Evaluation of the One Health-Ness of 20 Years of Antimicrobial Resistance Surveillance in Norway
Abstract
1. Introduction
2. Results
3. Discussion
3.1. Organisation
3.2. Operation
3.3. Impact
4. Materials and Methods
4.1. Definition of the One Health System of AMR Surveillance
4.2. Description of the Different Sectors of the One Health System of AMR Surveillance
4.3. Methodology
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; UNEP; WHO; WOAH. One Health Joint Plan. of Action. (2022–2026). Working Together for the Health of Humans, Animals, Plants and the Environment; FAO: Rome, Italy, 2022. [Google Scholar]
- Aenishaenslin, C.; Hasler, B.; Ravel, A.; Parmley, E.J.; Mediouni, S.; Bennani, H.; Stark, K.D.C.; Buckeridge, D.L. Evaluating the Integration of One Health in Surveillance Systems for Antimicrobial Use and Resistance: A Conceptual Framework. Front. Vet. Sci. 2021, 8, 611931. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. NORM-VET—The Monitoring Program for Antimicrobial Resistance in the Veterinary and Food Production Sectors. Available online: https://www.vetinst.no/en/surveillance-programmes/norm-norm-vet-report (accessed on 26 April 2023).
- NORM/NORM-VET 2021. Usage of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Norway. Tromsø/Oslo/Ås, Norway. 2022. ISSN 1502-2307 (print)/1890-9965 (electronic). Available online: https://www.vetinst.no/en/surveillance-programmes/norm-norm-vet-report (accessed on 29 March 2023).
- Bennani, H.; Cornelsen, L.; Stark, K.D.C.; Hasler, B. Characterisation and mapping of the surveillance system for antimicrobial resistance and antimicrobial use in the United Kingdom. Vet. Rec. 2021, 188, e10. [Google Scholar] [CrossRef] [PubMed]
- Calba, C.; Goutard, F.L.; Hoinville, L.; Hendrikx, P.; Lindberg, A.; Saegerman, C.; Peyre, M. Surveillance systems evaluation: A systematic review of the existing approaches. BMC Public. Health 2015, 15, 448. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, M.; Hesp, A.; Aenishaenslin, C.; Bordier, M.; Bennani, H.; Bergwerff, U.; Chantziaras, I.; De Meneghi, D.; Ellis-Iversen, J.; Filippizi, M.E.; et al. Assessment of Evaluation Tools for Integrated Surveillance of Antimicrobial Use and Resistance Based on Selected Case Studies. Front. Vet. Sci. 2021, 8, 620998. [Google Scholar] [CrossRef] [PubMed]
- Rüegg, S.; Antoine-Mosseaux, N.; Aenishaensilin, C.; Alban, L.; Bordier, M.; Bennani, H.; Schauer, B.; Arnold, J.; Gabain, I.; Sauter-Louis, C.; et al. Guidance for evaluating integrated surveillance of antimicrobial use and resistance. CABI One Health 2022, ohcs20220007. [Google Scholar] [CrossRef]
- Tegegne, H.A.; Bogaardt, C.; Collineau, L.; Cazeau, G.; Lailler, R.; Reinhardt, J.; Freeth, F.T.A.; Taylor, E.; Prada, J.M.; Henaux, V. OH-EpiCap: A semi-quantitative tool for the evaluation of One Health epidemiological surveillance capacities and capabilities. Front. Public Health 2023, 11, 1053986. [Google Scholar] [CrossRef]
- Consortium, M. OH-EpiCap Tool. Available online: https://freddietafreeth.shinyapps.io/OH-EpiCap (accessed on 26 April 2023).
