Bioinspired Design of 3D-Printed Cellular Metamaterial Prosthetic Liners for Enhanced Comfort and Stability
Abstract
:1. Introduction
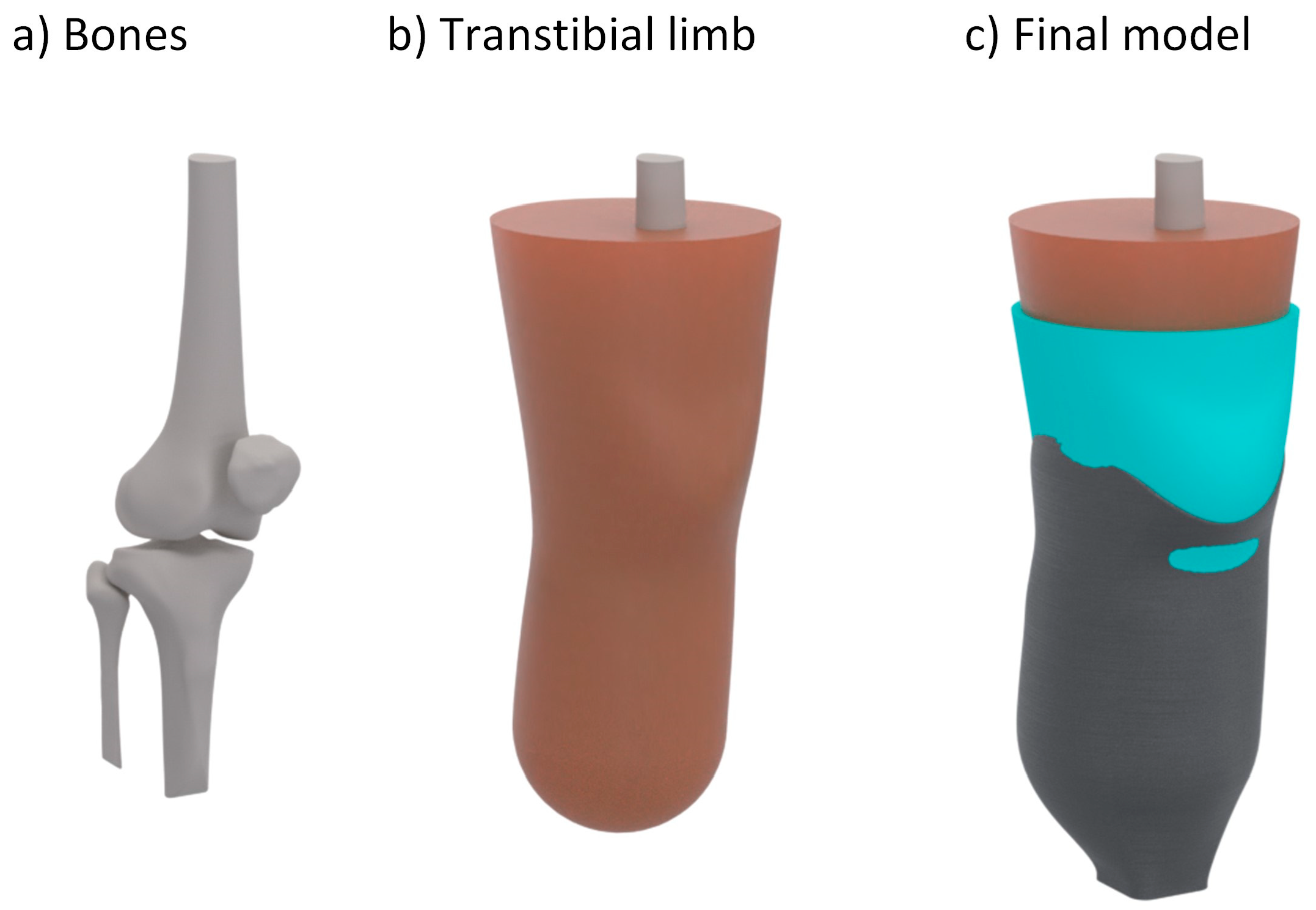
2. Materials and Methods
2.1. Metamaterial Unit Cell Identification
- The gyroid structure;
- The Schwarz structure;
- The Neovius structure;
- The diamond structure.
- Simple cubic unit cell;
- Octet unit cell;
- Beam diamond unit cell;
- Re-entrant unit cell.
