (Figure 1). The radiologist performed volumetric analyses on the treatment planning system while maintaining a blind approach to the clinical data.
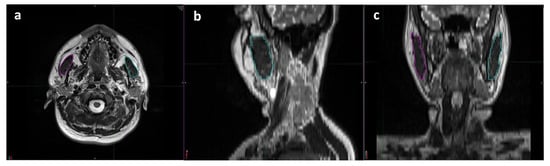
Figure 1.
Representative delineation process used for volumetric measurements of each masseter muscle using T2 weighted magnetic resonance imaging scans: (a) axial, (b) sagittal, and (c) coronal planes (magenta: right masseter muscle; cyan: left masseter muscle).
2.5. Baseline and Follow-Up Oral Evaluation and the Determination of RIT
An oral and maxillofacial surgeon with extensive experience (ES) conducted all oral assessments and MMO measurements. RIT was defined as having an MMO of 35 mm or less according to the standards set by Dijkstra et al. [20]. To measure MMO, we utilized Therabite® from Atos Medical AB in Hörby, Sweden [21]. Patients were instructed to open their mouths as widely as possible while wearing the Therabite® motion scale to measure the distance between the lower edge of one of the upper central incisors and the corresponding upper edge of one of the mandibular central incisors. The mean MMO was determined as the average numerical value of three consecutive measurements during each session. Measurements of MMO were repeated for each patient at 1, 3, 6, 9, and 12 months after C-CRT to evaluate the status of RIT. Subsequent measurements were carried out as required, or at every scheduled appointment thereafter.
2.6. Statistical Analysis
The primary intent of this retrospective research was to investigate the potential association between pre-TMMV readings and RIT rates. The last day of C-CRT was used as a reference point for determining the timing of the RIT diagnosis. The patients’ data were censored in cases where there was a diagnosis of RIT, a loss of follow-up, or death. The categorical data were displayed via percentage frequency distributions, while the continuous variables were shown utilizing median or mean values and ranges, as indicated. We conducted a receiver operating characteristic (ROC) curve analysis to determine the cutoff value(s) for continuous variables, which would allow us to divide the research cohort into two groups with substantially different outcomes. Logistic regression analysis was used to evaluate the individual significance of each variable in the multivariate analysis. All tests utilized a two-tailed p-value with a significance level of <0.05.
3. Results
The present retrospective cohort study examined the data of 112 patients diagnosed with LA-NPC who underwent C-CRT. As shown in Table 1, the median age was 60 years (range: 18–79), and 80 (71.4%) patients were male. Patients who had previously used tobacco products or alcohol made up 67.0% and 30.4%, respectively. The majority of patients had T3-4 (N = 65, 58.0%) or N2-3 (N = 75, 67.0%) advanced disease stages. The median pre-C-CRT MMO measurement was 41.6 mm (with a range of 37.0–46.8 mm). After the final post-C-CRT measurements, the median MMO decreased by 2.6 mm, which represents a decrease of 6.25% (Table 1 and Table 2). Based on Dijkstra’s widely accepted MMO ≤ 35 mm criterion, 28 patients were diagnosed with RIT, resulting in a prevalence rate of 25.0% [22]. The median time from the completion of C-CRT to the RIT diagnosis was 9 months (range: 4–18 months).

Table 1.
Baseline and treatment characteristics for all patients and per total masseter muscle volume groups.

Table 2.
Treatment characteristics and outcomes per total masseter muscle volume groups.
Using ROC curve analysis, we sought a possible significant link between RIT incidence rates and pre-C-CRT TMMV measurements. The ideal TMMV cutoff was 35.0 cc [area under the curve (AUC): 79.5%; sensitivity: 75.0%; specificity: 78.6%; Youden index: 0.536] (Figure 2). As a result, the cutoff value was used to divide the entire research population into two groups: Group 1: TMMV ≤ 35.0 cc (N = 43) and Group 2: TMMV > 35.0 cc (N = 69). The two TMMV groups had similar distributions of baseline and treatment characteristics (Table 1 and Table 2). However, the incidence of RIT was significantly higher in patients with TMMV ≤ 35.0 cc compared to those with TMMV > 35.0 cc [51.2% vs. 8.7%; HR: 6.79; p < 0.001] (Table 3). Further analysis revealed that the mean C-CRT to RIT diagnosis interval was also significantly shorter in the TMMV ≤ 35.0 cc group compared to the TMMV > 35.0 cc group (9.6 months vs. 13.8 months; p = 0.02) (Table 2).
