Influence of Trabecular Bone Presence on Osseodensification Instrumentation: An In Vivo Study in Sheep
Abstract
1. Introduction
2. Methods
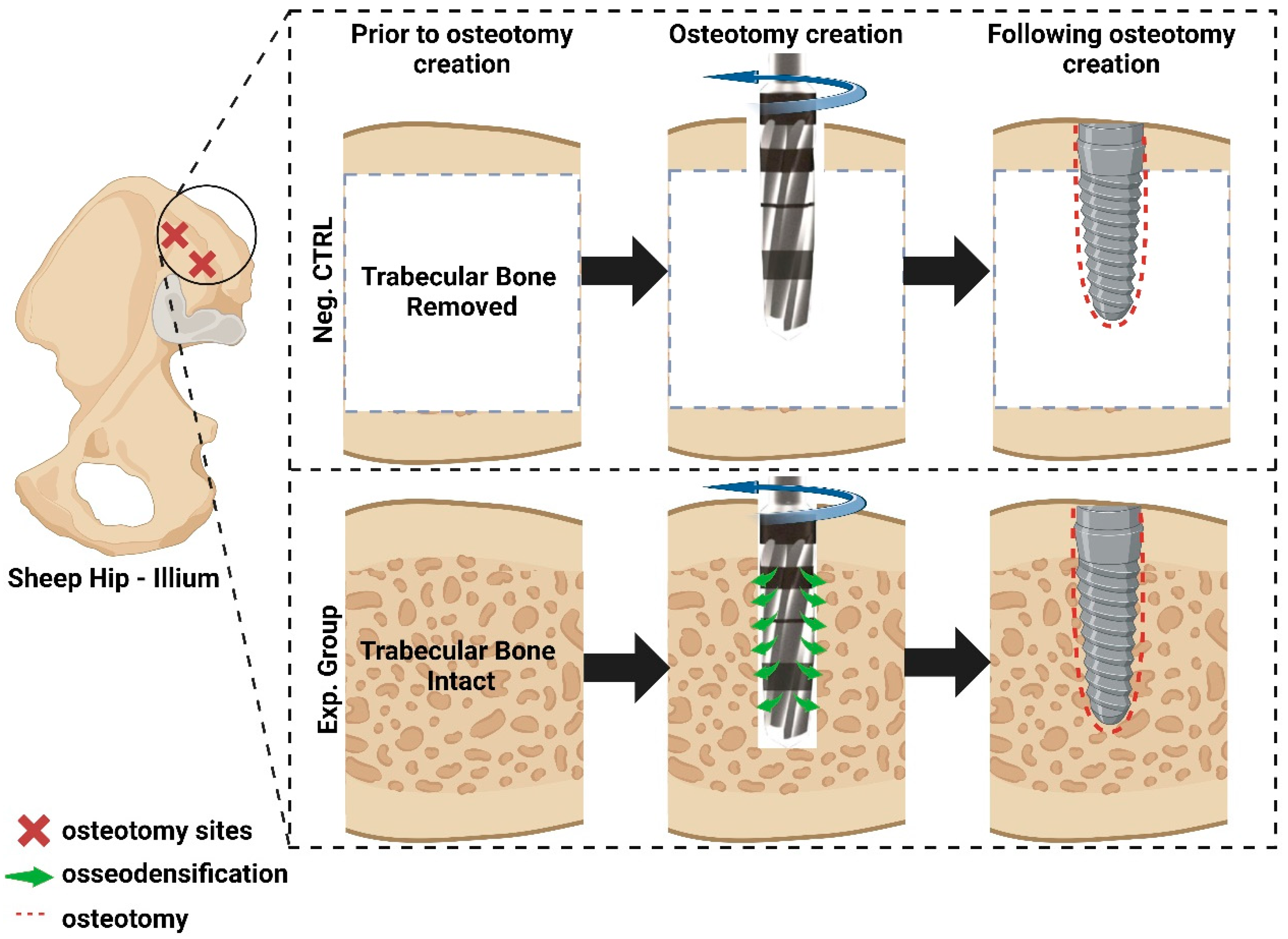
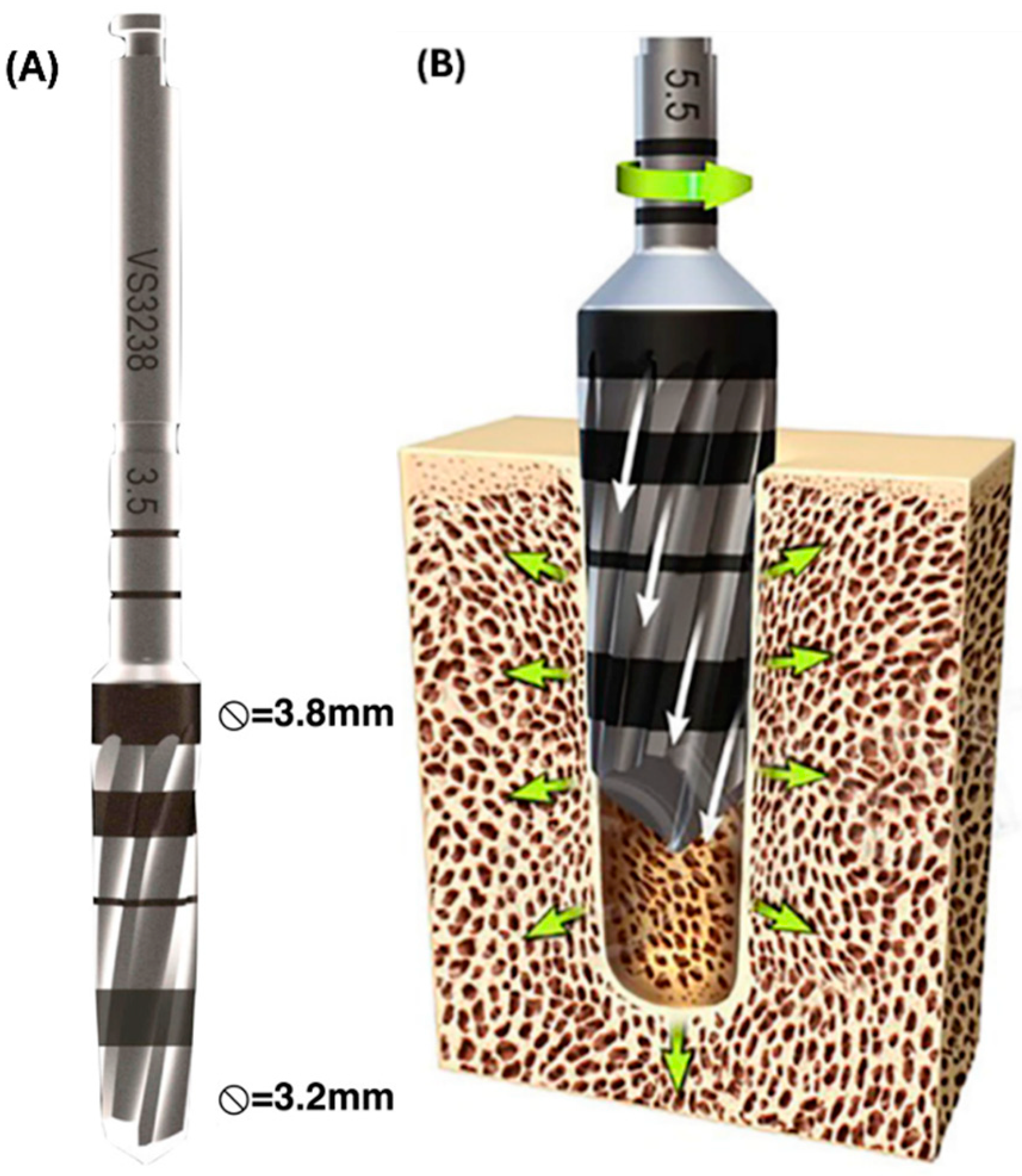
2.1. Pre-Clinical Experiments
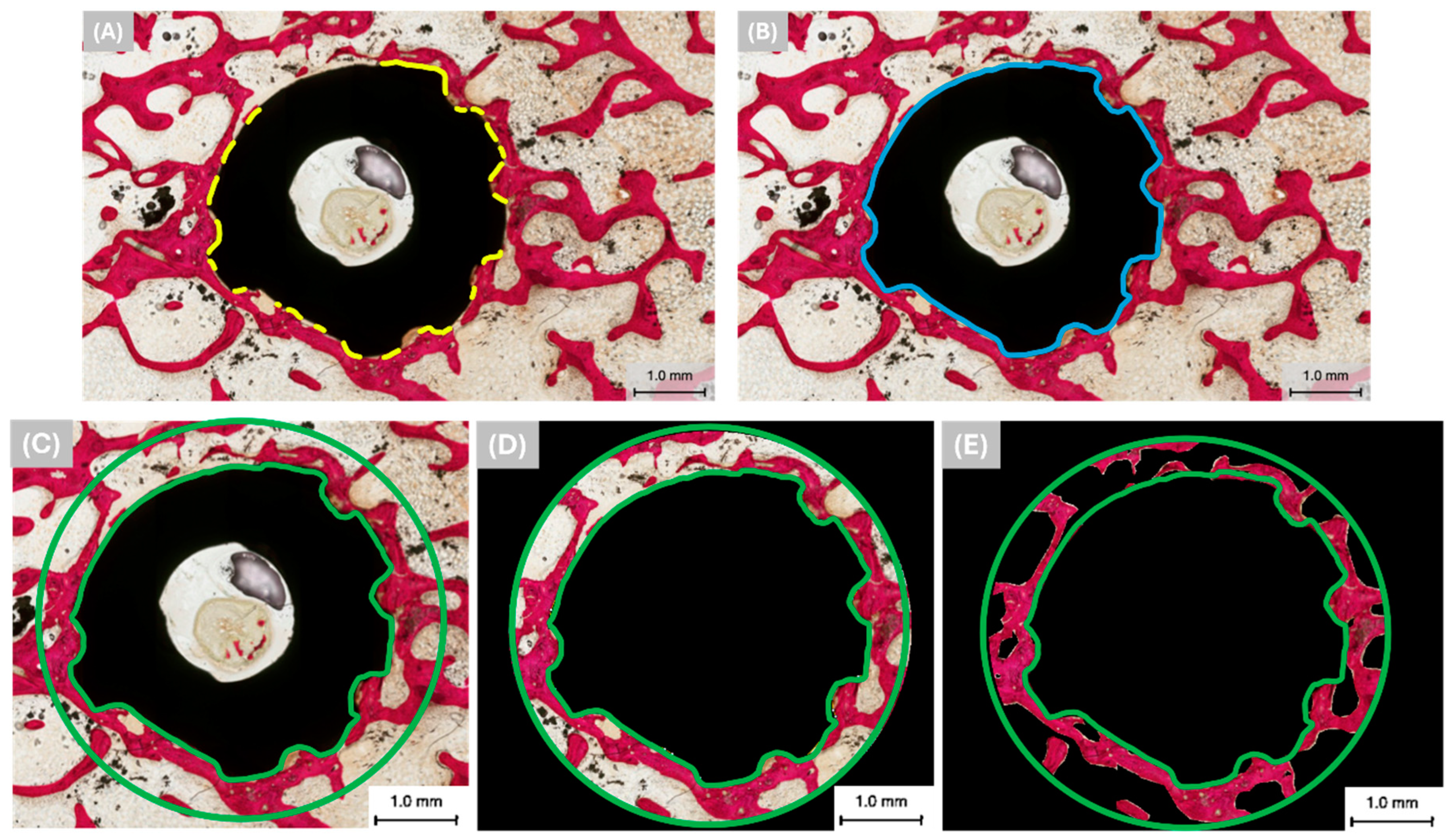
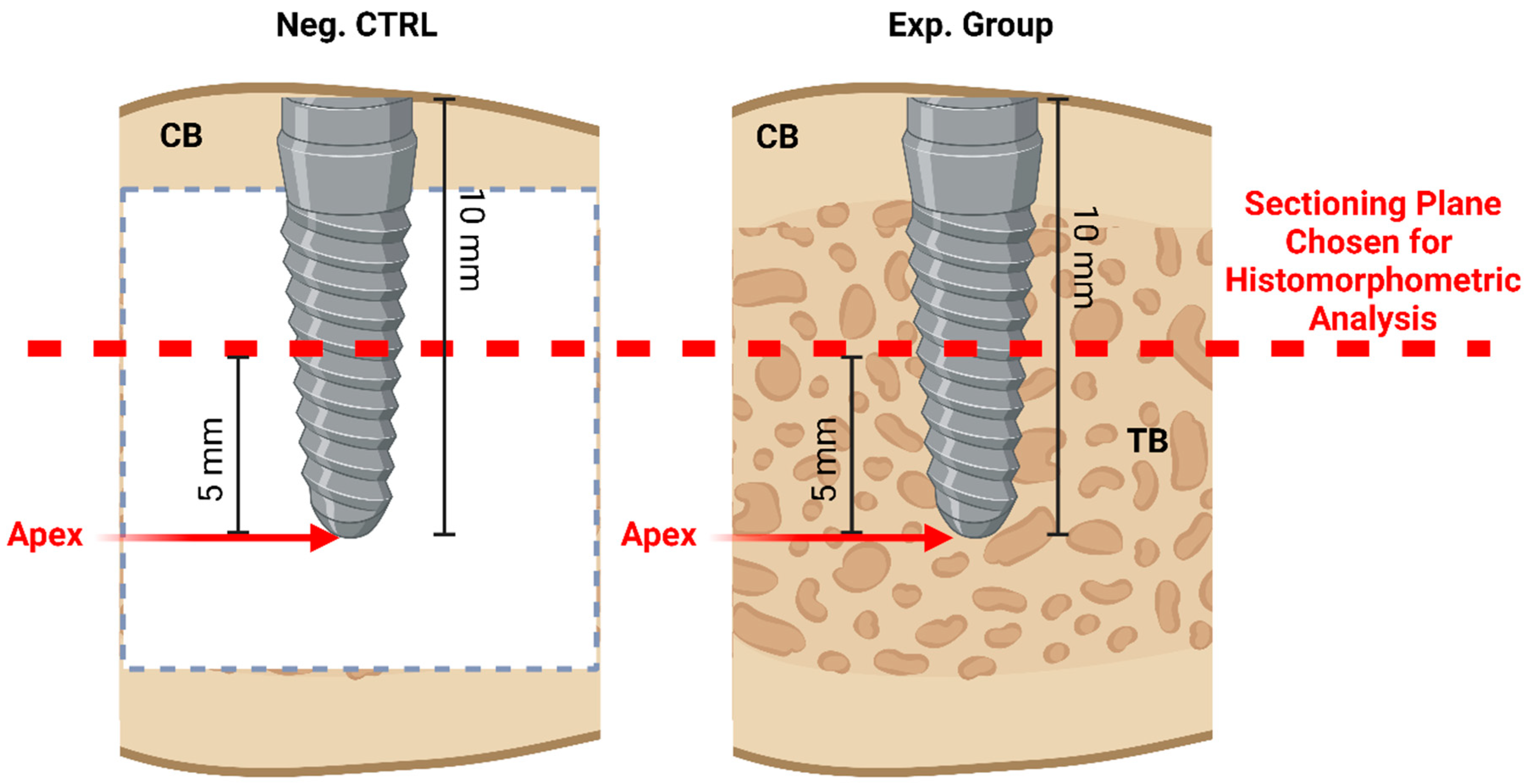
2.2. Histomorphometric Analysis
2.3. Statistical Analysis
3. Results
3.1. Qualitative Histologic Findings
3.2. Histomorphometric Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, L.F. The Current and Future Treatment of Edentulism. J. Prosthodont. 2009, 18, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Romandini, M.; Baima, G.; Antonoglou, G.; Bueno, J.; Figuero, E.; Sanz, M. Periodontitis, edentulism, and risk of mortality: A systematic review with meta-analyses. J. Dent. Res. 2021, 100, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Felton, D.A.; Jemt, T.; Koka, S. Rehabilitation of edentulism and mortality: A systematic review. J. Prosthodont. 2019, 28, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Shin, K.S.; Jung, J.H.; Cho, H.W.; Kwon, K.H.; Kim, Y.L. Clinical study on screw loosening in dental implant prostheses: A 6-year retrospective study. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-C.; Small, P.-N.; Elian, N.; Tarnow, D.J.I.d. Screw loosening for standard and wide diameter implants in partially edentulous cases: 3- to 7-year longitudinal data. Implant. Dent. 2004, 13, 245–250. [Google Scholar] [CrossRef]
