Association of Myelofibrosis Phenotypes with Clinical Manifestations, Molecular Profiles, and Treatments
Abstract
Simple Summary
Abstract
1. Introduction
2. Driver and Non-Driver Mutations, Clonal Evolution, and Cooperating Mutations Associated with Disease Progression and Survival
3. Genes Associated with the Myeloproliferative and Myelodepletive Phenotypes
4. Molecular Profiles of MF Phenotypes and Prognostication
5. Prognostic Relevance of Cytopenias Encountered in the Myelodepletive Phenotype and Patient Outcomes
6. Phenotypes, Molecular Profiles, and Differentiated Efficacy of Treatments in MF
| JAK Inhibitor | Clinical Trial NCT Number | Phase | Lowest Platelet Counts of Enrolled Patients | Reference |
|---|---|---|---|---|
| Ruxolitinib | EXPAND (NCT01317875) | 1b | 50 to <100 × 109/L | [112] |
| Ruxolitinib | JUMP (NCT01493414) | 3b | 50 to <100 × 109/L | [113] |
| Fedratinib | Pooled analysis of JAKARTA (NCT01437787) and JAKARTA2 (NCT01523171) | JAKARTA (phase 3) JAKARTA2 (phase 2) | 50 to <100 × 109/L | [114] |
| Pacritinib | PERSIST-1 (NCT01773187) | 3 | No lower limit | [129] |
| Pacritinib | PERSIST-2 (NCT02055781) | 3 | ≤100 × 109/L | [127] |
| Pacritinib | PAC203 (NCT04884191) | 2 | <50 × 109/L | [128] |
| Pacritinib | PACIFICA (NCT03165734) | 3 | <50 × 109/L | [133] |
| Momelotinib | SIMPLIFY-1 (NCT01969838) | 3 | ≥50 × 109/L | [140] |
| Momelotinib | SIMPLIFY-2 (NCT02101268) | 3 | No lower limit | [140] |
| Momelotinib | MOMENTUM (NCT04173494) | 3 | ≥25 × 109/L | [136,141] |
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bose, P.; Masarova, L.; Amin, H.M.; Verstovsek, S. Philadelphia chromosome-negative myeloproliferative neoplasms (Chapter 6). In The MD Anderson Manual of Medical Oncology, 4th ed.; Kantarjian, H.M., Wolff, R.A., Rieber, A.G., Eds.; McGraw-Hill, LLC: China, 2022; pp. 119–162. [Google Scholar]
- Marcellino, B.K.; Verstovsek, S.; Mascarenhas, J. The myelodepletive phenotype in myelofibrosis: Clinical relevance and therapeutic implication. Clin. Lymphoma Myeloma Leuk. 2020, 20, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, J.; Gleitz, H.F.E.; Chifotides, H.T.; Harrison, C.N.; Verstovsek, S.; Vannucchi, A.M.; Rampal, R.K.; Kiladjian, J.-J.; Vainchenker, W.; Hoffman, R.; et al. Biological drivers of clinical phenotype in myelofibrosis. Leukemia 2023, 37, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, D.; Mesa, R.; Scott, B.; Buckley, S.; Roman-Torres, K.; Verstovsek, S.; Mascarenhas, J. Pacritinib demonstrates spleen volume reduction in patients with myelofibrosis independent of JAK2 V617F allele burden. Blood Adv. 2020, 4, 5929–5935. [Google Scholar] [PubMed]
- Scotch, A.H.; Kosiorek, H.; Scherber, R.; Dueck, A.C.; Slot, S.; Zweegman, S.; W te Boekhorst, P.A.; Commandeur, S.; Schouten, H.; Sackmann, F.; et al. Symptom burden profile in myelofibrosis patients with thrombocytopenia: Lessons and unmet needs. Leuk. Res. 2017, 63, 34–40. [Google Scholar] [CrossRef]
- Masarova, L.; Bose, P.; Daver, N.; Pemmaraju, N.; Newberry, K.J.; Manshouri, T.; Cortes, J.; Kantarjian, H.M.; Verstovsek, S. Patients with post-essential thrombocythemia and post-polycythemia vera differ from patients with primary myelofibrosis. Leuk. Res. 2017, 59, 110–116. [Google Scholar] [CrossRef]
- Rampal, R.; Al-Shahrour, F.; Abdel-Wahab, O.; Patel, J.P.; Brunel, J.-P.; Mermel, C.H.; Bass, A.J.; Pretz, J.; Ahn, J.; Hricik, T.; et al. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood 2014, 123, e123–e133. [Google Scholar] [CrossRef]
- Bose, P.; Verstovsek, S. Mutational profiling in myelofibrosis: Implications for management. Inter. J. Hematol. 2020, 111, 192–199. [Google Scholar] [CrossRef]
- Rotunno, G.; Pacilli, A.; Artusi, V.; Rumi, E.; Maffioli, M.; Delaini, F.; Brogi, G.; Fanelli, T.; Pancrazzi, A.; Pietra, D.; et al. Epidemiology and clinical relevance of mutations in postpolycythemia vera and postessential thromobocythemia myelofibrosis: A study on 359 patients of the AGIMM group. Am. J. Hematol. 2016, 91, 681–686. [Google Scholar] [CrossRef]
- Guglielmelli, P.; Barosi, G.; Pieri, L.; Antonioli, E.; Bose, A.; Vannucchi, A.M. JAK2V617F mutational status and allele burden have little influence on clinical phenotype and prognosis in patients with post-polycythemia vera and post-essential thrombocythemia myelofibrosis. Haematologica 2009, 94, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Coltro, G.; Mannelli, F.; Loscocco, G.G.; Mannarelli, C.; Rotunno, G.; Maccari, C.; Pancani, F.; Atanasio, A.; Tefferi, A.; Vannucchi, A.; et al. A myelodepletive phenotype is associated with distinctive molecular features and outcomes in patients with myelofibrosis. Blood 2021, 138 (Suppl. 1), 1498. [Google Scholar] [CrossRef]
- Vainchenker, W.; Kralovics, R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood 2017, 129, 667–679. [Google Scholar] [CrossRef]
- Schieber, M.; Crispino, J.D.; Stein, B. Myelofibrosis in 2019: Moving beyond JAK2 inhibition. Blood Cancer J. 2019, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, G.; McPherson, S.; Mills, K.; McMullin, M.F. The ruxolitinib effect: Understanding how molecular pathogenesis and epigenetic dysregulation impact therapeutic efficacy in myeloproliferative neoplasms. J. Transl. Med. 2018, 16, 360. [Google Scholar] [CrossRef]
- Pasca, S.; Chifotides, H.T.; Verstovsek, S.; Bose, P. Mutational landscape of blast phase myeloproliferative neoplasms (BP-MPN) and antecedent MPN. Inter. Rev. Cell Mol. Biol. 2022, 366, 83–124. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, A.J.; Rampal, R.K.; Levine, R. Leukemia secondary to myeloproliferative neoplasms. Blood 2020, 136, 61–70. [Google Scholar] [CrossRef]
- Grinfeld, J.; Nangalia, J.; Baxter, E.J.; Wedge, D.C.; Angelopoulos, N.; Cantrill, R.; Godfrey, A.L.; Papaemmanuil, E.; Gundem, G.; MacLean, C.; et al. Classification and personalized prognosis in myeloproliferative neoplasms. N. Engl. J. Med. 2018, 379, 1416–1430. [Google Scholar] [CrossRef]
- Paz, D.L.; Kralovics, R.; Skoda, R.C. Genetic basis and molecular profiling in myeloproliferative neoplasms. Blood 2023, 141, 1909–1921. [Google Scholar] [CrossRef] [PubMed]