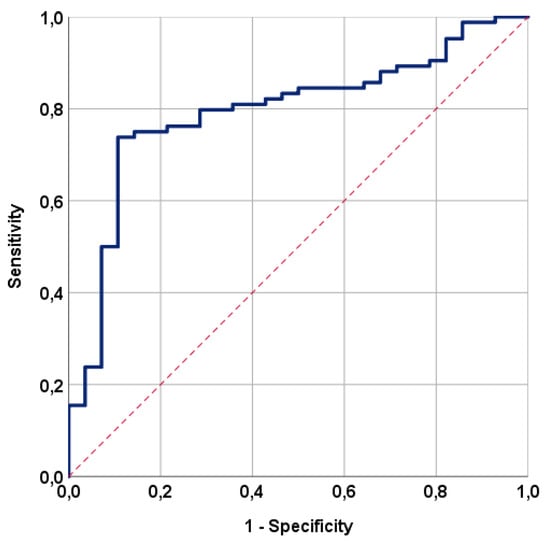
Figure 2.
The outcomes of a receiver operating characteristic (ROC) curve analysis examining the correlation between the pre-treatment total masseter muscle volume and radiation-induced trismus rates following concurrent chemoradiotherapy [area under the curve (AUC): 79.5%; sensitivity: 75.0%; specificity: 78.6%; Youden index: 0.536].

Table 3.
Results of univariate and multivariate analysis.
Univariate analyses revealed a significant relationship between RIT incidences and pre-C-CRT groups of MMO (38.6% vs. 10.9% for >41.6 mm, p = 0.001), MAD V56.5 group (14.3% vs. 38.1% for ≥34% Gy, p = 0.002), and TMMV group (51.2% vs. 8.7% for >35 cc, p ˂ 0.001), with the former groups exhibiting higher RIT rates (Table 3). Multivariate logistic regression analyses confirmed that all three characteristics were independent and significant predictors of RIT in this patient group (p < 0.05 for each) (Table 3, Figure 3).
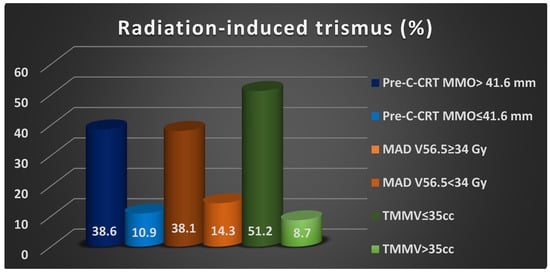
Figure 3.
The bar chart depicting the rates of radiation-induced trismus rates per factor demonstrated independent significance in multivariate analyses. Abbreviations: C-CRT; concurrent chemoradiotherapy, MMO; maximum mouth opening, MAD; masticatory apparatus dose, V; volume, TMMV; total masseter muscle volume, cc; cubic centimeter.
4. Discussion
The main objective of this retrospective study was to determine the feasibility of utilizing pre-C-CRT TMMV levels as a predictor for the occurrence of RIT in patients with LA-NPC. Our most remarkable findings were the demonstration of a robust and independent association between pre-C-CRT TMMV 35.0 cc and a higher incidence of RIT [51.2% vs. 8.7% for TMMV > 35.0 cc; HR: 6.79; p < 0.001] and a significantly shorter mean C-CRT to RIT diagnosis interval (9.6 months vs. 13.8 months for TMMV > 35.0 cc; p = 0.02).
Pre-C-CRT MMO and MAD measures are two significant factors associated with RIT incidence rates following RT or C-CRT [22,23,24]. In this regard, our findings demonstrated a pre-C-CRT MMO ≤ 41.6 mm (38.6% vs. 10.9% for pre-C-CRT MMO > 41.6 mm, p = 0.001) and a MAD V56 > 34% (38.1% vs. 14.3% for MAD V56 ≤ 34%, p = 0.002), confirming narrower baseline MMO and higher MAD measurements as strong predictors of RIT in this patient group. Similar to our findings, Kraaijenga et al. [23], Owosho et al. [22], and Somay et al. [9] reported baseline MMO measurements of <46 mm, <40 mm, and ≤40.7 mm as significant predictors of increased RIT rates. While there is limited research available on MAD, studies have indicated that higher mean doses of RT administered to the components of the masticatory apparatus (measured as a dosimetric parameter for 100% volume) are linked to higher rates of radiation-induced toxicity such as RIT [9,11]. Based on the findings of the limited number of studies currently available and our research, it can be deduced that the mean and Vx (volume receiving X Gy) MAD values have the potential to function as indicators for assessing the extent of damage in the masticatory apparatus following RT, which is a functional parallel organ in terms of radiobiology. Therefore, every effort should be made to achieve as low a MAD value as feasible during the RT planning process to reduce the RIT rates.
