From Nature to Technology: Exploring the Potential of Plant-Based Materials and Modified Plants in Biomimetics, Bionics, and Green Innovations
Abstract
1. Introduction
2. Plants in Materials Science
2.1. Plants as Inspiration in Developing Materials
2.2. Phyto-Nanotechnology
2.3. Camouflage Inspiration from Green Vegetation
3. Plant-Based Sources for Tissue Engineering
3.1. Decellularization Process
3.2. Applications of Decellularized Plants in Bone Tissue Engineering
3.3. Challenges of Decellularized Plant Scaffolds for Transplantation
3.4. Applications of Decellularized Plants in Drug Testing
4. Plants in Green Electronics. Bionic Light-Emitting Plants
4.1. Plants in Dye-Sensitized Solar Cells (DSSCs)
4.2. Insights into Natural Pigments for DSSCs
4.3. Plants in Bioelectronics: Natural/Plant-Based DSSCs
4.4. Light-Emitting Plants
4.5. Engineered/Bionic Plants for Detection of Explosives and Pollutants
5. Plants in the Agricultural Sector
6. Phyto-Based Approaches in Wastewater Treatments
7. Botanical Robots
8. Concluding Remarks and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamers, J.; van der Meer, T.; Testerink, C. How Plants Sense and Respond to Stressful Environments. Plant Physiol. 2020, 182, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Leopold, A.C. Smart plants: Memory and communication without brains. Plant Signal. Behav. 2014, 9, e972268. [Google Scholar] [CrossRef] [PubMed]
- Cvrčková, F.; Lipavská, H.; Žárský, V. Plant intelligence. Plant Signal. Behav. 2009, 4, 394–399. [Google Scholar] [CrossRef][Green Version]
- Machín, A.; Fontánez, K.; Arango, J.C.; Ortiz, D.; De León, J.; Pinilla, S.; Nicolosi, V.; Petrescu, F.I.; Morant, C.; Márquez, F. One-Dimensional (1D) Nanostructured Materials for Energy Applications. Materials 2021, 14, 2609. [Google Scholar] [CrossRef] [PubMed]
- Arazoe, H.; Miyajima, D.; Akaike, K.; Araoka, F.; Sato, E.; Hikima, T.; Kawamoto, M.; Aida, T. An autonomous actuator driven by fluctuations in ambient humidity. Nat. Mater. 2016, 15, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Barbinta-Patrascu, M.-E.; Nichita, C.; Antohe, S. Innovative Strategy Based on Green Nanotechnology for Elimination and Reduction of Aquatic Weeds. Rom. Rep. Phys. 2023, 75, 604. [Google Scholar] [CrossRef]
- Albrecht, M.A.; Evans, C.W.; Raston, C.L. Green chemistry and the health implications of nanoparticles. Green Chem. 2006, 8, 417–432. [Google Scholar] [CrossRef]
- Mohebbi, S.; Nezhad, M.N.; Zarrintaj, P.; Jafari, S.H.; Gholizadeh, S.S.; Saeb, M.R.; Mozafari, M. Chitosan in Biomedical Engineering: A Critical Review. Curr. Stem Cell Res. Ther. 2019, 14, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Ansoms, P.; Barzegari, M.; Sloten, J.V.; Geris, L. Coupling biomechanical models of implants with biodegradation models: A case study for biodegradable mandibular bone fixation plates. J. Mech. Behav. Biomed. Mater. 2023, 147, 106120. [Google Scholar] [CrossRef]
- Mironescu, M.; Lazea-Stoyanova, A.; Barbinta-Patrascu, M.E.; Virchea, L.-I.; Rexhepi, D.; Mathe, E.; Georgescu, C. Green Design of Novel Starch-Based Packaging Materials Sustaining Human and Environmental Health. Polymers 2021, 13, 1190. [Google Scholar] [CrossRef]
- Navasingh, R.J.H.; Gurunathan, M.K.; Nikolova, M.P.; Królczyk, J.B. Sustainable Bioplastics for Food Packaging Produced from Renewable Natural Sources. Polymers 2023, 15, 3760. [Google Scholar] [CrossRef] [PubMed]
- Kouassi, M.-C.; Grisel, M.; Gore, E. Multifunctional active ingredient-based delivery systems for skincare formulations: A review. Colloids Surf. B Biointerfaces 2022, 217, 112676. [Google Scholar] [CrossRef] [PubMed]
- Asadollahi, A.; Khoobdel, M.; Zahraei-Ramazani, A.; Azarmi, S.; Mosawi, S.H. Effectiveness of plant-based repellents against different Anopheles species: A systematic review. Malar. J. 2019, 18, 436. [Google Scholar] [CrossRef] [PubMed]
- Boonyuan, W.; Panthawong, A.; Thannarin, T.; Kongratarporn, T.; Khamvarn, V.; Chareonviriyaphap, T.; Nararak, J. Irritant and repellent behaviors of sterile male Aedes aegypti (L.)(Diptera: Culicidae) mosquitoes are crucial in the development of disease control strategies applying sterile insect technique. PeerJ 2024, 12, e17038. [Google Scholar] [CrossRef] [PubMed]
- Fasani, E.; Manara, A.; Martini, F.; Furini, A.; DalCorso, G. The potential of genetic engineering of plants for the remediation of soils contaminated with heavy metals. Plant Cell Environ. 2018, 41, 1201–1232. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Q.; Zhang, Y.; Li, C.; He, Z.; Choy, W.C.H.; Low, P.J.; Sonar, P.; Kyaw, A.K.K. Biodegradable Materials and Green Processing for Green Electronics. Adv. Mater. 2020, 32, 2001591. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef]
- Hughes-Riley, T.; Dias, T.; Cork, C. A historical review of the development of electronic textiles. Fibers 2018, 6, 34. [Google Scholar] [CrossRef]
- Beek, L.; Skirde, J.-E.; Akdere, M.; Gries, T. Bio-Inspired Textiles for Self-Driven Oil–Water Separation—A Simulative Analysis of Fluid Transport. Biomimetics 2024, 9, 261. [Google Scholar] [CrossRef]
- Reichenauer, T.G.; Germida, J.J. Phytoremediation of Organic Contaminants in Soil and Groundwater. ChemSusChem 2008, 1, 708–717. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Osibote, A.O. Green Synthesis of Metal Oxide Nanoparticles, and Their Various Applications. J. Hazard. Mater. Adv. 2024, 13, 100401. [Google Scholar] [CrossRef]
- Karban, R. Plant behaviour and communication. Ecol. Lett. 2008, 11, 727–739. [Google Scholar] [CrossRef]
- Niklas, K.J.; Spatz, H.-C. Plant Physics; University of Chicago Press: Chicago, IL, USA, 2012. [Google Scholar]
- Speck, O.; Speck, T. Biomimetics in Botanical Gardens—Educational Trails and Guided Tours. Biomimetics 2023, 8, 303. [Google Scholar] [CrossRef]
- Vierstraete, M.; Chastan, P.; Meneghin, A.; Muysoms, F. History of the Creation of Self-Gripping Mesh. J. Abdom. Wall Surg. 2023, 2, 11330. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.; Kan, M.M.P.; Cheng, H.M.H.; De Carvalho, D.E.; Anwer, S.; Li, H.; Wong, A.Y.L. Effects of Using a Shoulder/Scapular Brace on the Posture and Muscle Activity of Healthy University Students during Prolonged Typing—A Randomized Controlled Cross-Over Trial. Healthcare 2023, 11, 1555. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lake-Thompson, G.; Keim, O.; Brook, P.; Sutcliffe, S.; Tudor, J.; Yang, K. Durability Testing of Knitted E-Textiles and Design of a User-Friendly E-Sleeve for Stroke Arm and Hand Rehabilitation. Eng. Proc. 2024, 52, 18. [Google Scholar] [CrossRef]
- De Grave, A.; Botija, P.; Hansen, H.N.; Tang, P.T. Manufacturing and characterization of water repellent surfaces. In 4M 2006—Second International Conference on Multi-Material Micro Manufacture; Menz, W., Dimov, S., Fillon, B., Eds.; Elsevier: Oxford, UK, 2006; pp. 281–284. [Google Scholar] [CrossRef]
- Collins, C.M.; Safiuddin, M. Lotus-Leaf-Inspired Biomimetic Coatings: Different Types, Key Properties, and Applications in Infrastructures. Infrastructures 2022, 7, 46. [Google Scholar] [CrossRef]
- Barthlott, W. Self-Cleaning Surfaces in Plants: The Discovery of the Lotus Effect as a Key Innovation for Biomimetic Technologies. In Handbook of Self-Cleaning Surfaces and Materials; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 359–369. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Ma, X.; Li, W.; Yang, W. A Bionic Venus Flytrap Soft Microrobot Driven by Multiphysics for Intelligent Transportation. Biomimetics 2023, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hou, Y.; Zhong, S.; Zhu, J.; Guan, C. Biomimetic venus flytrap structures using smart composites: A review. Materials 2023, 16, 6702. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Li, H.; Shen, J.; Zhang, F.; He, J.; Lin, J.; Wang, B.; Niu, S.; Han, Z.; et al. Bioinspired hydrogel actuator for soft robotics: Opportunity and challenges. Nano Today 2023, 49, 101764. [Google Scholar] [CrossRef]
- Hagihara, T.; Toyota, M. Mechanical Signaling in the Sensitive Plant Mimosa pudica L. Plants 2020, 9, 587. [Google Scholar] [CrossRef] [PubMed]
- Sankaewthong, S.; Miyata, K.; Horanont, T.; Xie, H.; Karnjana, J. Mimosa Kinetic Façade: Bio-Inspired Ventilation Leveraging the Mimosa Pudica Mechanism for Enhanced Indoor Air Quality. Biomimetics 2023, 8, 603. [Google Scholar] [CrossRef]
- Cummins, C.; Seale, M.; Macente, A.; Certini, D.; Mastropaolo, E.; Viola, I.M.; Nakayama, N. A separated vortex ring underlies the flight of the dandelion. Nature 2018, 562, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Budholiya, S.; Bhat, A.; Raj, S.A.; Sultan, M.T.H.; Shah, A.U.M.; Basri, A.A. State of the Art Review about Bio-Inspired Design and Applications: An Aerospace Perspective. Appl. Sci. 2021, 11, 5054. [Google Scholar] [CrossRef]
- Iyer, V.; Gaensbauer, H.; Daniel, T.L.; Gollakota, S. Wind dispersal of battery-free wireless devices. Nature 2022, 603, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Zgura, I.; Badea, N.; Enculescu, M.; Maraloiu, V.-A.; Ungureanu, C.; Barbinta-Patrascu, M.-E. Burdock-Derived Composites Based on Biogenic Gold, Silver Chloride and Zinc Oxide Particles as Green Multifunctional Platforms for Biomedical Applications and Environmental Protection. Materials 2023, 16, 1153. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.-E.; Gorshkova, Y.; Ungureanu, C.; Badea, N.; Bokuchava, G.; Lazea-Stoyanova, A.; Bacalum, M.; Zhigunov, A.; Petrovic, S. Characterization and Antitumoral Activity of Biohybrids Based on Turmeric and Silver/Silver Chloride Nanoparticles. Materials 2021, 14, 4726. [Google Scholar] [CrossRef] [PubMed]
- Barbinta-Patrascu, M.-E.; Nichita, C.; Bita, B.; Antohe, S. Biocomposite Materials Derived from Andropogon halepensis: Eco-Design and Biophysical Evaluation. Materials 2024, 17, 1225. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 2000. [Google Scholar] [CrossRef]
- Filho, S.A.; dos Santos, M.S.; dos Santos, O.A.L.; Backx, B.P.; Soran, M.-L.; Opriş, O.; Lung, I.; Stegarescu, A.; Bououdina, M. Biosynthesis of Nanoparticles Using Plant Extracts and Essential Oils. Molecules 2023, 28, 3060. [Google Scholar] [CrossRef]
- Radulescu, D.-M.; Surdu, V.-A.; Ficai, A.; Ficai, D.; Grumezescu, A.-M.; Andronescu, E. Green Synthesis of Metal and Metal Oxide Nanoparticles: A Review of the Principles and Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 15397. [Google Scholar] [CrossRef]
- Balusamy, S.R.; Joshi, A.S.; Perumalsamy, H.; Mijakovic, I.; Singh, P. Advancing sustainable agriculture: A critical review of smart and eco-friendly nanomaterial applications. J. Nanobiotechnol. 2023, 21, 372. [Google Scholar] [CrossRef] [PubMed]
- Alhalili, Z. Metal Oxides Nanoparticles: General Structural Description, Chemical, Physical, and Biological Synthesis Methods, Role in Pesticides and Heavy Metal Removal through Wastewater Treatment. Molecules 2023, 28, 3086. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ahmad, S.; Ali, S.; Esa, M.; Khan, A.; Yan, H. Recent Advancements and Unexplored Biomedical Applications of Green Synthesized Ag and Au Nanoparticles: A Review. Int. J. Nanomed. 2024, 19, 3187–3215. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Rajput, V.D.; Kumari, A.; Mandzhieva, S.S.; Sushkova, S.; Prazdnova, E.V.; Zargar, S.M.; Raza, A.; Minkina, T.; Chung, G. Nanobionics in Crop Production: An Emerging Approach to Modulate Plant Functionalities. Plants 2022, 11, 692. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.E.; Chilom, C.; Nichita, C.; Zgura, I.; Iftimie, S.; Antohe, S. Biophysical Insights on Jack Bean Urease in the Presence of Silver Chloride Phytonanoparticles Generated from Mentha piperita L. Leaves. Rom. Rep. Phys. 2022, 74, 605. [Google Scholar]
- Shad, S.; Lynch, I.; Shah, S.W.H.; Bashir, N. Remediation of Water Using a Nanofabricated Cellulose Membrane Embedded with Silver Nanoparticles. Membranes 2022, 12, 1035. [Google Scholar] [CrossRef] [PubMed]
- Packialakshmi, P.; Gobinath, P.; Ali, D.; Alarifi, S.; Ravindran, B.; Idhayadhulla, A.; Surendrakumar, R. Novel Chitosan Polymer Design, Synthesis Using Mentha piperita of ZnO NPs as a Catalyst: Antibacterial Evaluation against Gram-Negative Multidrug-Resistant Pathogens. J. Nanomater. 2021, 2021, e8804837. [Google Scholar] [CrossRef]
- Erol, I.; Sivrier, M.; Cigerci, I.H.; Özkara, A.; Akyıl, D. ZnO-containing nanocomposites produced from Mentha pulegium L. of a new HEMA-based methacrylate copolymer: Improvement the thermal and antimicrobial effect. J. Polym. Res. 2023, 30, 103. [Google Scholar] [CrossRef]
- Stoyanova, D.; Stambolova, I.; Blaskov, V.; Georgieva, P.; Shipochka, M.; Zaharieva, K.; Dimitrov, O.; Markov, P.; Dyakova, V.; Kostova, Y.; et al. Modified Approach Using Mentha arvensis in the Synthesis of ZnO Nanoparticles—Textural, Structural, and Photocatalytic Properties. Appl. Sci. 2022, 12, 1096. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Al-Qahtani, K.M.; Alflaij, S.O.; Al-Qahtani, S.F.; Alsamhan, F.A. Green copper oxide nanoparticles for lead, nickel, and cadmium removal from contaminated water. Sci. Rep. 2021, 11, 12547. [Google Scholar] [CrossRef]