- Kreissl, M.E.; Gerds, T.; Muche, R.; Heydecke, G.; Strub, J.R. Technical complications of implant-supported fixed partial dentures in partially edentulous cases after an average observation period of 5 years. Clin. Oral Implant. Res. 2007, 18, 720–726. [Google Scholar] [CrossRef]
- Alsubaiy, E.F. Abutment screw loosening in implants: A literature review. J. Fam. Med. Prim. Care 2020, 9, 5490–5494. [Google Scholar] [CrossRef]
- Kanneganti, K.C.; Vinnakota, D.N.; Pottem, S.R.; Pulagam, M. Comparative effect of implant-abutment connections, abutment angulations, and screw lengths on preloaded abutment screw using three-dimensional finite element analysis: An in vitro study. J. Indian Prosthodont. Soc. 2018, 18, 161–167. [Google Scholar]
- Calderon, P.S.; Dantas, P.M.; Montenegro, S.C.; Carreiro, A.F.; Oliveira, Â.G.; Dantas, E.M.; Gurgel, B.C. Technical complications with implant-supported dental prostheses. J. Oral Sci. 2014, 56, 179–184. [Google Scholar] [CrossRef]
- Levin, L. Dealing with dental implant failures. J. Appl. Oral Sci. 2008, 16, 171–175. [Google Scholar] [CrossRef]
- Paquette, D.W.; Brodala, N.; Williams, R.C. Risk factors for endosseous dental implant failure. Dent. Clin. 2006, 50, 361–374. [Google Scholar] [CrossRef]
- Hsieh, M.-K.; Liu, M.-Y.; Chen, J.-K.; Tsai, T.-T.; Lai, P.-L.; Niu, C.-C.; Tai, C.-L. Use of longer sized screws is a salvage method for broken pedicles in osteoporotic vertebrae. Sci. Rep. 2020, 10, 10441. [Google Scholar] [CrossRef]