- σ [Pa]: −6 × 1 stress vector
- ε [-]: −6 × 1 strain vector
- C [Pa]: −6 × 6 stiffness matrix
- S [Pa−1]: −6 × 6 compliance matrix
2.2. Multilinear Elastic Material Model
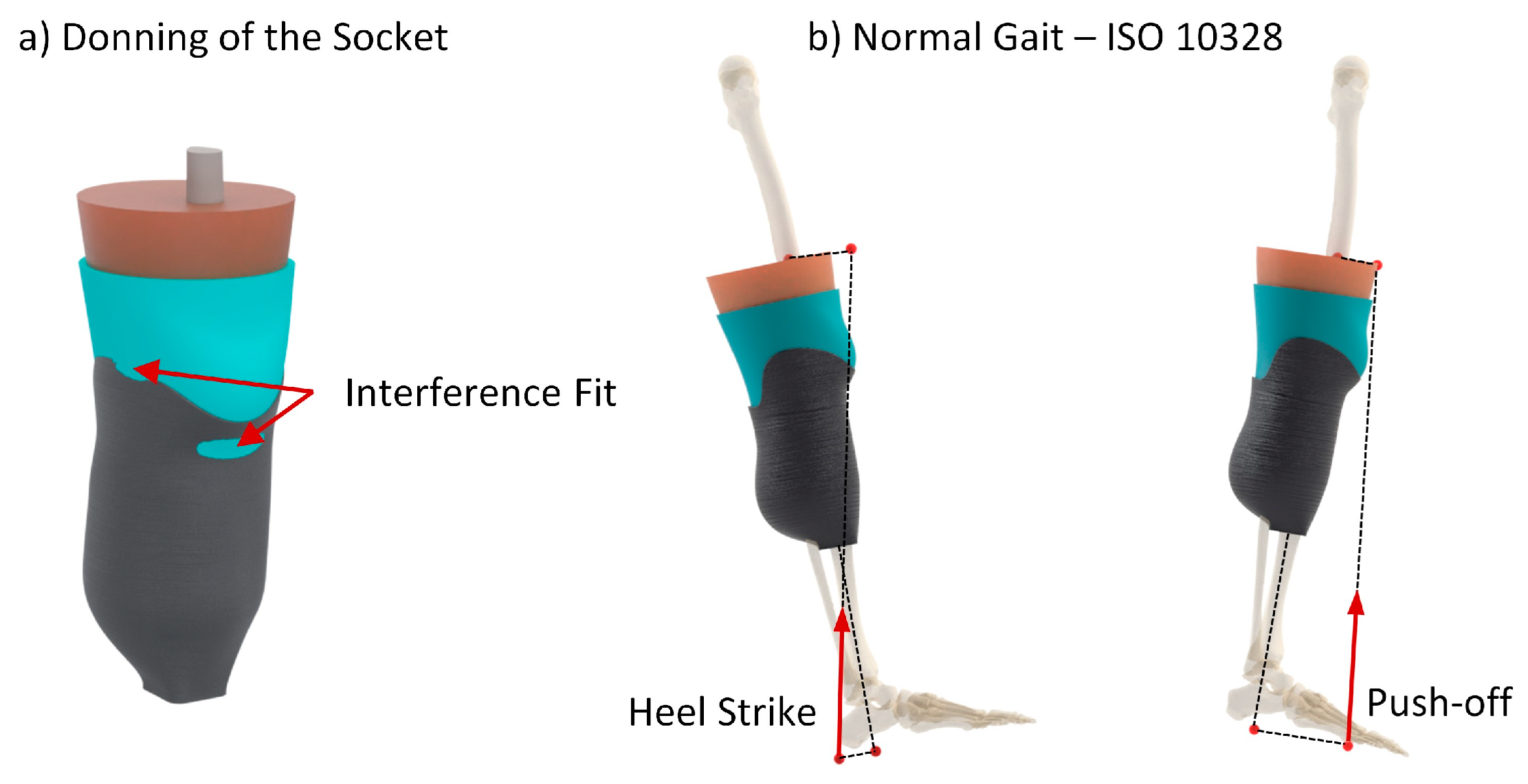
2.3. Numerical Analysis
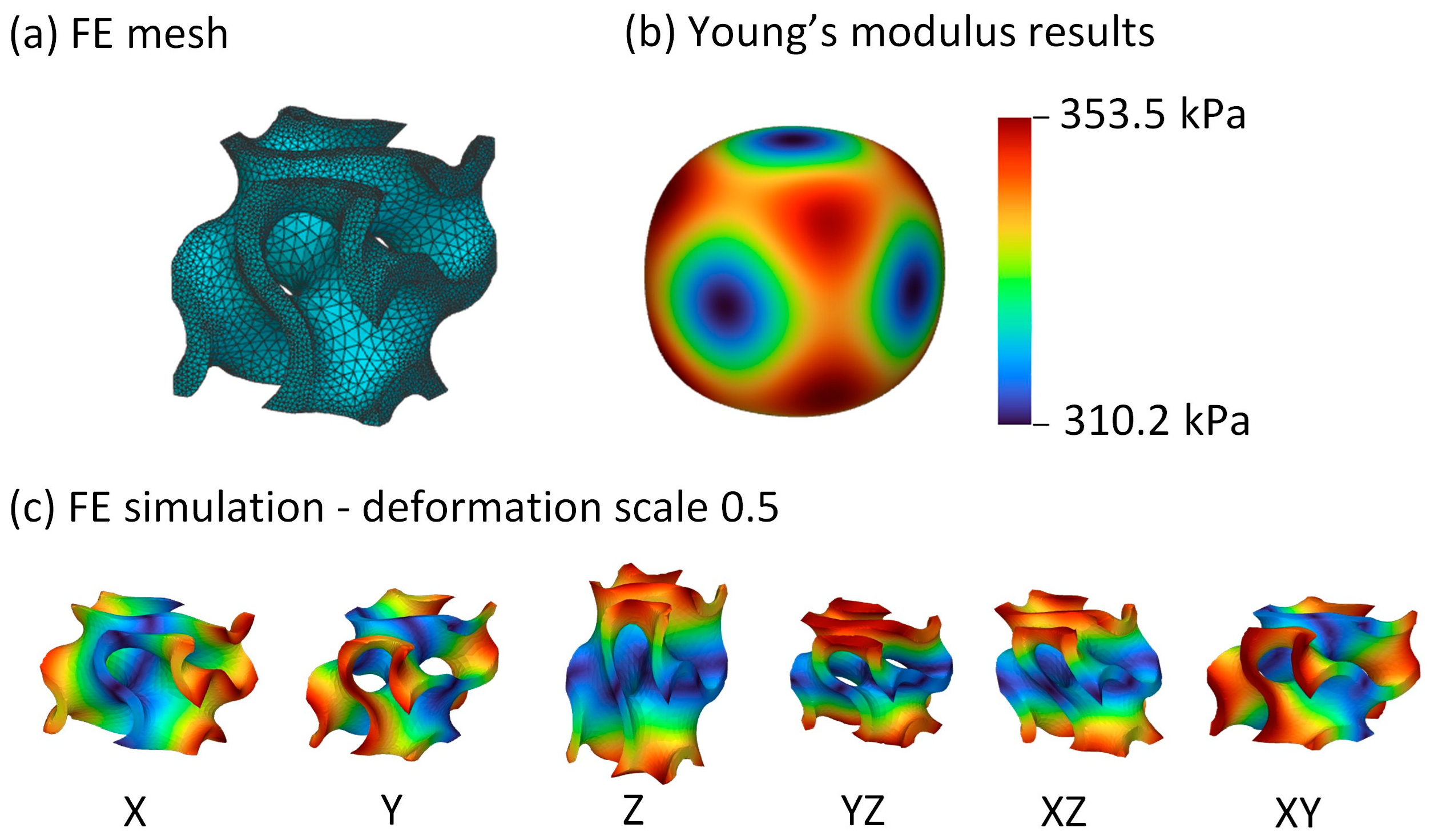
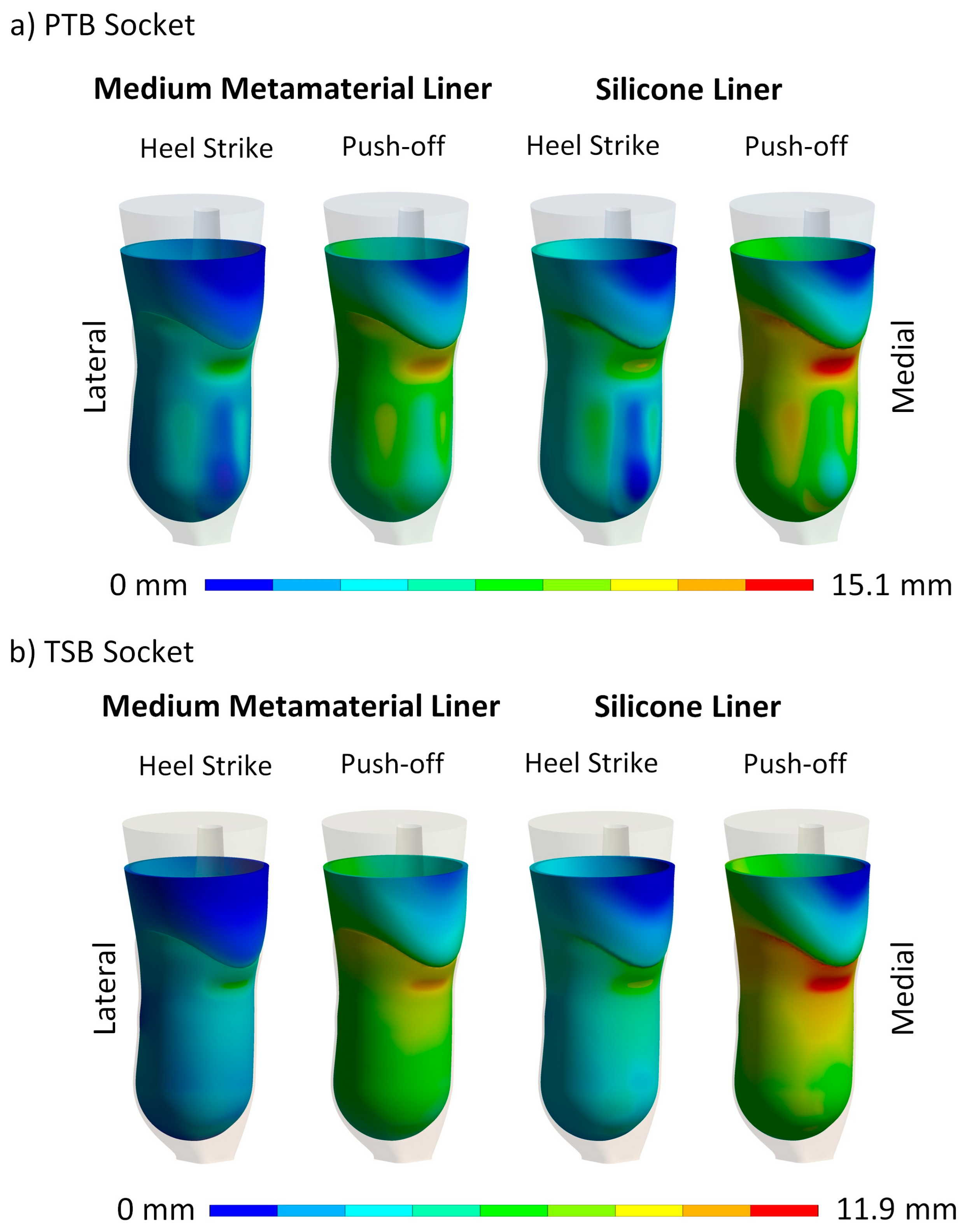
3. Results
4. Discussion
4.1. Unit Cell Selection
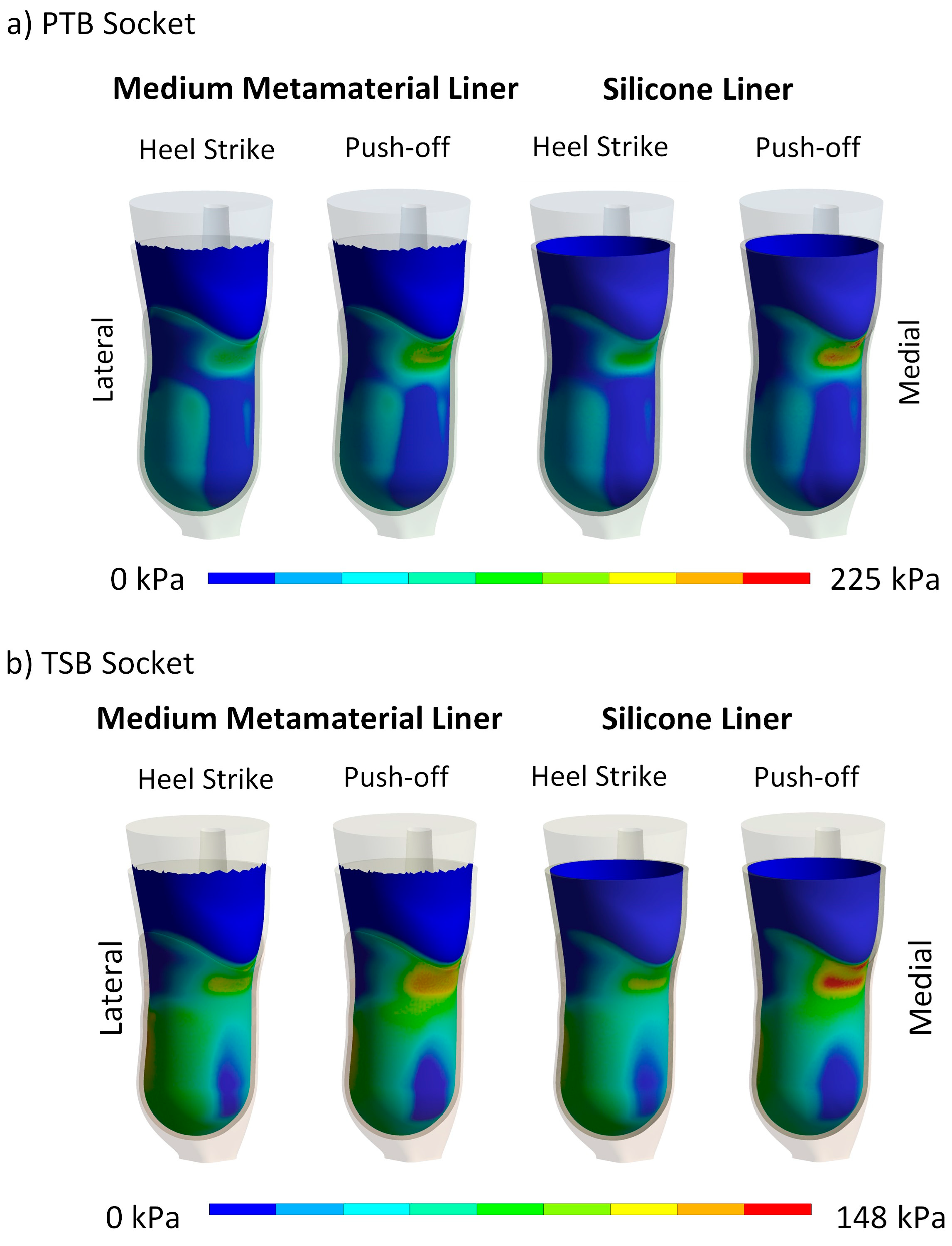
4.2. Maximum Contact Pressure and Maximum Liner Deformation
4.3. Contact Pressure Distribution and Liner Deformation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kolossváry, E.; Björck, M.; Behrendt, C.-A. Lower Limb Major Amputation Data as a Signal of an East/West Health Divide Across Europe. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 645–646. [Google Scholar] [CrossRef]
- Eskridge, S.L.; Dougherty, A.L.; Watrous, J.R.; McCabe, C.T.; Cancio, J.M.; Mazzone, B.N.; Galarneau, M.R. Prosthesis satisfaction and quality of life in US service members with combat-related major lower-limb amputation. Prosthet. Orthot. Int. 2022, 46, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Krajbich, J.I.; Pinzur, M.S.; Potter, B.K.; Stevens, P.M. (Eds.) Atlas of Amputations and Limb Deficiencies: Surgical, Prosthetic, and Rehabilitation Principles, 4th ed.; Lippincott Williams & Wilkins: Rosemont, IL, USA, 2016. [Google Scholar]
- Manz, S.; Valette, R.; Damonte, F.; Avanci Gaudio, L.; Gonzalez-Vargas, J.; Sartori, M.; Dosen, S.; Rietman, J. A review of user needs to drive the development of lower limb prostheses. J. Neuroeng. Rehabil. 2022, 19, 119. [Google Scholar] [CrossRef] [PubMed]
- Baars, E.C.; Schrier, E.; Dijkstra, P.U.; Geertzen, J.H.B. Prosthesis satisfaction in lower limb amputees: A systematic review of associated factors and questionnaires. Medicine 2018, 97, e12296. [Google Scholar] [CrossRef]
- Speckman, R.A.; Biggs, W.T. Prosthetic Componentry in Lower Limb Prosthetic Restoration. Phys. Med. Rehabil. Clin. N. Am. 2024; in press. [Google Scholar] [CrossRef]
- Barrios-Muriel, J.; Romero-Sánchez, F.; Alonso-Sánchez, F.J.; Rodríguez Salgado, D. Advances in Orthotic and Prosthetic Manufacturing: A Technology Review. Materials 2020, 13, 295. [Google Scholar] [CrossRef] [PubMed]
- Pezzin, L.E.; Dillingham, T.R.; MacKenzie, E.J.; Ephraim, P.; Rossbach, P. Use and satisfaction with prosthetic limb devices and related services. Arch. Phys. Med. Rehabil. 2004, 85, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-P.; Zheng, Y.-P. Measurement of Soft Tissue Elasticity In Vivo: Techniques and Applications; CRC Press: New York, NY, USA, 2015. [Google Scholar]