- Iliger, K.S.; Sofi, T.A.; Bhat, N.A.; Ahanger, F.A.; Sekhar, J.C.; Elhendi, A.Z.; Al-Huqail, A.A.; Khan, F. Copper nanoparticles: Green synthesis and managing fruit rot disease of chilli caused by Colletotrichum capsici. Saudi J. Biol. Sci. 2021, 28, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Moirangthem, M.; Schenning, A.P.H.J. Full Color Camouflage in a Printable Photonic Blue-Colored Polymer. ACS Appl. Mater. Interfaces 2018, 10, 4168–4172. [Google Scholar] [CrossRef]
- Yamamoto, N.; Sota, T. Evolutionary fine-tuning of background-matching camouflage among geographical populations in the sandy beach tiger beetle. Proc. R. Soc. B Biol. Sci. 2020, 287, 20202315. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Liu, Z.; Wu, X.; Li, G.; Fang, D.; Zhang, X. Nanofibrous Kevlar Aerogel Films and Their Phase-Change Composites for Highly Efficient Infrared Stealth. ACS Nano 2019, 13, 2236–2245. [Google Scholar] [CrossRef] [PubMed]
- Dian, R.; Li, S.; Sun, B.; Guo, A. Recent advances and new guidelines on hyperspectral and multispectral image fusion. Inf. Fusion 2021, 69, 40–51. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, H.; Peng, S. Study on identification of altered rock in hyperspectral imagery using spectrum of field object. Ore Geol. Rev. 2014, 56, 584–595. [Google Scholar] [CrossRef]
- Kishore, S.R.; Sridharan, A.P.; Chadha, U.; Narayanan, D.; Mishra, M.; Selvaraj, S.K.; Patterson, A.E. Natural fiber biocomposites via 4D printing technologies: A review of possibilities for agricultural bio-mulching and related sustainable applications. Prog. Addit. Manuf. 2024, 9, 37–67. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.N.; Leporatti, S.; El-Kemary, M.A. Extraction of chlorophyll and carotenoids loaded into chitosan as potential targeted therapy and bio imaging agents for breast carcinoma. Int. J. Biol. Macromol. 2021, 182, 1150–1160. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, X.; Yang, P.; Långvik, O.; Wang, X.; Zhang, Y.; Cheng, F.; Österberg, M.; Willför, S.M.; Xu, C. Surface Engineered Biomimetic Inks Based on UV Cross-Linkable Wood Biopolymers for 3D Printing. ACS Appl. Mater. Interfaces 2019, 11, 12389–12400. [Google Scholar] [CrossRef]
- Gregor, J.; Maršálek, B. Freshwater phytoplankton quantification by chlorophyll a: A comparative study of in vitro, in vivo and in situ methods. Water Res. 2004, 38, 517–522. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Baranoski, G.V.G.; Rokne, J.G. A practical approach for estimating the red edge position of plant leaf reflectance. Int. J. Remote Sens. 2005, 26, 503–521. [Google Scholar] [CrossRef]
- Ansari, H.H.; Siddiqui, A.; Wajid, D.; Tabassum, S.; Umar, M.; Siddiqui, Z.S. Profiling of energy compartmentalization in photosystem II (PSII), light harvesting complexes and specific energy fluxes of primed maize cultivar (P1429) under salt stress environment. Plant Physiol. Biochem. 2022, 170, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Kalirajan, C.; Dukle, A.; Nathanael, A.J.; Oh, T.-H.; Manivasagam, G. A Critical Review on Polymeric Biomaterials for Biomedical Applications. Polymers 2021, 13, 3015. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Ju, W.; Zhan, W.; Cheng, T.; Qiu, F.; Wang, J. A new spectral similarity water index for the estimation of leaf water content from hyperspectral data of leaves. Remote Sens. Environ. 2017, 196, 13–27. [Google Scholar] [CrossRef]
- Gao, Y.; Ye, H. Bionic membrane simulating solar spectrum reflection characteristics of natural leaf. Int. J. Heat Mass Transf. 2017, 114, 115–124. [Google Scholar] [CrossRef]
- Ai, S.; Zheng, H.; Yu, J. Preparation and Reflectance Spectrum Modulation of Cr2O3 Green Pigment by Solution Combustion Synthesis. Materials 2020, 13, 1540. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, S.; Wang, L.; Ye, H. Bionic coating imitating reflection characteristics of plant leaf in solar spectrum waveband for hyperspectral camouflage. Infrared Phys. Technol. 2022, 127, 104477. [Google Scholar] [CrossRef]
- Gao, Y.; Tang, B.; Ji, G.; Chen, K.; Wang, Z.; Ye, H. A camouflage coating with similar solar spectrum reflectance to leaves based on polymeric inorganic composite. Mater. Res. Express 2021, 8, 066404. [Google Scholar] [CrossRef]
- Hu, A.; Li, M.; Zhang, L.; Wang, C.; Fu, S. Polyurethane-based bionic material simulating the Vis-NIR spectrum and thermal infrared properties of vegetation. RSC Adv. 2019, 9, 41438–41446. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Zu, M.; Li, M.; Liu, D.; Wang, Z.; Li, Q.; Cheng, H. A Hyperspectral Camouflage Colorant Inspired by Natural Leaves. Adv. Mater. 2023, 35, e2302973. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.-O.; Choi, Y.; Kim, D.-W.; Jeong, H.-S.; Park, J.P.; Weitz, D.A.; Lee, S.-J.; Lee, H.; Choi, C.-H. Cell-Inspired Hydrogel Microcapsules with a Thin Oil Layer for Enhanced Retention of Highly Reactive Antioxidants. ACS Appl. Mater. Interfaces 2022, 14, 2597–2604. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Guan, Y.; Fu, S. Self-driven super water vapor-absorbing calcium alginate-based bionic leaf for Vis-NIR spectral simulation. Carbohydr. Polym. 2022, 296, 119932. [Google Scholar] [CrossRef] [PubMed]
- Kräutler, B. Phyllobilins—The abundant bilin-type tetrapyrrolic catabolites of the green plant pigment chlorophyll. Chem. Soc. Rev. 2014, 43, 6227–6238. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhu, S.; Jiao, B.; Duan, M.; Meng, Q.; Ma, N.; Lv, W. SlSGRL, a tomato SGR-like protein, promotes chlorophyll degradation downstream of the ABA signaling pathway. Plant Physiol. Biochem. 2020, 157, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Long, L.; Gao, Y.; Tang, Z.; Zhang, J.; Xu, K.; Ye, H.; Liu, M. A Color-Changing Biomimetic Material Closely Resembling the Spectral Characteristics of Vegetation Foliage. Small 2023, 20, e2303966. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, L.; Ye, H. Heat and mass transfer characteristics of bionic material simulating the thermal effect of natural leaf transpiration. Int. J. Heat Mass Transf. 2021, 179, 121736. [Google Scholar] [CrossRef]
- O’Brien, C.M.; Holmes, B.; Faucett, S.; Zhang, L.G. Three-Dimensional Printing of Nanomaterial Scaffolds for Complex Tissue Regeneration. Tissue Eng. Part B Rev. 2015, 21, 103–114. [Google Scholar] [CrossRef]
- Pramanik, S.; Kharche, S.; More, N.; Ranglani, D.; Singh, G.; Kapusetti, G. Natural Biopolymers for Bone Tissue Engineering: A Brief Review. Eng. Regen. 2023, 4, 193–204. [Google Scholar] [CrossRef]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Stolarska, M.A.; Othmer, H.G. The role of the microenvironment in tumor growth and invasion. Prog. Biophys. Mol. Biol. 2011, 106, 353–379. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Xue, W.; Du, J.; Li, Q.; Wang, Y.; Lu, Y.; Fan, J.; Yu, S.; Yang, Y. Preparation, Properties, and Application of Graphene-Based Materials in Tissue Engineering Scaffolds. Tissue Eng. Part B Rev. 2022, 28, 1121–1136. [Google Scholar] [CrossRef] [PubMed]
- Banihashemian, A.; Benisi, S.Z.; Hosseinzadeh, S.; Shojaei, S. Biomimetic biphasic scaffolds in osteochondral tissue engineering: Their composition, structure and consequences. Acta Histochem. 2023, 125, 152023. [Google Scholar] [CrossRef] [PubMed]
- Budak, K.; Sogut, O.; Sezer, U.A. A review on synthesis and biomedical applications of polyglycolic acid. J. Polym. Res. 2020, 27, 208. [Google Scholar] [CrossRef]
- Taib, N.-A.A.B.; Rahman, R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bin Bakri, M.K.; Bin Julaihi, M.R.M.; Khan, A. A review on poly lactic acid (PLA) as a biodegradable polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Chavan, Y.R.; Tambe, S.M.; Jain, D.D.; Khairnar, S.V.; Amin, P.D. Redefining the importance of polylactide-co-glycolide acid (PLGA) in drug delivery. Ann. Pharm. Fr. 2022, 80, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.M.; Yusoh, K. A Review on the Recent Research of Polycaprolactone (PCL). Adv. Mater. Res. 2016, 1134, 249–255. [Google Scholar] [CrossRef]
- Kim, T.G.; Shin, H.; Lim, D.W. Biomimetic Scaffolds for Tissue Engineering. Adv. Funct. Mater. 2012, 22, 2446–2468. [Google Scholar] [CrossRef]
- Valdoz, J.C.; Johnson, B.C.; Jacobs, D.J.; Franks, N.A.; Dodson, E.L.; Sanders, C.; Cribbs, C.G.; Van Ry, P.M. The ECM: To Scaffold, or Not to Scaffold, That Is the Question. Int. J. Mol. Sci. 2021, 22, 12690. [Google Scholar] [CrossRef] [PubMed]
- Ergun, C.; Parmaksiz, M.; Vurat, M.T.; Elçin, A.E.; Elçin, Y.M. Decellularized liver ECM-based 3D scaffolds: Compositional, physical, chemical, rheological, thermal, mechanical, and in vitro biological evaluations. Int. J. Biol. Macromol. 2022, 200, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Dutta, R.C.; Dey, M.; Dutta, A.K.; Basu, B. Competent processing techniques for scaffolds in tissue engineering. Biotechnol. Adv. 2017, 35, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Pei, Y.; Tang, K.; Albu-Kaya, M.G. Structure, extraction, processing, and applications of collagen as an ideal component for biomaterials—A review. Collagen Leather 2023, 5, 20. [Google Scholar] [CrossRef]
- Aazmi, A.; Zhang, D.; Mazzaglia, C.; Yu, M.; Wang, Z.; Yang, H.; Huang, Y.Y.S.; Ma, L. Biofabrication methods for reconstructing extracellular matrix mimetics. Bioact. Mater. 2024, 31, 475–496. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, H.M.; Mady, E.A.; Usui, T.; Ishihara, Y.; Yoshida, T.; Kobayashi, M.; Sasaki, K.; Ma, D.; Yairo, A.; Mandour, A.S.; et al. Adipose Stem Cell-Seeded Decellularized Porcine Pericardium: A Promising Functional Biomaterial to Synergistically Restore the Cardiac Functions Post-Myocardial Infarction. Vet. Sci. 2023, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, H.M.; Mady, E.A.; Kaneda, M.; Shimada, K.; Nakazawa, Y.; Usui, T.; Elbadawy, M.; Ishihara, Y.; Hirose, M.; Kamei, Y.; et al. Comparison of Bovine- and Porcine-Derived Decellularized Biomaterials: Promising Platforms for Tissue Engineering Applications. Pharmaceutics 2023, 15, 1906. [Google Scholar] [CrossRef]
- Cho, S.; Lee, S.; Ahn, S.I. Design and engineering of organ-on-a-chip. Biomed. Eng. Lett. 2023, 13, 97–109. [Google Scholar] [CrossRef]
- Lepowsky, E.; Muradoglu, M.; Tasoglu, S. Towards preserving post-printing cell viability and improving the resolution: Past, present, and future of 3D bioprinting theory. Bioprinting 2018, 11, e00034. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, S.; Yenilmez, B.; Anand, S.; Tasoglu, S. Photocrosslinking-based bioprinting: Examining crosslinking schemes. Bioprinting 2017, 5, 10–18. [Google Scholar] [CrossRef]
- Indurkar, A.; Pandit, A.; Jain, R.; Dandekar, P. Plant-based biomaterials in tissue engineering. Bioprinting 2021, 21, e00127. [Google Scholar] [CrossRef]
- Tarrahi, R.; Khataee, A.; Karimi, A.; Yoon, Y. The latest achievements in plant cellulose-based biomaterials for tissue engineering focusing on skin repair. Chemosphere 2022, 288, 132529. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.R.; Kar, D. An Insight into Plant Nanobionics and Its Applications. In Plant Nanobionics: Volume 1, Advances in the Understanding of Nanomaterials Research and Applications; Prasad, R., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 65–82. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.E.; Ungureanu, C.; Badea, N.; Bacalum, M.; Lazea-Stoyanova, A.; Zgura, I.; Negrila, C.; Enculescu, M.; Burnei, C. Novel Ecogenic Plasmonic Biohybrids as Multifunctional Bioactive Coatings. Coatings 2020, 10, 659. [Google Scholar] [CrossRef]
- Fontana, G.; Gershlak, J.; Adamski, M.; Lee, J.-S.; Matsumoto, S.; Le, H.D.; Binder, B.; Wirth, J.; Gaudette, G.; Murphy, W.L. Biofunctionalized Plants as Diverse Biomaterials for Human Cell Culture. Adv. Healthc. Mater. 2017, 6, 1601225. [Google Scholar] [CrossRef]
- Modulevsky, D.J.; Cuerrier, C.M.; Leblanc-Latour, M.; Hickey, R.J.; Obhi, R.-J.K.; Shore, I.; Galuta, A.; Walker, K.L.A.; Tsai, E.C.; Pelling, A.E. Plant Scaffolds Support Motor Recovery and Regeneration in Rats after Traumatic Spinal Cord Injury. bioRxiv 2022. [Google Scholar] [CrossRef]
- Bilirgen, A.C.; Toker, M.; Odabas, S.; Yetisen, A.K.; Garipcan, B.; Tasoglu, S. Plant-Based Scaffolds in Tissue Engineering. ACS Biomater. Sci. Eng. 2021, 7, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. Plants and plant-based polymers as scaffolds for tissue engineering. Green Chem. 2019, 21, 4839–4867. [Google Scholar] [CrossRef]
- Ahmadian, Z.; Correia, A.; Hasany, M.; Figueiredo, P.; Dobakhti, F.; Eskandari, M.R.; Hosseini, S.H.; Abiri, R.; Khorshid, S.; Hirvonen, J.; et al. A Hydrogen-Bonded Extracellular Matrix-Mimicking Bactericidal Hydrogel with Radical Scavenging and Hemostatic Function for pH-Responsive Wound Healing Acceleration. Adv. Healthc. Mater. 2021, 10, 2001122. [Google Scholar] [CrossRef]
- Taylor, D.A.; Sampaio, L.C.; Ferdous, Z.; Gobin, A.S.; Taite, L.J. Decellularized matrices in regenerative medicine. Acta Biomater. 2018, 74, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.M.; Bhaw-Luximon, A.; Jhurry, D. Cell-matrix mechanical interaction in electrospun polymeric scaffolds for tissue engineering: Implications for scaffold design and performance. Acta Biomater. 2017, 50, 41–55. [Google Scholar] [CrossRef]