- Alban, L.; Bordier, M.; Häsler, B.; Collineau, L.; Thomassone, L.; Benan, H.; Aenishaenslin, C.; Norström, M.; Aragrande, M.; Fillipitzi, M.E.; et al. Capturing systematically users’ experience of evaluation tools for integrated AMU and AMR surveillance. Front. Vet. Sci. 2023, 10, 1107122. [Google Scholar] [CrossRef]
- Moura, P.C.L.; Sandberg, M.; Tomassone, L.; De Meneghi, D.; Norström, M.; Bennani, H.; Häsler, B.; Colomb-Cotinat, M.; Bourély, C.; Filippitzi, M.-E.; et al. Users’perception of the OH-EpiCap evaluation tool based on its application to nine national antimicrobial resistance surveillance systems. Front. Public Health 2023, 11, 1138645. [Google Scholar] [CrossRef]
- Norwegian Ministries. National Strategy against Antibiotic Resistance 2015–2020; Norwegian Ministries: Oslo, Norway, 2015. [Google Scholar]
- Norström, M.; Hofshagen, M.; Stavnes, T.; Schau, J.; Lassen, J.; Kruse, H. Antimicrobial resistance in Campylobacter jejuni from humans and broilers in Norway. Epidemiol. Infect. 2006, 134, 127–130. [Google Scholar] [CrossRef]
- Berg, E.S.; Wester, A.L.; Ahrenfeldt, J.; Mo, S.S.; Slettemeas, J.S.; Steinbakk, M.; Samuelsen, O.; Grude, N.; Simonsen, G.S.; Lohr, I.H.; et al. Norwegian patients and retail chicken meat share cephalosporin-resistant Escherichia coli and IncK/blaCMY-2 resistance plasmids. Clin. Microbiol. Infect. 2017, 23, 407.e9–407.e15. [Google Scholar] [CrossRef]
- Mattilsynet. Samfunnsøkonomisk Analyse Av Aktuelle Tiltak for å Forebygge Spredning Av Mrsa I Norsk Svinehold; Mattilsynet: Oslo, Norway, 2016. [Google Scholar]
- Elstrøm, P.; Grøntvedt, C.A.; Gabrielsen, C.; Stegger, M.; Angen, O.; Amdal, S.; Enger, H.; Urdahl, A.M.; Jore, S.; Steinbakk, M.; et al. Livestock-Associated MRSA CC1 in Norway; Introduction to Pig Farms, Zoonotic Transmission, and Eradication. Front. Microbiol. 2019, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- NORM/NORM-VET 2019. Usage of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Norway. Tromsø/Oslo, Norway. 2020. ISSN 1502-2307 (print)/1890-9965 (electronic). Available online: https://www.vetinst.no/en/surveillance-programmes/norm-norm-vet-report (accessed on 29 March 2023).
- NORM/NORM-VET 2018. Usage of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Norway. Tromsø/Oslo, Norway. 2019. ISSN 1502-2307 (print)/1890-9965 (electronic). Available online: https://www.vetinst.no/en/surveillance-programmes/norm-norm-vet-report (accessed on 29 March 2023).
- NORM/NORM-VET 2020. Usage of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Norway. Tromsø/Oslo, Norway. 2021. ISSN 1502-2307 (print)/1890-9965 (electronic). Available online: https://www.vetinst.no/en/surveillance-programmes/norm-norm-vet-report (accessed on 29 March 2023).
- Ministry of Agriculture and Food; Ministry of Trade and Fisheries. Forskrift Om Dyrehelse (Dyrehelseforskriften); Ministry of Agriculture and Food: Oslo, Norway; Ministry of Trade and Fisheries: Oslo, Norway, 2022.