- Lee, W.C.C. Computational and Experimental Analysis of the Use of Lower-Limb Prostheses, Concerning Comfort, Structural Design and Gait Performance; ProQuest Dissertations Publishing: Ann Arbor, MI, USA, 2006. [Google Scholar]
- Lee, W.; Zhang, M. Using computational simulation to aid in the prediction of socket fit: A preliminary study. Med. Eng. Phys. 2007, 29, 923–929. [Google Scholar] [CrossRef]
- Wu, C.L.; Chang, C.H.; Hsu, A.T.; Lin, C.C.; Chen, S.I.; Chang, G.L. A proposal for the pre-evaluation protocol of below-knee socket design—Integration pain tolerance with finite element analysis. J. Chin. Inst. Eng. 2003, 26, 853–860. [Google Scholar] [CrossRef]
- Frauziols, F.; Chassagne, F.; Badel, P.; Navarro, L.; Molimard, J.; Curt, N.; Avril, S. In vivo Identification of the Passive Mechanical Properties of Deep Soft Tissues in the Human Leg. Strain 2016, 52, 400–411. [Google Scholar] [CrossRef]
- Zheng, Y.; Mak, A.F.T. Effective elastic properties for lower limb soft tissues from manual indentation experiment. IEEE Trans. Rehabil. Eng. 1999, 7, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Paterno, L.; Ibrahimi, M.; Gruppioni, E.; Menciassi, A.; Ricotti, L. Sockets for Limb Prostheses: A Review of Existing Technologies and Open Challenges. IEEE Trans. Biomed. Eng. 2018, 65, 1996–2010. [Google Scholar] [CrossRef] [PubMed]
- Meulenbelt, H.E.J.; Geertzen, J.H.B.; Jonkman, M.F.; Dijkstraa, P.U. Skin Problems of the Stump in Lower Limb Amputees: 2. Influence on Functioning in Daily Life. Acta Derm.-Venereol. 2011, 91, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Safari, R. Lower limb prosthetic interfaces: Clinical and technological advancement and potential future direction. Prosthet. Orthot. Int. 2020, 44, 384–401. [Google Scholar] [CrossRef]
- Bombek, M.; Vesenjak, U.; Pisek, M.; Vidmar, G.; Knez, S.; Medved, S. Mechanical testing of laminated composite materials for prosthetic sockets. Mater. Technol. 2021, 55, 655–661. [Google Scholar] [CrossRef]
- Garg, V. System and Method of Manufacturing Prostheses. US11311370B2, 26 April 2022. [Google Scholar]
- Yang, X.; Zhao, R.; Solav, D.; Yang, X.; Lee, D.R.; Sparrman, B.; Fan, Y.; Herr, H. Material, design, and fabrication of custom prosthetic liners for lower-extremity amputees: A review. Med. Nov. Technol. Devices 2022, 17, 100197. [Google Scholar] [CrossRef]
- Wang, M.; Nong, Q.; Liu, Y.; Yu, H. Design of lower limb prosthetic sockets: A review. Expert Rev. Med. Devices 2022, 19, 63–73. [Google Scholar] [CrossRef]