- Gershlak, J.R.; Hernandez, S.; Fontana, G.; Perreault, L.R.; Hansen, K.J.; Larson, S.A.; Binder, B.Y.; Dolivo, D.M.; Yang, T.; Dominko, T.; et al. Crossing kingdoms: Using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials 2017, 125, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Phan, N.V.; Wright, T.; Rahman, M.M.; Xu, J.; Coburn, J.M. In Vitro Biocompatibility of Decellularized Cultured Plant Cell-Derived Matrices. ACS Biomater. Sci. Eng. 2020, 6, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Toker, M.; Rostami, S.; Kesici, M.; Gul, O.; Kocaturk, O.; Odabas, S.; Garipcan, B. Decellularization and characterization of leek: A potential cellulose-based biomaterial. Cellulose 2020, 27, 7331–7348. [Google Scholar] [CrossRef]
- Courtenay, J.C.; Sharma, R.I.; Scott, J.L. Recent Advances in Modified Cellulose for Tissue Culture Applications. Molecules 2018, 23, 654. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.F.; Lacombe, J.; Zenhausern, F. The Emerging Role of Decellularized Plant-Based Scaffolds as a New Biomaterial. Int. J. Mol. Sci. 2021, 22, 12347. [Google Scholar] [CrossRef] [PubMed]
- Predeina, A.L.; Dukhinova, M.S.; Vinogradov, V.V. Bioreactivity of decellularized animal, plant, and fungal scaffolds: Perspectives for medical applications. J. Mater. Chem. B 2020, 8, 10010–10022. [Google Scholar] [CrossRef] [PubMed]
- Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-Specific Decellularization Methods: Rationale and Strategies to Achieve Regenerative Compounds. Int. J. Mol. Sci. 2020, 21, 5447. [Google Scholar] [CrossRef]
- Narayanan, D.; Bhat, S.; Baranwal, G. Characterization of innately decellularised micropattern pseudostem of Musa balbisiana—A non-surface functionalized 3D economic biomaterial scaffold. Appl. Biol. Chem. J. 2021, 2, 76–88. [Google Scholar] [CrossRef]
- Harris, A.F.; Lacombe, J.; Liyanage, S.; Han, M.Y.; Wallace, E.; Karsunky, S.; Abidi, N.; Zenhausern, F. Supercritical carbon dioxide decellularization of plant material to generate 3D biocompatible scaffolds. Sci. Rep. 2021, 11, 3643. [Google Scholar] [CrossRef] [PubMed]
- Adamski, M.; Fontana, G.; Gershlak, J.R.; Gaudette, G.R.; Le, H.D.; Murphy, W.L. Two Methods for Decellularization of Plant Tissues for Tissue Engineering Applications. J. Vis. Exp. 2018, 135, e57586. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Hrebikova, I.; Jelinek, L.; Silveirinha, M.G. Embedded energy state in an open semiconductor heterostructure. Phys. Rev. B 2015, 92, 155303. [Google Scholar] [CrossRef]
- Deng, H.; Li, X.-F.; Cheng, W.-D.; Zhu, Y.-G. Resistance and resilience of Cu-polluted soil after Cu perturbation, tested by a wide range of soil microbial parameters. FEMS Microbiol. Ecol. 2009, 70, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Negrini, N.C.; Toffoletto, N.; Farè, S.; Altomare, L. Plant Tissues as 3D Natural Scaffolds for Adipose, Bone and Tendon Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 723. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Hickey, R.J.; Pelling, A.E. Cellulose Biomaterials for Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Robbins, E.R.; Pins, G.D.; Laflamme, M.A.; Gaudette, G.R. Creation of a contractile biomaterial from a decellularized spinach leaf without ECM protein coating: An in vitro study. J. Biomed. Mater. Res. Part A 2020, 108, 2123–2132. [Google Scholar] [CrossRef]
- Lacombe, J.; Harris, A.F.; Zenhausern, R.; Karsunsky, S.; Zenhausern, F. Plant-Based Scaffolds Modify Cellular Response to Drug and Radiation Exposure Compared to Standard Cell Culture Models. Front. Bioeng. Biotechnol. 2020, 8, 932. [Google Scholar] [CrossRef]
- Dikici, S.; Claeyssens, F.; MacNeil, S. Decellularised baby spinach leaves and their potential use in tissue engineering applications: Studying and promoting neovascularisation. J. Biomater. Appl. 2019, 34, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-W.; Shiwarski, D.J.; Ball, R.L.; Whitehead, K.A.; Feinberg, A.W.W. Engineering Aligned Skeletal Muscle Tissue Using Decellularized Plant-Derived Scaffolds. ACS Biomater. Sci. Eng. 2020, 6, 3046–3054. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E. RGD And Other Recognition Sequences for Integrins. Annu. Rev. Cell Dev. Biol. 1996, 12, 697–715. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, Y.; Zhang, H.; Wang, X.; Chen, T.; Wu, Y.; Xu, X.; Yang, S.; Ji, P.; Song, J. Natural Plant Tissue with Bioinspired Nano Amyloid and Hydroxyapatite as Green Scaffolds for Bone Regeneration. Adv. Healthc. Mater. 2022, 11, e2102807. [Google Scholar] [CrossRef]
- Mahendiran, B.; Muthusamy, S.; Janani, G.; Mandal, B.B.; Rajendran, S.; Krishnakumar, G.S. Surface Modification of Decellularized Natural Cellulose Scaffolds with Organosilanes for Bone Tissue Regeneration. ACS Biomater. Sci. Eng. 2022, 8, 2000–2015. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dominko, T.; Weathers, P.J. Using decellularized grafted leaves as tissue engineering scaffolds for mammalian cells. Vitr. Cell. Dev. Biol. Plant 2020, 56, 765–774. [Google Scholar] [CrossRef]
- Walawalkar, S.; Almelkar, S. Fabricating a pre-vascularized large-sized metabolically-supportive scaffold using Brassica oleracea leaf. J. Biomater. Appl. 2021, 36, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Modulevsky, D.J.; Lefebvre, C.; Haase, K.; Al-Rekabi, Z.; Pelling, A.E. Apple Derived Cellulose Scaffolds for 3D Mammalian Cell Culture. PLoS ONE 2014, 9, e97835. [Google Scholar] [CrossRef] [PubMed]
- James, B.D.; Ruddick, W.N.; Vasisth, S.E.; Dulany, K.; Sulekar, S.; Porras, A.; Marañon, A.; Nino, J.C.; Allen, J.B. Palm readings: Manicaria saccifera palm fibers are biocompatible textiles with low immunogenicity. Mater. Sci. Eng. C 2020, 108, 110484. [Google Scholar] [CrossRef]
- Harris, A.F.; Lacombe, J.; Sanchez-Ballester, N.M.; Victor, S.; Curran, K.A.J.; Nordquist, A.R.; Thomas, B.; Gu, J.; Veuthey, J.-L.; Soulairol, I.; et al. Decellularized Spinach Biomaterials Support Physiologically Relevant Mechanical Cyclic Strain and Prompt a Stretch-Induced Cellular Response. ACS Appl. Bio Mater. 2022, 5, 5682–5692. [Google Scholar] [CrossRef]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef]
- Esmaeili, J.; Jadbabaee, S.; Far, F.M.; Lukolayeh, M.E.; Kırboğa, K.K.; Rezaei, F.S.; Barati, A. Decellularized Alstroemeria flower stem modified with chitosan for tissue engineering purposes: A cellulose/chitosan scaffold. Int. J. Biol. Macromol. 2022, 204, 321–332. [Google Scholar] [CrossRef]
- Bai, H.; Xie, B.; Wang, Z.; Li, M.; Sun, P.; Wei, S.; Wang, W.; Wu, H.; Bai, L.; Li, J. Application of the Tissue-Engineered Plant Scaffold as a Vascular Patch. ACS Omega 2021, 6, 11595–11601. [Google Scholar] [CrossRef]
- Kondziolka, D.; Gobbel, G.T.; Fellows-Mayle, W.; Chang, Y.-F.; Uram, M. Injection Parameters Affect Cell Viability and Implant Volumes in Automated Cell Delivery for the Brain. Cell Transplant. 2011, 20, 1901–1906. [Google Scholar] [CrossRef]
- Richard, P.-L.; Gosselin, C.; Laliberté, T.; Paradis, M.; Goulet, M.; Tremblay, J.P.; Skuk, D. A First Semimanual Device for Clinical Intramuscular Repetitive Cell Injections. Cell Transplant. 2010, 19, 67–78. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, D.; Wang, T. Hierarchical Structures of Bone and Bioinspired Bone Tissue Engineering. Small 2016, 12, 4611–4632. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Lange, C.; Reichert, J.; Berner, A.; Chen, F.; Fratzl, P.; Schantz, J.-T.; Hutmacher, D.W. Bone tissue engineering: From bench to bedside. Mater. Today 2012, 15, 430–435. [Google Scholar] [CrossRef]
- Antunes, M.; Bonani, W.; Reis, R.L.; Migliaresi, C.; Ferreira, H.; Motta, A.; Neves, N.M. Development of alginate-based hydrogels for blood vessel engineering. Mater. Sci. Eng. C 2022, 134, 112588. [Google Scholar] [CrossRef] [PubMed]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef]
- Zafar, M.J.; Zhu, D.; Zhang, Z. 3D Printing of Bioceramics for Bone Tissue Engineering. Materials 2019, 12, 3361. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [PubMed]
- Yunos, D.M.; Bretcanu, O.; Boccaccini, A.R. Polymer-bioceramic composites for tissue engineering scaffolds. J. Mater. Sci. 2008, 43, 4433–4442. [Google Scholar] [CrossRef]
- Sinha, R.; Sanchez, A.; Camara-Torres, M.; Uriszar-Aldaca, I.C.; Calore, A.R.; Harings, J.; Gambardella, A.; Ciccarelli, L.; Vanzanella, V.; Sisani, M.; et al. Additive Manufactured Scaffolds for Bone Tissue Engineering: Physical Characterization of Thermoplastic Composites with Functional Fillers. ACS Appl. Polym. Mater. 2021, 3, 3788–3799. [Google Scholar] [CrossRef] [PubMed]
- Boyetey, M.-J.B.; Torgbo, S.; Sukyai, P. Bio-scaffold for bone tissue engineering with focus on bacterial cellulose, biological materials for hydroxyapatite synthesis and growth factors. Eur. Polym. J. 2023, 194, 112168. [Google Scholar] [CrossRef]
- Goyal, R.; Guvendiren, M.; Freeman, O.; Mao, Y.; Kohn, J. Optimization of Polymer-ECM Composite Scaffolds for Tissue Engineering: Effect of Cells and Culture Conditions on Polymeric Nanofiber Mats. J. Funct. Biomater. 2017, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Metwally, S.; Ferraris, S.; Spriano, S.; Krysiak, Z.J.; Kaniuk, Ł.; Marzec, M.M.; Kim, S.K.; Szewczyk, P.K.; Gruszczyński, A.; Wytrwal-Sarna, M.; et al. Surface potential and roughness controlled cell adhesion and collagen formation in electrospun PCL fibers for bone regeneration. Mater. Des. 2020, 194, 108915. [Google Scholar] [CrossRef]
- Salehi, A.; Mobarhan, M.A.; Mohammadi, J.; Shahsavarani, H.; Shokrgozar, M.A.; Alipour, A. Efficient mineralization and osteogenic gene overexpression of mesenchymal stem cells on decellularized spinach leaf scaffold. Gene 2020, 757, 144852. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, X.; Ho, S.-H. Microalgae as a solution of third world energy crisis for biofuels production from wastewater toward carbon neutrality: An updated review. Chemosphere 2021, 291, 132863. [Google Scholar] [CrossRef]
- Mateti, T.; Laha, A.; Shenoy, P. Artificial Meat Industry: Production Methodology, Challenges, and Future. JOM 2022, 74, 3428–3444. [Google Scholar] [CrossRef]
- Chen, C.-C.; Shih, W.-P.; Chang, P.-Z.; Lai, H.-M.; Chang, S.-Y.; Huang, P.-C.; Jeng, H.-A. Onion artificial muscles. Appl. Phys. Lett. 2015, 106, 183702. [Google Scholar] [CrossRef]
- Zhang, X.; Aziz, S.; Salahuddin, B.; Zhu, Z. Thermoresponsive hydrogel artificial muscles. Matter 2023, 6, 2735–2775. [Google Scholar] [CrossRef]
- Predeina, A.L.; Prilepskii, A.Y.; de Zea Bermudez, V.; Vinogradov, V.V. Bioinspired In Vitro Brain Vasculature Model for Nanomedicine Testing Based on Decellularized Spinach Leaves. Nano Lett. 2021, 21, 9853–9861. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Hosseini, S.; Alipour, A.; Jahanfar, M.; Farrokhi, N.; Homaeigohar, S.; Shahsavarani, H. In vitro modeling of hepatocellular carcinoma niche on decellularized tomato thorny leaves: A novel natural three-dimensional (3D) scaffold for liver cancer therapeutics. Front. Bioeng. Biotechnol. 2023, 11, 1189726. [Google Scholar] [CrossRef] [PubMed]
- Fadhilah, N.; Pratama, D.Y.; Sawitri, D.; Risanti, D.D. Preparation of Au@TiO2@SiO2 core-shell nanostructure and their light harvesting capability on DSSC (dye sensitized solar cells). AIP Conf. Proc. 2019, 2088, 060007. [Google Scholar] [CrossRef]
- Kumar, K.A.; Pandurangan, A.; Arumugam, S.; Sathiskumar, M. Effect of Bi-functional Hierarchical Flower-like CoS Nanostructure on its Interfacial Charge Transport Kinetics, Magnetic and Electrochemical Behaviors for Supercapacitor and DSSC Applications. Sci. Rep. 2019, 9, 1228. [Google Scholar] [CrossRef] [PubMed]
- Rajamanickam, N.; Isogami, S.; Ramachandran, K. Effect of Co doping for improved photovoltaic performance of dye-sensitized solar cells in BaSnO3 nanostructures. Mater. Lett. 2020, 275, 128139. [Google Scholar] [CrossRef]
- Aksoy, S.; Polat, O.; Gorgun, K.; Caglar, Y.; Caglar, M. Li doped ZnO based DSSC: Characterization and preparation of nanopowders and electrical performance of its DSSC. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 121, 114127. [Google Scholar] [CrossRef]
- Castillo-Robles, J.A.; Rocha-Rangel, E.; Ramírez-De-León, J.A.; Caballero-Rico, F.C.; Armendáriz-Mireles, E.N. Advances on Dye-Sensitized Solar Cells (DSSCs) Nanostructures and Natural Colorants: A Review. J. Compos. Sci. 2021, 5, 288. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Chuang, P.-Y.; Hsu, H.-L. Adding graphene nanosheets in liquid electrolytes to improve the efficiency of dye-sensitized solar cells. Mater. Chem. Phys. 2018, 207, 154–160. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, W.; Li, N.; Ding, R.; Hong, K. Hetero-seed meditated method to synthesize ZnO/TiO2 multipod nanostructures with ultra-high yield for dye-sensitized solar cells. J. Alloys Compd. 2019, 805, 868–872. [Google Scholar] [CrossRef]
- Ramakrishnan, V.M.; Muthukumarasamy, N.; Balraju, P.; Pitchaiya, S.; Velauthapillai, D.; Pugazhendhi, A. Transformation of TiO2 nanoparticles to nanotubes by simple solvothermal route and its performance as dye-sensitized solar cell (DSSC) photoanode. Int. J. Hydrogen Energy 2020, 45, 15441–15452. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.J.; Saravanan, A. A review on photochemical, biochemical and electrochemical transformation of CO2 into value-added products. J. CO2 Util. 2019, 33, 131–147. [Google Scholar] [CrossRef]
