Development and Evaluation of Fucoidan-Loaded Electrospun Polyvinyl Alcohol/Levan Nanofibers for Wound Dressing Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Halomonas Levan’s Production and Purification
2.3. Preparation of Electrospun Fibrous Mats
2.4. Morphological Characterization
2.5. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
2.6. Differential Scanning Calorimetry
2.7. Tensile Testing
2.8. Biological Characterization and Cell Viability Tests
2.9. In Vitro Drug Release Tests
2.10. Statistical Analysis
3. Results and Discussion
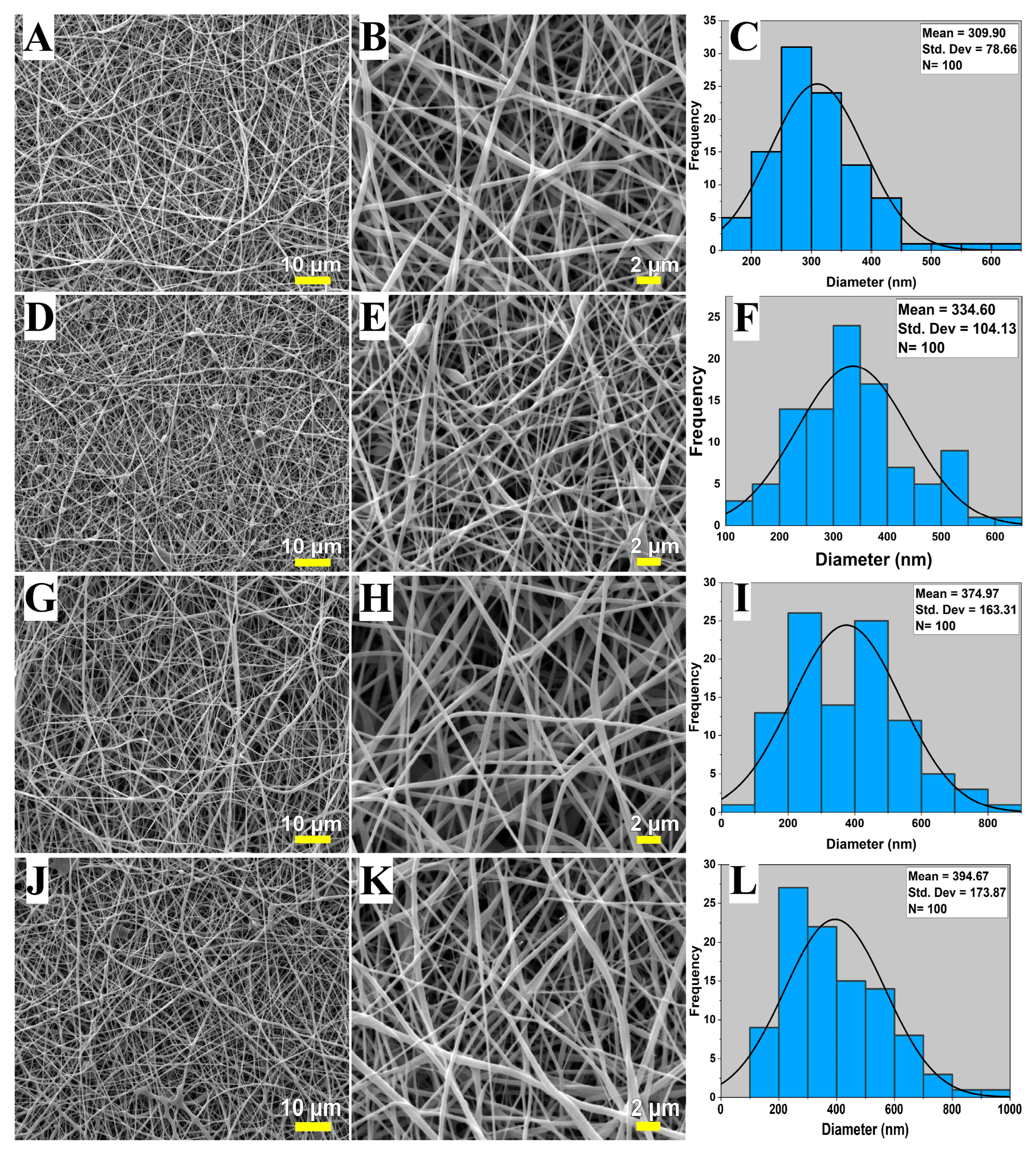
3.1. SEM Analysis
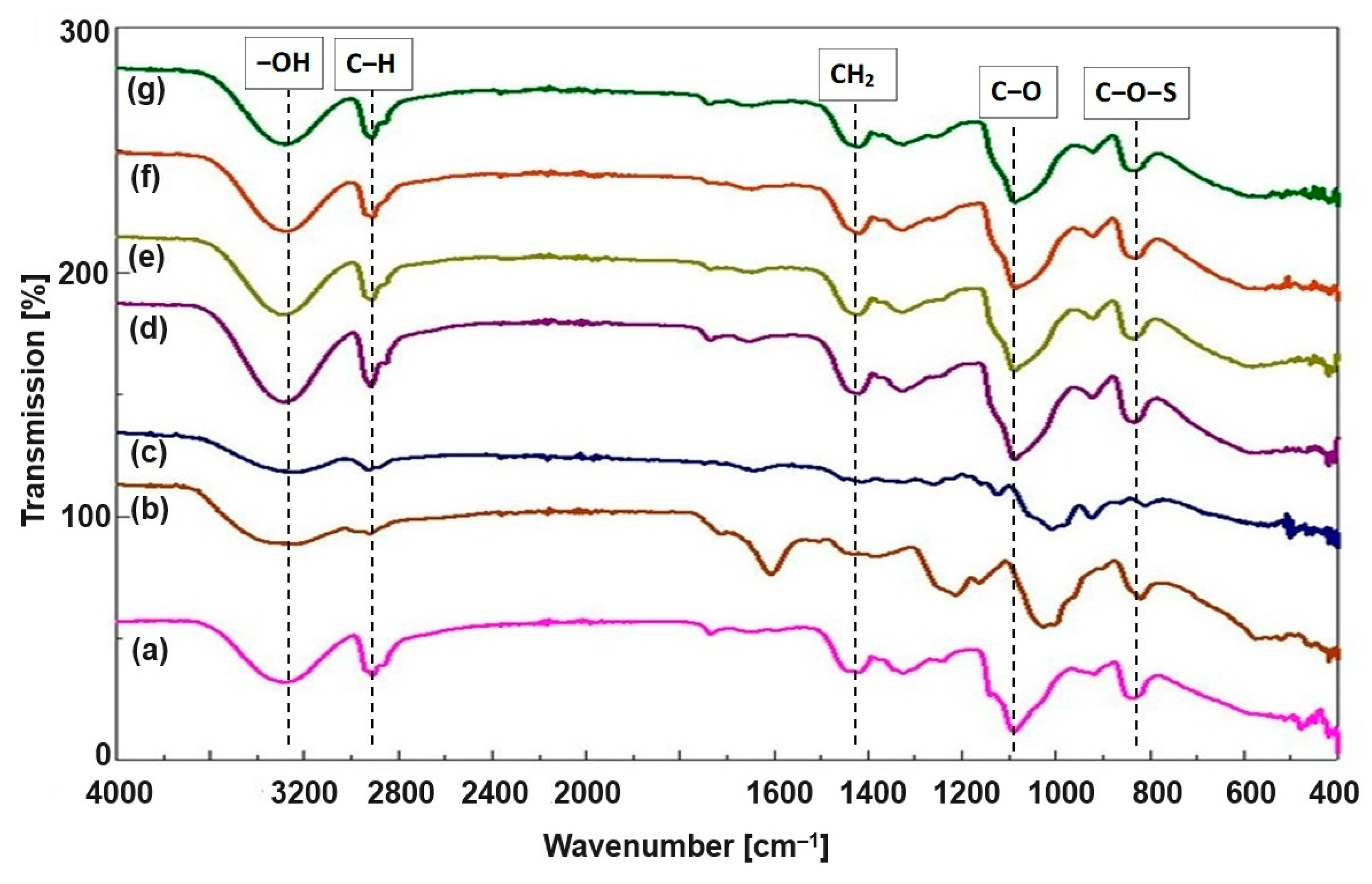
3.2. FTIR Analysis
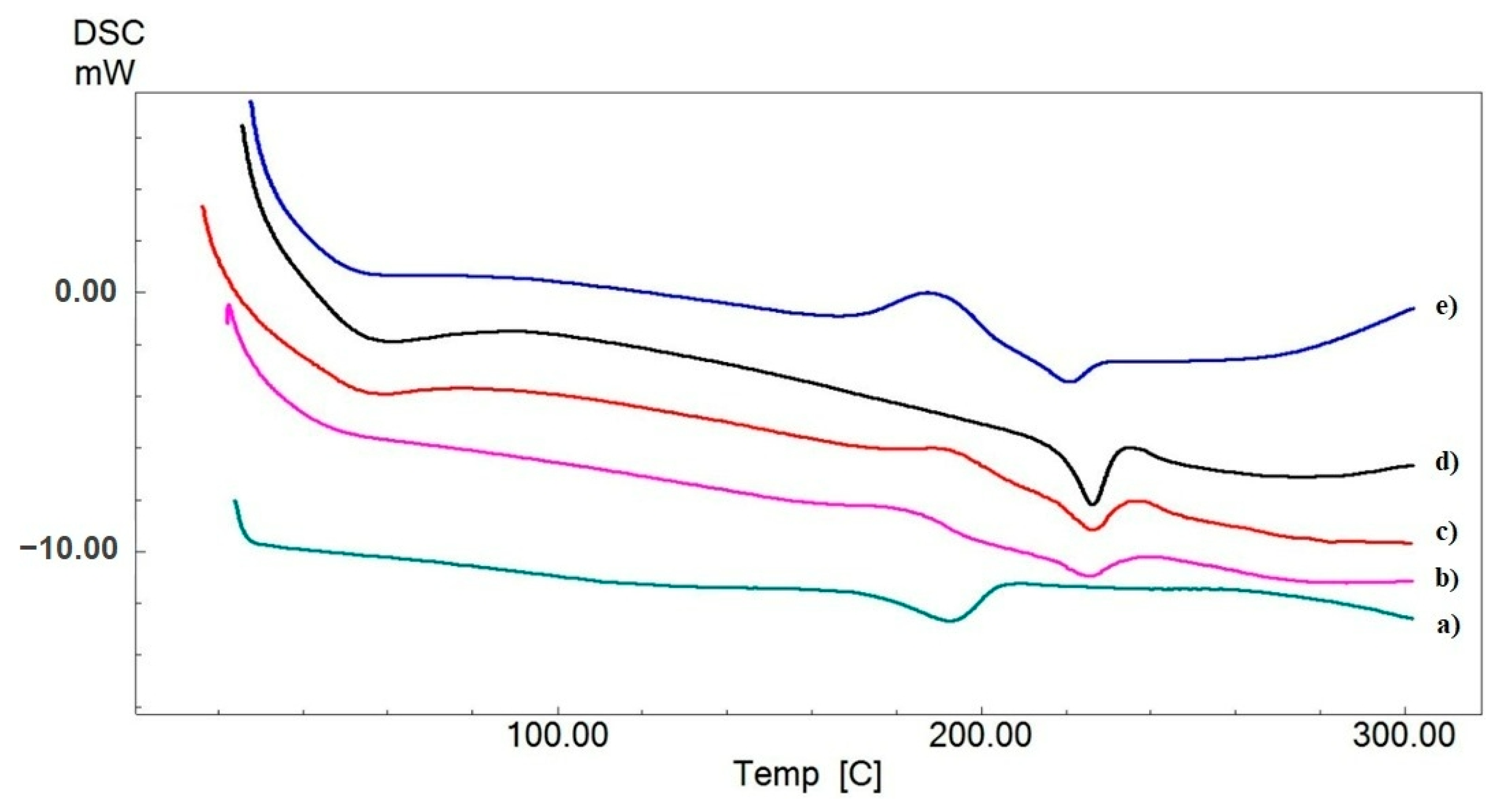
3.3. Differential Scanning Calorimetry Analysis
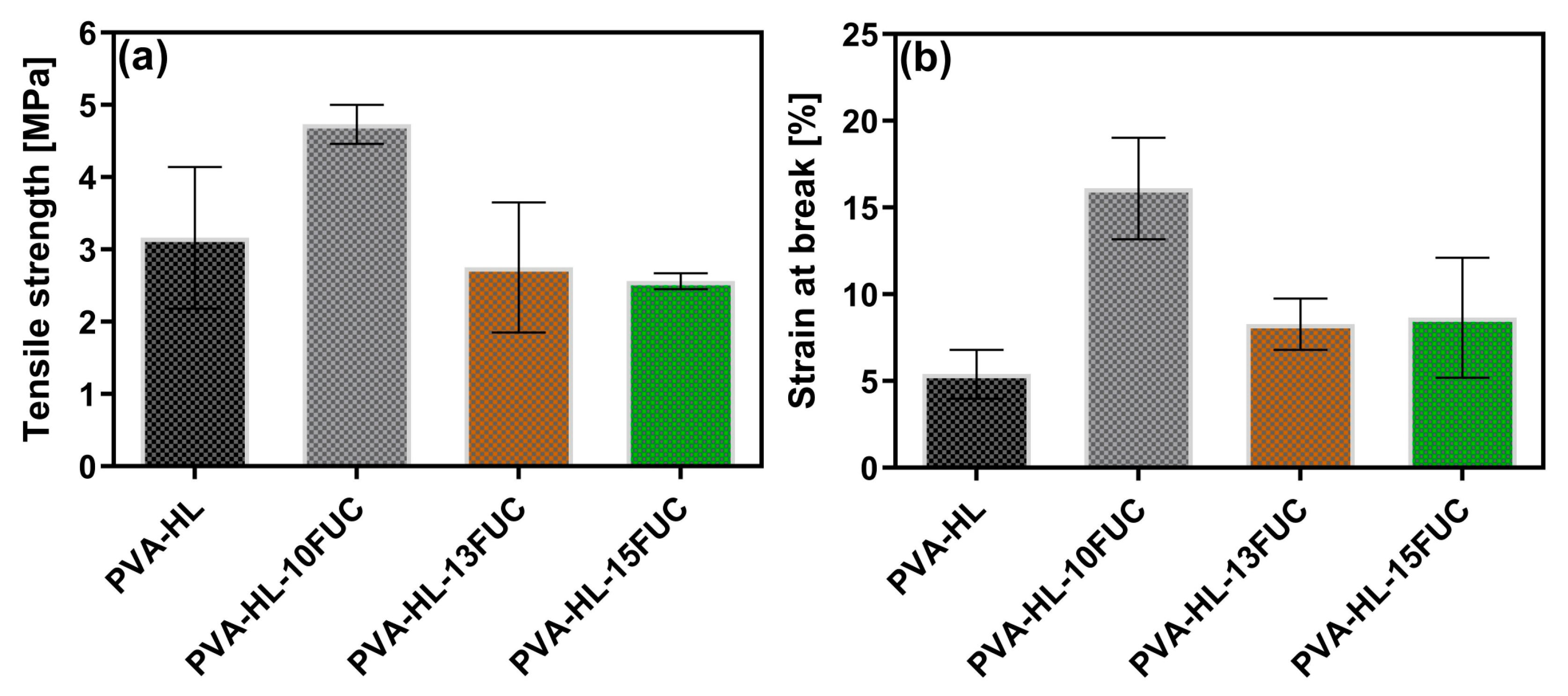
3.4. Tensile Strength
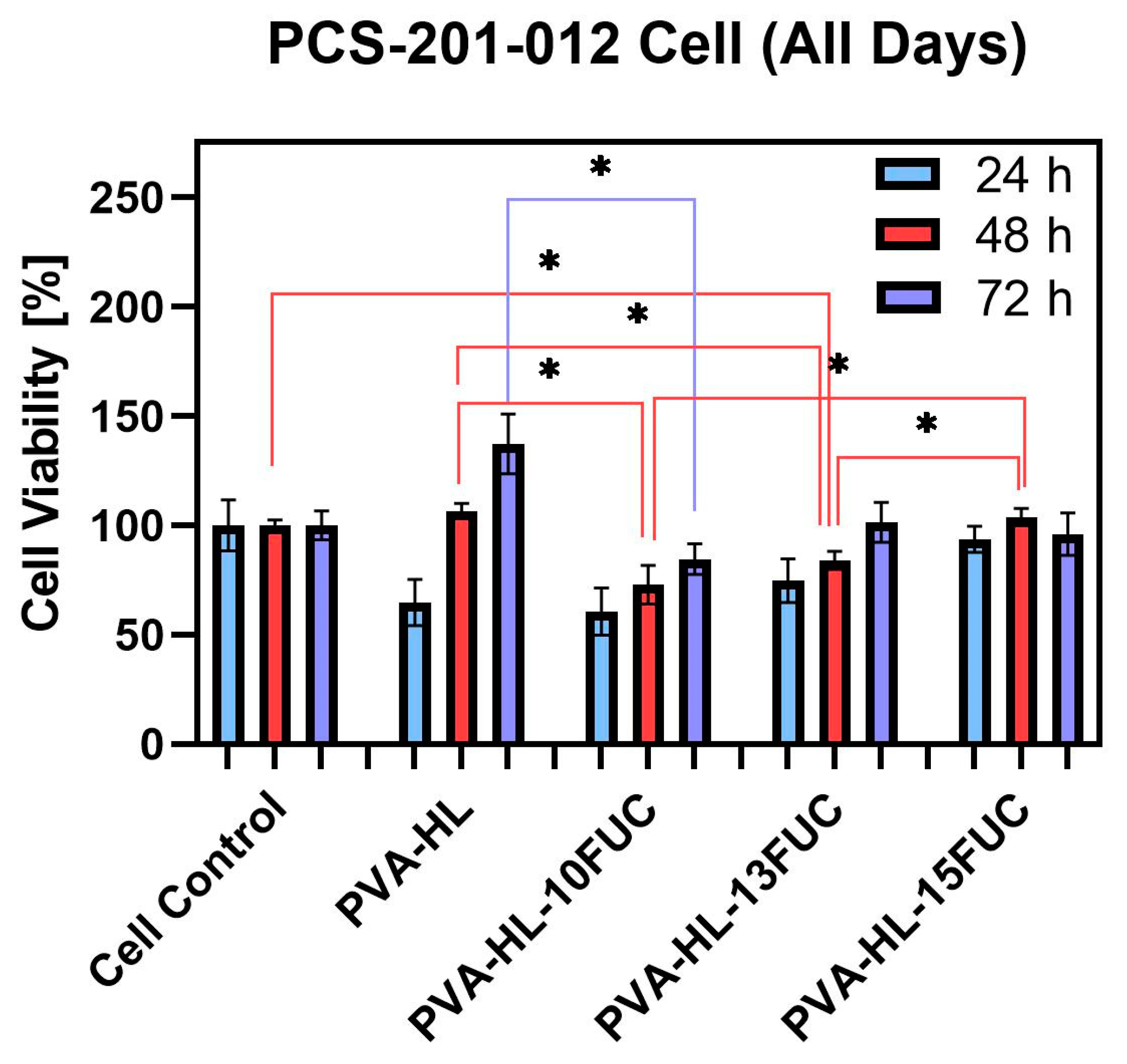
3.5. Cell Viability
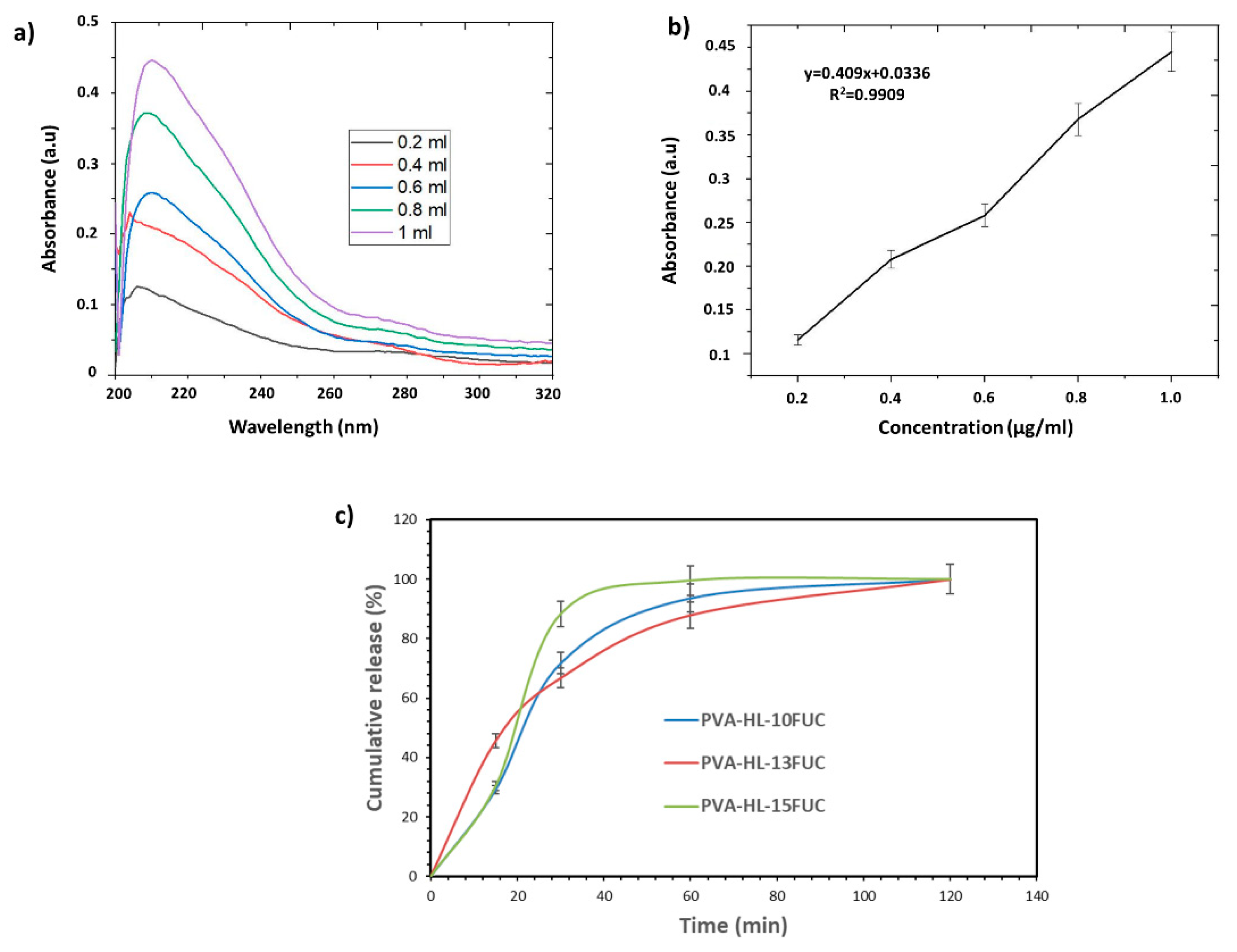
3.6. Release Behavior of the FUC from the PVA-HL Nanofibers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borda, L.J.; Macquhae, F.E.; Kirsner, R.S. Wound Dressings: A Comprehensive Review. Curr. Dermatol. Rep. 2016, 5, 287–297. [Google Scholar] [CrossRef]
- Ovington, L.G. Advances in Wound Dressings. Clin. Dermatol. 2007, 25, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Babalska, Z.Ł.; Korbecka-Paczkowska, M.; Karpiński, T.M. Wound Antiseptics and European Guidelines for Antiseptic Application in Wound Treatment. Pharmaceuticals 2021, 14, 1253. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in Nanofibers for Wound Dressing: A Review. Eur. Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- Sandri, G.; Rossi, S.; Bonferoni, M.C.; Caramella, C.; Ferrari, F. Electrospinning Technologies in Wound Dressing Applications. In Therapeutic Dressings and Wound Healing Applications; Wiley Online Library: Hoboken, NJ, USA, 2020. [Google Scholar]