The most notable discovery of our research was that patients presenting with a TMMV ≤ 35.0 cc had higher RIT incidence rates than those with a TMMV > 35.0 cc [51.2% vs. 8.7%; p < 0.001]. To our best knowledge, this finding is the first to establish a significant connection between RIT rates and TMMV in LA-NPC patients treated with definitive C-CRT. Previous research has linked radiation-induced inflammation to the difficult-to-predict RIT. It has been proposed that biomarkers derived from pre-treatment blood parameters could be used to predict RIT rates [9,11]. Previous research has not yet explored the value of pretreatment masseter muscle volume in predicting the rate of RIT. However, it is well recognized that masseter muscle abnormalities are established factors in the development of trismus, regardless of the cause, including RIT. In the first study of its kind, using the masseter muscle cross-sectional area (MMCSA) method, Wallace et al. [25] investigated the masseter muscle, which revealed a strong correlation between sarcopenia and MMCSA. Based on the findings of this research, McGoldrick et al. [26] assessed the usefulness of the same methodology in head and neck cancer patients. Their investigation found that sarcopenia, as indicated by MMCSA, may have prognostic significance in senior patients (HR: 1.74, p = 0.042). However, regardless of the methodological differences, these collective findings consistently highlight the dependability of masseter muscle measures as a novel biomarker for predicting treatment-related toxicities and survival outcomes.
Muscle wasting in cancer patients is a poor prognostic factor frequently associated with pre-cachexia or cachexia [27]. This condition is characterized by the unintentional loss of muscle mass in the absence of surgical or other physical damage to the muscle fibers, nerves, and/or blood vessels. The loss of skeletal muscle mass experienced during the development of cancer cachexia is mainly caused by a combination of protein degradation and reduced protein synthesis. This process is triggered by the increased production of inflammatory mediators and the activation of specific transcription factors and signaling pathways [28,29]. Given these facts, the significant association between TMMV and RIT rates in our patient cohort manifesting with a pre-C-CRT TMMMV < 35 cc may imply a condition of cancer cachexia in progress and related weakened muscle mass and strength-inducing trismus in this particular patient cohort. This implication is indirectly supported by a recently reported study by Pehlivan et al., where the authors discovered significant associations between lower pre-treatment TMMV values and worse survival outcomes in LA-NPC patients treated with definitive C-CRT, as a pre-cachectic condition may have impacted the results [16]. Another logical explanation could be the collaboration of two hyperinflammatory states, namely cancer cachexia and radiation-induced tissue injury, which resulted in increased inflammatory mediator secretion, increased proteolysis, facilitated sarcopenia and fibrosis in the masseter muscles, and ultimately RIT development [30,31].
Another key finding in our study was the demonstration of a significant correlation between a smaller TMMV and a shorter mean duration between the completion of C-CRT and RIT manifestation (9.6 months vs. 13.8 months for TMMV ≤ 35.0 cc; p = 0.02). While the precise explanation requires more investigation, this discovery might potentially be attributed to the possibility that a reduced TMMV may serve as an early indicator of a currently underway insult affecting the muscle mass, such as cancer cachexia. Furthermore, it is possible that the impact of radiation-induced damage on muscle fibers, vasculature, and nerves was more significant in the smaller volume and, conceivably, the weaker masseter muscles, which might have led to an earlier onset of muscle fibrosis and resultant RIT in these individuals [32]. Regardless of the precise underlying causes, based on our current knowledge, the present finding indicates that most instances of RIT will occur within a time frame of less than one year in individuals presenting with smaller baseline TMMV measures. Despite the need for confirmatory research results, we contend that providing nutritional assistance and implementing preventative measures for RIT at an earlier stage might be a prudent strategy to reduce the incidences of cancer cachexia and RIT in such patients.