- Nielsen, K.M.; Wasteson, Y.; Yazdankhah, S.; Bergh, Ø.; Eklo, O.M.; Joner, E.; Madslien, E.H.; Trosvik, P.; Ytrehus, B. Antimicrobial Resistance (AMR) from an Environmental Perspective—A Short Summary of Assessments Prepared by VKM in the Period 2015–2020; Norwegian Scientific Committee for Food and Environment (VKM): Oslo, Norway, 2020; ISBN 978-82-8259-348-9. ISSN 2535-4019. [Google Scholar]
- EUR-Lex. Regulation (EC) No 2160/2003 of the European Parliament and of the Council of 17 November 2003 on the Control of Salmonella and other Specified Food-Borne Zoonotic Agents; EUR-Lex: Luxembourg, 2003. [Google Scholar]
- EUR-Lex. Commission Implementing Decision (EU) 2020/1729 of 17 November 2020 on the Monitoring and Reporting of Antimicrobial Resistance in Zoonotic and Commensal Bacteria and Repealing Implementing Decision 2013/652/EU (Notified under Document C(2020) 7894); EUR-Lex: Luxembourg, 2020. [Google Scholar]
- European Food Safety Authority. Technical specifications on randomised sampling for harmonised monitoring of antimicrobial resistance in zoonotic and commensal bacteria. EFSA J. 2014, 12, 33. [Google Scholar] [CrossRef]
- Urdahl, A.M.; Norström, M.; Sunde, M.; Grøntvedt, C.A. The Surveillance Programme for Methicillin Resistant Staphylococcus aureus in Pigs in Norway 2022; Norwegian Veterinary Institute: Ås, Norway, 2023. Available online: www.vetinst.no (accessed on 10 May 2023).


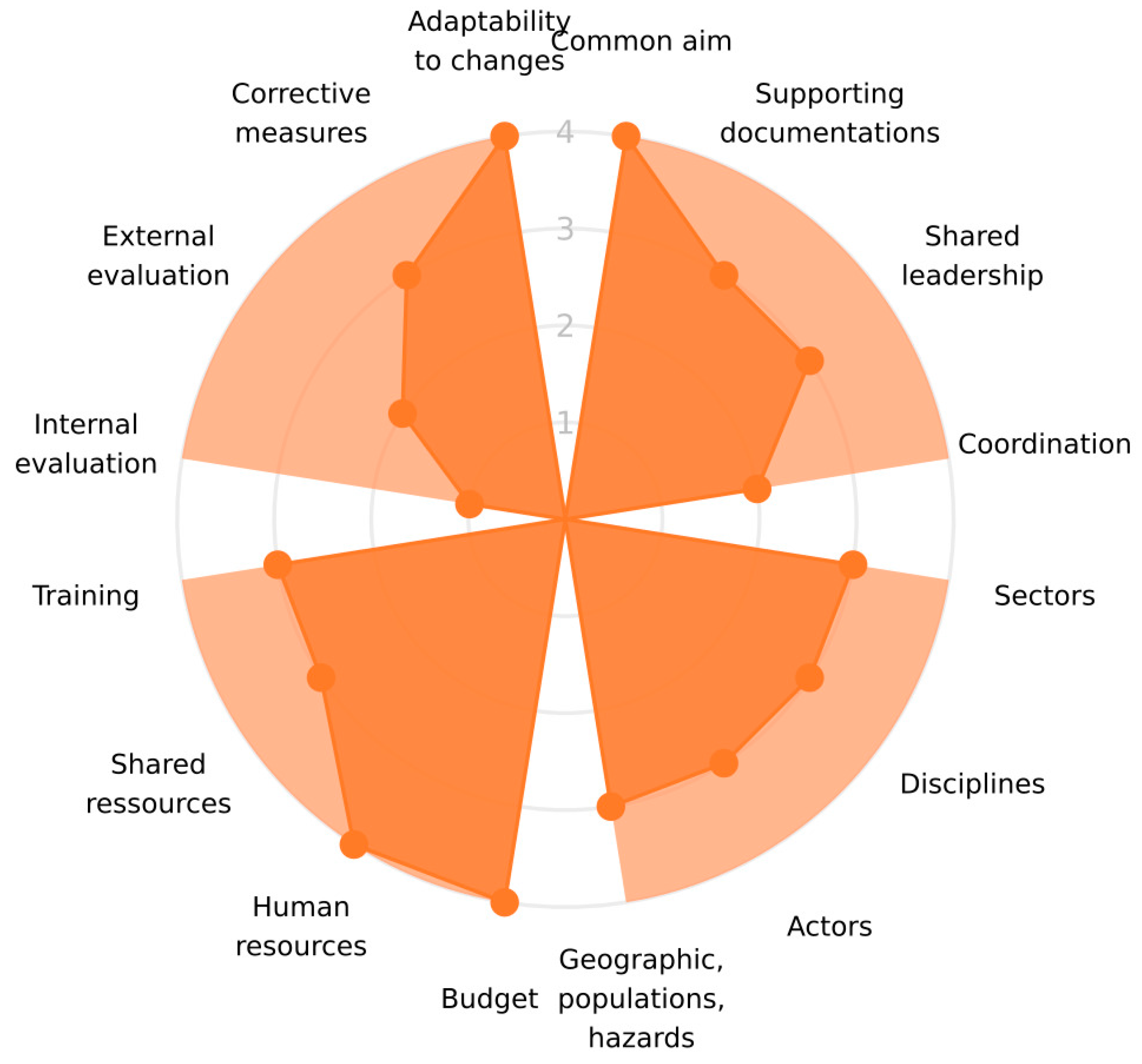
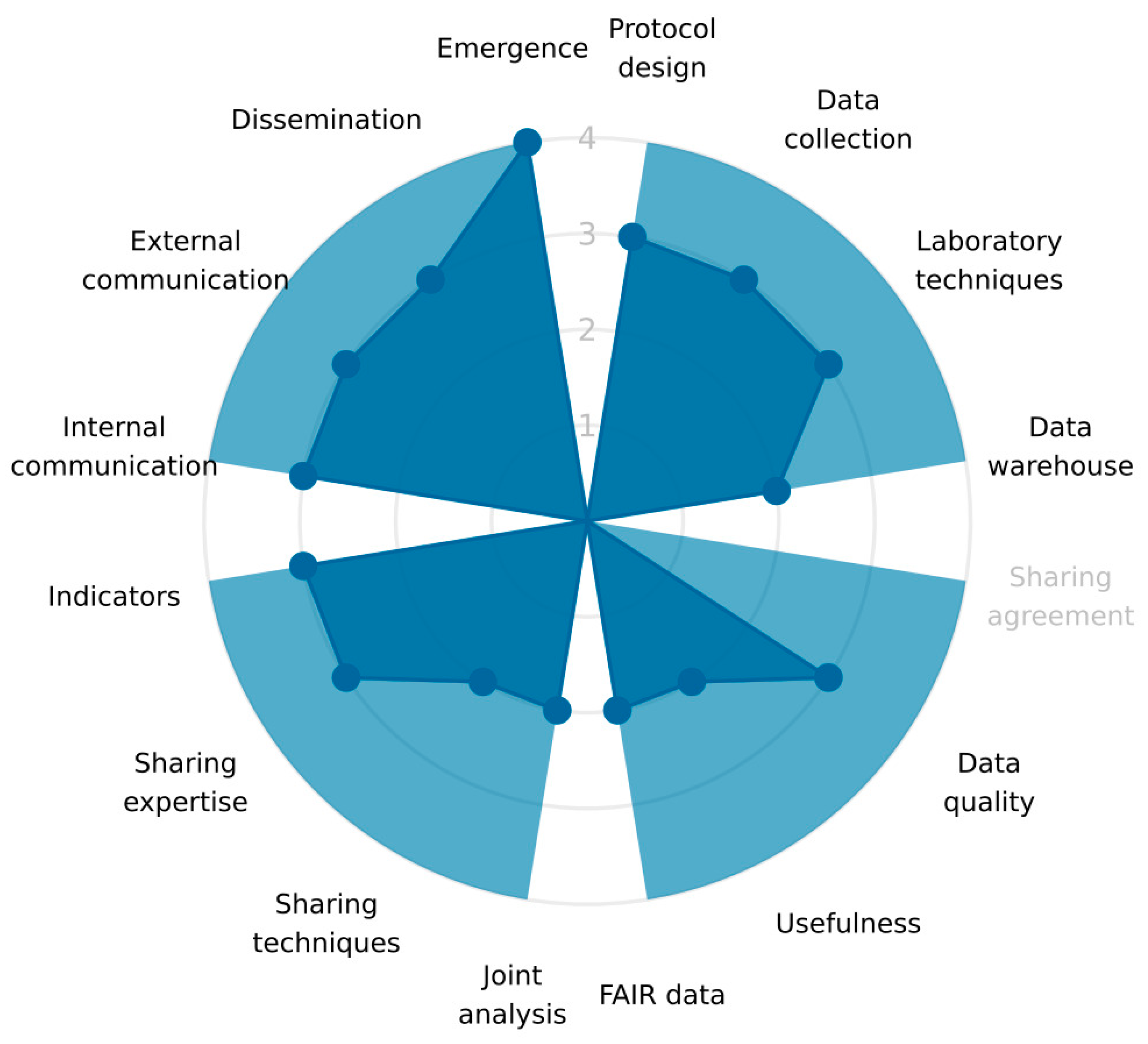
| Dimensions | |||
|---|---|---|---|
| Description | Organisation | Operation | Impact |