- Kaya, S.; Derman, S. Properties of ideal wound dressing. J. Fac. Pharm. Ank. Univ. 2023, 47, 1119–1131. [Google Scholar] [CrossRef]
- De Luca, I.; Pedram, P.; Moeini, A.; Cerruti, P.; Peluso, G.; Di Salle, A.; Germann, N. Nanotechnology Development for Formulating Essential Oils in Wound Dressing Materials to Promote the Wound-Healing Process: A Review. Appl. Sci. 2021, 11, 1713. [Google Scholar] [CrossRef]
- Sezer, A.D. (Ed.) Application of Nanotechnology in Drug Delivery; InTech: Houston TX, USA, 2014; ISBN 978-953-51-1628-8. [Google Scholar]
- Zhang, Y.; Su, B.; Venugopal, J.; Ramakrishna, S.; Lim, C. Biomimetic and Bioactive Nanofibrous Scaffolds from Electrospun Composite Nanofibers. Int. J. Nanomed. 2007, 2, 623–638. [Google Scholar]
- Islam, M.S.; Ang, B.C.; Andriyana, A.; Afifi, A.M. A Review on Fabrication of Nanofibers via Electrospinning and Their Applications. SN Appl. Sci. 2019, 1, 1248. [Google Scholar] [CrossRef]
- Si, Y.; Shi, S.; Hu, J. Applications of Electrospinning in Human Health: From Detection, Protection, Regulation to Reconstruction. Nano Today 2023, 48, 101723. [Google Scholar] [CrossRef]
- Hamzeh, S.; Miraftab, M.; Yoosefinejad, A. Study of Electrospun Nanofibre Formation Process and Their Electrostatic Analysis. J. Ind. Text. 2014, 44, 147–158. [Google Scholar] [CrossRef]
- Dehnad, D.; Ghorani, B.; Emadzadeh, B.; Zhang, F.; Yang, N.; Jafari, S.M. Electrospinning of Legume Proteins: Fundamentals, Fiber Production, Characterization, and Applications with a Focus on Soy Proteins. Food Hydrocoll. 2024, 151, 109795. [Google Scholar] [CrossRef]