- Miles, L.A.; Bowman, R.L.; Merlinsky, T.R.; Csete, I.; Ooi, A.T.; Durruthy, R.; Bowman, M.; Famulare, C.; Patel, M.A.; Mendez, P.; et al. Single-cell mutation analysis of clonal evolution in myeloid malignancies. Nature 2020, 587, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, E.; Yoshida, K.; Frick, M.; Hoyer, K.; Christen, F.; Kaeda, J.; Obenaus, M.; Nörenberg, D.; Hennch, C.; Chan, W.; et al. Single-cell analysis based dissection of clonality in myelofibrosis. Nat. Commun. 2020, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.; Lee, J.; Mitchell, E.; Moore, L.; Baxter, E.J.; Hewinson, J.; Dawson, K.J.; Menzies, A.; Godfrey, A.L.; Green, A.R.; et al. Life histories of myeloproliferative neoplasms inferred from phylogenies. Nature 2022, 602, 162–168. [Google Scholar] [CrossRef]
- Dunbar, A.; Park, Y.; Levine, R. Epigenetic dysregulation of myeloproliferative neoplasms. Hematol. Oncol. Clin. N. Am. 2021, 35, 237–251. [Google Scholar] [CrossRef]
- Greenfield, G.; McMullin, M.F.; Mills, K. Molecular pathogenesis of myeloproliferative neoplasms. J. Hematol. Oncol. 2021, 14, 103. [Google Scholar] [CrossRef]
- Loscocco, G.; Coltro, G.; Guglielmelli, P.; Vannucchi, A.M. Integration of molecular information in risk assessment of patients with myeloproliferative neoplasms. Cells 2021, 10, 1962. [Google Scholar] [CrossRef]
- Tefferi, A.; Lasho, T.R.; Finke, C.M.; Elala, Y.; Hanson, C.A.; Kettering, R.P.; Gangat, N.; Pardanani, A. Targeted deep sequencing in primary myelofibrosis. Blood Adv. 2016, 1, 105–111. [Google Scholar] [CrossRef]
- Vannucchi, A.M.; Lasho, T.L.; Guglielmelli, P.; Biamonte, F.; Pereira, A.; Finke, C.; Score, J.; Gangat, N.; Mannarelli, C.; Ketterling, R.P.; et al. Mutations and prognosis in primary myelofibrosis. Leukemia 2013, 27, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, O.; Manshouri, T.; Patel, J.; Harris, K.; Yao, J.J.; Hedvat, C.; Heguy, A.; Bueso-Ramos, C.; Kantarjian, H.; Levine , R.L.; et al. Genetic analysis of transforming events that convert chronic myeloproliferative neoplasms to leukemias. Cancer Res. 2010, 70, 447–452. [Google Scholar] [CrossRef]
- Paz, D.L.; Riou, J.; Verger, E.; Cassinat, B.; Chauveau, A.; Ianotto, J.-C.; Dupriez, B.; Boyer, F.; Renard, M.; Mansier, O.; et al. Genomic analysis of primary and secondary myelofibrosis redefines the prognostic impact of ASXL1 mutations: A FIM study. Blood Adv. 2021, 5, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Guglielmelli, P.; Coltro, G.; Mannelli, F.; Rotynno, G.; Loscocco, G.G.; Mannarelli, C.; Maccari, C.; Paoli, C.; Romagnoli, S.; Bartalucii, N.; et al. ASXL1 mutations are prognostically significant in PMF but not MF following essential thrombocythemia or polycythemia vera. Blood Adv. 2022, 6, 2927–2931. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Lin, C.-C.; Lee, S.H.; Tsai, C.-H.; Wu, S.-J.; Hou, H.-A.; Huang, T.-C.; Kuo, Y.-Y.; Yao, M.; Chang, K.; et al. ASXL1 mutation confers poor prognosis in primary myelofibrosis patients with low JAK2V617F allele burden but not in those with high allele burden. Blood Cancer J. 2020, 99, 2454. [Google Scholar] [CrossRef] [PubMed]
- Vallapureddy, R.R.; Mudireddy, M.; Penna, D.; Lasho, T.L.; Finke, C.M.; Hanson, C.A.; Ketterling, R.P.; Begna, K.H.; Gangat, N.; Pardanani, A.; et al. Leukemic transformation among 1306 patients with primary myelofibrosis: Risk factors and development of a predictive model. Blood Cancer J. 2019, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Lasho, T.L.; Jimma, T.; Finke, C.M.; Patnaik, M.; Hanson, C.A.; Ketterling, R.P.; Pardanani, A.; Tefferi, A. SRSF2 mutations in primary myelofibrosis: Significant clustering with IDH mutations and independent association with inferior overall and leukemia-free survival. Blood 2012, 120, 4158–4171. [Google Scholar] [CrossRef]
- Loscocco, G.G.; Mannelli, F.; Bartalucci, N.; Vannucchi, A.M. SF3B1 mutations in primary and secondary myelofibrosis: Clinical, molecular and prognostic correlates. Am. J. Hematol. 2022, 97, E347–E349. [Google Scholar] [CrossRef] [PubMed]
- Shahin, O.A.; Chifotides, H.T.; Bose, P.; Masarova, L.; Verstovsek, S. Accelerated phase of myeloproliferative neoplasms. Acta Haematologica 2021, 144, 484–499. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Jimma, T.; Sulai, N.H.; Lasho, T.L.; Finke, C.M.; Knudson, R.A.; McClure, R.F.; Pardanani, A. IDH mutations in primary myelofibrosis predict leukemic transformation and shortened survival: Clinical evidence for leukemogenic collaboration with JAK2 V617F. Leukemia 2012, 26, 475–480. [Google Scholar] [CrossRef] [PubMed]
- McKenney, A.S.; Allison, N.L.; Hanasoge Somasundra, A.V.; Spitzer, B.; Interkofer, A.M.; Ahn, J.; Shank, K.; Rapaport, F.T.; Patel, M.A.; Papalexi, E.; et al. JAK2/IDH-mutant–driven myeloproliferative neoplasm is sensitive to combined targeted inhibition. J. Clin. Investig. 2018, 128, 789–804. [Google Scholar] [CrossRef]
- Shimizu, T.; Kubovcakoya, L.; Nienhold, R.; Zmajkovic, J.; Meyer, S.C.; Hao-Shen, H.; Geier, F.; Dirnhofer, S.; Guglielmelli, P.; Vannucchi, A.M.; et al. Loss of Ezh2 synergizes with JAK2-V617F in initiating myeloproliferative neoplasms and promoting myelofibrosis. J. Exp. Med. 2016, 213, 1479–1496. [Google Scholar] [CrossRef]
- Bartels, S.; Vogtmann, J.; Schipper, E.; Büsche, G.; Schlue, J.; Lehmann, U.; Kreipe, H. Combination of myeloproliferative neoplasm driver gene activation with mutations of splice factor or epigenetic modifier genes increases risk of rapid blastic progression. Eur. J. Haematol. 2021, 106, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Marcellino, B.K.; Hoffman, R.; Tripodi, J.; Lu, M.; Kosiorek, H.; Mascarenhas, J.; Rampal, R.K.; Dueck, A.; Najfeld, V. Advanced forms of MPNs are accompanied by chromosomal abnormalities that lead to dysregulation of TP53. Blood Adv. 2018, 2, 3581–3589. [Google Scholar] [CrossRef]
- Lundberg, P.; Karow, A.; Nienhold, R.; Looser, R.; Hao-Shen, H.; Nissen, I.; Girsberger, S.; Lehmann, T.; Passweg, J.; Stern, M.; et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood 2014, 123, 2220–2228. [Google Scholar] [CrossRef]
- Rampal, R.; Ahn, J.; Abdel-Wahab, O.; Nahas, M.; Wang, K.; Lipson, D.; Otto, G.A.; Yelensky, R.; Hricik, T.; McKenney, A.S.; et al. Genomic and functional analysis of leukemic transformation of myeloproliferative neoplasms. Proc. Natl. Acad. Sci. USA 2014, 111, E5401–E5410. [Google Scholar] [CrossRef]