| Indicators showing good adherence to the OH principles | common aim budget human resources adaptability to changes | emergence | improved knowledge OH team strategy preparedness interventions advocacy awareness research policy changes |
| Indicators that could be improved according to the One Health principles | internal evaluation | none | operational costs |
| Dimensions | Targets | Questions About: |
|---|---|---|
| Organisation | formalization | “the objectives, supporting documentation, coordination roles, leadership” |
| coverage | “whether all relevant actors and disciplines, sectors geography, populations and hazards are covered” | |
| resources | “availability of financial and human resources, training and sharing of the available operational resources”. | |
| evaluation and resilience | “internal and external evaluation, implementation of corrective measures and the capacity to adapt to changes” | |
| Operations | data collection and methods of sharing | “multisectoral collaborations in the design of surveillance protocols, data collection, harmonisation of laboratory techniques and data warehousing” |
| data sharing | “data sharing agreements, evaluation of data quality, use of shared data, compliance with the FAIR principle”. | |
| data analysis and interpretation | “multisectoral integration of data analysis, sharing of statistical techniques and scientific expertise, harmonization of indicators” | |
| communication | “internal and external communication processes, dissemination to decision-makers, information sharing in case of suspicion” | |
| Impact | technical outputs | “Timely detection of emergence, knowledge improvement on hazard epidemiological situations, increased effectiveness of surveillance, reduction of operational costs” |