- Abdulhussain, R.; Adebisi, A.; Conway, B.R.; Asare-Addo, K. Electrospun Nanofibers: Exploring Process Parameters, Polymer Selection, and Recent Applications in Pharmaceuticals and Drug Delivery. J. Drug Deliv. Sci. Technol. 2023, 90, 105156. [Google Scholar] [CrossRef]
- Öner, E.T.; Hernández, L.; Combie, J. Review of Levan Polysaccharide: From a Century of Past Experiences to Future Prospects. Biotechnol. Adv. 2016, 34, 827–844. [Google Scholar] [CrossRef]
- Avsar, G.; Agirbasli, D.; Agirbasli, M.A.; Gunduz, O.; Oner, E.T. Levan Based Fibrous Scaffolds Electrospun via Co-Axial and Single-Needle Techniques for Tissue Engineering Applications. Carbohydr. Polym. 2018, 193, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Oner, E.T.; Eroglu, M.S. Novel Levan and PNIPA Temperature Sensitive Hydrogels for 5-ASA Controlled Release. Carbohydr. Polym. 2017, 165, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, R.; Reddy, C.H.S.S.S.; Siddartha, G.; Ramaiah, M.J.; Uppuluri, K.B. Review on Production, Characterization and Applications of Microbial Levan. Carbohydr. Polym. 2015, 120, 102–114. [Google Scholar] [CrossRef]
- Abdillah, B.F.; Nasir, M.; Mozef, T.; Hertadi, R. Fabrication of Physically Crosslink Levan-Lsbl-Bk1/PVA Electrospun Nanofiber. Eur. Polym. J. 2023, 195, 112237. [Google Scholar] [CrossRef]
- Ranjha, N.M.; Khan, S. Chitosan/Poly (Vinyl Alcohol) Based Hydrogels for Biomedical Applications: A Review. J. Pharm. Altern. Med. 2013, 2, 30–41. [Google Scholar]
- Lee, J.S.; Choi, K.H.; Ghim, H.D.; Kim, S.S.; Chun, D.H.; Kim, H.Y.; Lyoo, W.S. Role of Molecular Weight of Atactic Poly(Vinyl Alcohol) (PVA) in the Structure and Properties of PVA Nanofabric Prepared by Electrospinning. J. Appl. Polym. Sci. 2004, 93, 1638–1646. [Google Scholar] [CrossRef]