Our study possesses several strengths: first, the study consisted of a select group of LA-NPC patients treated with the same concurrent C-CRT and medication regimen. Second, while CT is commonly used in RT planning, incorporating co-registered CT-MRI scans may have enabled a more precise determination of TMMV by facilitating the identification of exact muscle borders. However, our study has some drawbacks as well: first, it has a retrospective design with a small cohort size, which may introduce unintentional selection bias-related issues. Second, as a consequence of the limited number of participants in our study, we were unable to validate the findings by using either external or internal control groups. Third, no probable correlation was examined between the pretreatment TMMV and biomarkers of cancer cachexia, such as cytokines and acute phase reactants. Fourth, because our study was limited to LA-NPC patients, it becomes arduous to draw inferences regarding TMMV’s ability to predict RIT and other toxicities in other head and neck cancers. We believe that a decrease in the volume of the masseter muscles, which are the strongest muscles used for chewing, may indicate the patient’s poor nutritional status and/or a pre-cachectic status with a potential to progress towards irreversibly fatal cancer cachexia. Despite being sensible, we believe that well-designed research on this vital subject is required to substantiate our opinion. And fifth, due to the lack of dynamic measurement of masseter muscle volume during and after treatment in the current study, the optimal threshold value could not be established. Hence, future research should use dynamic measurements to establish the best cutoff value, which may better stratify patients according to RIT and, presumably, other toxicity risks. Given these facts, we may have missed the chance to discover the exact processes that connect the first TMMV measurements with RIT rates. Undoubtedly, conducting additional well-designed and large-scale research is essential to address the issues associated with RIT in patients at a higher risk after RT or C-CRT. Such research outcomes can help initiate individualized nutritional support and timely preventive measures, which can play a pivotal role in mitigating the incapacitating effects of RIT.
5. Conclusions
Our research findings indicate that pre-treatment TMMV values can be used as an innovative biological predictor of RIT rates in LA-NPC patients who undergo definitive C-CRT treatment. If additional studies confirm these findings, we may be able to categorize these patients based on their risk of RIT and develop follow-up protocols tailored to their specific risk levels.
Author Contributions
Conceptualization, E.S., E.T., U.A.P., B.Y., A.A.B., H.M., B.P. and U.S.; methodology, E.S., E.T., U.A.P., B.Y., A.A.B., H.M., B.P. and U.S.; validation, E.S., E.T., U.A.P., B.Y., A.A.B., H.M., B.P. and U.S.; formal analysis, E.S., E.T., U.A.P., B.Y., A.A.B., H.M., B.P. and U.S.; investigation, E.S., E.T., U.A.P., B.Y., A.A.B., H.M., B.P. and U.S.; resources E.S., E.T., U.A.P., B.Y., A.A.B., H.M., B.P. and U.S.; data curation, E.S., E.T., U.A.P., B.Y., A.A.B., H.M., B.P. and U.S.; writing—original draft preparation, E.S., E.T., U.A.P., B.Y., A.A.B., H.M., B.P. and U.S.; writing—review and editing, E.S., E.T., U.A.P., B.Y., A.A.B., H.M., B.P. and U.S.; supervision, E.S., E.T., U.A.P., B.Y., A.A.B., H.M., B.P. and U.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and has received approval from the Baskent University Institutional Review Board under Project No: KA23/196. (21 March 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The present data belong to and are stored at the Baskent University Faculty of Medicine and cannot be shared without permission. For researchers who meet the following criteria for access to confidential data, contact the Baskent University Corporate Data Access/Ethics Board: adanabaskent@baskent.edu.tr.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Adoga, A.A.; Kokong, D.D.; Ma’an, N.D.; Silas, O.A.; Dauda, A.M.; Yaro, J.P.; Mugu, J.G.; Mgbachi, C.J.; Yabak, C.J. The epidemiology, treatment, and determinants of outcome of primary head and neck cancers at the Jos University Teaching Hospital. South Asian J. Cancer 2018, 7, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, P.; Nguyen, F.; Moya-Plan, A.; Pignon, J.P.; Even, C.; Bidault, F.; Temam, S.; Ruffier, A.; Tao, Y. New developments in the management of nasopharyngeal carcinoma. Cancer Radiother. 2018, 22, 492–495. [Google Scholar] [CrossRef]