- Machín, A.; Cotto, M.; Ducongé, J.; Márquez, F. Artificial Photosynthesis: Current Advancements and Future Prospects. Biomimetics 2023, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.-I.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef]
- Unni, G.E.; Sasi, S.; Nair, A.S. Higher open-circuit voltage set by cobalt redox shuttle in SnO2 nanofibers-sensitized CdTe quantum dot solar cells. J. Energy Chem. 2016, 25, 481–488. [Google Scholar] [CrossRef]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef]
- Yadagiri, B.; Kaliamurthy, A.K.; Yoo, K.; Kang, H.C.; Ryu, J.; Asiam, F.K.; Lee, J. Molecular Engineering of Photosensitizers for Solid-State Dye-Sensitized Solar Cells: Recent Developments and Perspectives. ChemistryOpen 2023, 12, e202300170. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Boschloo, G.; Hagfeldt, A. Molecular Devices for Solar Energy Conversion and Storage; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Elangovan, N.K.; Kannadasan, R.; Beenarani, B.; Alsharif, M.H.; Kim, M.-K.; Inamul, Z.H. Recent developments in perovskite materials, fabrication techniques, band gap engineering, and the stability of perovskite solar cells. Energy Rep. 2024, 11, 1171–1190. [Google Scholar] [CrossRef]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.-K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.-P.; et al. Efficient perovskite solar cells via improved carrier management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef]
- Green, M.; Dunlop, E.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Hao, X. Solar cell efficiency tables (version 57). Prog. Photovolt. Res. Appl. 2021, 29, 3–15. [Google Scholar] [CrossRef]
- Morselli, G.; Villa, M.; Fermi, A.; Critchley, K.; Ceroni, P. Luminescent copper indium sulfide (CIS) quantum dots for bioimaging applications. Nanoscale Horiz. 2021, 6, 676–695. [Google Scholar] [CrossRef] [PubMed]
- Hashemkhani, M.; Loizidou, M.; MacRobert, A.J.; Acar, H.Y. One-Step Aqueous Synthesis of Anionic and Cationic AgInS2 Quantum Dots and Their Utility in Improving the Efficacy of ALA-Based Photodynamic Therapy. Inorg. Chem. 2022, 61, 2846–2863. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-García, A.B.; Benesperi, I.; Boschloo, G.; Concepcion, J.J.; Delcamp, J.H.; Gibson, E.A.; Meyer, G.J.; Pavone, M.; Pettersson, H.; Hagfeldt, A.; et al. Dye-sensitized solar cells strike back. Chem. Soc. Rev. 2021, 50, 12450–12550. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Du, P.; Xiong, J.; Ko, F.; Cui, C. Efficiency enhancement of dye-sensitized solar cells by optimization of electrospun ZnO nanowire/nanoparticle hybrid photoanode and combined modification. Electrochim. Acta 2015, 163, 330–337. [Google Scholar] [CrossRef]
- Saxena, S.; Raja, A.S.M. Natural Dyes: Sources, Chemistry, Application and Sustainability Issues. In Roadmap to Sustainable Textiles and Clothing: Eco-Friendly Raw Materials, Technologies, and Processing Methods; Muthu, S.S., Ed.; Springer: Singapore, 2014; pp. 37–80. [Google Scholar] [CrossRef]
- Ndeze, U.I.; Aidan, J.; Ezike, S.C.; Wansah, J.F. Comparative performances of nature-based dyes extracted from Baobab and Shea leaves photo-sensitizers for dye-sensitized solar cells (DSSCs). Curr. Res. Green Sustain. Chem. 2021, 4, 100105. [Google Scholar] [CrossRef]
- Mahajan, U.; Prajapat, K.; Dhonde, M.; Sahu, K.; Shirage, P.M. Natural dyes for dye-sensitized solar cells (DSSCs): An overview of extraction, characterization and performance. Nano-Struct. Nano-Objects 2024, 37, 101111. [Google Scholar] [CrossRef]
- Sahu, K.; Dhonde, M.; Murty, V.V.S. Efficiency of Solar Cells Based on Natural Dyes with Plasmonic Nanoparticle-Based Photo Anode. Int. J. Nanosci. 2019, 18, 1850042. [Google Scholar] [CrossRef]
- Freitag, M.; Boschloo, G. The revival of dye-sensitized solar cells. Curr. Opin. Electrochem. 2017, 2, 111–119. [Google Scholar] [CrossRef]
- Shalini, S.; Balasundaraprabhu, R.; Kumar, T.S.; Sivakumaran, K.; Kannan, M.D. Synergistic effect of sodium and yeast in improving the efficiency of DSSC sensitized with extract from petals of Kigelia Africana. Opt. Mater. 2018, 79, 210–219. [Google Scholar] [CrossRef]
- Shalini, S.; Kumar, T.S.; Prasanna, S.; Balasundaraprabhu, R. Investigations on the effect of co-doping in enhancing the performance of nanostructured TiO2 based DSSC sensitized using extracts of Hibiscus Sabdariffa calyx. Optik 2020, 212, 164672. [Google Scholar] [CrossRef]
- Sinha, D.; De, D.; Goswami, D.; Ayaz, A. Fabrication of DSSC with Nanostructured ZnO Photo Anode and Natural Dye Sensitizer. Mater. Today Proc. 2018, 5, 2056–2063. [Google Scholar] [CrossRef]
- Bashar, H.; Bhuiyan, M.; Hossain, M.; Kabir, F.; Rahaman, M.; Manir, M.; Ikegami, T. Study on combination of natural red and green dyes to improve the power conversion efficiency of dye sensitized solar cells. Optik 2019, 185, 620–625. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.E.; Tugulea, L.; Meghea, A. Procaine Effects on Model Membranes with Chlorophylla. Rev. Chim. 2009, 60, 337–341. [Google Scholar]
- Barbinta-Patrascu, M.; Tugulea, L.; Meghea, A.; Popescu, A. Oxidative stress on liposomes with chlorophyll a monitored by spectral studies. Optoelectron. Adv. Mater.—Rapid Commun. 2008, 2, 113–116. [Google Scholar]
- Barbinta-Patrascu, M.E.; Badea, N.; Constantin, M.; Ungureanu, C.; Nichita, C.; Iordache, S.M.; Vlad, A.; Antohe, S. Bio-Activity of Organic/Inorganic Phyto-Generated Composites in Bio-Inspired Systems. Rom. J. Phys. 2018, 63, 702. [Google Scholar]
- Kabir, F.; Bhuiyan, M.; Manir, M.; Rahaman, M.; Khan, M.; Ikegami, T. Development of dye-sensitized solar cell based on combination of natural dyes extracted from Malabar spinach and red spinach. Results Phys. 2019, 14, 102474. [Google Scholar] [CrossRef]
- Das, S.; Debnath, N.; Pradhan, S.; Goswami, A. Enhancement of photon absorption in the light-harvesting complex of isolated chloroplast in the presence of plasmonic gold nanosol—A nanobionic approach towards photosynthesis and plant primary growth augmentation. Gold Bull. 2017, 50, 247–257. [Google Scholar] [CrossRef]
- Daria, M.; Krzysztof, L.; Jakub, M. Characteristics of biodegradable textiles used in environmental engineering: A comprehensive review. J. Clean. Prod. 2020, 268, 122129. [Google Scholar] [CrossRef]
- Horton, P.; Horton, P.; Horton, B.P.; Horton, B.P. Re-defining Sustainability: Living in Harmony with Life on Earth. One Earth 2019, 1, 86–94. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Parthasarthy, V.; Bose, D.; Gulia, K.; Srivastava, S.; Roshan, K.R.; Shankar, R. Overview of the advances in plant microbial fuel cell technology for sustainable energy recovery from rhizodeposition. Biotechnol. Bioeng. 2023, 120, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Xuan, D.; Poon, C.S.; Zheng, W. Management and sustainable utilization of processing wastes from ready-mixed concrete plants in construction: A review. Resour. Conserv. Recycl. 2018, 136, 238–247. [Google Scholar] [CrossRef]
- Egamberdiev, E.; Akmalova, G.; Rahmonberdiev, G. Obtaining paper products from cellulose-containing plants and researching its field of application. IOP Conf. Ser. Earth Environ. Sci. 2023, 1142, 012054. [Google Scholar] [CrossRef]
- Khan, M.R.; Rizvi, T.F. Nanotechnology: Scope and Application in Plant Disease Management. Plant Pathol. J. 2014, 13, 214–231. [Google Scholar] [CrossRef]
- Bouzroud, S.; El Maaiden, E.; Sobeh, M.; Merghoub, N.; Boukcim, H.; Kouisni, L.; El Kharrassi, Y. Biotechnological Approaches to Producing Natural Antioxidants: Anti-Ageing and Skin Longevity Prospects. Int. J. Mol. Sci. 2023, 24, 1397. [Google Scholar] [CrossRef]
- Giraldo, J.P.; Landry, M.P.; Faltermeier, S.M.; McNicholas, T.P.; Iverson, N.M.; Boghossian, A.A.; Reuel, N.F.; Hilmer, A.J.; Sen, F.; Brew, J.A.; et al. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 2014, 13, 400–408. [Google Scholar] [CrossRef]
- Yan, Q.; Fong, S.S. Challenges and Advances for Genetic Engineering of Non-model Bacteria and Uses in Consolidated Bioprocessing. Front. Microbiol. 2017, 8, 2060. [Google Scholar] [CrossRef] [PubMed]
- Presti, D.L.; Di Tocco, J.; Massaroni, C.; Cimini, S.; De Gara, L.; Singh, S.; Raucci, A.; Manganiello, G.; Woo, S.L.; Schena, E.; et al. Current understanding, challenges and perspective on portable systems applied to plant monitoring and precision agriculture. Biosens. Bioelectron. 2023, 222, 115005. [Google Scholar] [CrossRef]
- Li, Y.; Park, S.; Sarang, K.; Mei, H.; Tseng, C.-P.; Hu, Z.; Zhu, D.; Li, X.; Lutkenhaus, J.; Verduzco, R. Mixed Ionic–Electronic Conduction Increases the Rate Capability of Polynaphthalenediimide for Energy Storage. ACS Polym. Au 2023, 3, 267–275. [Google Scholar] [CrossRef]
- Marks, R.A.; Amézquita, E.J.; Percival, S.; Rougon-Cardoso, A.; Chibici-Revneanu, C.; Tebele, S.M.; Farrant, J.M.; Chitwood, D.H.; VanBuren, R. A critical analysis of plant science literature reveals ongoing inequities. Proc. Natl. Acad. Sci. USA 2023, 120, e2217564120. [Google Scholar] [CrossRef]
- Salazar-Iribe, A.; De-La-Peña, C. Auxins, the hidden player in chloroplast development. Plant Cell Rep. 2020, 39, 1595–1608. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.; Barros, A.N.; Rosa, E.; Antunes, L. Enhancing Health Benefits through Chlorophylls and Chlorophyll-Rich Agro-Food: A Comprehensive Review. Molecules 2023, 28, 5344. [Google Scholar] [CrossRef] [PubMed]
- Koifman, O.I.; Stuzhin, P.A.; Travkin, V.V.; Pakhomov, G.L. Chlorophylls in thin-film photovoltaic cells, a critical review. RSC Adv. 2021, 11, 15131–15152. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Sasaki, S.; Song, J.; Wang, C.; Tamiaki, H.; Tian, W.; Chen, G.; Miyasaka, T.; Wang, X. Dopant-Free Zinc Chlorophyll Aggregates as an Efficient Biocompatible Hole Transporter for Perovskite Solar Cells. ChemSusChem 2016, 9, 2862–2869. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, W.; Li, M.; Chen, G.; Wang, X.; Fu, X.; Kitao, O.; Tamiaki, H.; Sakai, K.; Ikeuchi, T.; et al. Chlorophyll-Based Organic–Inorganic Heterojunction Solar Cells. Chem.—Eur. J. 2017, 23, 10886–10892. [Google Scholar] [CrossRef]
- Lv, J.; Xie, J.; Mohamed, A.G.A.; Zhang, X.; Feng, Y.; Jiao, L.; Zhou, E.; Yuan, D.; Wang, Y. Solar utilization beyond photosynthesis. Nat. Rev. Chem. 2023, 7, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Croce, R.; van Amerongen, H. Light harvesting in oxygenic photosynthesis: Structural biology meets spectroscopy. Science 2020, 369, eaay2058. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, D. Biochemistry and Molecular Biology of Plants; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Prabavathy, N.; Shalini, S.; Balasundaraprabhu, R.; Velauthapillai, D.; Prasanna, S.; Muthukumarasamy, N. Enhancement in the photostability of natural dyes for dye-sensitized solar cell (DSSC) applications: A review. Int. J. Energy Res. 2017, 41, 1372–1396. [Google Scholar] [CrossRef]
- Buscemi, G.; Vona, D.; Trotta, M.; Milano, F.; Farinola, G.M. Chlorophylls as Molecular Semiconductors: Introduction and State of Art. Adv. Mater. Technol. 2022, 7, 2100245. [Google Scholar] [CrossRef]
- Al-Alwani, M.A.; Ludin, N.A.; Mohamad, A.B.; Kadhum, A.A.H.; Mukhlus, A. Application of dyes extracted from Alternanthera dentata leaves and Musa acuminata bracts as natural sensitizers for dye-sensitized solar cells. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2018, 192, 487–498. [Google Scholar] [CrossRef]
- Arifin, Z.; Soeparman, S.; Widhiyanuriyawan, D.; Suyitno, S.; Setyaji, A.T. Improving Stability of Chlorophyll as Natural Dye for Dye-Sensitized Solar Cells. J. Teknol. 2017, 80, 1. [Google Scholar] [CrossRef]
- Bachtiar, M.I.; Agustina, M.N.P.; Hariyani, L.W.; Nurosyid, F. Effect of dye variation on DSSC efficiency. J. Phys. Conf. Ser. 2019, 1153, 012097. [Google Scholar] [CrossRef]
- Güzel, E.; Arslan, B.S.; Durmaz, V.; Cesur, M.; Tutar, F.; Sarı, T.; İşleyen, M.; Nebioğlu, M.; Şişman, I. Photovoltaic performance and photostability of anthocyanins, isoquinoline alkaloids and betalains as natural sensitizers for DSSCs. Sol. Energy 2018, 173, 34–41. [Google Scholar] [CrossRef]
- Ruhane, T.; Islam, M.T.; Rahaman, S.; Bhuiyan, M.; Islam, J.M.; Newaz, M.; Khan, K.; Khan, M.A. Photo current enhancement of natural dye sensitized solar cell by optimizing dye extraction and its loading period. Optik 2017, 149, 174–183. [Google Scholar] [CrossRef]
- Kocak, Y.; Atli, A.; Atilgan, A.; Yildiz, A. Extraction method dependent performance of bio-based dye-sensitized solar cells (DSSCs). Mater. Res. Express 2019, 6, 095512. [Google Scholar] [CrossRef]
- Maurya, I.C.; Singh, S.; Srivastava, P.; Maiti, B.; Bahadur, L. Natural dye extract from Cassia fistula and its application in dye-sensitized solar cell: Experimental and density functional theory studies. Opt. Mater. 2019, 90, 273–280. [Google Scholar] [CrossRef]
- Ammar, A.M.; Mohamed, H.S.H.; Yousef, M.M.K.; Abdel-Hafez, G.M.; Hassanien, A.S.; Khalil, A.S.G. Dye-Sensitized Solar Cells (DSSCs) Based on Extracted Natural Dyes. J. Nanomater. 2019, 2019, e1867271. [Google Scholar] [CrossRef]