- Najmi, Z.; Mlinarić, N.M.; Scalia, A.C.; Cochis, A.; Selmani, A.; Učakar, A.; Abram, A.; Zore, A.; Delač, I.; Jerman, I. Antibacterial evaluation of different prosthetic liner textiles coated by CuO nanoparticles. Heliyon 2024, 10, e23849. [Google Scholar] [CrossRef]
- Cagle, J.C.; Hafner, B.J.; Taflin, N.; Sanders, J.E. Characterization of prosthetic liner products for people with transtibial amputation. J. Prosthet. Orthot. 2018, 30, 187–199. [Google Scholar] [CrossRef]
- Ali, S.; Abu Osman, N.A.; Arifin, N.; Gholizadeh, H.; Abd Razak, N.A.; Abas, W.A.W. Comparative Study between Dermo, Pelite, and Seal-In X5 Liners: Effect on Patient’s Satisfaction and Perceived Problems. Sci. World J. 2014, 2014, 769810. [Google Scholar] [CrossRef]
- Sankaran, S.; Murugan, P.R.; Johnson, J.C.; Abdullah, H.J.S.; Raj, C.M.N.; Ashokan, D. Prevention of skin problems in patients using prosthetic limb: A review of current technologies and limitations. In Proceedings of the 2019 IEEE International Conference on Communication and Signal Processing, Chennai, India, 4–6 April 2019; pp. 77–81. [Google Scholar]
- Plesec, V.; Harih, G. Lower limb prosthesis: 3D generic model for numerical evaluation of the socket-liner system. In Proceedings of the 9th World Congress of Biomechanics, Taipei, Taiwan, 10–14 July 2022; pp. 2519–2520. [Google Scholar]
- Plesec, V.; Harih, G. Lower limb prosthesis: Computer methods for designing matamaterial liner. In Proceedings of the CMBBE2023 Symposium: Challenges in Computational Biomechanics for Tomorrow’s Healthcare Systems, Paris, France, 3–5 May 2023; p. 20. [Google Scholar]
- Plesec, V.; Harih, G. Numerical approach to improve socket-liner system using tailorable 3D printed metamaterials. In Proceedings of the ESB2022, Porto, Portugal, 26–29 June 2022; p. 636. [Google Scholar]
- Plesec, V.; Harih, G. Designing and evaluating tailorable 3D-printed metamaterials for lower limb prostheses. In Proceedings of the 32nd Annual Conference of the European Society for Biomaterials: Book of Abstracts, Bordeaux, France, 4–8 September 2022; p. 231. [Google Scholar]
- Colombo, G.; Rizzi, C.; Regazzoni, D.; Vitali, A. 3D interactive environment for the design of medical devices. Int. J. Interact. Des. Manuf. (IJIDeM) 2018, 12, 699–715. [Google Scholar] [CrossRef]
- Ribeiro, D.; Cimino, S.R.; Mayo, A.L.; Ratto, M.; Hitzig, S.L. 3D printing and amputation: A scoping review. Disabil. Rehabil. Assist. Technol. 2021, 16, 221–240. [Google Scholar] [CrossRef]