- do Nascimento, F.C.; de Aguiar, L.C.V.; Costa, L.A.T.; Fernandes, M.T.; Marassi, R.J.; Gomes, A.d.S.; de Castro, J.A. Formulation and Characterization of Crosslinked Polyvinyl Alcohol (PVA) Membranes: Effects of the Crosslinking Agents. Polym. Bull. 2021, 78, 917–929. [Google Scholar] [CrossRef]
- Sonker, A.K.; Rathore, K.; Nagarale, R.K.; Verma, V. Crosslinking of Polyvinyl Alcohol (PVA) and Effect of Crosslinker Shape (Aliphatic and Aromatic) Thereof. J. Polym. Environ. 2018, 26, 1782–1794. [Google Scholar] [CrossRef]
- Muppalaneni, S. Polyvinyl Alcohol in Medicine and Pharmacy: A Perspective. J. Dev. Drugs 2013, 2, 2. [Google Scholar] [CrossRef]
- Koski, A.; Yim, K.; Shivkumar, S. Effect of Molecular Weight on Fibrous PVA Produced by Electrospinning. Mater. Lett. 2004, 58, 493–497. [Google Scholar] [CrossRef]
- Jin, S.G. Production and Application of Biomaterials Based on Polyvinyl Alcohol (PVA) as Wound Dressing. Chem. Asian J. 2022, 17, e202200595. [Google Scholar] [CrossRef]
- Haddar, A.; Ben Ayed, E.; Sila, A.; Putaux, J.L.; Bougatef, A.; Boufi, S. Hybrid Levan-Ag/AgCl Nanoparticles Produced by UV-Irradiation: Properties, Antibacterial Efficiency and Application in Bioactive Poly(Vinyl Alcohol) Films. RSC Adv. 2021, 11, 38990–39003. [Google Scholar] [CrossRef]
- Puigmal, A.C.; Ayran, M.; Ulag, S.; Altan, E.; Guncu, M.M.; Aksu, B.; Durukan, B.K.; Sasmazel, H.T.; Perez, R.A.; Koc, E.; et al. Fucoidan-Loaded Electrospun Polyvinyl-Alcohol/Chitosan Nanofibers with Enhanced Antibacterial Activity for Skin Tissue Engineering. J. Mech. Behav. Biomed. Mater. 2023, 148, 106163. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef]