| collaborative added value | “strengthenings of the OH-team and network, international collaboration and common strategy” | |
| immediate and intermediate outcomes | “advocacy, awareness, preparedness, interventions based on the information generated by the OH-surveillance system” | |
| ultimate outcomes | “research opportunities, policy changes, behavioural changes, better health outcomes that are attributed to the OH-surveillance system” |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norström, M.; Simonsen, G.S.; Slettemeås, J.S.; Furberg, A.-S.; Urdahl, A.M. Evaluation of the One Health-Ness of 20 Years of Antimicrobial Resistance Surveillance in Norway. Antibiotics 2023, 12, 1080. https://doi.org/10.3390/antibiotics12071080
Norström M, Simonsen GS, Slettemeås JS, Furberg A-S, Urdahl AM. Evaluation of the One Health-Ness of 20 Years of Antimicrobial Resistance Surveillance in Norway. Antibiotics. 2023; 12(7):1080. https://doi.org/10.3390/antibiotics12071080
Chicago/Turabian StyleNorström, Madelaine, Gunnar Skov Simonsen, Jannice Schau Slettemeås, Anne-Sofie Furberg, and Anne Margrete Urdahl. 2023. "Evaluation of the One Health-Ness of 20 Years of Antimicrobial Resistance Surveillance in Norway" Antibiotics 12, no. 7: 1080. https://doi.org/10.3390/antibiotics12071080
APA StyleNorström, M., Simonsen, G. S., Slettemeås, J. S., Furberg, A.-S., & Urdahl, A. M. (2023). Evaluation of the One Health-Ness of 20 Years of Antimicrobial Resistance Surveillance in Norway. Antibiotics, 12(7), 1080. https://doi.org/10.3390/antibiotics12071080