- Paré, P.E.; Chappuis, J.L.; Rampersaud, R.; Agarwala, A.O.; Perra, J.H.; Erkan, S.; Wu, C. Biomechanical evaluation of a novel fenestrated pedicle screw augmented with bone cement in osteoporotic spines. Spine 2011, 36, E1210–E1214. [Google Scholar] [CrossRef]
- Kreve, S.; Ferreira, I.; da Costa Valente, M.L.; dos Reis, A.C. Relationship between dental implant macro-design and osseointegration: A systematic review. Oral Maxillofac. Surg. 2024, 28, 1–14. [Google Scholar] [CrossRef]
- Hiranmayi, V.K. Factors influencing implant stability. J. Dent. Implant. 2018, 8, 69–76. [Google Scholar] [CrossRef]
- Ivanova, V.; Chenchev, I.; Zlatev, S.; Mijiritsky, E. Correlation between primary, secondary stability, bone density, percentage of vital bone formation and implant size. Int. J. Environ. Res. Public Health 2021, 18, 6994. [Google Scholar] [CrossRef]
- Rabel, A.; Köhler, S.G.; Schmidt-Westhausen, A.M. Clinical study on the primary stability of two dental implant systems with resonance frequency analysis. Clin. Oral Investig. 2007, 11, 257–265. [Google Scholar] [CrossRef]
- Möhlhenrich, S.C.; Kniha, K.; Heussen, N.; Hölzle, F.; Modabber, A. Effects on primary stability of three different techniques for implant site preparation in synthetic bone models of different densities. Br. J. Oral Maxillofac. Surg. 2016, 54, 980–986. [Google Scholar] [CrossRef]
- Summers, R.B.J.C. A new concept in maxillary implant surgery: The osteotome technique. Compendium 1994, 15, 152, 154–156, 158 passim; quiz 162. [Google Scholar]