- Kabir, F.; Bhuiyan, M.; Hossain, M.; Bashar, H.; Rahaman, M.; Manir, M.; Ullah, S.; Uddin, S.; Mollah, M.; Khan, R.; et al. Improvement of efficiency of Dye Sensitized Solar Cells by optimizing the combination ratio of Natural Red and Yellow dyes. Optik 2018, 179, 252–258. [Google Scholar] [CrossRef]
- Najm, A.S.; Ludin, N.A.; Abdullah, M.F.; Almessiere, M.A.; Ahmed, N.M.; Al-Alwani, M.A.M. Areca catechu extracted natural new sensitizer for dye-sensitized solar cell: Performance evaluation. J. Mater. Sci. Mater. Electron. 2020, 31, 3564–3575. [Google Scholar] [CrossRef]
- Madnasri, S.; Wulandari, R.D.A.; Hadi, S.; Yulianti, I.; Edi, S.S.; Prastiyanto, D. Natural Dye of Musa acuminata bracts as Light Absorbing Sensitizer for Dye-Sensitized Solar Cell. Mater. Today Proc. 2019, 13, 246–251. [Google Scholar] [CrossRef]
- Rajaramanan, T.; Gourji, F.H.; Elilan, Y.; Yohi, S.; Senthilnanthanan, M.; Ravirajan, P.; Velauthapillai, D. Natural sensitizer extracted from Mussaenda erythrophylla for dye-sensitized solar cell. Sci. Rep. 2023, 13, 13844. [Google Scholar] [CrossRef] [PubMed]
- Iman, R.N.; Harrabi, K.; Younas, M.; Mekki, A. Fabrication of efficient natural dye-sensitized Solar Cells using Mediterranean olive leaves as natural dye sensitizer. J. Photochem. Photobiol. A Chem. 2024, 450, 115477. [Google Scholar] [CrossRef]
- Mahapatra, A.; Kumar, P.; Behera, A.K.; Sen, A.; Pradhan, B. Comparative study of natural dye-sensitized solar cells using inedible extracts from kumkum, kamala and malabar spinach fruits. J. Photochem. Photobiol. A Chem. 2023, 436, 114385. [Google Scholar] [CrossRef]
- Lin, B.; Huang, C. Promoting variable renewable energy integration: The moderating effect of digitalization. Appl. Energy 2023, 337, 120891. [Google Scholar] [CrossRef]
- Kanjanapokin, C.; Thiravetyan, P.; Krobthong, S.; Aonbangkhen, C.; Yingchutrakul, Y.; Kittipornkul, P.; Treesubsuntorn, C. Possibility to Apply Strontium Aluminate to Produce Light-Emitting Plants: Efficiency and Safety. Chem. Biodivers. 2023, 20, e202300552. [Google Scholar] [CrossRef] [PubMed]
- Krichevsky, A.; Meyers, B.; Vainstein, A.; Maliga, P.; Citovsky, V. Autoluminescent Plants. PLoS ONE 2010, 5, e15461. [Google Scholar] [CrossRef] [PubMed]
- Mitiouchkina, T.; Mishin, A.S.; Somermeyer, L.G.; Markina, N.M.; Chepurnyh, T.V.; Guglya, E.B.; Karataeva, T.A.; Palkina, K.A.; Shakhova, E.S.; Fakhranurova, L.I.; et al. Plants with genetically encoded autoluminescence. Nat. Biotechnol. 2020, 38, 944–946. [Google Scholar] [CrossRef] [PubMed]
- Lew, T.T.S.; Koman, V.B.; Gordiichuk, P.; Park, M.; Strano, M.S. The Emergence of Plant Nanobionics and Living Plants as Technology. Adv. Mater. Technol. 2019, 5, 1900657. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Y. Chapter 26—Nanomaterial-Based Biosensors for Detection of Pesticides and Explosives. In Nanotechnology Applications for Clean Water; Micro and Nano Technologies; Savage, N., Diallo, M., Duncan, J., Street, A., Sustich, R., Eds.; William Andrew Publishing: Boston, MA, USA, 2009; pp. 377–390. [Google Scholar] [CrossRef]
- Gohil, N.; Bhattacharjee, G.; Singh, V. 19—Genetic engineering approaches for detecting environmental pollutants. In Bioremediation of Pollutants; Pandey, V.C., Singh, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 387–401. [Google Scholar] [CrossRef]
- Cakaj, A.; Drzewiecka, K.; Hanć, A.; Lisiak-Zielińska, M.; Ciszewska, L.; Drapikowska, M. Plants as effective bioindicators for heavy metal pollution monitoring. Environ. Res. 2024, 256, 119222. [Google Scholar] [CrossRef]
- Cortés-Eslava, J.; Gómez-Arroyo, S.; Cortés, P.A.M.; Jiménez-García, L.F.; Lara-Martínez, R.; Arenas-Huertero, F.; Morton-Bermea, O.; Testillano, P.S. The wild plant Gnaphalium lavandulifolium as a sentinel for biomonitoring the effects of environmental heavy metals in the metropolitan area of México Valley. Environ. Monit. Assess. 2022, 195, 195. [Google Scholar] [CrossRef]
- Xiang, L.; Li, G.; Wen, L.; Su, C.; Liu, Y.; Tang, H.; Dai, J. Biodegradation of aromatic pollutants meets synthetic biology. Synth. Syst. Biotechnol. 2021, 6, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Rylott, E.L.; Bruce, N.C. How synthetic biology can help bioremediation. Curr. Opin. Chem. Biol. 2020, 58, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Moreno, J.-P.; Stepanova, A.N. Monitoring Ethylene in Plants: Genetically Encoded Reporters and Biosensors. Small Methods 2020, 4, 1900260. [Google Scholar] [CrossRef]
- Su, W.; Xu, M.; Radani, Y.; Yang, L. Technological Development and Application of Plant Genetic Transformation. Int. J. Mol. Sci. 2023, 24, 10646. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-H.; Yu, M.; Lai, E.-M. Agrobacterium-mediated plant transformation: Biology and applications. Arab. Book 2017, 15, e0186. [Google Scholar] [CrossRef] [PubMed]
- Nikoleli, G.-P.; Siontorou, C.G.; Nikolelis, D.P.; Karapetis, S.; Bratakou, S. Chapter 1—Prototype Biosensing Devices: Design and Microfabrication Based on Nanotechnological Tools for the Rapid in the Field Detection of Food Toxicants and Environmental Pollutants. In Nanotechnology and Biosensors; Advanced Nanomaterials; Nikolelis, D.P., Nikoleli, G.-P., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–28. [Google Scholar] [CrossRef]
- Kurbanoglu, S.; Ozkan, S.A.; Merkoçi, A. Nanomaterials-based enzyme electrochemical biosensors operating through inhibition for biosensing applications. Biosens. Bioelectron. 2017, 89, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Lew, T.T.S.; Park, M.; Cui, J.; Strano, M.S. Plant Nanobionic Sensors for Arsenic Detection. Adv. Mater. 2020, 33, e2005683. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Microbial-derived biosensors for monitoring environmental contaminants: Recent advances and future outlook. Process Saf. Environ. Prot. 2019, 124, 8–17. [Google Scholar] [CrossRef]
- Manginell, M.; Harper, J.; Arango, D.; Brozik, S.; Dolan, P. Viral Vectors for Gene Modification of Plants as Chem/Bio Sensors; SAND2006-6955, 902219; Sandia National Laboratories (SNL): Livermore, CA, USA, 2006. [Google Scholar] [CrossRef][Green Version]
- Ang, M.C.-Y.; Dhar, N.; Khong, D.T.; Lew, T.T.S.; Park, M.; Sarangapani, S.; Cui, J.; Dehadrai, A.; Singh, G.P.; Chan-Park, M.B.; et al. Nanosensor Detection of Synthetic Auxins In Planta using Corona Phase Molecular Recognition. ACS Sens. 2021, 6, 3032–3046. [Google Scholar] [CrossRef]
- Kwak, S.-Y.; Wong, M.H.; Lew, T.T.S.; Bisker, G.; Lee, M.A.; Kaplan, A.; Dong, J.; Liu, A.T.; Koman, V.B.; Sinclair, R.; et al. Nanosensor Technology Applied to Living Plant Systems. Annu. Rev. Anal. Chem. 2017, 10, 113–140. [Google Scholar] [CrossRef]
- Kenry; Duan, Y.; Liu, B. Recent Advances of Optical Imaging in the Second Near-Infrared Window. Adv. Mater. 2018, 30, e1802394. [Google Scholar] [CrossRef]
- Iverson, N.M.; Bisker, G.; Farias, E.; Ivanov, V.; Ahn, J.; Wogan, G.N.; Strano, M.S. Quantitative Tissue Spectroscopy of Near Infrared Fluorescent Nanosensor Implants. J. Biomed. Nanotechnol. 2016, 12, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Barone, P.W.; Baik, S.; Heller, D.A.; Strano, M.S. Near-infrared optical sensors based on single-walled carbon nanotubes. Nat. Mater. 2005, 4, 86–92. [Google Scholar] [CrossRef]
- Ansari, M.H.D.; Lavhale, S.; Kalunke, R.M.; Srivastava, P.L.; Pandit, V.; Gade, S.; Yadav, S.; Laux, P.; Luch, A.; Gemmati, D.; et al. Recent Advances in Plant Nanobionics and Nanobiosensors for Toxicology Applications. Curr. Nanosci. 2020, 16, 27–41. [Google Scholar] [CrossRef]
- Nanobionic Spinach Plants Can Detect Explosives. MIT News|Massachusetts Institute of Technology. Available online: https://news.mit.edu/2016/nanobionic-spinach-plants-detect-explosives-1031 (accessed on 12 May 2024).
- Wong, M.H.; Giraldo, J.P.; Kwak, S.-Y.; Koman, V.B.; Sinclair, R.; Lew, T.T.S.; Bisker, G.; Liu, P.; Strano, M.S. Nitroaromatic detection and infrared communication from wild-type plants using plant nanobionics. Nat. Mater. 2016, 16, 264–272. [Google Scholar] [CrossRef]
- Ju, K.-S.; Parales, R.E. Nitroaromatic Compounds, from Synthesis to Biodegradation. Microbiol. Mol. Biol. Rev. 2010, 74, 250–272. [Google Scholar] [CrossRef]
- Liang, J.; Zulkifli, M.Y.B.; Choy, S.; Li, Y.; Gao, M.; Kong, B.; Yun, J.; Liang, K. Metal–Organic Framework–Plant Nanobiohybrids as Living Sensors for On-Site Environmental Pollutant Detection. Environ. Sci. Technol. 2020, 54, 11356–11364. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Anderson, M.C.; Zhang, X.; Yang, Z.; Alfieri, J.G.; Kustas, W.P.; Mueller, R.; Johnson, D.M.; Prueger, J.H. Toward mapping crop progress at field scales through fusion of Landsat and MODIS imagery. Remote Sens. Environ. 2017, 188, 9–25. [Google Scholar] [CrossRef]
- Ballesteros, R.; Intrigliolo, D.S.; Ortega, J.F.; Ramírez-Cuesta, J.M.; Buesa, I.; Moreno, M.A. Vineyard yield estimation by combining remote sensing, computer vision and artificial neural network techniques. Precis. Agric. 2020, 21, 1242–1262. [Google Scholar] [CrossRef]
- Schweiger, A.K.; Schütz, M.; Risch, A.C.; Kneubühler, M.; Haller, R.; Schaepman, M.E. How to predict plant functional types using imaging spectroscopy: Linking vegetation community traits, plant functional types and spectral response. Methods Ecol. Evol. 2017, 8, 86–95. [Google Scholar] [CrossRef]
- Maes, W.H.; Steppe, K. Perspectives for Remote Sensing with Unmanned Aerial Vehicles in Precision Agriculture. Trends Plant Sci. 2019, 24, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Sareen, H.; Zheng, J.; Maes, P. Cyborg Botany: Augmented Plants as Sensors, Displays and Actuators. In Extended Abstracts of the 2019 CHI Conference on Human Factors in Computing Systems, CHI 19, Glasgow, UK, 4–9 May 2019; Association for Computing Machinery: New York, NY, USA, 2019; pp. 1–2. [Google Scholar] [CrossRef]
- Gechev, T.; Lyall, R.; Petrov, V.; Bartels, D. Systems biology of resurrection plants. Cell. Mol. Life Sci. 2021, 78, 6365–6394. [Google Scholar] [CrossRef] [PubMed]
- Peix, A.; Ramírez-Bahena, M.H.; Velázquez, E.; Bedmar, E.J. Bacterial Associations with Legumes. Crit. Rev. Plant Sci. 2015, 34, 17–42. [Google Scholar] [CrossRef]
- Reddy, G.C.; Goyal, R.K.; Puranik, S.; Waghmar, V.; Vikram, K.V.; Sruthy, K.S. Biofertilizers Toward Sustainable Agricultural Development. In Plant Microbe Symbiosis; Varma, A., Tripathi, S., Prasad, R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 115–128. [Google Scholar] [CrossRef]
- Bajguz, A.; Hayat, S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009, 47, 1–8. [Google Scholar] [CrossRef]
- Kumari, P.; Ginzburg, N.; Sayas, T.; Saphier, S.; Bucki, P.; Miyara, S.B.; Caldwell, D.L.; Iyer-Pascuzzi, A.S.; Kleiman, M. A biomimetic platform for studying root-environment interaction. Plant Soil 2020, 447, 157–168. [Google Scholar] [CrossRef]
- López-Bucio, J.; Cruz-Ramírez, A.; Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Sage, R.F. Autotrophs. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Academic Press: Oxford, UK, 2008; pp. 291–300. [Google Scholar] [CrossRef]
- Christin, P.-A.; Wood, D. C4 and CAM Photosynthesis in Land Plants, Evolution and Diversification of. In Encyclopedia of Evolutionary Biology; Kliman, R.M., Ed.; Academic Press: Oxford, UK, 2016; pp. 254–259. [Google Scholar] [CrossRef]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Elbehiry, A.; Abalkhail, A.; Marzouk, E.; Elmanssury, A.E.; Almuzaini, A.M.; Alfheeaid, H.; Alshahrani, M.T.; Huraysh, N.; Ibrahem, M.; Alzaben, F.; et al. An Overview of the Public Health Challenges in Diagnosing and Controlling Human Foodborne Pathogens. Vaccines 2023, 11, 725. [Google Scholar] [CrossRef]
- Lechenet, M.; Dessaint, F.; Py, G.; Makowski, D.; Munier-Jolain, N. Reducing pesticide use while preserving crop productivity and profitability on arable farms. Nat. Plants 2017, 3, 17008. [Google Scholar] [CrossRef]
- World Health Organization. Priority Medicines for Europe and the World. Art. No. WHO/EDM/PAR/2004.7. 2004. Available online: https://iris.who.int/handle/10665/68769 (accessed on 12 May 2024).
- Rehman, T.; Faheem, M.; Khan, A.U. An insight into the biophysical characterization of different states of cefotaxime hydrolyzing β-lactamase 15 (CTX-M-15). J. Biomol. Struct. Dyn. 2014, 33, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Jarman, W.M.; Ballschmiter, K. From coal to DDT: The history of the development of the pesticide DDT from synthetic dyes till Silent Spring. Endeavour 2012, 36, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Goswami, M.; Attri, K.; Goswami, I. Applications of Molecular Markers in Fruit Crops: A Review. Int. J. Econ. Plants 2022, 9, 121–126. [Google Scholar] [CrossRef]
- Sadasivaiah, S.; Tozan, Y.; Breman, J.G. Dichlorodiphenyltrichloroethane (DDT) for Indoor Residual Spraying in Africa: How Can It Be Used for Malaria Control? In Defining and Defeating the Intolerable Burden of Malaria III: Progress and Perspectives: Supplement to Volume 77(6) of American Journal of Tropical Medicine and Hygiene; American Society of Tropical Medicine and Hygiene: Arlington, VA, USA, 2007. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1724/ (accessed on 12 May 2024).