- Benton, C.B.; Boddu, P.C.; DiNardo, C.D.; Bose, P.; Wang, F.; Assi, R.; Pemmaraju, N.; Kc, D.; Pierce, S.; Patel, K.; et al. Janus kinase 2 variants associated with the transformation of myeloproliferative neoplasms into acute myeloid leukemia. Cancer 2019, 125, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Celik, H.; Koh, W.K.; Kramer, A.C.; Ostrander, E.L.; Mallaney, C.; Fisher, D.A.C.; Xiang, J.; Wilson, W.C.; Martens, A.; Kothari, A.; et al. JARID2 functions as a tumor suppressor in myeloid neoplasms by repressing self-renewal in hematopoietic progenitor cells. Cancer Cell 2018, 34, 741–756.e8. [Google Scholar] [CrossRef]
- Marinaccio, C.; Suraneni, P.; Celik, H.; Volk, A.; Wen, Q.J.; Ling, T.; Bulic, M.; Lasho, T.; Koche, R.P.; Famulare, C.A.; et al. LKB1/STK11 is a tumor suppressor in the progression of myeloproliferative neoplasms. Cancer Discov. 2021, 11, 1398–1410. [Google Scholar] [CrossRef]
- Guglielmelli, P.; Lasho, T.L.; Rotunno, G.; Score, G.; Mannarelli, C.; Pancrazzi, A.; Biamonte, F.; Pardanani, A.; Zoi, K.; Reiter, A.; et al. The number of prognostically detrimental mutations and prognosis in primary myelofibrosis: An international study of 797 patients. Leukemia 2018, 28, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.J.; Panzarella, T.; Kennedy, J.A.; Arruda, A.; Claudio, J.O.; Daher-Reyes, G.; Ho, J.; Siddiq, N.; Devlin, R.; Tsui, H.; et al. The mutational landscape of accelerated and blast-phase myeloproliferative neoplasms impacts patient outcomes. Blood Adv. 2018, 2, 2658–2671. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.P.S.; Getta, B.; Masarova, L.; Famulare, C.; Schulman, J.; Datoguia, T.S.; Puga, R.D.; de Melo Alves Paiva, R.; Arcila, M.E.; Hamerschiak, N.; et al. Prognostic impact of RAS-pathway mutations in patients with myelofibrosis. Leukemia 2020, 34, 799–810. [Google Scholar] [CrossRef]
- Coltro, G.; Rotunno, G.; Mannelli, L.; Mannarelli, C.; Fiaccabrino, S.; Romagnoli, S.; Bartalucci, N.; Ravenda, E.; Gelli, E.; Sant’Antonio, E.; et al. RAS/CBL mutations predict resistance to JAK inhibitors in myelofibrosis and are associated with poor prognostic features. Blood Adv. 2020, 4, 3677–3687. [Google Scholar] [PubMed]
- Tefferi, A.; Lasho, T.L.; Finke, C.M.; Knudson, R.A.; Ketterling, R.; Hanson, C.H.; Maffioli, M.; Caramazza, D.; Passamonti, F.; Pardanani, A. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: Clinical, cytogenetic and molecular comparisons. Leukemia 2014, 28, 1472–1477. [Google Scholar] [CrossRef]
- Kuykendall, A.T.; Tatalati, C.; Padron, E.; Sweet, K.; Lancet, J.E.; List, A.F.; Sallman, D.; Komrojki, R.S. Driver mutation-specific clinical and genomic correlates differ between primary and secondary myelofibrosis. Am. J. Hematol. 2019, 94, E314–E317. [Google Scholar] [CrossRef]
- Guglielmelli, P.; Barosi, G.; Speechia, G.; Rambaldi, A.; Lo Coco, F.; Antonioli, E.; Pieri, L.; Pancrazzi, A.; Ponziani, V.; Delaini, F.; et al. Identification of patients with poorer survival in primary myelofibrosis based on the burden of JAK2V617F mutated allele. Blood 2009, 114, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Rumi, E.; Pietra, D.; Pascutto, C.; Guglielmelli, P.; Martinez-Trillos, A.; Casetti, I.; Colomer, D.; Pieri, L.; Pratcorona, M.; Rotunno, G.; et al. Clinical effect of driver mutations of JAK2, CALR or MPL in primary myelofibrosis. Blood 2014, 124, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yamamoto, S.; Sheng, M.; Bai, J.; Zhang, P.; Chen, R.; Chen, S.; Shi, L.; Abdel-Wahab, O.; Xu, M.; et al. ASXL1 plays an important role in erythropoiesis. Sci. Rep. 2016, 6, 28789. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, O.; Gao, J.; Adli, M.; Dey, A.; Trimarchi, T.; Chung, Y.R.; Kuscu, C.; Hricik, T.; Ndiaye-Lobry, D.; Lafave, L.M.; et al. Deletion of Asxl1 results in myelodysplasia and severe developmental defects in vivo. J. Exp. Med. 2013, 210, 2641–2659. [Google Scholar] [CrossRef] [PubMed]
- Komeno, Y.; Huang, Y.-J.; Qiu, J.; Lin, L.; Xu, Y.; Zhou, Y.; Chen, L.; Monterozza, D.D.; Li, H.; DeKelver, R.C.; et al. SRSF2 is essential for hematopoiesis, and its myelodysplastic syndrome-related mutations dysregulate alternative pre-mRNA splicing. Mol. Cell Biol. 2015, 35, 3071–3082. [Google Scholar] [PubMed]
- Liang-Fei, D.; Zhen, T.; Durham, B.; Ferrarone, J.; Zhang, T.; Garrett, L.; Yoshimi, A.; Sbdel-Wahab, O.; Bradley, R.K.; Liu, P.; et al. Impaired hematopoiesis and leukemia development in mice with a conditional knock-in allele of a mutant splicing factor gene U2af1. Proc. Nat. Acad. Sci. USA 2018, 115, E10437–E10446. [Google Scholar] [CrossRef] [PubMed]
- Damm, F.; Kosmider, O.; Gelsi-Boyer, V.; Renneville, A.; Carbuccia, N.; Hidalgo-Curtis, C.; Della Valle, V.; Couronné, L.; Scourzic, L.; Chesnais, V.; et al. Mutations affecting mRNA spicing define distinct clinical phenotypes and correlate with patient outcome in myelodysplastic syndromes. Blood 2012, 119, 3211–3218. [Google Scholar] [CrossRef]
- Aujla, A.; Linder, K.; Iragavarapu, C.; Karass, M.; Liu, D. SRSF2 mutations in myelodysplasia/myeloproliferative neoplasms. Biomark. Res. 2018, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Senapati, J.; Verstovsek, S.; Masarova, L.; Pemmaraju, N.; Patel, K.P.; Montalban-Bravo, G.; Pierce, S.A.; Zhou, L.; Garcia-Manero, G.; Kantarjian, H.M.; et al. Impact of SF3B1 mutation in myelofibrosis. Leuk. Lymphoma 2022, 63, 2701–2705. [Google Scholar] [CrossRef]
- Tefferi, A.; Finke, C.M.; Lasho, T.L.; Wassie, E.A.; Knudson, R.; Ketterling, R.P.; Hanson, C.A.; Pardanani, A. U2AF1 mutations in primary myelofibrosis are strongly associated with anemia and thrombocytopenia despite clustering with JAK2V617F and normal karyotype. Leukemia 2014, 28, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Finke, C.M.; Lasho, T.L.; Hanson, C.A.; Ketterling, R.P.; Gangat, N.; Pardanani, A. U2AF1 mutation types in primary myelofibrosis: Phenotypic and prognostic distinctions. Leukemia 2018, 32, 2274–2278. [Google Scholar] [CrossRef] [PubMed]
- Kuykendall, A.T.; Mo, Q.; Sallman, D.A.; Al Ali, N.; Chan, O.; Yun, S.; Sweet, K.L.; Padron, E.; Lancet, J.E.; Komrokji, R.S. Disease-related thrombocytopenia in myelofibrosis is defined by distinct genetic etiologies and is associated with unique prognostic correlates. Cancer 2022, 128, 3495–3501. [Google Scholar] [CrossRef] [PubMed]