- Allafchian, A.; Hosseini, H.; Ghoreishi, S.M. Electrospinning of PVA-Carboxymethyl Cellulose Nanofibers for Flufenamic Acid Drug Delivery. Int. J. Biol. Macromol. 2020, 163, 1780–1786. [Google Scholar] [CrossRef]
- Serio, F.; da Cruz, A.F.; Chandra, A.; Nobile, C.; Rossi, G.R.; D’Amone, E.; Gigli, G.; del Mercato, L.L.; de Oliveira, C.C. Electrospun Polyvinyl-Alcohol/Gum Arabic Nanofibers: Biomimetic Platform for in Vitro Cell Growth and Cancer Nanomedicine Delivery. Int. J. Biol. Macromol. 2021, 188, 764–773. [Google Scholar] [CrossRef]
- Valizadeh, A.; Farkhani, S.M. Electrospinning and Electrospun Nanofibres. IET Nanobiotechnol. 2014, 8, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Phulmogare, G.; Rani, S.; Lodhi, S.; Patil, U.K.; Sinha, S.; Ajazuddin; Gupta, U. Fucoidan Loaded PVA/Dextran Blend Electrospun Nanofibers for the Effective Wound Healing. Int. J. Pharm. 2024, 650, 123722. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, L.; Ma, J.; Wang, X.; Wang, Y.; Ran, F.; Wang, Y.; Ma, H.; Yu, S. Electrospinning of Fucoidan/Chitosan/Poly(Vinyl Alcohol) Scaffolds for Vascular Tissue Engineering. Fibers Polym. 2017, 18, 922–932. [Google Scholar] [CrossRef]
- Mehrali, F.; Ziyadi, H.; Hekmati, M.; Faridi-Majidi, R.; Qomi, M. Kefiran/Poly(Vinyl Alcohol)/Poly(Vinyl Pyrrolidone) Composite Nanofibers: Fabrication, Characterization and Consideration of Effective Parameters in Electrospinning. SN Appl. Sci. 2020, 2, 895. [Google Scholar] [CrossRef]
- Kim, S.J.; Bae, P.K.; Choi, M.; Keem, J.O.; Chung, W.; Shin, Y.B. Fabrication and Application of Levan-PVA Hydrogel for Effective Influenza Virus Capture. ACS Appl. Mater. Interfaces 2020, 12, 29103–29109. [Google Scholar] [CrossRef]