- Garrido, G.; Rodeiro, I.; Hernández, I.; García, G.; Pérez, G.; Merino, N.; Núñez-Sellés, A.; Delgado, R. In vivo acute toxicological studies of an antioxidant extract from Mangifera indica L. (Vimang). Drug Chem. Toxicol. 2009, 32, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Rehwagen, C. WHO recommends DDT to control malaria. BMJ 2006, 333, 622. [Google Scholar] [CrossRef] [PubMed]
- Souto, A.L.; Sylvestre, M.; Tölke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrián-Torrejón, G. Plant-Derived Pesticides as an Alternative to Pest Management and Sustainable Agricultural Production: Prospects, Applications and Challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Bioinsecticides based on plant essential oils: A short overview. Z. Für Naturforschung C 2020, 75, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An Overview of Some Biopesticides and Their Importance in Plant Protection for Commercial Acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef]
- Jahan, N.; Hussain, N.; Touqeer, S.I.; Rahman, K.U.; Shamshad, H.; Abbas, N. Formulation of Mentha piperita-Based Nanobiopesticides and Assessment of the Pesticidal and Antimicrobial Potential. Life 2024, 14, 144. [Google Scholar] [CrossRef]
- Robles-Martínez, M.; Patiño-Herrera, R.; Pérez-Vázquez, F.J.; Montejano-Carrizales, J.M.; González, J.F.C.; Pérez, E. Mentha piperita as a natural support for silver nanoparticles: A new Anti- Candida albicans treatment. Colloid Interface Sci. Commun. 2020, 35, 100253. [Google Scholar] [CrossRef]
- Ahmad, N.; Ali, S.; Abbas, M.; Fazal, H.; Saqib, S.; Ali, A.; Ullah, Z.; Zaman, S.; Sawati, L.; Zada, A.; et al. Antimicrobial efficacy of Mentha piperata-derived biogenic zinc oxide nanoparticles against UTI-resistant pathogens. Sci. Rep. 2023, 13, 14972. [Google Scholar] [CrossRef] [PubMed]
- Koyande, A.K.; Chew, K.W.; Manickam, S.; Chang, J.-S.; Show, P.-L. Emerging algal nanotechnology for high-value compounds: A direction to future food production. Trends Food Sci. Technol. 2021, 116, 290–302. [Google Scholar] [CrossRef]
- Ahmar, S.; Mahmood, T.; Fiaz, S.; Mora-Poblete, F.; Shafique, M.S.; Chattha, M.S.; Jung, K.-H. Advantage of Nanotechnology-Based Genome Editing System and Its Application in Crop Improvement. Front. Plant Sci. 2021, 12, 663849. [Google Scholar] [CrossRef]
- Landa, P. Positive effects of metallic nanoparticles on plants: Overview of involved mechanisms. Plant Physiol. Biochem. 2021, 161, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Hassanisaadi, M.; Barani, M.; Rahdar, A.; Heidary, M.; Thysiadou, A.; Kyzas, G.Z. Role of agrochemical-based nanomaterials in plants: Biotic and abiotic stress with germination improvement of seeds. Plant Growth Regul. 2022, 97, 375–418. [Google Scholar] [CrossRef]
- Amiri, M.S.; Mohammadzadeh, V.; Yazdi, M.E.T.; Barani, M.; Rahdar, A.; Kyzas, G.Z. Plant-Based Gums and Mucilages Applications in Pharmacology and Nanomedicine: A Review. Molecules 2021, 26, 1770. [Google Scholar] [CrossRef] [PubMed]
- Nongbet, A.; Mishra, A.K.; Mohanta, Y.K.; Mahanta, S.; Ray, M.K.; Khan, M.; Baek, K.-H.; Chakrabartty, I. Nanofertilizers: A Smart and Sustainable Attribute to Modern Agriculture. Plants 2022, 11, 2587. [Google Scholar] [CrossRef] [PubMed]
- Fatehi, S.F.; Oraei, M.; Gohari, G.; Akbari, A.; Faramarzi, A. Proline-Functionalized Graphene Oxide Nanoparticles (GO–Pro NPs) Mitigate Salt-Induced Adverse Effects on Morpho-Physiological Traits and Essential Oils Constituents in Moldavian Balm (Dracocephalum moldavica L.). J. Plant Growth Regul. 2021, 41, 2818–2832. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C. ZnO Nanoparticle Biosynthesis and Its Effect on Phosphorous-Mobilizing Enzyme Secretion and Gum Contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef]
- Niyigaba, E.; Twizerimana, A.; Mugenzi, I.; Ngnadong, W.A.; Ye, Y.P.; Wu, B.M.; Hai, J.B. Winter Wheat Grain Quality, Zinc and Iron Concentration Affected by a Combined Foliar Spray of Zinc and Iron Fertilizers. Agronomy 2019, 9, 250. [Google Scholar] [CrossRef]
- Abou-Salem, E.; Ahmed, A.R.; Elbagory, M.; Omara, A.E.-D. Efficacy of Biological Copper Oxide Nanoparticles on Controlling Damping-Off Disease and Growth Dynamics of Sugar Beet (Beta vulgaris L.) Plants. Sustainability 2022, 14, 12871. [Google Scholar] [CrossRef]
- Al-Saif, A.M.; Mosa, W.F.A.; Saleh, A.A.; Ali, M.M.; Sas-Paszt, L.; Abada, H.S.; Abdel-Sattar, M. Yield and Fruit Quality Response of Pomegranate (Punica granatum) to Foliar Spray of Potassium, Calcium and Kaolin. Horticulturae 2022, 8, 946. [Google Scholar] [CrossRef]
- Wohlmuth, J.; Tekielska, D.; Čechová, J.; Baránek, M. Interaction of the Nanoparticles and Plants in Selective Growth Stages—Usual Effects and Resulting Impact on Usage Perspectives. Plants 2022, 11, 2405. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Fedenia, L.N.; Sharifan, H.; Ma, X.; Lombardini, L. Effects of foliar application of zinc sulfate and zinc nanoparticles in coffee (Coffea arabica L.) plants. Plant Physiol. Biochem. 2019, 135, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Pullagurala, V.L.R.; Adisa, I.O.; Rawat, S.; Kalagara, S.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. ZnO nanoparticles increase photosynthetic pigments and decrease lipid peroxidation in soil grown cilantro (Coriandrum sativum). Plant Physiol. Biochem. 2018, 132, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Lovecká, P.; Macůrková, A.; Záruba, K.; Hubáček, T.; Siegel, J.; Valentová, O. Genomic Damage Induced in Nicotiana tabacum L. Plants by Colloidal Solution with Silver and Gold Nanoparticles. Plants 2021, 10, 1260. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.; Gopal, J.; Kim, D.-H.; Sivanesan, I. Reviewing the Impact of Vehicular Pollution on Road-Side Plants—Future Perspectives. Sustainability 2021, 13, 5114. [Google Scholar] [CrossRef]
- Jayakodi, S.; Kim, H.; Menon, S.; Shanmugam, V.K.; Choi, I.; Sekhar, M.R.; Bhaskar, R.; Han, S.S. Preparation of Novel Nanoformulation to Enhance Efficacy in the Treatment of Cardiovascular Disease. Biomimetics 2022, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, R.; Han, S.S.; Ramasamy, M.S. Piezoelectric Biosensors and Nanomaterials-based Therapeutics for Coronavirus and Other Viruses: A Mini-review. Curr. Top. Med. Chem. 2023, 23, 115–127. [Google Scholar] [CrossRef]