- Masarova, L.; Bose, P.; Pemmaraju, N.; Daver, N.G.; Zhou, L.; Pierce, S.; Sasaki, K.; Kantarjian, H.; Estrov, Z.; Verstovsek, S. Prognostic value of blasts in peripheral blood in myelofibrosis in the ruxolitinib era. Cancer 2020, 126, 4322–4331. [Google Scholar] [CrossRef] [PubMed]
- Courtier, F.; Garnier, S.; Carbuccia, N.; Guille, A.; Adélaide, J.; Chaffanet, M.; Hirsch, P.; Paz, D.L.; Slama, B.; Vey, N.; et al. Targeted molecular characterization shows differences between primary and secondary myelofibrosis. Genes Chromosomes Cancer 2019, 59, 30–39. [Google Scholar] [CrossRef]
- Guglielmelli, P.; Biamonte, F.; Score, J.; Hidalgo-Curtis, C.; Cervantes, F.; Maffiloi, M.; Fanelli, T.; Ernst, T.; Winkelman, N.; Jones, A.V.; et al. EZH2 mutational status predicts poor survival in myelofibrosis. Blood 2011, 118, 5227–5234. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, Y.; Zhao, Y.; Yang, Y.; Cai, Y.; Zhang, P.; Xu, Z.; Qin, T.; Qu, S.; Pan, L.; et al. ASXL1 mutations accelerate bone marrow fibrosis vis EGR1-TNFA axis mediated neoplastic fibrocyte generation in myeloproliferative neoplasms. Haematologica 2023, 108, 1359–1373. [Google Scholar] [CrossRef]
- Yan, X.; Xu, Z.; Zhang, P.; Sun, Q.; Jia, Y.; Qu, S.; Pan, L.; Li, Z.; Liu, J.; Song, Z.; et al. Non-driver mutations landscape in different stages of primary myelofibrosis determined ASXL1 mutations play a critical role in disease progression. Blood Cancer J. 2023, 13, 56. [Google Scholar] [CrossRef]
- Marcault, C.; Zhao, L.-P.; Maslah, N.; Verger, E.; de Oliveira, R.D.; Soret-Dulphy, J.; Cazaux, M.; Gauthier, N.; Roux, B.; Clappier, E.; et al. Impact of NFE2 mutations on AML transformation and overall survival in patients with myeloproliferative neoplasms. Blood 2021, 138, 2141–2148. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Patel, K.P.; Kantarjian, H.; Luthra, R.; Pierce, S.; Cortes, J.; Verstovsek, S. JAK2 p.617F detection and allele burden measurement in peripheral blood and bone marrow aspirates in patients with myeloproliferative neoplasms. Blood 2013, 122, 3784–3786. [Google Scholar] [CrossRef]
- Grinfeld, J.; Nangalia, J.; Green, A.R. Molecular determinants of pathogenesis and clinical phenotype in myeloproliferative neoplasms. Haematologica 2017, 102, 7–17. [Google Scholar] [CrossRef]
- Senín, A.; Fernández-Rodríguez, C.; Bellosillo, B.; Camacho, L.; Longarón, R.; Angona, A.; Besses, C.; Álvarez-Larrán, A. Non-driver mutations in patients with JAK2V617F-mutated polycythemia vera or essential thrombocythemia with long-term molecular follow-up. Ann. Hematol. 2018, 97, 443–451. [Google Scholar] [CrossRef]
- Barosi, G.; Bergamaschi, G.; Vannucchi, A.M.; Guglielmelli, P.; Antonioli, E.; Massa, M.; Rosti, V.; Campanelli, R.; Villani, L.; Viarengo, G.; et al. JAK2V617F mutational status predicts progression to large splenomegaly and leukemic transformation in primary myelofibrosis. Blood 2007, 110, 4030–4036. [Google Scholar] [CrossRef] [PubMed]
- Rozovski, U.; Verstovsek, S.; Manshouri, T.; Dembitz, V.; Bozinovic, K.; Newberry, K.; Zhang, Y.; Bove, J.E.; Pierce, S.; Kantarjian, H.; et al. An accurate simple prognostic model consisting of age, JAK2, CALR and MPL mutation status for patients with primary myelofibrosis. Haematologica 2017, 102, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Lasho, T.L.; Huang, J. Low JAK2V617F allele burden in primary myelofibrosis, compared to either a higher allele burden or unmutated status, is associated with inferior overall and leukemia-free survival. Leukemia 2008, 22, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Guglielmelli, P.; Maccari, C.; Sordi, B.; Balliu, M.; Atanasio, A.; Mannarelli, C.; Capecchi, G.; Sestini, I.; Coltro, G.; Loscocco, G.G.; et al. Phenotypic correlations of CALR mutation variant allele frequency in patients with myelofibrosis. Blood Cancer J. 2023, 13, 21. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Han, Y.; Jang, J.H.; Jung, C.W.; Kim, S.-H.; Kim, H.-J. Effects of CALR-mutant type and burden on the phenotype of myeloproliferative neoplasms. Diagnostics 2022, 12, 2570. [Google Scholar] [CrossRef]
- Loscocco, G.G.; Guglielmelli, P.; Vannucchi, A.M. Impact of mutational profile on the management of myeloproliferative neoplasms: A short review of the emerging data. OncoTargets Ther. 2020, 13, 12367–12382. [Google Scholar] [CrossRef]
- Vannucchi, A.M.; Guglielmelli, P. Molecular prognostication in Ph-negative MPNs in 2022. Hematol. Am. Soc. Hematol. Educ. Program 2022, 2022, 225–234. [Google Scholar] [CrossRef]
- Passamonti, F.; Mora, B. Myelofibrosis. Blood 2023, 141, 1954–1970. [Google Scholar] [CrossRef]
- How, J.; Hobbs, G.S. A practical guide for using myelofibrosis prognostic models in the clinic. J. Natl. Camp. Netw. 2020, 18, 1271–1278. [Google Scholar] [CrossRef]
- Mosquera-Orgueira, A.; Pérez-Encinas, M.; Hernández-Sánchez, A.; González-Martinez, T.; Arellano-Rodrigo, E.; Martínez-Elicegui, J.; Villaverde-Ramiro, A.; Raya, J.-M.; Ferrer-Marin, F.; Fox, M.-L.; et al. Machine learning improves risk stratification in myelofibrosis: An analysis of the Spanish, Registry of Myelofibrosis. HemaSphere 2023, 7, e818. [Google Scholar] [CrossRef]
- Barbui, T.; Ghirardi, A.; Carobbio, A.; Masciulli, A.; Carioli, G.; Rambaldi, A.; Finazzi, M.C.; Bellini, M.; Rumi, E.; Vanni, D.; et al. Increased risk of thrombosis in JAK2 V617F-positive patients with primary myelofibrosis and interaction of the mutation with the IPSS score. Blood Cancer J. 2022, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Chifotides, H.T.; Masarova, L.; Alfayez, M.; Daver, N.; Alvarado, Y.; Jabbour, E.; Konopleva, M.; Kantarjian, M.; Patel, K.P.; DiNardo, C.D.; et al. Outcome of patients with IDH1/2-mutated post-myeloproliferative neoplasm AML in the era of IDH inhibitors. Blood Adv. 2020, 4, 5336–5342. [Google Scholar] [CrossRef] [PubMed]
- Bewersdorf, J.P.; Rampal, R.K. Hitting the brakes on accelerated and blast-phase myeloproliferative neoplasms: Current and emerging concepts. Hematol. Am. Soc. Hematol. Educ. Program 2022, 2022, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.A.; Cahill, K.; Charnot-Katsikas, A.; Liu, H.; Gurbuxani, S.; Thirman, M.; Kosuri, S.; Artz, A.S.; Larson, R.A.; Stock, W.; et al. Clinical outcomes of IDH2-mutated advanced phase Ph-negative myeloproliferative neoplasm treated with enasidenib. Br. J. Haematol. 2020, 190, e48–e51. [Google Scholar] [CrossRef] [PubMed]