- Friberg, B.; Ekestubbe, A.; Mellström, D.; Sennerby, L. Brånemark implants and osteoporosis: A clinical exploratory study. Clin. Implant. Dent. 2001, 3, 50–56. [Google Scholar] [CrossRef]
- Elsayyad, A.A.; Osman, R.B. Osseodensification in implant dentistry: A critical review of the literature. Implant. Dent. 2019, 28, 306–312. [Google Scholar] [CrossRef]
- Trisi, P.; Berardini, M.; Falco, A.; Podaliri Vulpiani, M. New Osseodensification Implant Site Preparation Method to Increase Bone Density in Low-Density Bone: In Vivo Evaluation in Sheep. Implant. Dent. 2016, 25, 24–31. [Google Scholar] [CrossRef]
- Huwais, S.; Meyer, E.G. A Novel Osseous Densification Approach in Implant Osteotomy Preparation to Increase Biomechanical Primary Stability, Bone Mineral Density, and Bone-to-Implant Contact. Int. J. Oral Maxillofac. Implant. 2017, 32, 27–36. [Google Scholar] [CrossRef]
- Padhye, N.M.; Padhye, A.M.; Bhatavadekar, N.B. Osseodensification––A systematic review and qualitative analysis of published literature. J. Oral Biol. Craniofacial Res. 2020, 10, 375–380. [Google Scholar] [CrossRef]
- Gaspar, J.; Proença, L.; Botelho, J.; Machado, V.; Chambrone, L.; Neiva, R.; João Mendes, J. Implant Stability of Osseodensification Drilling Versus Conventional Surgical Technique: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2021, 36, 1104. [Google Scholar] [CrossRef]