- Ulag, S.; Ilhan, E.; Demirhan, R.; Sahin, A.; Yilmaz, B.K.; Aksu, B.; Sengor, M.; Ficai, D.; Titu, A.M.; Ficai, A. Propolis-Based Nanofiber Patches to Repair Corneal Microbial Keratitis. Molecules 2021, 26, 2577. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Kazak, H.; Gürleyendağ, B.; Tommonaro, G.; Pieretti, G.; Öner, E.T.; Nicolaus, B. High Level Synthesis of Levan by a Novel Halomonas Species Growing on Defined Media. Carbohydr. Polym. 2009, 78, 651–657. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, Y. Effect of Basic Fibroblast Growth Factor Released from Chitosan–Fucoidan Nanoparticles on Neurite Extension. J. Tissue Eng. Regen. Med. 2016, 10, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S. Phytochemical Constituents of Fucoidan (Padina tetrastromatica) and Its Assisted AgNPs for Enhanced Antibacterial Activity. IET Nanobiotechnol. 2017, 11, 292–299. [Google Scholar] [CrossRef]
- Siddaiah, T.; Ojha, P.; Kumar, N.O.; Ramu, C. Structural, Optical and Thermal Characterizations of PVA/MAA: EA Polyblend Films. Mater. Res. 2018, 21, e20170987. [Google Scholar] [CrossRef]
- Agrawal, S.L.; Awadhia, A. DSC and Conductivity Studies on PVA Based Proton Conducting Gel Electrolytes. Bull. Mater. Sci. 2004, 27, 523–527. [Google Scholar] [CrossRef]
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and Characterization of Chitosan/Gelatin/PVA Hydrogel for Wound Dressings. Carbohydr. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Kilmartin, P.A.; Versari, A. Application of Fourier Transform Infrared (FTIR) Spectroscopy in the Characterization of Tannins. Appl. Spectrosc. Rev. 2015, 50, 407–442. [Google Scholar] [CrossRef]