- Bar-Natan, M.; Mascarenhas, J.; Gerds, A.T.; Mesa, R.; Gupta, V.; Kremyanskaya, M.; Dougherty, M.; Fabris, F.; Johnson, K.; Yu, A.; et al. Molecularly targeted combination therapy for advanced phase myeloproliferative neoplasm: MPN-RC 119. Blood 2022, 140 (Suppl. 1), 3988–3990. [Google Scholar] [CrossRef]
- Rolles, B.; Mullally, A. Molecular pathogenesis of myeloproliferative neoplasms. Curr. Hematol. Malig. Rep. 2022, 17, 319–329. [Google Scholar] [CrossRef]
- Vachhani, P.; Verstovsek, S.; Bose, P. “Disease modification” in myelofibrosis: An elusive goal? J. Clin. Oncol. 2022, 40, 1147–1154. [Google Scholar] [CrossRef]
- Rumi, E.; Trotti, C.; Vanni, D.; Casetti, I.C.; Pietra, D.; Sant’Antonio, E. The genetic basis of primary myelofibrosis and its clinical relevance. Int. J. Mol. Sci. 2020, 21, 8885. [Google Scholar] [CrossRef]
- Bose, P.; Verstovsek, S. SOHO State of the Art, Updates and Next, Questions: Identifying and treating “progression” in myelofibrosis. Clin. Lymphoma Myeloma Leuk. 2021, 21, 641–649. [Google Scholar] [CrossRef]
- Bose, P.; Verstovsek, S. JAK inhibition for the treatment of myelofibrosis: Limitations and future perspectives. HemaSphere 2020, 4, e424. [Google Scholar] [CrossRef]
- Gerds, A.; Bose, P.; Hobbs, G.S.; Kuykendall, A.T.; Neilson, L.M.; Song, J.; Klencke, B.; Harrison, C.N. Treating anemic patients with myelofibrosis in the new Janus kinase inhibitor era: Current evidence and real-world implications. HemaSphere 2022, 6, e778. [Google Scholar] [CrossRef] [PubMed]
- Bose, P.; Verstovsek, S. Management of myelofibrosis-related cytopenias. Curr. Hematol. Malig. Rep. 2018, 13, 164–172. [Google Scholar] [CrossRef]
- Verstovsek, S. How I manage anemia related to myelofibrosis and its treatment regimens. Ann. Hematol. 2023, 102, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, F.; Harrison, C.N.; Mesa, R.A. Anemia in myelofibrosis: Current and emerging treatment options. Crit. Rev. Oncol. Hematol. 2022, 180, 103862. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Boluda, J.-C.; Correa, J.-G.; Alvarez-Larrán, A.; Ferrer-Marín, F.; Raya, J.-M.; Martinez-López, J.; Velez, P.; Pérez-Encinas, M.; Estrada, N.; García-Gutiérrez, V.; et al. Clinical characteristics, prognosis and treatment of myelofibrosis patients with severe thrombocytopenia. Br. J. Haematol. 2018, 181, 397–400. [Google Scholar] [CrossRef]
- Coltro, G.; Mannelli, F.; Loscocco, G.G.; Mannarelli, C.; Rotunno, G.; Maccari, C.; Pancani, F.; Atanasio, A.; Vannucchi, A.M.; Guglielmelli, P. Differential prognostic impact of cytopenic phenotype in prefibrotic vs overt primary myelofibrosis. Blood Cancer J. 2022, 12, 116. [Google Scholar] [CrossRef]
- Naymagon, L.; Mascarenhas, J. Myelofibrosis-related anemia: Current and emerging therapeutic strategies. HemaSphere 2017, 1, e1. [Google Scholar] [CrossRef]
- Nicolosi, M.; Mudireddy, M.; Lasho, T.L.; Hanson, C.A.; Kettering, R.P.; Gangat, N.; Pardanani, A.; Tefefri, A. Sex and degree of severity influence the prognostic impact of anemia in primary myelofibrosis: Analysis based on 1109 consecutive patients. Leukemia 2018, 32, 1254–1258. [Google Scholar] [CrossRef]
- Elena, C.; Passamonti, F.; Rumi, E.; Malcovati, L.; Arcaini, L.; Boveri, E.; Merli, M.; Pietra, D.; Pascutto, C.; Lazzarino, M. Red blood cell transfusion-dependency implies a poor survival in primary myelofibrosis irrespective of IPSS and DIPSS. Haematologica 2011, 96, 167–170. [Google Scholar] [CrossRef]
- Tefferi, A.; Lasho, T.L.; Jimma, T.; Finke, C.M.; Gangat, N.; Vaidya, R.; Begna, K.H.; Al-Kali, A.; Ketterling, R.P.; Hanson, C.A.; et al. One thousand patients with primary myelofibrosis: The Mayo clinic experience. Mayo Clin. Proc. 2012, 87, 25–33. [Google Scholar] [CrossRef]
- Masarova, L.; Alhuraiji, A.; Bose, P.; Daver, N.; Pemmaraju, N.; Cortes, J.; Pierce, S.; Kantarjian, H.; Verstovsek, S. Significance of thrombocytopenia in patients with primary and post-essential thrombocythemia/polycythemia vera myelofibrosis. Eur. J. Haematol. 2018, 100, 257–263. [Google Scholar] [CrossRef]
- Sastow, D.; Mascarenhas, J.; Tremblay, D. Thrombocytopenia in patients with myelofibrosis: Pathogenesis, prevalence, prognostic impact, and treatment. Clin. Lymphoma Myeloma Leuk. 2022, 22, e507–e520. [Google Scholar] [CrossRef]
- Tam, C.S.; Kantarjian, H.; Cortes, J.; Lynn, A.; Pierce, S.; Zhou, L.; Keating, M.J.; Thomas, D.A.; Verstovsek, S. Dynamic model for predicting death within 12 months in patients with primary or post-polycythemia vera/essential thrombocythemia myelofibrosis. J. Clin. Oncol. 2009, 27, 5587–5593. [Google Scholar] [CrossRef]
- Patel, K.P.; Newberry, K.J.; Luthra, R.; Jabbour, E.; Pierce, S.; Cortes, J.; Singh, R.; Mehrotra, M.; Routbort, M.J.; Luthra, M.; et al. Correlation of mutation profile and response in patients with myelofibrosis treated with ruxolitinib. Blood 2015, 126, 790–797. [Google Scholar] [CrossRef]
- Spiegel, J.Y.; McNamara, C.; Kennedy, J.A.; Panzarella, T.; Arruda, A.; Stockley, T.; Sukhai, M.; Thomas, M.; Bartoszko, J.; Ho, J.; et al. Impact of genomic alterations on outcomes in myelofibrosis patients receiving JAK1/2 inhibitor therapy. Blood 2017, 1, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Pacilli, A.; Rotunno, G.; Mannarelli, C.; Fanelli, T.; Panrazzi, A.; Contini, E.; Mannelli, F.; Gesullo, F.; Bartalucci, N.; Corbizi Fattori, G.; et al. Mutation landscape in patients with myelofibrosis receiving ruxolitinib or hydroxyurea. Blood Cancer J. 2018, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Newberry, K.J.; Patel, K.P.; Masarova, L.; Luthra, R.; Manshouri, T.; Jabbour, E.; Bose, P.; Daver, N.; Cortes, J.; Kantarjian, H.; et al. Clonal evolution and outcomes in myelofibrosis after ruxolitinib discontinuation. Blood 2017, 130, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Barosi, G.; Klersy, C.; Villani, L.; Bonetti, E.; Catarsi, P.; Poletto, V.; Campanelli, R.; Impera, S.; Latagliata, R.; Viarengo, G.; et al. JAK2(V617F) allele burden ≥ 50% is associated with response to ruxolitinib in persons with MPN-associated myelofibrosis and splenomegaly requiring therapy. Leukemia 2016, 30, 1772–1775. [Google Scholar] [CrossRef] [PubMed]