- van der Stelt, M.; Grobusch, M.P.; Koroma, A.R.; Papenburg, M.; Kebbie, I.; Masanga 3D Prosthesis Printing Research Group; Slump, C.H.; Maal, T.J.J.; Brouwers, L. Pioneering low-cost 3D-printed transtibial prosthetics to serve a rural population in Sierra Leone—An observational cohort study. EClinicalMedicine 2021, 35, 100874. [Google Scholar] [CrossRef] [PubMed]
- Moerman, K.; Sengeh, D.; Herr, H. Automated and data-driven computational design of patient-specific biomechanical interfaces. engrXiv 2016. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, Q.; Pu, F.; Boone, D.; Zhang, M. A Review of the Application of Additive Manufacturing in Prosthetic and Orthotic Clinics from a Biomechanical Perspective. Engineering 2020, 6, 1258–1266. [Google Scholar] [CrossRef]
- Vitali, A.; Colombo, G.; Regazzoni, D.; Rizzi, C. Virtual prototyping and physical experimentation of lower limb prosthesis. In Proceedings of the 5th Workshop on ICTs for improving Patients Rehabilitation Research Techniques, Popayan, Columbia, 11–13 September 2019; pp. 111–117. [Google Scholar]
- Meng, Z.; Wong, D.W.-C.; Zhang, M.; Leung, A.K.-L. Analysis of compression/release stabilized transfemoral prosthetic socket by finite element modelling method. Med. Eng. Phys. 2020, 83, 123–129. [Google Scholar] [CrossRef]
- Steer, J.W.; Worsley, P.R.; Browne, M.; Dickinson, A.S. Predictive prosthetic socket design: Part 1—Population-based evaluation of transtibial prosthetic sockets by FEA-driven surrogate modelling. Biomech. Model. Mechanobiol. 2019, 19, 1331–1346. [Google Scholar] [CrossRef] [PubMed]
- Moerman, K.M.; van Vijven, M.; Solis, L.R.; van Haaften, E.E.; Loenen, A.C.Y.; Mushahwar, V.K.; Oomens, C.W.J. On the importance of 3D, geometrically accurate, and subject-specific finite element analysis for evaluation of in-vivo soft tissue loads. Comput. Methods Biomech. Biomed. Eng. 2017, 20, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, A.S.; Steer, J.W.; Worsley, P.R. Finite element analysis of the amputated lower limb: A systematic review and recommendations. Med. Eng. Phys. 2017, 43, 1–18. [Google Scholar] [CrossRef]
- Plesec, V.; Harih, G. Development of a Generic Numerical Transtibial Model for Limb–Prosthesis System Evaluation. Appl. Sci. 2023, 13, 2339. [Google Scholar] [CrossRef]
- Cerveri, P.; Belfatto, A.; Manzotti, A. Predicting Knee Joint Instability Using a Tibio-Femoral Statistical Shape Model. Front. Bioeng. Biotechnol. 2020, 8, 253–264. [Google Scholar] [CrossRef]
- Ramalho, A.; Ferraz, M.; Gaspar, M.; Capela, C. Development of a preliminary finite element model to assess the effects of friction on the residual limb of a transfemoral amputee. Mater. Today Proc. 2020, 33, 1859–1863. [Google Scholar] [CrossRef]