- Emin, M.; Ertas, B.; Alenezi, H.; Hazar-yavuz, A.N.; Cesur, S.; Sinemcan, G.; Ekentok, C.; Guler, E.; Katsakouli, C.; Demirbas, Z.; et al. Materials Science & Engineering C Accelerated Diabetic Wound Healing by Topical Application of Combination Oral Antidiabetic Agents-Loaded Nanofibrous Scaffolds: An in Vitro and in Vivo Evaluation Study. Mater. Sci. Eng. C 2021, 119, 111586. [Google Scholar] [CrossRef]
- Tobin, D.J. Introduction to Skin Aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef]
- Zahouani, H.; Pailler-Mattei, C.; Sohm, B.; Vargiolu, R.; Cenizo, V.; Debret, R. Characterization of the Mechanical Properties of a Dermal Equivalent Compared with Human Skin in Vivo by Indentation and Static Friction Tests. Skin. Res. Technol. 2009, 15, 68–76. [Google Scholar] [CrossRef]
- Chang, T.; Yin, H.; Yu, X.; Wang, L.; Fan, L.; Xin, J.H.; Yu, H. 3D PCL/Collagen Nanofibrous Medical Dressing for One-Time Treatment of Diabetic Foot Ulcers. Colloids Surf. B Biointerfaces 2022, 214, 112480. [Google Scholar] [CrossRef]
- Ilhan, E.; Cesur, S.; Sulutas, R.B.; Pilavci, E.; Dalbayrak, B.; Kaya, E.; Arisan, E.D.; Tinaz, G.B.; Sengor, M.; Kijeńska-Gawrońska, E.; et al. The Role of Multilayer Electrospun Poly(Vinyl Alcohol)/Gelatin Nanofibers Loaded with Fluconazole and Cinnamaldehyde in the Potential Treatment of Fungal Keratitis. Eur. Polym. J. 2022, 176, 111390. [Google Scholar] [CrossRef]
- Aydin, A.; Ulag, S.; Sahin, A.; Aksu, B.; Gunduz, O.; Ustundag, C.B.; Marinas, I.C.; Georgescu, M.; Chifiriuc, M.C. Biocompatible polyvinyl alcohol nanofibers loaded with amoxicillin and salicylic acid to prevent wound infections. Biomed. Mater. 2023, 18, 055029. [Google Scholar] [CrossRef] [PubMed]
| Solution Codes | PVA | HL | FUC | Distilled Water |
|---|---|---|---|---|
| PVA-HL | 2.4 g | - | - | 20 mL |
| PVA-HL-10FUC | 2.4 g | 24 mg | 10 mg | 20 mL |