- Palandri, F.; Bartoletti, D.; Breccia, M.; Auteri, G.; Elli, E.M.; Trawinska, M.M.; Polverelli, N.; Tiribelli, M.; Benevolo, G.; Iurlo, A.; et al. P1010: Ruxolitinib in myelodepletive myelofibrosis: Response, toxicity and outcome. HemaSphere 2022, 6, 900–901. [Google Scholar] [CrossRef]
- Palandri, F.; Breccia, M.; Mazzoni, C.; Auteri, G.; Elli, E.M.; Trawinska, M.M.; Polverelli, N.; Tiribelli, M.; Benevolo, G.; Iurlo, A.; et al. Ruxolitinib in cytopenic myelofibrosis: Response, toxicity, drug discontinuation, and outcome. Cancer 2023, 129, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Guglielmelli, P.; Kiladjian, J.-J.; Vannucchi, A.M.; Duan, M.; Meng, H.; Pan, L.; He, G.; Verstovsek, S.; Boyer, F.; Barraco, F.; et al. Efficacy and safety of ruxolitinib in patients with myelofibrosis and low platelet count (50 × 109/L to < 100 × 109/L) at baseline: The final analysis of EXPAND. Ther. Adv. Hematol. 2022, 13, 20406207221118429. [Google Scholar] [CrossRef] [PubMed]
- Al-Ali, H.K.; Griesshammer, M.; Foltz, L.; Palumbo, G.A.; Martino, B.; Palandri, F.; Liberati, A.M.; le Coutre, P.; García-Hernández, C.; Zaritskey, A.; et al. Primary analysis of JUMP a phase 3b, expanded-access study evaluating the safety and efficacy of ruxolitinib in patients with myelofibrosis, including those with low platelet counts. Br. J. Haematol. 2020, 189, 888–903. [Google Scholar] [CrossRef]
- Harrison, C.N.; Schaap, N.; Vannucchi, A.M.; Kiladjian, J.-J.; Passamonti, F.; Zweegman, S.; Talpaz, M.; Verstovsek, S.; Rose, S.; Zhang, J.; et al. Safety and efficacy of fedratinib, a selective oral inhibitor of Janus kinase-2 (JAK2), in patients with myelofibrosis and low pretreatment platelet counts. Br. J. Haematol. 2022, 198, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Palandri, F.; Breccia, M.; Bonifacio, M.; Polverelli, N.; Elli, E.M.; Benevolo, G.; Tribelli, M.; Iurlo, A.; Heidel, F.H.; Bergamaschi, M.; et al. Life after ruxolitinib: Reasons for discontinuation, impact of disease phase, and outcomes in 218 patients with myelofibrosis. Cancer 2020, 126, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Maffioli, M.; Mora, B.; Ball, S.; Iurlo, A.; Elli, E.M.; Finazzi, M.C.; Polverelli, N.; Rumi, E.; Caramella, M.; Carraro, M.C.; et al. A prognostic model to predict survival after 6 months of ruxolitinib in patients with myelofibrosis. Blood Adv. 2022, 6, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Chifotides, H.T.; Bose, P.; Masarova, L.; Pemmaraju, N.; Verstovsek, S. SOHO State of the Art, Updates and Next, Questions: Novel therapies in development for myelofibrosis. Clin. Lymphoma Myeloma Leuk. 2022, 22, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Chifotides, H.T.; Masarova, L.; Verstovsek, S. SOHO State of the Art, Updates and Next, Questions: Novel, therapeutic strategies in development for myelofibrosis. Clin. Lymphoma Myeloma Leuk. 2023, 23, 219–231. [Google Scholar] [CrossRef]
- How, J.; Garcia, J.S.; Mullally, A. Biology and therapeutic targeting of molecular mechanisms in MPNs. Blood 2023, 141, 1922–1933. [Google Scholar] [CrossRef]
- Waksal, J.A.; Mascarenhas, J. Novel therapies in myelofibrosis: Beyond, JAK myelofibrosis. Curr. Hematol. Malig. Rep. 2022, 17, 140–154. [Google Scholar] [CrossRef]
- Bose, P.; Kuykendall, A.T.; Miller, K.; Kurtin, S.; Farina, K.; Harting, D.M.; Mascarenhas, J.O.; Mesa, R.A. Moving beyond ruxolitinib failure in myelofibrosis: Evolving strategies for second line therapy. Expert Opin. Pharmacother. 2023, 24, 1091–1100. [Google Scholar] [CrossRef]
- Oh, S.T.; Talpaz, M.; Gerds, A.T.; Gupta, V.; Verstovsek, S.; Mesa, R.; Miller, C.B.; Rivera, C.E.; Fleischman, A.G.; Goel, S.; et al. ACVR1/JAK1/JAK2 inhibitor momelotinib reverses transfusion dependency and suppresses hepcidin in myelofibrosis phase 2 trial. Blood Adv. 2020, 4, 4282–4291. [Google Scholar] [CrossRef] [PubMed]
- Chifotides, H.T.; Bose, P.; Verstovsek, S. Momelotinib: An emerging treatment for myelofibrosis patients with anemia. J. Hematol. Oncol. 2021, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.T.; Mesa, R.; Harrison, C.; Bose, P.; Gerds, A.T.; Heaney, M.L.; Gupta, V.; Scott, B.L.; Kiladjian, J.-J.; Lucchesi, A.; et al. Pacritinib is a potent ACVR1 inhibitor with significant anemia benefit in patients with myelofibrosis. Blood 2022, 140 (Suppl. 1), 1518–1521. [Google Scholar] [CrossRef]
- Mascarenhas, J. Pacritinib for the treatment of patients with myelofibrosis and thrombocytopenia. Exp. Rev. Hematol. 2022, 15, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, S.; Mascarenhas, J. The, Odyssey of pacritinib in myelofibrosis. Blood Adv. 2022, 6, 4905–4913. [Google Scholar] [CrossRef]
- Mascarenhas, J.; Hoffman, R.; Talpaz, M.; Gerds, A.T.; Stein, B.; Gupta, V.; Szoke, A.; Drummond, M.; Pristupa, A.; Granston, T.; et al. Pacritinib vs. best available therapy, including ruxolitinib, in patients with myelofibrosis: A randomized clinical trial. JAMA Oncol. 2018, 4, 652–659. [Google Scholar] [CrossRef]
- Gerds, A.T.; Savona, M.R.; Scott, B.L.; Talpaz, M.; Egyed, M.; Harrison, C.N.; Yacoub, A.; Vannucchi, A.M.; Mead, A.J.; Kiladjian, J.-J.; et al. Determining the recommended dose of pacritinib: Results from the PAC203 dose-finding trial in advanced myelofibrosis. Blood Adv. 2020, 4, 5825–5835. [Google Scholar] [CrossRef]
- Mesa, R.A.; Vannucchi, A.M.; Mead, A.; Egyed, M.; Szoke, A.; Suvorov, A.; Jakucs, J.; Perkins, A.; Prasad, R.; Mayer, J.; et al. Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): An international, randomised, phase 3 trial. Lancet Haematol. 2017, 4, e225–e236. [Google Scholar] [CrossRef]
- Vannucchi, A.M.; Mead, A.; Egyed, M.; Szoke, A.; Suvorov, A.; Perkins, A.; Mayer, J.; Ganly, P.; Schouten, H.C.; Tosi, P.; et al. Relationship of JAK2V617F allelic burden (AB) to demographics, disease characteristics, and response to therapy in PERSIST-1, a randomized phase III study of pacritinib (PAC) versus best available therapy (BAT) in patients (pts) with primary and secondary myelofibrosis (MF). Blood 2016, 128, 3131. [Google Scholar] [CrossRef]