- Zhao, F.; Xin, X.; Cao, Y.; Su, D.; Ji, P.; Zhu, Z.; He, Z. Use of Carbon Nanoparticles to Improve Soil Fertility, Crop Growth and Nutrient Uptake by Corn (Zea mays L.). Nanomaterials 2021, 11, 2717. [Google Scholar] [CrossRef]
- Shankramma, K.; Yallappa, S.; Shivanna, M.B.; Manjanna, J. Fe2O3 magnetic nanoparticles to enhance S. lycopersicum (tomato) plant growth and their biomineralization. Appl. Nanosci. 2015, 6, 983–990. [Google Scholar] [CrossRef]
- Singh, P.; Singh, R.; Borthakur, A.; Srivastava, P.; Srivastava, N.; Tiwary, D.; Mishra, P.K. Effect of nanoscale TiO2-activated carbon composite on Solanum lycopersicum (L.) and Vigna radiata (L.) seeds germination. Energy Ecol. Environ. 2016, 1, 131–140. [Google Scholar] [CrossRef]
- Saxena, M.; Maity, S.; Sarkar, S. Carbon nanoparticles in ‘biochar’ boost wheat (Triticum aestivum) plant growth. RSC Adv. 2014, 4, 39948–39954. [Google Scholar] [CrossRef]
- Wang, X.; Han, H.; Liu, X.; Gu, X.; Chen, K.; Lu, D. Multi-walled carbon nanotubes can enhance root elongation of wheat (Triticum aestivum) plants. J. Nanopart. Res. 2012, 14, 841. [Google Scholar] [CrossRef]
- Song, J.; Zhao, H.; Zhao, G.; Xiang, Y.; Liu, Y. Novel Semi-IPN Nanocomposites with Functions of both Nutrient Slow-Release and Water Retention. 1. Microscopic Structure, Water Absorbency, and Degradation Performance. J. Agric. Food Chem. 2019, 67, 7587–7597. [Google Scholar] [CrossRef] [PubMed]
- Adisa, I.O.; Pullagurala, V.L.R.; Peralta-Videa, J.R.; Dimkpa, C.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environ. Sci. Nano 2019, 6, 2002–2030. [Google Scholar] [CrossRef]
- Monreal, C.M.; DeRosa, M.; Mallubhotla, S.C.; Bindraban, P.S.; Dimkpa, C. Nanotechnologies for increasing the crop use efficiency of fertilizer-micronutrients. Biol. Fertil. Soils 2016, 52, 423–437. [Google Scholar] [CrossRef]
- Lobo, M.A.; Cardoso, J.M.P.; Rocha, P.R.F. Electrical sensing of the plant Mimosa pudica under environmental temperatures. In Proceedings of the 2023 IEEE 7th Portuguese Meeting on Bioengineering (ENBENG), Porto, Portugal, 22–23 June 2023; pp. 191–194. [Google Scholar] [CrossRef]
- Wang, A.; Kang, F.; Wang, Z.; Shao, Q.; Li, Z.; Zhu, G.; Lu, J.; Li, Y.Y. Facile Synthesis of Nitrogen-Rich Carbon Dots as Fertilizers for Mung Bean Sprouts. Adv. Sustain. Syst. 2018, 3, 1800132. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Synthetic apatite nanoparticles as a phosphorus fertilizer for soybean (Glycine max). Sci. Rep. 2014, 4, 5686. [Google Scholar] [CrossRef]
- Calicioglu, O.; Flammini, A.; Bracco, S.; Bellù, L.; Sims, R. The Future Challenges of Food and Agriculture: An Integrated Analysis of Trends and Solutions. Sustainability 2019, 11, 222. [Google Scholar] [CrossRef]
- Oren, S.; Ceylan, H.; Schnable, P.S.; Dong, L. High-Resolution Patterning and Transferring of Graphene-Based Nanomaterials onto Tape toward Roll-to-Roll Production of Tape-Based Wearable Sensors. Adv. Mater. Technol. 2017, 2, 1700223. [Google Scholar] [CrossRef]
- Al-Jadabi, N.; Laaouan, M.; El Hajjaji, S.; Mabrouki, J.; Benbouzid, M.; Dhiba, D. The Dual Performance of Moringa Oleifera Seeds as Eco-Friendly Natural Coagulant and as an Antimicrobial for Wastewater Treatment: A Review. Sustainability 2023, 15, 4280. [Google Scholar] [CrossRef]
- Samdin, S.M.; Peng, L.H.; Marzuki, M. Investigation of Coconut Shells Activated Carbon as the Cost Effective Absorbent in Drinking Water Filter. J. Teknol. 2013, 77, 22. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Conradie, J. Banana peel as a biosorbent for the decontamination of water pollutants. A review. Environ. Chem. Lett. 2020, 18, 1085–1112. [Google Scholar] [CrossRef]
- Choudhary, M.; Ray, M.B.; Neogi, S. Evaluation of the potential application of cactus (Opuntia ficus-indica) as a bio-coagulant for pre-treatment of oil sands process-affected water. Sep. Purif. Technol. 2019, 209, 714–724. [Google Scholar] [CrossRef]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Zularisam, A.; Nasrullah, M. Water hyacinth (Eichhornia crassipes) for organic contaminants removal in water—A review. J. Hazard. Mater. Adv. 2022, 7, 100092. [Google Scholar] [CrossRef]
- Anand, S.; Bharti, S.K.; Dviwedi, N.; Barman, S.C.; Kumar, N. Macrophytes for the Reclamation of Degraded Waterbodies with Potential for Bioenergy Production. In Phytoremediation Potential of Bioenergy Plants; Bauddh, K., Singh, B., Korstad, J., Eds.; Springer: Singapore, 2017; pp. 333–351. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Hemavathy, R.; Jeevanantham, S.; Harikumar, P.; Priyanka, G.; Devakirubai, D.R.A. A comprehensive review on sources, analysis and toxicity of environmental pollutants and its removal methods from water environment. Sci. Total. Environ. 2021, 812, 152456. [Google Scholar] [CrossRef] [PubMed]
- Hand, S.; Cusick, R.D. Electrochemical Disinfection in Water and Wastewater Treatment: Identifying Impacts of Water Quality and Operating Conditions on Performance. Environ. Sci. Technol. 2021, 55, 3470–3482. [Google Scholar] [CrossRef] [PubMed]
- Peydayesh, M.; Mezzenga, R. Protein nanofibrils for next generation sustainable water purification. Nat. Commun. 2021, 12, 3248. [Google Scholar] [CrossRef]
- Wei, G.; Zhang, J.; Usuelli, M.; Zhang, X.; Liu, B.; Mezzenga, R. Biomass vs inorganic and plastic-based aerogels: Structural design, functional tailoring, resource-efficient applications and sustainability analysis. Prog. Mater. Sci. 2022, 125, 100915. [Google Scholar] [CrossRef]
- Ji, Y.-L.; Gu, B.; Xie, S.; Yin, M.; Qian, W.; Zhao, Q.; Hung, W.; Lee, K.; Zhou, Y.; An, Q.; et al. Superfast Water Transport Zwitterionic Polymeric Nanofluidic Membrane Reinforced by Metal–Organic Frameworks. Adv. Mater. 2021, 33, 2102292. [Google Scholar] [CrossRef]
- Amibo, T.A.; Beyan, S.M.; Damite, T.M. Production and Optimization of Bio-Based Silica Nanoparticle from Teff Straw (Eragrostis tef) Using RSM-Based Modeling, Characterization Aspects, and Adsorption Efficacy of Methyl Orange Dye. J. Chem. 2022, 2022, e9770520. [Google Scholar] [CrossRef]
- Ansari, M.J.; Jasim, S.A.; Bokov, D.O.; Thangavelu, L.; Yasin, G.; Khalaji, A.D. Preparation of new bio-based chitosan/Fe2O3/NiFe2O4 as an efficient removal of methyl green from aqueous solution. Int. J. Biol. Macromol. 2022, 198, 128–134. [Google Scholar] [CrossRef]