- Alifarag, A.M.; Lopez, C.D.; Neiva, R.F.; Tovar, N.; Witek, L.; Coelho, P.G. Atemporal osseointegration: Early biomechanical stability through osseodensification. J. Orthop. Res. 2018, 36, 2516–2523. [Google Scholar] [CrossRef]
- Fontes Pereira, J.; Costa, R.; Nunes Vasques, M.; Salazar, F.; Mendes, J.M.; Infante da Câmara, M. Osseodensification: An Alternative to Conventional Osteotomy in Implant Site Preparation: A Systematic Review. J. Clin. Med. 2023, 12, 7046. [Google Scholar] [CrossRef]
- Koutouzis, T.; Huwais, S.; Hasan, F.; Trahan, W.; Waldrop, T.; Neiva, R. Alveolar Ridge Expansion by Osseodensification-Mediated Plastic Deformation and Compaction Autografting: A Multicenter Retrospective Study. Implant. Dent. 2019, 28, 349–355. [Google Scholar] [CrossRef]
- Torroni, A.; Lima Parente, P.E.; Witek, L.; Hacquebord, J.H.; Coelho, P.G. Osseodensification drilling vs. conventional manual instrumentation technique for posterior lumbar fixation: Ex-vivo mechanical and histomorphological analysis in an ovine model. J. Orthop. Res. 2021, 39, 1463–1469. [Google Scholar] [CrossRef]
- Maglio, M.; Salamanna, F.; Brogini, S.; Borsari, V.; Pagani, S.; Nicoli Aldini, N.; Giavaresi, G.; Fini, M. Histological, Histomorphometrical, and Biomechanical Studies of Bone-Implanted Medical Devices: Hard Resin Embedding. Biomed. Res. Int. 2020, 2020, 1804630. [Google Scholar] [CrossRef]