- Verstovsek, S.; Scott, B.L.; Taylor, J.A.; Mascarenhas, J. The oral JAK2/IRAK1 inhibitor pacritinib demonstrates spleen volume reduction in myelofibrosis patients independent of JAK2V617F allele burden. Blood 2019, 134 (Suppl. 1), 1674. [Google Scholar] [CrossRef]
- Verstovsek, S.; Mesa, R.; Talpaz, M.; Kiladjian, J.-J.; Harrison, C.N.; Oh, S.T.; Vannucchi, A.M.; Rampal, R.; Scott, B.L.; Buckley, S.A.; et al. Retrospective analysis of pacritinib in patients with myelofibrosis and severe thrombocytopenia. Haematologica 2022, 107, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, J.; Gerds, A.T.; Kiladjian, J.-J.; Dohner, K.; Buckley, S.; Smith, J.; Craig, A.; Singh, S.; Verstovsek, S.; Harrison, C. MPN-038, PACIFICA: A randomized, controlled phase 3 study of pacritinib versus physician’s choice in patients with primary or secondary myelofibrosis with severe thrombocytopenia. Clin. Lymphoma Myeloma Leuk. 2021, 21, S352–S353. [Google Scholar] [CrossRef]
- Mesa, R.; Hudgens, S.; Floden, L.; Harrison, C.N.; Palmer, J.; Gupta, V.; McLornan, D.P.; McMullin, M.F.; Kiladjian, J.-J.; Foltz, L.; et al. Symptomatic benefit of momelotinib in patients with myelofibrosis: Results from the SIMPLIFY phase III studies. Cancer Med. 2023, 12, 10612–10624. [Google Scholar] [CrossRef] [PubMed]
- Verstovsek, S.; Chen, C.-C.; Egyed, M.; Ellis, M.; Fox, L.; Goh, Y.T.; Gupta, V.; Harrison, C.; Kiladjian, J.-J.; Lazaroiu, M.C.; et al. MOMENTUM: Momelotinib vs. danazol in patients with myelofibrosis previously treated with JAKi who are symptomatic and anemic. Future Oncol. 2021, 17, 1449–1458. [Google Scholar] [CrossRef]
- Verstovsek, S.; Gerds, A.T.; Vannucchi, A.M.; Al-Ali, H.K.; Lavie, D.; Kuykendall, A.T.; Grosicki, S.; Iurlo, A.; Goh, Y.T.; Lazaroiu, M.C.; et al. Momelotinib versus danazol in symptomatic patients with anaemia and myelofibrosis (MOMENTUM): Results from an international, double-blind, randomised, controlled phase 3 study. Lancet 2023, 401, 269–280. [Google Scholar] [CrossRef]
- Mesa, R.A.; Kiladjian, J.-J.; Catalano, J.V.; Devos, T.; Egyed, M.; Hellman, A.; McLornan, D.; Shimoda, K.; Winton, E.F.; Deng, W.; et al. SIMPLIFY-1, A phase III randomized trial of momelotinib versus ruxolitinib in Janus kinase inhibitor-naïve patients with myelofibrosis. J. Clin. Oncol. 2017, 35, 3844–3850. [Google Scholar] [CrossRef]
- Harrison, C.N.; Vannucchi, A.M.; Platzbecker, U.; Cervantes, F.; Gupta, V.; Lavie, D.; Passamonti, F.; Winton, E.F.; Dong, H.; Kawashima, J.; et al. Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY 2): A randomized, open-label, phase 3 trial. Lancet Haematol. 2018, 5, e73–e81. [Google Scholar] [CrossRef]
- Verstovsek, S.; Mesa, R.A.; Gupta, V.; Lavie, D.; Dubruille, V.; Cambier, N.; Platzbecker, U.; Hus, M.; Xicoy, B.; Oh, S.T.; et al. Momelotinib long-term safety and survival in myelofibrosis: Integrated analysis of phase 3 randomized-controlled trials. Blood Adv. 2023, in press. [Google Scholar] [CrossRef]
- Kiladjian, J.-J.; Platzbecker, U.; Mayer, J.; Illés, A.; Prejzner, W.; Wozny, T.; Tzvetkov, N.; Vannucchi, A.M.; Kirgner, I.; Nagy, Z.; et al. Momelotinib’s spleen, symptom and anemia efficacy is maintained in intermediate/high-risk myelofibrosis patients with thrombocytopenia. Blood 2020, 136 (Suppl. 1), 43–44. [Google Scholar] [CrossRef]
- Gerds, A.; Verstovsek, S.; Vannucchi, A.; Al-Ali, H.K.; Lavie, D.; Kuykendall, A.; Grosicki, S.; Iurlo, A.; Goh, Y.T.; Lazaroiu, M.; et al. MPN-483 Thrombocytopenic myelofibrosis (MF) patients previously treated with a JAK inhibitor in a phase 3 randomized study of momelotinib (MMB) versus danazol (DAN) [MOMENTUM]. Clin. Lymphoma Myeloma Leuk. 2022, 22 (Suppl. 2), S340. [Google Scholar] [CrossRef]
- Hatzimichael, E.; Timotheatou, D.; Koumpis, E.; Benetatos, L.; Makris, A. Luspatercept: A new tool for the treatment of anemia related to b-thalassemia, myelodysplastic syndromes and primary myelofibrosis. Diseases 2022, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Gerds, A.T.; Vannucchi, A.M.; Passamonti, F.; Kremyanskaya, M.; Gotlib, J.; Palmer, J.M.; McCaul, K.; Ribrag, V.; Mead, A.J.; Harrison, C.; et al. Duration of response to luspatercept in patients (pts) requiring red blood cell (RBC) transfusions with myelofibrosis (MF)—Updated data from the phase 2 ACE-536-MF-001 study. Blood 2020, 136 (Suppl. 1), 47–48. [Google Scholar] [CrossRef]
- Mesa, R.; Barosi, G.; Harrison, C.; Kiladjian, J.-J.; Verstovsek, S.; Gerike, T.G.; Chia, V.; Shetty, J.K.; Wang, Y.; Marks, H.; et al. Efficacy and safety of luspatercept versus placebo in patients with myeloproliferative neoplasm-associated myelofibrosis on JAK2 inhibitor therapy and requiring RBC transfusions (INDEPENDENCE trial). HemaSphere 2021, 6 (Suppl. 2), 805–806. [Google Scholar]
- Verstovsek, S.; Al-Ali, H.K.; Mascarenhas, J.; Perkins, A.; Vannucchi, A.M.; Mohan, S.R.; Scott, B.L.; Woszczyk, D.; Koschmieder, S.; García-Delgado, R.; et al. BOREAS: A global, phase III study of the MDM2 inhibitor navtemadlin (KRT-232) in relapsed/refractory myelofibrosis. Future Oncol. 2022, 18, 4059–4069. [Google Scholar] [CrossRef] [PubMed]
- Vachhani, P.; Perkins, A.; Mascarenhas, J.; Al-Ali, H.; Kiladjian, J.-J.; Cerquozzi, S.; Dybko, J.; Garcia Delgardo, R.; Hernández Rivas, J.M.; Hebart, H.; et al. Disease modifying activity of navtemadlin (nvtm) correlated with survival outcomes in Janus kinase inhibitor (JAKi) relapsed/refractory (R/R) myelofibrosis patients. HemaSphere 2023, 7, abstract S214. [Google Scholar]
- Gupta, V.; Kremyanskaya, M.; Mascarenhas, J.; Palandri, F.; Patriarca, A.; Devos, T.; Harrison, C.; Passamonti, F.; Rampal, R.; Mead, A.; et al. Clinical benefit of pelabresib (CPI-0610) in combination with ruxolitinib in JAK inhibitor treatment naïve myelofibrosis patients: Interim efficacy subgroup analysis from Arm 3 of the MANIFEST phase 2 study. Clin. Lymphoma Myeloma Leuk. 2021, 21 (Suppl. 1), S362. [Google Scholar] [CrossRef]
- Mascarenhas, J.; Kremyanskaya, M.; Patriarca, A.; Palandri, F.; Devos, T.; Passamonti, F.; Rampal, R.; Mead, A.J.; Hobbs, G.; Scandura, J.M.; et al. MANIFEST: Pelabresib in combination with ruxolitinib for Janus kinase inhibitor treatment-naïve myelofibrosis. J. Clin. Oncol. 2023, in press. [Google Scholar] [CrossRef]