- Qamar, M.A.; Javed, M.; Shahid, S.; Shariq, M.; Fadhali, M.M.; Ali, S.K.; Khan, M.S. Synthesis and applications of graphitic carbon nitride (g-C3N4) based membranes for wastewater treatment: A critical review. Heliyon 2023, 9, e12685. [Google Scholar] [CrossRef]
- Karić, N.; Maia, A.S.; Teodorović, A.; Atanasova, N.; Langergraber, G.; Crini, G.; Ribeiro, A.R.; Đolić, M. Bio-waste valorisation: Agricultural wastes as biosorbents for removal of (in)organic pollutants in wastewater treatment. Chem. Eng. J. Adv. 2022, 9, 100239. [Google Scholar] [CrossRef]
- Kumar, P.S.; Saravanan, A.; Kumar, K.A.; Yashwanth, R.; Visvesh, S. Removal of toxic zinc from water/wastewater using eucalyptus seeds activated carbon: Non-linear regression analysis. IET Nanobiotechnol. 2016, 10, 244–253. [Google Scholar] [CrossRef]
- Qamar, M.A.; Javed, M.; Shahid, S.; Sher, M. Fabrication of g-C3N4/transition metal (Fe, Co, Ni, Mn and Cr)-doped ZnO ternary composites: Excellent visible light active photocatalysts for the degradation of organic pollutants from wastewater. Mater. Res. Bull. 2022, 147, 111630. [Google Scholar] [CrossRef]
- Qamar, M.A.; Al-Gethami, W.; Alaghaz, A.-N.M.; Shariq, M.; Mohammed, A.; Areshi, A.A.; Khan, Z.; Qayyum, W. Progress in the development of phyto-based materials for adsorption of dyes from wastewater: A review. Mater. Today Commun. 2024, 38, 108385. [Google Scholar] [CrossRef]
- Manimegalai, S.; Vickram, S.; Deena, S.R.; Rohini, K.; Thanigaivel, S.; Manikandan, S.; Subbaiya, R.; Karmegam, N.; Kim, W.; Govarthanan, M. Carbon-based nanomaterial intervention and efficient removal of various contaminants from effluents—A review. Chemosphere 2023, 312, 137319. [Google Scholar] [CrossRef]
- Rando, G.; Sfameni, S.; Galletta, M.; Drommi, D.; Cappello, S.; Plutino, M.R. Functional Nanohybrids and Nanocomposites Development for the Removal of Environmental Pollutants and Bioremediation. Molecules 2022, 27, 4856. [Google Scholar] [CrossRef]
- Cambié, D.; Bottecchia, C.; Straathof, N.J.W.; Hessel, V.; Noël, T. Applications of Continuous-Flow Photochemistry in Organic Synthesis, Material Science, and Water Treatment. Chem. Rev. 2016, 116, 10276–10341. [Google Scholar] [CrossRef]
- Buglioni, L.; Raymenants, F.; Slattery, A.; Zondag, S.D.A.; Noël, T. Technological Innovations in Photochemistry for Organic Synthesis: Flow Chemistry, High-Throughput Experimentation, Scale-up, and Photoelectrochemistry. Chem. Rev. 2021, 122, 2752–2906. [Google Scholar] [CrossRef]
- Candish, L.; Collins, K.D.; Cook, G.C.; Douglas, J.J.; Gómez-Suárez, A.; Jolit, A.; Keess, S. Photocatalysis in the Life Science Industry. Chem. Rev. 2022, 122, 2907–2980. [Google Scholar] [CrossRef]
- Kasbaji, M.; Ibrahim, I.; Mennani, M.; Abuelalla, O.A.; Fekry, S.S.; Mohamed, M.M.; Salama, T.M.; Moneam, I.A.; Mbarki, M.; Moubarik, A.; et al. Future trends in dye removal by metal oxides and their Nano/Composites: A comprehensive review. Inorg. Chem. Commun. 2023, 158, 111546. [Google Scholar] [CrossRef]
- Alhalili, Z. Green synthesis of copper oxide nanoparticles CuO NPs from Eucalyptus Globoulus leaf extract: Adsorption and design of experiments. Arab. J. Chem. 2022, 15, 103739. [Google Scholar] [CrossRef]
- Yamada, Y. Feasibility Study on Botanical Robotics: Ophiocordyceps-like Biodegradable Laminated Foam-Based Soft Actuators with Germination Ability. IEEE Robot. Autom. Lett. 2021, 6, 3777–3784. [Google Scholar] [CrossRef]
- Shrestha, B.; Zhang, W.; Zhang, Y.; Liu, X. What is the Chinese caterpillar fungus Ophiocordyceps sinensis (Ophiocordycipitaceae)? Mycology 2010, 1, 228–236. [Google Scholar] [CrossRef]
- Evans, H.C.; Elliot, S.L.; Hughes, D.P. Hidden Diversity Behind the Zombie-Ant Fungus Ophiocordyceps unilateralis: Four New Species Described from Carpenter Ants in Minas Gerais, Brazil. PLoS ONE 2011, 6, e17024. [Google Scholar] [CrossRef]
- Frazier, P.A.; Jamone, L.; Althoefer, K.; Calvo, P. Plant Bioinspired Ecological Robotics. Front. Robot. AI 2020, 7, 79. [Google Scholar] [CrossRef]

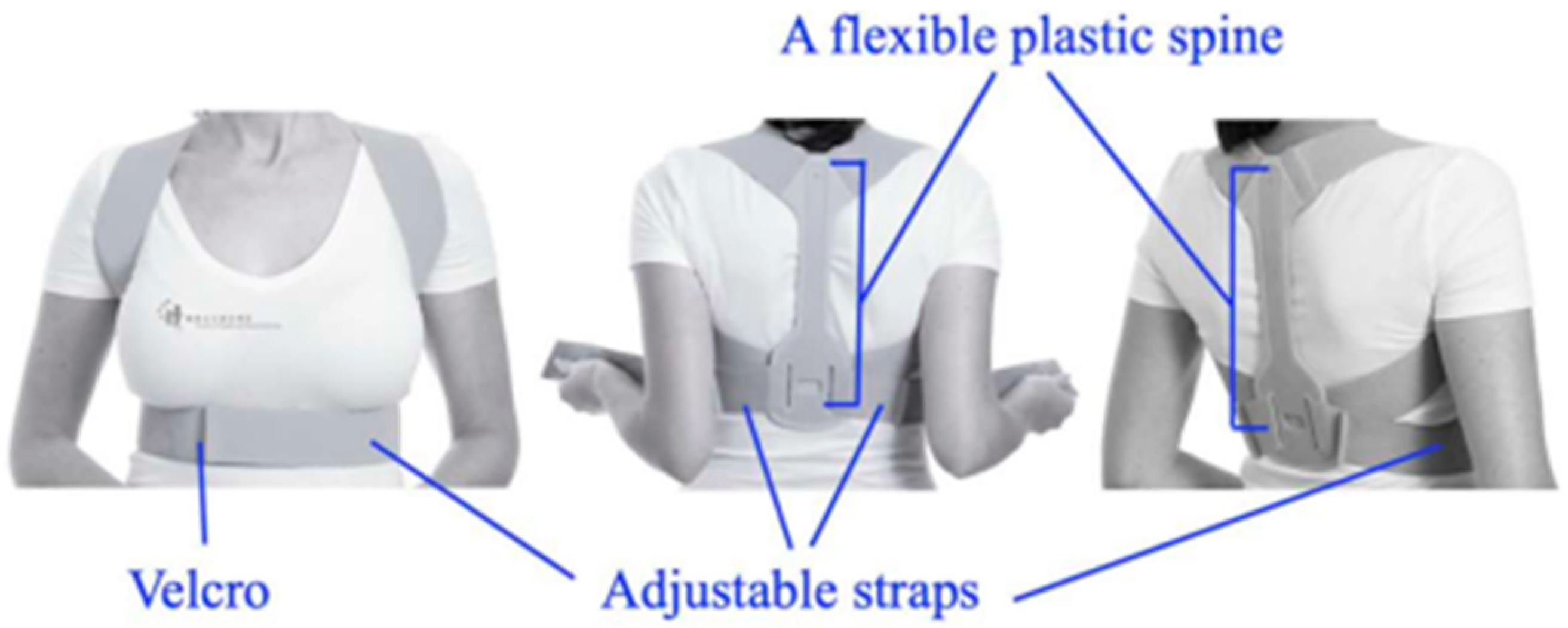
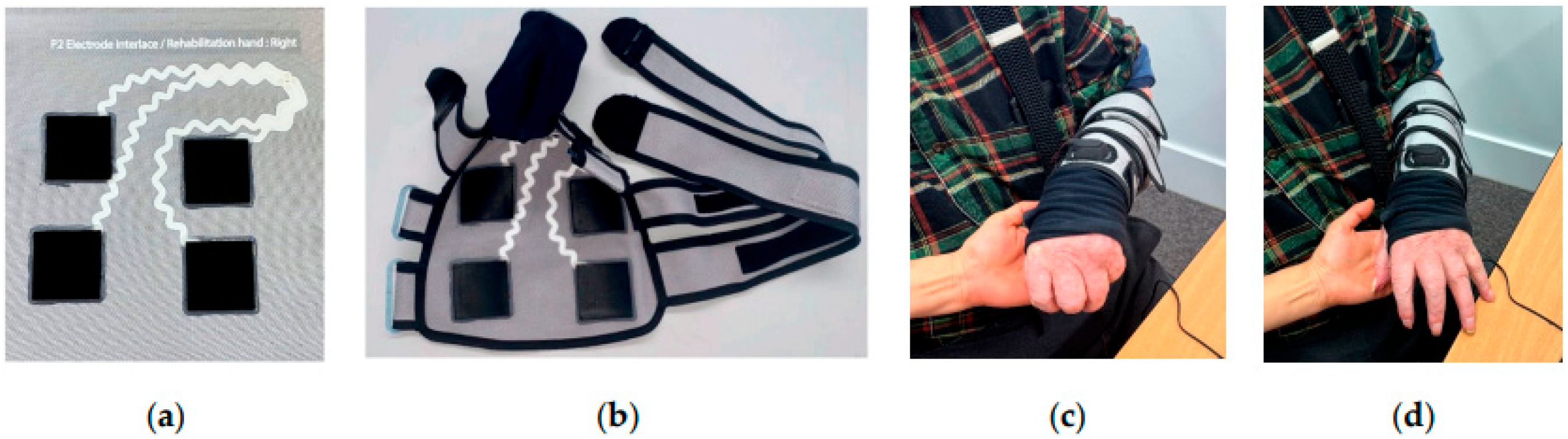

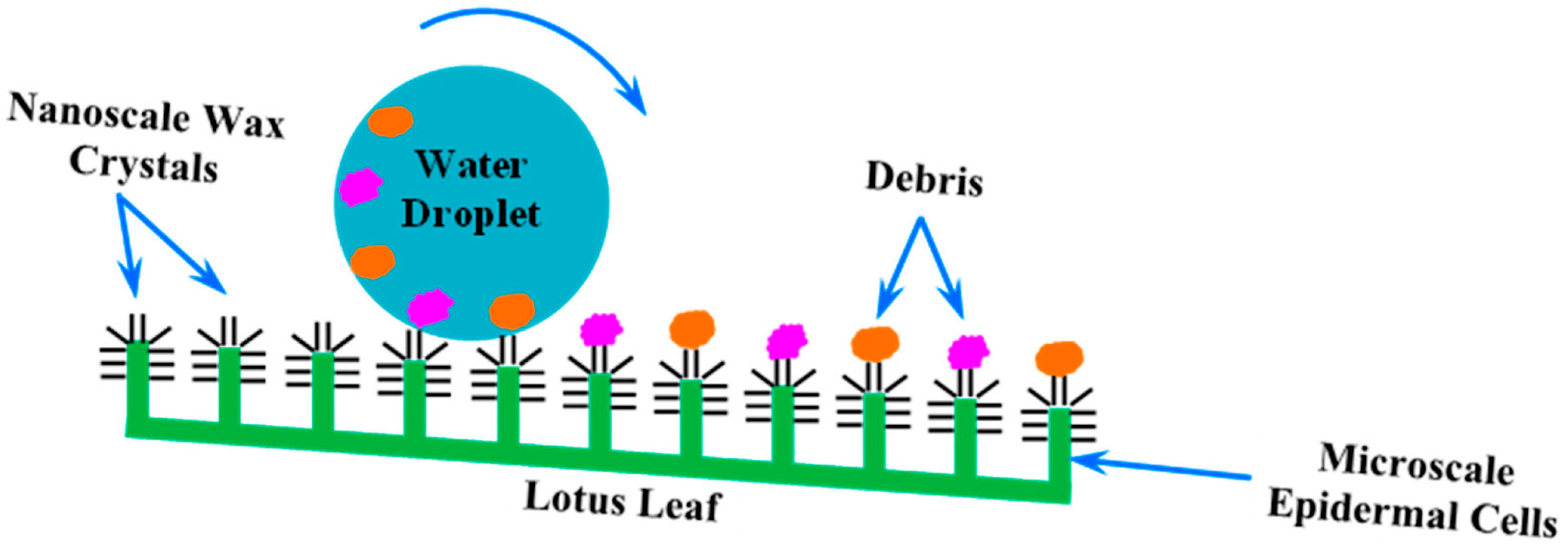

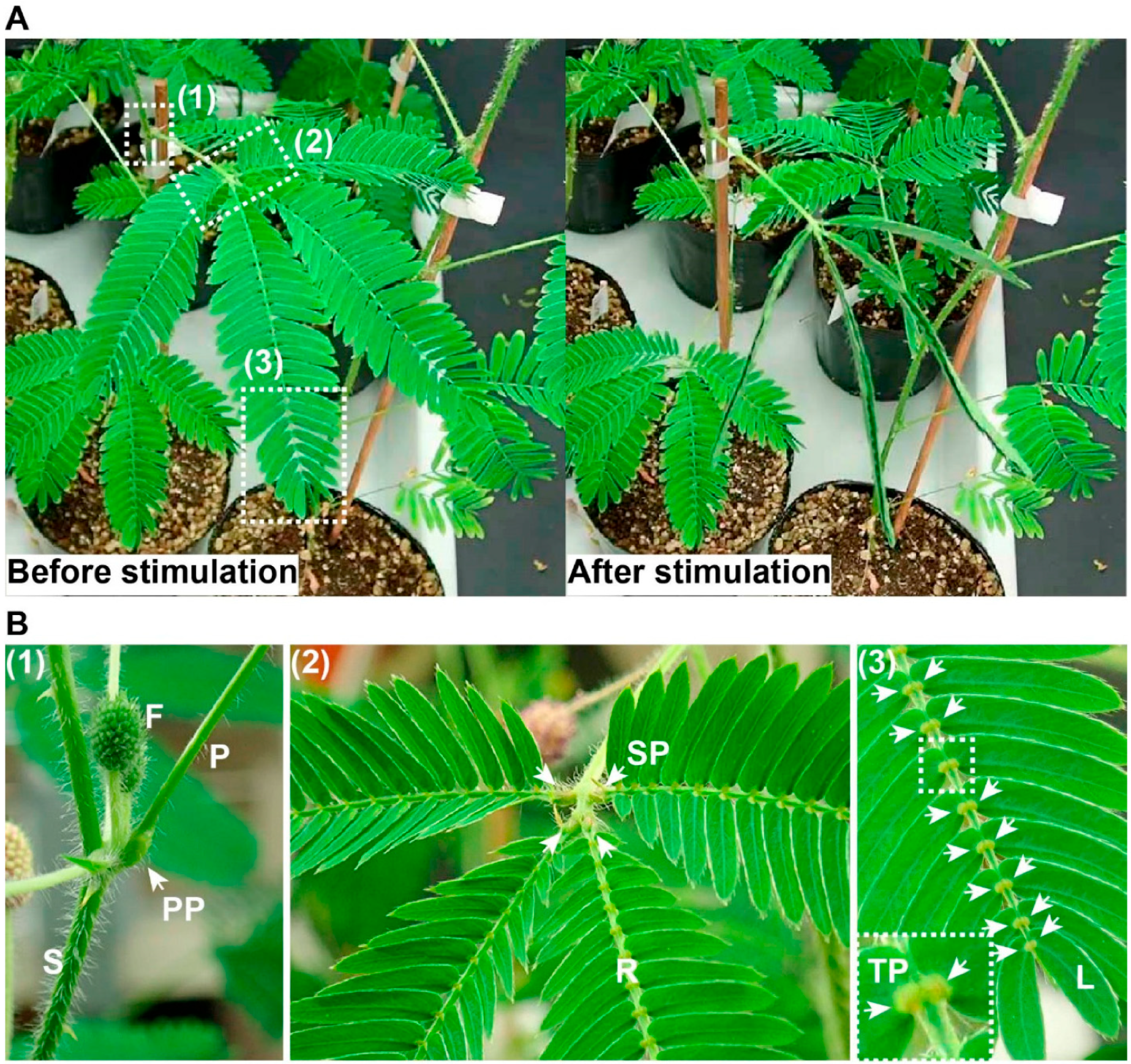
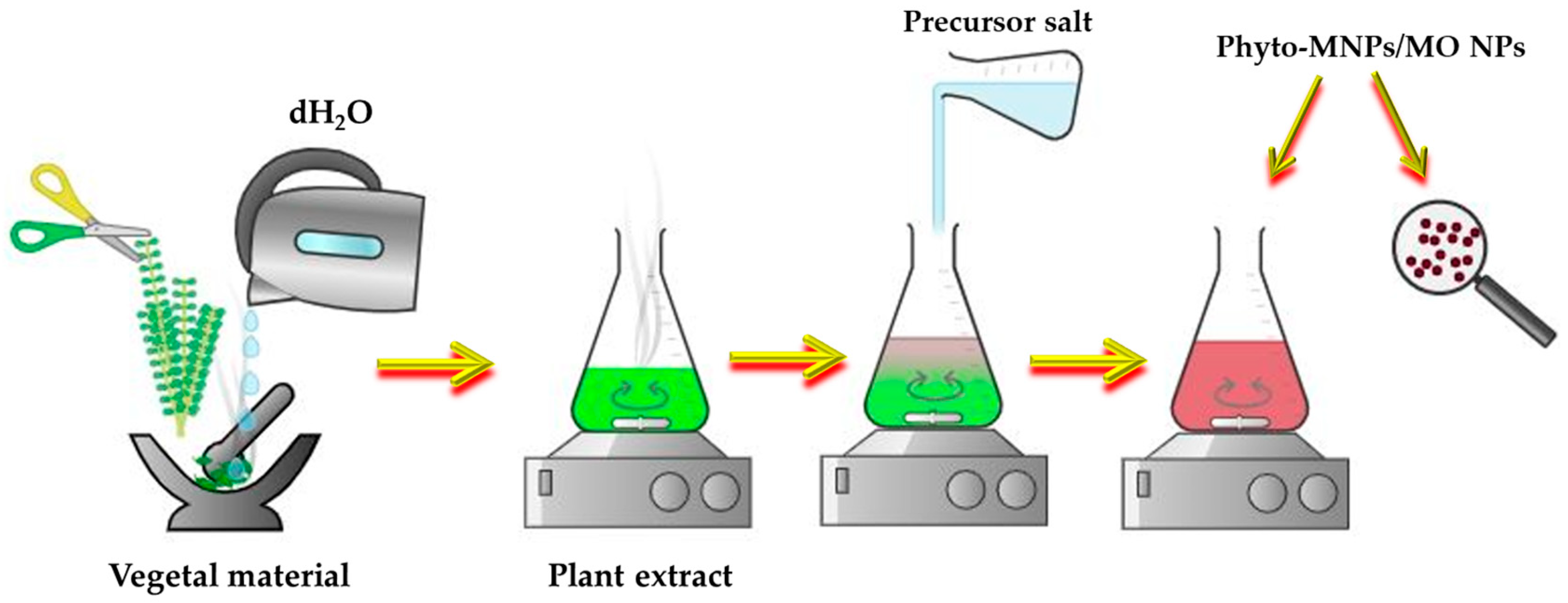
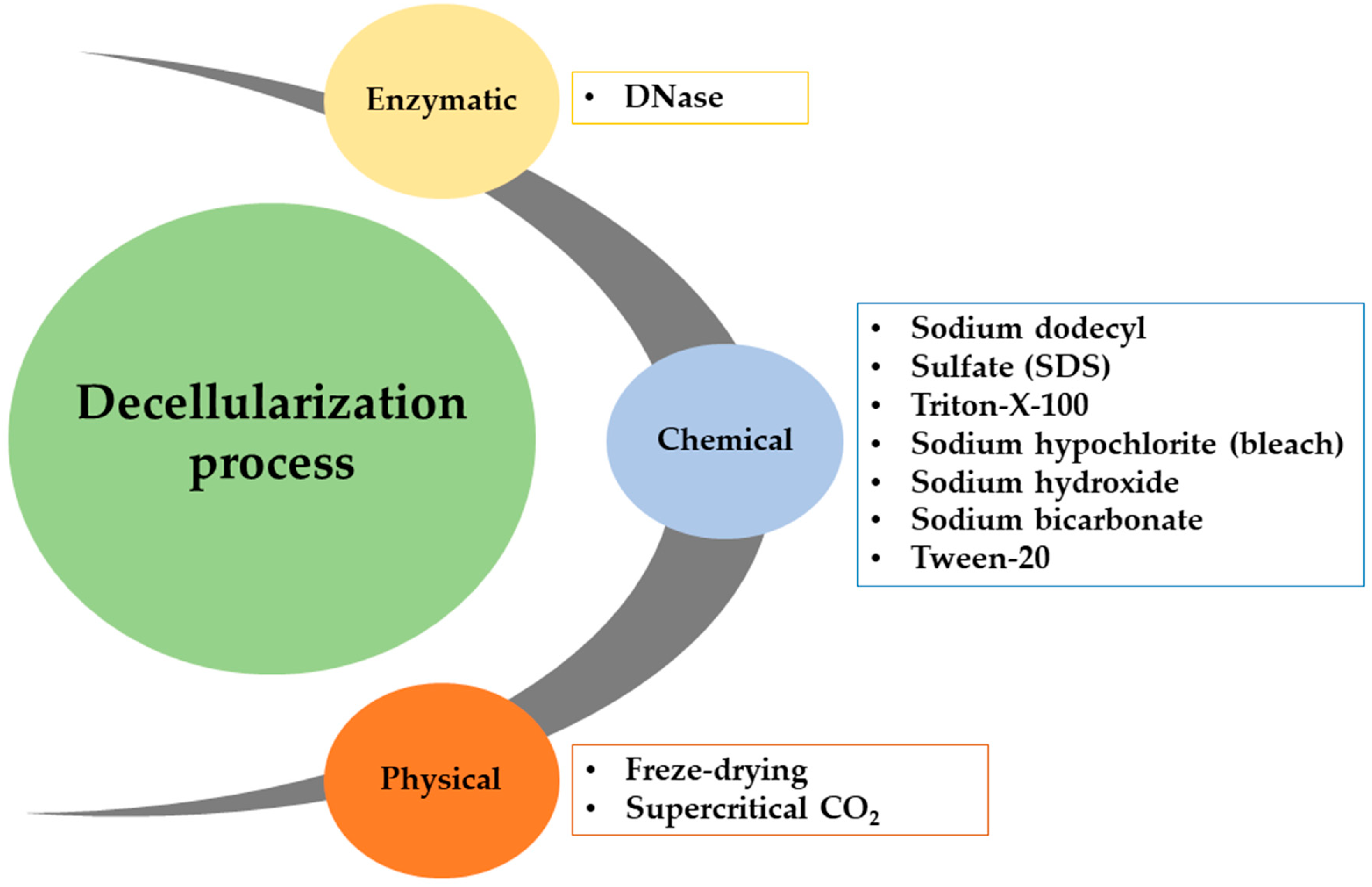
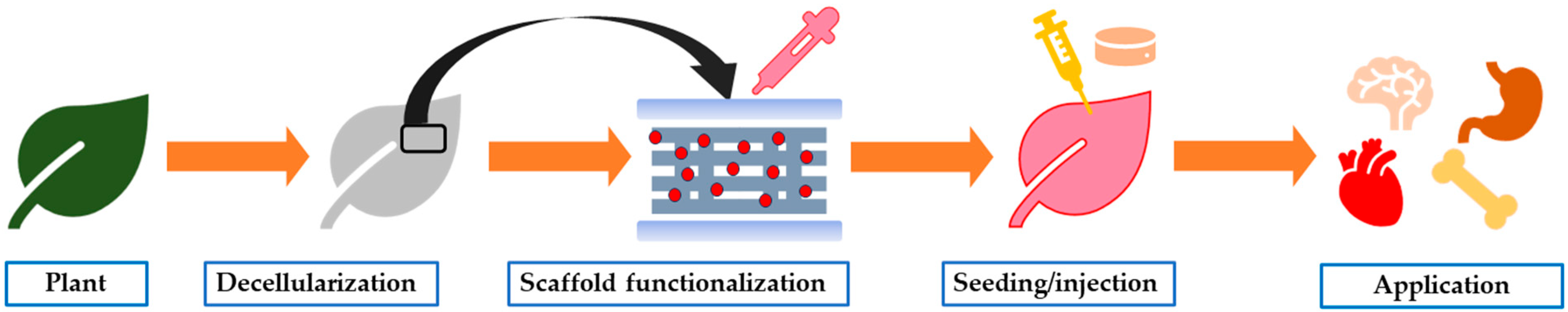
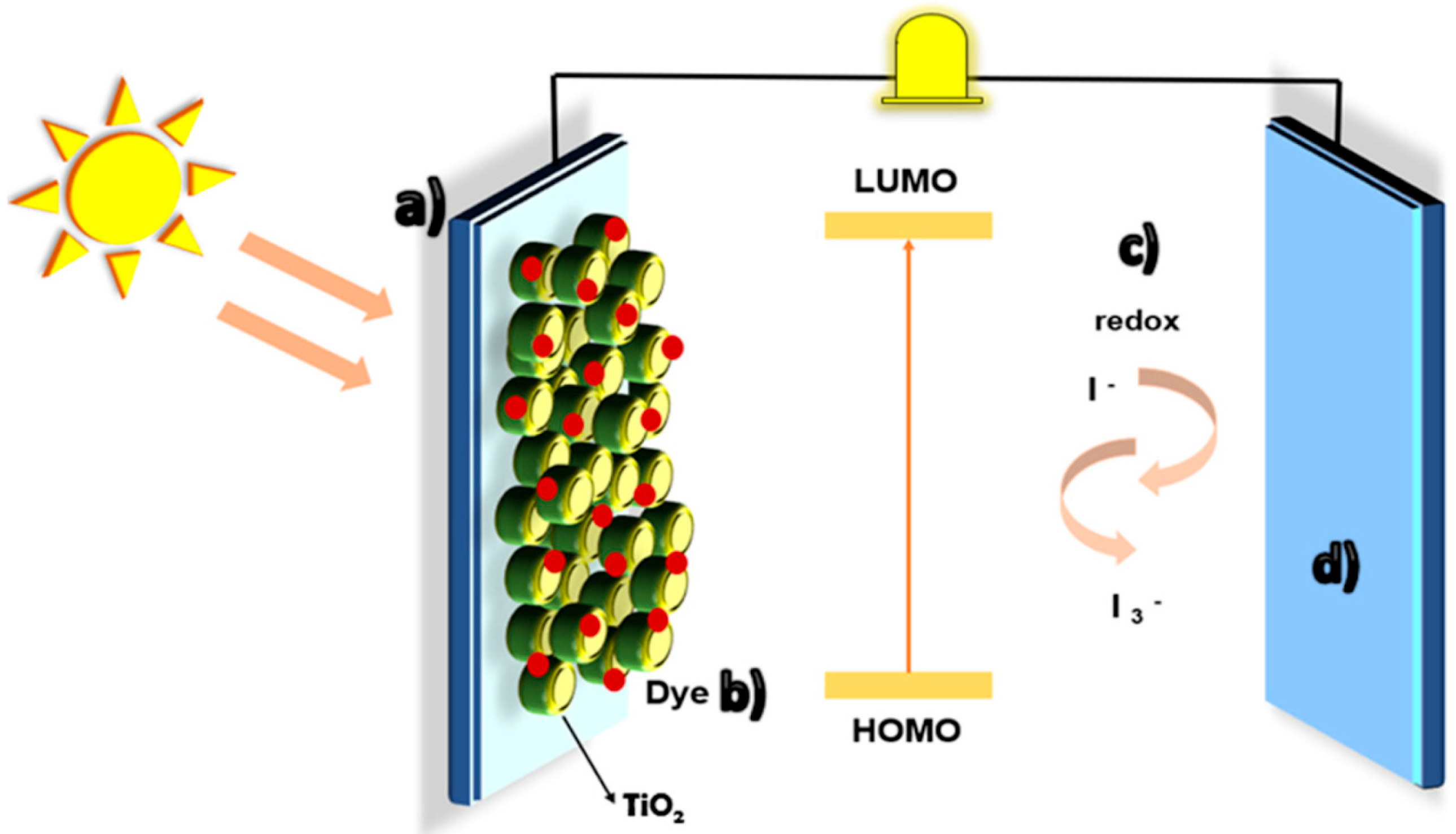
| Method | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Chemical-based |
|
| [122] |
| Physical |
|
| [123] |
| Enzymatic |
|
| [122] |
| Plant Type | Treatment/Coating | Cells | Scaffold Properties | Outcome | Potential Application | Ref. |
|---|---|---|---|---|---|---|
| Onion (Allium cepa) | - | hBM-MSC | Low surface roughness, high and regular porosity | Enhanced osteogenic differentiation | Non-load bearing | [161] |
| Carrot (Daucus carota) | Poly-L-lysine | MC3T3 | Heterogeneous structure, non-homogeneous pores distribution | Enhanced pre-osteoblasts adhesion proliferation and osteogenic differentiation | Bone filler, non-load bearing | [161] |
| Asian palmyra palm (Borassus flabellifer) | Organosilanes (APTES, OTS) | MG63 | Fibrous interconnected structure | Improved osteoblast differentiation and mineralization | Bone filler, non-load bearing | [140] |
| Alstroemeria flower stem | Chitosan | MC3T3 | Innate vasculature, high surface area | Improved mechanical behavior | Fluid transfer in BTE | [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbinta-Patrascu, M.-E.; Bita, B.; Negut, I. From Nature to Technology: Exploring the Potential of Plant-Based Materials and Modified Plants in Biomimetics, Bionics, and Green Innovations. Biomimetics 2024, 9, 390. https://doi.org/10.3390/biomimetics9070390
Barbinta-Patrascu M-E, Bita B, Negut I. From Nature to Technology: Exploring the Potential of Plant-Based Materials and Modified Plants in Biomimetics, Bionics, and Green Innovations. Biomimetics. 2024; 9(7):390. https://doi.org/10.3390/biomimetics9070390
Chicago/Turabian StyleBarbinta-Patrascu, Marcela-Elisabeta, Bogdan Bita, and Irina Negut. 2024. "From Nature to Technology: Exploring the Potential of Plant-Based Materials and Modified Plants in Biomimetics, Bionics, and Green Innovations" Biomimetics 9, no. 7: 390. https://doi.org/10.3390/biomimetics9070390
APA StyleBarbinta-Patrascu, M.-E., Bita, B., & Negut, I. (2024). From Nature to Technology: Exploring the Potential of Plant-Based Materials and Modified Plants in Biomimetics, Bionics, and Green Innovations. Biomimetics, 9(7), 390. https://doi.org/10.3390/biomimetics9070390