- Lee, N.; Harris, J.; Garden, A.S.; Straube, W.; Glisson, B.; Xia, P.; Bosch, W.; Morrison, W.H.; Quivey, J.; Thorstad, W.; et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: Radiation therapy oncology group phase II trial 0225. J. Clin. Oncol. 2009, 27, 3684–3690. [Google Scholar] [CrossRef] [PubMed]
- Skiba-Tatarska, M.; Kusa-Podkańska, M.; Surtel, A.; Wysokińska-Miszczuk, J. The side-effects of head and neck tumors radiotherapy. Pol. Merkur. Lekarski. 2016, 41, 47–49. [Google Scholar]
- Pauli, N.; Johnson, J.; Finizia, C.; Andréll, P. The incidence of trismus and long-term impact on health-related quality of life in patients with head and neck cancer. Acta Oncol. 2013, 52, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Garnett, M.J.; Nohl, F.S.; Barclay, S.C. Management of patients with reduced oral aperture and mandibular hypomobility (trismus) and implications for operative dentistry. Br. Dent. J. 2008, 204, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Musha, A.; Shimada, H.; Kubo, N.; Kawamura, H.; Okano, N.; Miyasaka, Y.; Sato, H.; Shirai, K.; Saitoh, J.I.; Yokoo, S.; et al. Evaluation of Carbon Ion Radiation-Induced Trismus in Head and Neck Tumors Using Dose-Volume Histograms. Cancers 2020, 12, 3116. [Google Scholar] [CrossRef]
- Chou, C.; Chen, C.C.; Lai, C.S.; Lin, S.D.; Kuo, Y.R. Simultaneous double free radial forearm flaps combined with coronoidectomy and myotomy to release bilateral severe trismus: A case report. Microsurgery 2017, 37, 831–835. [Google Scholar] [CrossRef]
- Somay, E.; Yilmaz, B.; Topkan, E.; Kucuk, A.; Haksoyler, V.; Pehlivan, B.; Selek, U.; Araz, K. Hemoglobin-to-platelet ratio in predicting the incidence of trismus after concurrent chemoradiotherapy. Oral Dis. 2022, 29, 2962–2970. [Google Scholar] [CrossRef]
- Somay, E.; Yilmaz, B.; Topkan, E.; Pehlivan, B.; Selek, U. Low hemoglobin levels predict increased radiation-induced trismus rates in nasopharyngeal cancer. Oral Dis. 2023, 8, 1–9. [Google Scholar] [CrossRef]
- Somay, E.; Yilmaz, B.; Topkan, E.; Kucuk, A.; Pehlivan, B.; Selek, U. Initial neutrophil-to-lymphocyte ratio predicts radiation-induced trismus in parotid gland cancer. Oral Dis. 2022, 29, 2772–2779. [Google Scholar] [CrossRef]
- Anjanappa, M.; Corden, M.; Green, A.; Roberts, D.; Hoskin, P.; McWilliam, A.; Choudhury, A. Sarcopenia in cancer: Risking more than muscle loss. Tech. Innov. Patient Support Radiat. Oncol. 2020, 16, 50–57. [Google Scholar] [CrossRef]
- Douglas, E.; McMillan, D.C. Towards a simple objective framework for the investigation and treatment of cancer cachexia: The Glasgow Prognostic Score. Cancer Treat. Rev. 2014, 40, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ma, J.; Li, L.; Zhu, X.D. Severe muscle loss during radical chemoradiotherapy for non-metastatic nasopharyngeal carcinoma predicts poor survival. Cancer Med. 2019, 8, 6604–6613. [Google Scholar] [CrossRef]