- Cagle, J. A Computational Tool to Enhance Clinical Selection of Prosthetic Liners for People with Lower Limb Amputation. Ph.D. Thesis, University of Washington, Ann Arbor, MI, USA, 2016. [Google Scholar]
- Cagle, J.C.; Reinhall, P.G.; Allyn, K.J.; McLean, J.; Hinrichs, P.; Hafner, B.J.; Sanders, J.E. A finite element model to assess transtibial prosthetic sockets with elastomeric liners. Med. Biol. Eng. Comput. 2018, 56, 1227–1240. [Google Scholar] [CrossRef] [PubMed]
- Palay, M.; Sotelo, R. Design of an affordable lower limb bionic prosthesis. In Proceedings of the 2022 IEEE Global Humanitarian Technology Conference (GHTC), Santa Clara, CA, USA, 8–11 September 2022; pp. 429–432. [Google Scholar]
- Xu, K.; Qin, S.-F. 3D Printing, Limb Prosthetics and Generative Design: A Scoping Review. In Proceedings of the 2022 27th International Conference on Automation and Computing (ICAC), Bristol, UK, 1–3 September 2022; pp. 1–6. [Google Scholar]
- Rosický, J.; Bouma, T.; Grygar, A. 3D Printed Prosthetic Socket for Residual Limb. US20220265444A1, 25 August 2022. [Google Scholar]
- Nicoloso, L.G.D.V.; Pelz, J.; Barrack, H.; Kuester, F. Towards 3D printing of a monocoque transtibial prosthesis using a bio-inspired design workflow. Rapid Prototyp. J. 2021, 27, 67–80. [Google Scholar] [CrossRef]
- Gonabadi, H.; Chen, Y.; Bull, S. Investigation of the effects of volume fraction, aspect ratio and type of fibres on the mechanical properties of short fibre reinforced 3D printed composite materials. Prog. Addit. Manuf. 2024, 1–17. [Google Scholar] [CrossRef]
- Gonabadi, H.; Hosseini, S.F.; Chen, Y.; Bull, S. Structural analysis of small-scale 3D printed composite tidal turbine blades. Ocean Eng. 2024, 306, 118057. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- van der Stelt, M.; Verhamme, L.; Slump, C.H.; Brouwers, L.; Maal, T.J. Strength testing of low-cost 3D-printed transtibial prosthetic socket. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2022, 236, 367–375. [Google Scholar] [CrossRef]
- Brown, N.; Owen, M.K.; Garland, A.; DesJardins, J.D.; Fadel, G.M. Design of a single layer metamaterial for pressure offloading of transtibial amputees. J. Biomech. Eng. 2021, 143, 15. [Google Scholar] [CrossRef]
- Hudak, Y.F.; Li, J.-S.; Cullum, S.; Strzelecki, B.M.; Richburg, C.; Kaufman, G.E.; Abrahamson, D.; Heckman, J.T.; Ripley, B.; Telfer, S.; et al. A novel workflow to fabricate a patient-specific 3D printed accommodative foot orthosis with personalized latticed metamaterial. Med. Eng. Phys. 2022, 104, 103802. [Google Scholar] [CrossRef]