- Oftadeh, R.; Perez-Viloria, M.; Villa-Camacho, J.C.; Vaziri, A.; Nazarian, A. Biomechanics and mechanobiology of trabecular bone: A review. J. Biomech. Eng. 2015, 137, 0108021–01080215. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, J.; Wiesnerova, L.; Chocholata, P.; Kulda, V.; Landsmann, L.; Cedikova, M.; Kripnerova, M.; Eberlova, L.; Babuska, V. Human cells with osteogenic potential in bone tissue research. Biomed. Eng. Online 2023, 22, 33. [Google Scholar] [CrossRef] [PubMed]
- Das, N. The new bone drilling concept: Osseodensification (Hydrodynamic Bone Preparation). EC Dent. Sci. 2019, 18, 2345–2355. [Google Scholar]
- Javed, F.; Ahmed, H.B.; Crespi, R.; Romanos, G.E. Role of primary stability for successful osseointegration of dental implants: Factors of influence and evaluation. Interv. Med. Appl. Sci. 2013, 5, 162–167. [Google Scholar] [CrossRef]
- Büchter, A.; Kleinheinz, J.; Wiesmann, H.P.; Kersken, J.; Nienkemper, M.; Weyhrother, H.v.; Joos, U.; Meyer, U. Biological and biomechanical evaluation of bone remodelling and implant stability after using an osteotome technique. Clin. Oral Implant. Res. 2005, 16, 1–8. [Google Scholar] [CrossRef]
- Kalra, J.; Dhawan, P.; Jain, N. Implant stability and crestal bone level in osseodensification and conventional drilling protocols: A systematic review and meta-analysis. J. Prosthet. Dent. 2024; in press. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Xhajanka, E.; Romeo, D.M.; Romeo, M.; Zappone, C.M.F.; Malcangi, G.; Scarano, A.; Lorusso, F.; et al. The Effectiveness of Osseodensification Drilling Protocol for Implant Site Osteotomy: A Systematic Review of the Literature and Meta-Analysis. Materials 2021, 14, 1147. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stauber, Z.; Wu, S.; Herbert, J.E.; Willers, A.; Bergamo, E.T.P.; Nayak, V.V.; Mirsky, N.A.; Castellano, A.; Jabori, S.K.; Parra, M.V.; et al. Influence of Trabecular Bone Presence on Osseodensification Instrumentation: An In Vivo Study in Sheep. Biomimetics 2024, 9, 568. https://doi.org/10.3390/biomimetics9090568
Stauber Z, Wu S, Herbert JE, Willers A, Bergamo ETP, Nayak VV, Mirsky NA, Castellano A, Jabori SK, Parra MV, et al. Influence of Trabecular Bone Presence on Osseodensification Instrumentation: An In Vivo Study in Sheep. Biomimetics. 2024; 9(9):568. https://doi.org/10.3390/biomimetics9090568
Chicago/Turabian StyleStauber, Zachary, Shangtao Wu, Justin E. Herbert, Amanda Willers, Edmara T. P. Bergamo, Vasudev Vivekanand Nayak, Nicholas A. Mirsky, Arthur Castellano, Sinan K. Jabori, Marcelo V. Parra, and et al. 2024. "Influence of Trabecular Bone Presence on Osseodensification Instrumentation: An In Vivo Study in Sheep" Biomimetics 9, no. 9: 568. https://doi.org/10.3390/biomimetics9090568
APA StyleStauber, Z., Wu, S., Herbert, J. E., Willers, A., Bergamo, E. T. P., Nayak, V. V., Mirsky, N. A., Castellano, A., Jabori, S. K., Parra, M. V., Bonfante, E. A., Witek, L., & Coelho, P. G. (2024). Influence of Trabecular Bone Presence on Osseodensification Instrumentation: An In Vivo Study in Sheep. Biomimetics, 9(9), 568. https://doi.org/10.3390/biomimetics9090568