- Kamada, T.; Ohdaira, H.; Ito, E.; Takahashi, J.; Nakashima, K.; Nakaseko, Y.; Suzuki, N.; Yoshida, M.; Eto, K.; Suzuki, Y. Association between masseter muscle sarcopenia and postoperative pneumonia in patients with esophageal cancer. Sci. Rep. 2022, 12, 16374. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, U.A.; Somay, E.; Yilmaz, B.; Besen, A.A.; Mertsoylu, H.; Selek, U.; Topkan, E. Pretreatment Masseter Muscle Volume Predicts Survival in Locally Advanced Nasopharyngeal Carcinoma Patients Treated with Concurrent Chemoradiotherapy. J. Clin. Med. 2023, 12, 6863. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Mao, Y.P.; Tang, L.L.; Chen, L.; Sun, Y.; Ma, J. The evolution of nasopharyngeal carcinoma staging. Br. J. Radiol. 2019, 92, 20190244. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.B.; Possebon, A.P.D.R.; Schuster, A.J.; Marcello-Machado, R.M.; de Rezende Pinto, L.; Faot, F. Relationship Between Masticatory Function Impairment and Oral Health-Related Quality of Life of Edentulous Patients: An interventional Study. J. Prosthodont. 2019, 28, 634–642. [Google Scholar] [CrossRef]
- Yilmaz, B.; Somay, E.; Selek, U.; Topkan, E. Pretreatment Systemic Immune-Inflammation Index Predict Needs for Teeth Extractions for Locally Advanced Head and Neck Cancer Patients Undergoing Concurrent Chemoradiotherapy. Ther. Clin. Risk Manag. 2021, 17, 1113–1121. [Google Scholar] [CrossRef]
- Dijkstra, P.U.; Kalk, W.W.; Roodenburg, J.L. Trismus in head and neck oncology: A systematic review. Oral. Oncol. 2004, 40, 879–889. [Google Scholar] [CrossRef]
- Bhargava, D.; Jain, M.; Deshpande, A.; Singh, A.; Jaiswal, J. Temporomandibular joint arthrocentesis for internal derangement with disc displacement without reduction. J. Maxillofac. Oral Surg. 2015, 14, 454–459. [Google Scholar] [CrossRef]
- Owosho, A.A.; Pedreira Ramalho, L.M.; Rosenberg, H.I.; Yom, S.K.; Drill, E.; Riedel, E.; Tsai, C.J.; Lee, N.Y.; Huryn, J.M.; Estilo, C.L. Objective assessment of trismus in oral and oropharyngeal cancer patients treated with intensity-modulated radiation therapy (IMRT). J. Cranio-Maxillofac. Surg. 2016, 44, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Kraaijenga, S.A.; Hamming-Vrieze, O.; Verheijen, S.; Lamers, E.; van der Molen, L.; Hilgers, F.J.; van den Brekel, M.W.; Heemsbergen, W.D. Radiation dose to the masseter and medial pterygoid muscle in relation to trismus after chemoradiotherapy for advanced head and neck cancer. Head Neck 2019, 41, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Rajalalitha, P.; Vali, S. Molecular pathogenesis of oral submucous fibrosis–a collagen metabolic disorder. J. Oral Pathol. Med. 2005, 34, 321–328. [Google Scholar] [CrossRef]
- Wallace, J.D.; Calvo, R.Y.; Lewis, P.R.; Brill, J.B.; Shackford, S.R.; Sise, M.J.; Sise, C.B.; Bansal, V. Sarcopenia as a predictor of mortality in elderly blunt trauma patients: Comparing the masseter to the psoas using computed tomography. J. Trauma Acute Care Surg. 2017, 82, 65–72. [Google Scholar] [CrossRef]
- McGoldrick, D.M.; Yassin Alsabbagh, A.; Shaikh, M.; Pettit, L.; Bhatia, S.K. Masseter muscle defined sarcopenia and survival in head and neck cancer patients. Br. J. Oral Maxillofac. Surg. 2022, 60, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Huang, J.; Wu, H.; Wang, Y.; Du, Z.; Ling, Y.; Wang, W.; Wu, Q.; Gao, W. Molecular mechanisms of cancer cachexia-induced muscle atrophy (Review). Mol. Med. Rep. 2020, 22, 4967–4980. [Google Scholar] [CrossRef]
- Peixoto da Silva, S.; Santos, J.M.O.; Costa, E.; Silva, M.P.; Gil da Costa, R.M.; Medeiros, R. Cancer cachexia and its pathophysiology: Links with sarcopenia, anorexia and asthenia. J. Cachexia Sarcopenia Muscle 2020, 11, 619–635. [Google Scholar] [CrossRef]
- Gould, D.W.; Lahart, I.; Carmichael, A.R.; Koutedakis, Y.; Metsios, G.S. Cancer cachexia prevention via physical exercise: Molecular mechanisms. J. Cachexia Sarcopenia Muscle 2013, 4, 111–124. [Google Scholar] [CrossRef]
- Muthanandam, S.; Muthu, J. Understanding Cachexia in Head and Neck Cancer. Asia Pac. J. Oncol. Nurs. 2021, 8, 527–538. [Google Scholar] [CrossRef]
- Topkan, E.; Yavuz, A.A.; Ozyilkan, O. Cancer cachexia: Pathophysiologic aspects and treatment options. Asian Pac. J. Cancer Prev. 2007, 8, 445–451. [Google Scholar] [PubMed]
- Straub, J.M.; New, J.; Hamilton, C.D.; Lominska, C.; Shnayder, Y.; Thomas, S.M. Radiation-induced fibrosis: Mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol. 2015, 141, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).