- Robinson, M.; Soe, S.; McShane, G.; Celeghini, R.; Burek, R.; Alves, M.; Hanna, B.; Theobald, P. Developing elastomeric cellular structures for multiple head impacts. In Proceedings of the Conference Proceedings International Research Council on the Biomechanics of Injury, IRCOBI, Antwerp, Belgium, 13–15 September 2017; pp. 182–189. [Google Scholar]
- Soe, S.; Martin, P.; Jones, M.; Robinson, M.; Theobald, P. Feasibility of optimising bicycle helmet design safety through the use of additive manufactured TPE cellular structures. Int. J. Adv. Manuf. Technol. 2015, 79, 1975–1982. [Google Scholar] [CrossRef]
- Cupar, A.; Kaljun, J.; Dolšak, B.; Harih, G. 3D printed deformable product handle material for improved ergonomics. Int. J. Ind. Ergon. 2021, 82, 103080. [Google Scholar] [CrossRef]
- Altenbach, H.; Öchsner, A. Cellular and Porous Materials in Structures and Processes; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; Volume 521. [Google Scholar]
- Zhang, X.M.; Wang, Y.C.; Su, M.N. Experimental, numerical and analytical study to develop a design method for bending and shear resistances of 3D printed beetle elytron inspired sandwich plate (beetle elytron plate). Thin-Walled Struct. 2023, 183, 110371. [Google Scholar] [CrossRef]
- Yang, T.; Wu, Z.; Chen, H.; Zhu, Y.; Li, L. Quantitative 3D structural analysis of the cellular microstructure of sea urchin spines (I): Methodology. Acta Biomater. 2020, 107, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Wang, Y.C.; Foster, A.S.J.; Su, M.N. Elastic local buckling behaviour of beetle elytron plate. Thin-Walled Struct. 2021, 165, 107922. [Google Scholar] [CrossRef]
- Novak, N.; Al-Ketan, O.; Krstulović-Opara, L.; Rowshan, R.; Abu Al-Rub, R.K.; Vesenjak, M.; Ren, Z. Quasi-static and dynamic compressive behaviour of sheet TPMS cellular structures. Compos. Struct. 2021, 266, 113801. [Google Scholar] [CrossRef]
- Yang, E.; Leary, M.; Lozanovski, B.; Downing, D.; Mazur, M.; Sarker, A.; Khorasani, A.; Jones, A.; Maconachie, T.; Bateman, S.; et al. Effect of geometry on the mechanical properties of Ti-6Al-4V Gyroid structures fabricated via SLM: A numerical study. Mater. Des. 2019, 184, 108165. [Google Scholar] [CrossRef]
- Han, L.; Che, S. An Overview of Materials with Triply Periodic Minimal Surfaces and Related Geometry: From Biological Structures to Self-Assembled Systems. Adv. Mater. 2018, 30, 1705708. [Google Scholar] [CrossRef]
- nTop. Available online: https://www.ntop.com/resources/blog/architected-materials-impact-absorption (accessed on 14 July 2024).