- Harrison, C.; Gupta, V.; Gerds, A.T.; Rampal, R.; Verstovsek, S.; Talpaz, M.; Kiladjian, J.-J.; Mesa, R.; Kuykendall, A.T.; Vannucchi, A.M.; et al. Phase, III MANIFEST-2, Pelabresib + ruxolitinib vs. placebo + ruxolitinib in JAK-inhibitor treatment-naïve myelofibrosis. Future Oncol. 2022, 18, 2987–2997. [Google Scholar] [CrossRef]
- Pemmaraju, N.; Garcia, J.S.; Potluri, J.; Harb, J.G.; Sun, Y.; Jung, P.; Qin, Q.Q.; Tantravahi, S.K.; Verstovsek, S.; Harrison, C. Addition of navitoclax to ongoing ruxolitinib treatment in patients with myelofibrosis (REFINE): A post-hoc analysis of molecular markers in a phase 2 study. Lancet Haematol. 2022, 9, e434–e444. [Google Scholar] [CrossRef]
- Passamonti, F.; Foran, J.; Tandra, A.; De Stefano, V.; Fox, M.L.; Mattour, A.H.; McMullin, M.F.; Perkins, A.; Rodríguez-Macias, G.; Sibai, H.; et al. Navitoclax plus ruxolitinib in JAK-inhibitor naïve patients with myelofibrosis: Preliminary safety and efficacy in a multicenter, open-label phase 2 study. HemaSphere 2022, 6 (Suppl. 3), 98–99. [Google Scholar] [CrossRef]
- Passamonti, F.; Foran, J.; Tandra, A.; De Stefano, V.; Fox, M.L.; Mattour, A.H.; McMullin, M.F.; Perkins, A.C.; Rodriguez-Macias, G.; Sibai, H.; et al. The combination of navitoclax and ruxolitinib in JAK inhibitor-naïve patients with myelofibrosis mediates responses suggestive of disease modification. Blood 2022, 140 (Suppl. 1), 583–585. [Google Scholar] [CrossRef]
- Mascarenhas, J.; Harrison, C.; Kiladjian, J.-J.; Komrokji, R.S.; Koschmieder, S.; Vannucchi, A.M.; Berry, T.; Redding, D.; Sherman, L.; Dougherty, S.; et al. Imetelstat in intermediate-2 or high-risk myelofibrosis (MF) refractory to Janus kinase inhibitor: IMpactMF phase III study design. Future Oncol. 2022, 18, 2393–2402. [Google Scholar] [CrossRef]
- Mascarenhas, J.; Komrokji, R.S.; Palandri, F.; Martino, B.; Niederwieser, D.; Reiter, A.; Scott, B.L.; Baer, M.R.; Hoffman, R.; Odenike, O.; et al. Randomized, single-blind, multicenter phase II study of two doses of imetelstat in relapsed or refractory myelofibrosis. J. Clin. Oncol. 2021, 39, 2881–2892. [Google Scholar] [CrossRef] [PubMed]
- Vachhani, P.; Verstovsek, S.; Bose, P. Cytopenic myelofibrosis: Prevalence, relevance, and treatment. Expert Opin. Pharmacother. 2023, 24, 901–912. [Google Scholar] [CrossRef]
- Reynolds, S.B.; Pettit, K. New approaches to tackle cytopenic myelofibrosis. Hematol. Am. Soc. Hematol. Educ. Program 2022, 2022, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Verstovsek, S.; Gotlib, J.; Mesa, R.A.; Vannucchi, A.M.; Kiladjian, J.-J.; Cervantes, F.; Harrison, C.N.; Paquette, R.; Sun, W.; Naim, A.; et al. Long-term survival in patients treated with ruxolitinib for myelofibrosis: COMFORT-I and -II pooled analyses. J. Hematol. Oncol. 2017, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Masarova, L.; Bose, P.; Pemmaraju, N.; Daver, N.G.; Sasaki, K.; Chifotides, H.T.; Zhou, L.; Kantarjian, H.M.; Estrov, Z.; Verstovsek, S. Improved survival of patients with myelofibrosis in the last decade: Single-center experience. Cancer 2022, 128, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Verstovsek, S.; Parasuraman, S.; Yu, J.; Shah, A.; Kumar, S.; Xi, A.; Harrison, C. Real-world survival of US patients with intermediate- to high-risk myelofibrosis: Impact of ruxolitinib approval. Ann. Hematol. 2022, 101, 131–137. [Google Scholar] [CrossRef]
- Verstovsek, S.; Kiladjian, J.J.; Vannucchi, A.M.; Mesa, R.A.; Squier, P.; Hamer-Maansson, J.E.; Harrison, C. Early intervention in myelofibrosis and impact on outcomes: A pooled analysis of the COMFORT-I and COMFORT-II studies. Cancer 2023, 129, 1681–1690. [Google Scholar] [CrossRef]
- Masarova, L.; Bose, P.; Pemmaraju, N.; Daver, N.G.; Sasaki, K.; Chifotides, H.T.; Zhou, L.; Kantarjian, H.M.; Estrov, Z.; Verstovsek, S. The role of therapy in the outcome of patients with myelofibrosis. Cancer 2023, in press. [Google Scholar] [CrossRef]
- Mesa, R.; Harrison, C.; Oh, S.; Gerds, A.T.; Gupta, V.; Catalano, J.; Cervantes, F.; Devos, T.; Hus, M.; Kiladjian, J.-J.; et al. Overall survival in the SIMPLIFY-1 and SIMPLIFY-2 phase 3 trials of momelotinib in patients with myelofibrosis. Leukemia 2022, 36, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
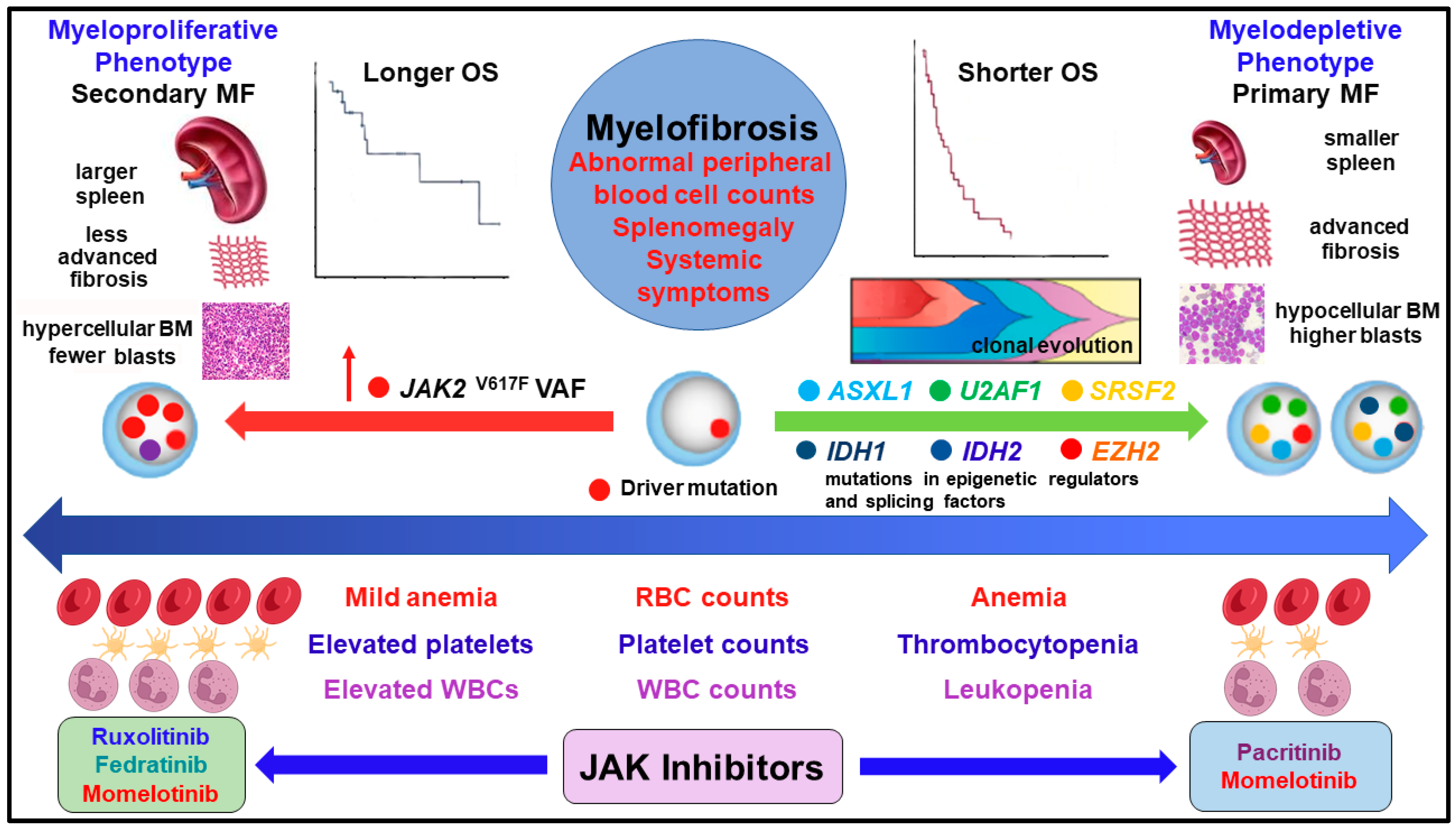
| Clinical Features | Myeloproliferative Phenotype | Myelodepletive Phenotype |
|---|---|---|
| MF subtype (not exclusive) | More secondary MF | Usually primary MF |
| Peripheral blood cell counts | Normal or mildly elevated | ≥2 cytopenias |
| RBC counts, hemoglobin | Mild or no anemia | Prominent anemia |
| Platelet counts | Normal or high | Moderate (50–100 × 109/L) or severe (< 50 × 109/L) thrombocytopenia |
| WBC counts | Leukocytosis | Leukopenia |
| RBC transfusion dependence | Usually independent or minimal | More likely to be dependent |
| Spleen volume | Larger | Smaller |
| Constitutional symptoms | Abdominal pain, night sweats | Fatigue |
| Bone marrow fibrosis grade | <2 | ≥2 |
| Bone marrow cellularity | Usually hypercellular | More likely to be hypocellular |
| JAK2 V617F VAF | Higher median (≥50%) | Lower median (<25%) |
| HMR mutations * (epigenetic or mRNA splicing) | 0–1 | Multiple |
| Blast counts | Fewer blasts | Higher blasts |
| Median overall survival | Longer | Shorter |
| Risk of leukemic transformation | Lower | Higher |
| Response to ruxolitinib | High | Limited |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chifotides, H.T.; Verstovsek, S.; Bose, P. Association of Myelofibrosis Phenotypes with Clinical Manifestations, Molecular Profiles, and Treatments. Cancers 2023, 15, 3331. https://doi.org/10.3390/cancers15133331
Chifotides HT, Verstovsek S, Bose P. Association of Myelofibrosis Phenotypes with Clinical Manifestations, Molecular Profiles, and Treatments. Cancers. 2023; 15(13):3331. https://doi.org/10.3390/cancers15133331
Chicago/Turabian StyleChifotides, Helen T., Srdan Verstovsek, and Prithviraj Bose. 2023. "Association of Myelofibrosis Phenotypes with Clinical Manifestations, Molecular Profiles, and Treatments" Cancers 15, no. 13: 3331. https://doi.org/10.3390/cancers15133331
APA StyleChifotides, H. T., Verstovsek, S., & Bose, P. (2023). Association of Myelofibrosis Phenotypes with Clinical Manifestations, Molecular Profiles, and Treatments. Cancers, 15(13), 3331. https://doi.org/10.3390/cancers15133331