| PVA-HL-13FUC | 2.4 g | 24 mg | 13 mg | 20 mL |
| PVA-HL-15FUC | 2.4 g | 24 mg | 15 mg | 20 mL |
| Comparison | Time Interval (hours) | p-Value |
|---|---|---|
| Cell control vs. PVA-HL-13FUC | 48 | 0.03 |
| PVA-HL vs. PVA-HL-10FUC | 48 | 0.04 |
| PVA-HL vs. PVA-HL-10FUC | 72 | 0.04 |
| PVA-HL vs. PVA-HL-13FUC | 48 | 0.03 |
| PVA-HL-10FUC vs. PVA-HL-15FUC | 48 | 0.05 |
| PVA-HL-13FUC vs. PVA-HL-15FUC | 48 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismayilova, N.; Zia, M.K.; Akkaya, H.S.; Ulag, S.; Guldorum, Y.; Oner, E.T.; Ince, E.; Duta, L.; Gunduz, O. Development and Evaluation of Fucoidan-Loaded Electrospun Polyvinyl Alcohol/Levan Nanofibers for Wound Dressing Applications. Biomimetics 2024, 9, 508. https://doi.org/10.3390/biomimetics9090508
Ismayilova N, Zia MK, Akkaya HS, Ulag S, Guldorum Y, Oner ET, Ince E, Duta L, Gunduz O. Development and Evaluation of Fucoidan-Loaded Electrospun Polyvinyl Alcohol/Levan Nanofibers for Wound Dressing Applications. Biomimetics. 2024; 9(9):508. https://doi.org/10.3390/biomimetics9090508
Chicago/Turabian StyleIsmayilova, Natavan, Muhammad Khaqan Zia, Hatice Selen Akkaya, Songul Ulag, Yeliz Guldorum, Ebru Toksoy Oner, Erol Ince, Liviu Duta, and Oguzhan Gunduz. 2024. "Development and Evaluation of Fucoidan-Loaded Electrospun Polyvinyl Alcohol/Levan Nanofibers for Wound Dressing Applications" Biomimetics 9, no. 9: 508. https://doi.org/10.3390/biomimetics9090508
APA StyleIsmayilova, N., Zia, M. K., Akkaya, H. S., Ulag, S., Guldorum, Y., Oner, E. T., Ince, E., Duta, L., & Gunduz, O. (2024). Development and Evaluation of Fucoidan-Loaded Electrospun Polyvinyl Alcohol/Levan Nanofibers for Wound Dressing Applications. Biomimetics, 9(9), 508. https://doi.org/10.3390/biomimetics9090508