- efunda. Available online: https://www.efunda.com/formulae/solid_mechanics/mat_mechanics/hooke_isotropic.cfm (accessed on 19 July 2024).
- Singh, A.; Chaudhary, H.; Nayak, C. Topology optimisation of transtibial prosthesis socket using finite element analysis. Int. J. Biomed. Eng. Technol. 2017, 24, 323–337. [Google Scholar] [CrossRef]
- Kallin, S.; Rashid, A.; Salomonsson, K.; Hansbo, P. Comparison of mechanical conditions in a lower leg model with 5 or 6 tissue types while exposed to prosthetic sockets applying finite element analysis. arXiv 2019, arXiv:1907.13340. [Google Scholar] [CrossRef]
- Plesec, V.; Humar, J.; Dobnik-Dubrovski, P.; Harih, G. Numerical Analysis of a Transtibial Prosthesis Socket Using 3D-Printed Bio-Based PLA. Materials 2023, 16, 1985. [Google Scholar] [CrossRef]
- Lacroix, D.; Patiño, J.F.R. Finite element analysis of donning procedure of a prosthetic transfemoral socket. Ann. Biomed. Eng. 2011, 39, 2972–2983. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Zhang, M.; Jia, X.; Cheung, J.T.M. Finite element modeling of the contact interface between trans-tibial residual limb and prosthetic socket. Med. Eng. Phys. 2004, 26, 655–662. [Google Scholar] [CrossRef] [PubMed]
- ISO 10328:2016; Prosthetics. Structural Testing of Lower-Limb Prostheses. Requirements and Test Methods. British Standards Institution: London, UK, 2016.







| Metamaterial Unit Cell | Maximum Range of Young’s Modulus |
|---|---|
| TPMS Structures | |
| Gyroid | 12.2% |
| Schwarz | 45.8% |
| Neovius | 11.5% |
| Diamond | 17.7% |
| Beam Structures | |
| Simple Cubic | 96.3% |
| Octet | 33.7% |
| Beam Diamond | 50.7% |
| Re-entrant | 92.1% |
| Soft (6% MELAS) | Medium (10% MELAS) | Hard (14% MELAS) | ||||
|---|---|---|---|---|---|---|
| Points | σ [kPa] | ε [-] | σ [kPa] | ε [-] | σ [kPa] | ε [-] |
| P0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P1 | 35 | 0.2 | 70 | 0.1 | 250 | 0.12 |
| P2 | 50 | 0.5 | 90 | 0.44 | 280 | 0.41 |
| P3 | 220 | 0.65 | 500 | 0.62 | 650 | 0.57 |
| Component | Material Model | Reference | Parameters |
|---|---|---|---|
| Soft tissue | Ogden 1st order | Kallin et al. [68] | μ1 = 0.012 MPa α1 = 14 d1 = 1.67 MPa−1 |
| Silicone liner | Yeoh 3rd order | Cagle et al. [44] | c10 = 0.02014 MPa c20 = −0.00115 MPa c30 = 0.00041 MPa d1 = 3 MPa−1 |
| Socket | Linear–elastic | Plesec et al. [69] | E = 4991 MPa ν = 0.3 |
| Peak Contact Pressure [kPa] | Maximum Deformation [mm] | |||||
|---|---|---|---|---|---|---|
| PTB Socket | Donning | Heel Strike | Push-off | Donning | Heel Strike | Push-off |
| Soft | 54 | 127 | 217 | 3.8 | 13.2 | 17 |
| Medium | 74 | 115 | 160 | 3.7 | 9.7 | 13.6 |
| Hard | 94 | 153 | 200 | 3.5 | 8.3 | 11.6 |
| Silicone | 66 | 128 | 225 | 3.6 | 11.2 | 15.1 |
| TSB Socket | ||||||
| Soft | 63 | 90 | 172 | 5.1 | 10.3 | 14.9 |
| Medium | 106 | 129 | 133 | 5.1 | 6.8 | 10.1 |
| Hard | 123 | 148 | 160 | 5.1 | 5.9 | 8.2 |
| Silicone | 91 | 119 | 148 | 5.0 | 8.2 | 11.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plesec, V.; Harih, G. Bioinspired Design of 3D-Printed Cellular Metamaterial Prosthetic Liners for Enhanced Comfort and Stability. Biomimetics 2024, 9, 540. https://doi.org/10.3390/biomimetics9090540
Plesec V, Harih G. Bioinspired Design of 3D-Printed Cellular Metamaterial Prosthetic Liners for Enhanced Comfort and Stability. Biomimetics. 2024; 9(9):540. https://doi.org/10.3390/biomimetics9090540
Chicago/Turabian StylePlesec, Vasja, and Gregor Harih. 2024. "Bioinspired Design of 3D-Printed Cellular Metamaterial Prosthetic Liners for Enhanced Comfort and Stability" Biomimetics 9, no. 9: 540. https://doi.org/10.3390/biomimetics9090540
APA StylePlesec, V., & Harih, G. (2024). Bioinspired Design of 3D-Printed Cellular Metamaterial Prosthetic Liners for Enhanced Comfort and Stability. Biomimetics, 9(9), 540. https://doi.org/10.3390/biomimetics9090540






