Efficient Inhibition of Deep Conversion of Partial Oxidation Products in C-H Bonds’ Functionalization Utilizing O2 via Relay Catalysis of Dual Metalloporphyrins on Surface of Hybrid Silica Possessing Capacity for Product Exclusion
Abstract
1. Introduction
2. Experimental Section
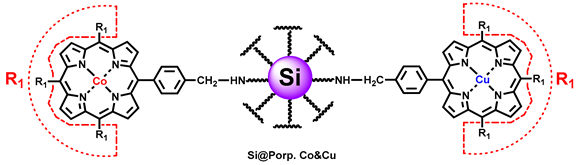
2.1. Immobilization of Metalloporphyrins on Hybrid Silica
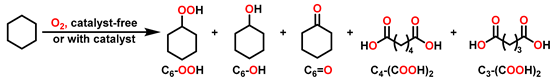
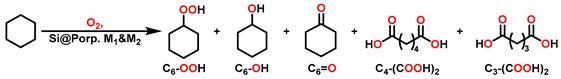
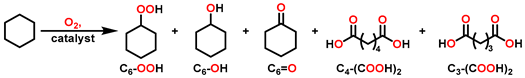
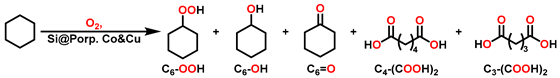
2.2. Partial Oxidation of C-H Bonds with O2
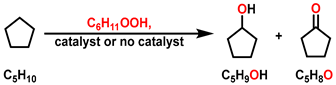
2.3. Relay Catalysis Study
2.4. UV-Vis Measurement
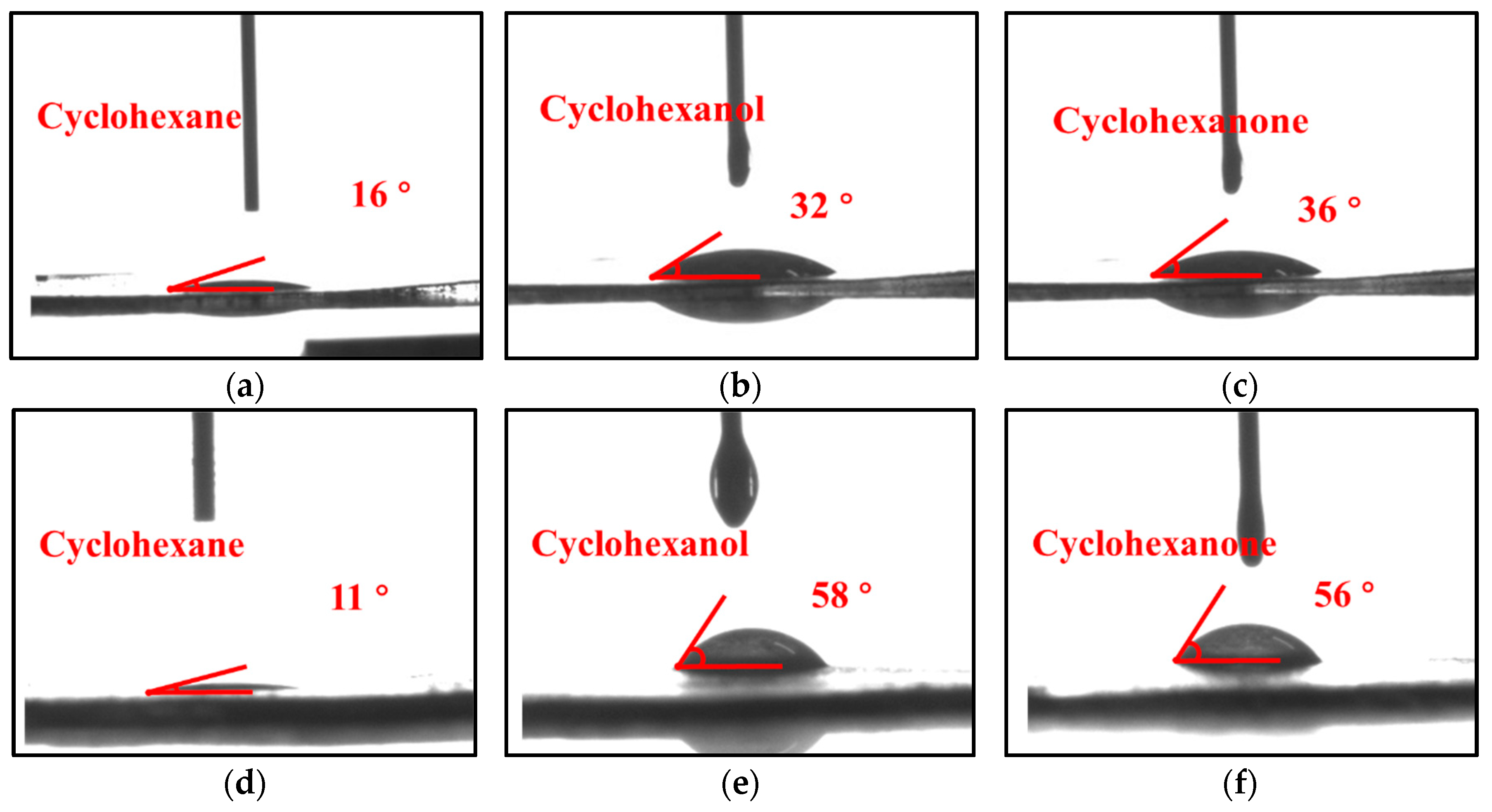
2.5. Contact Angle Measurement
3. Results and Discussion
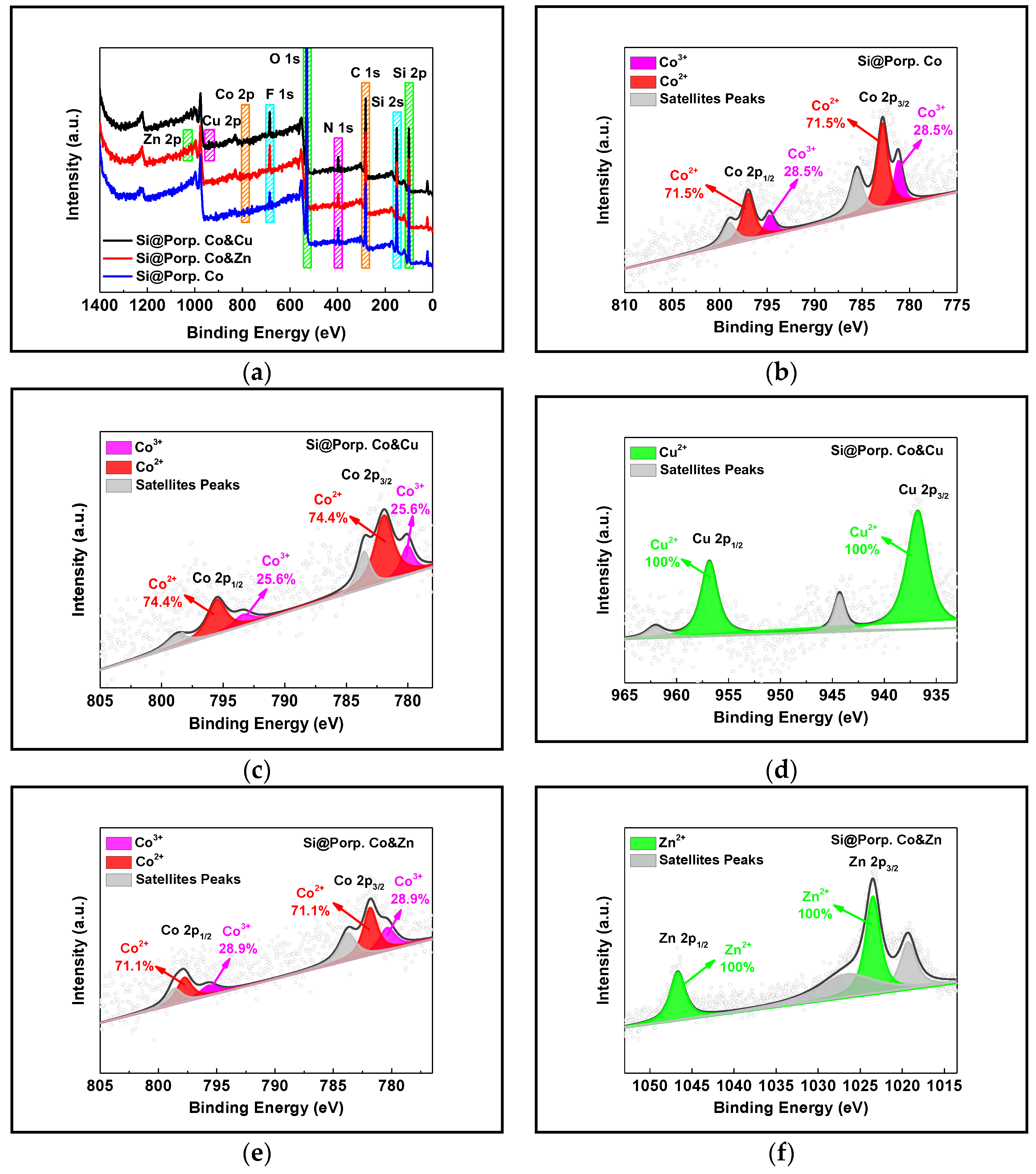
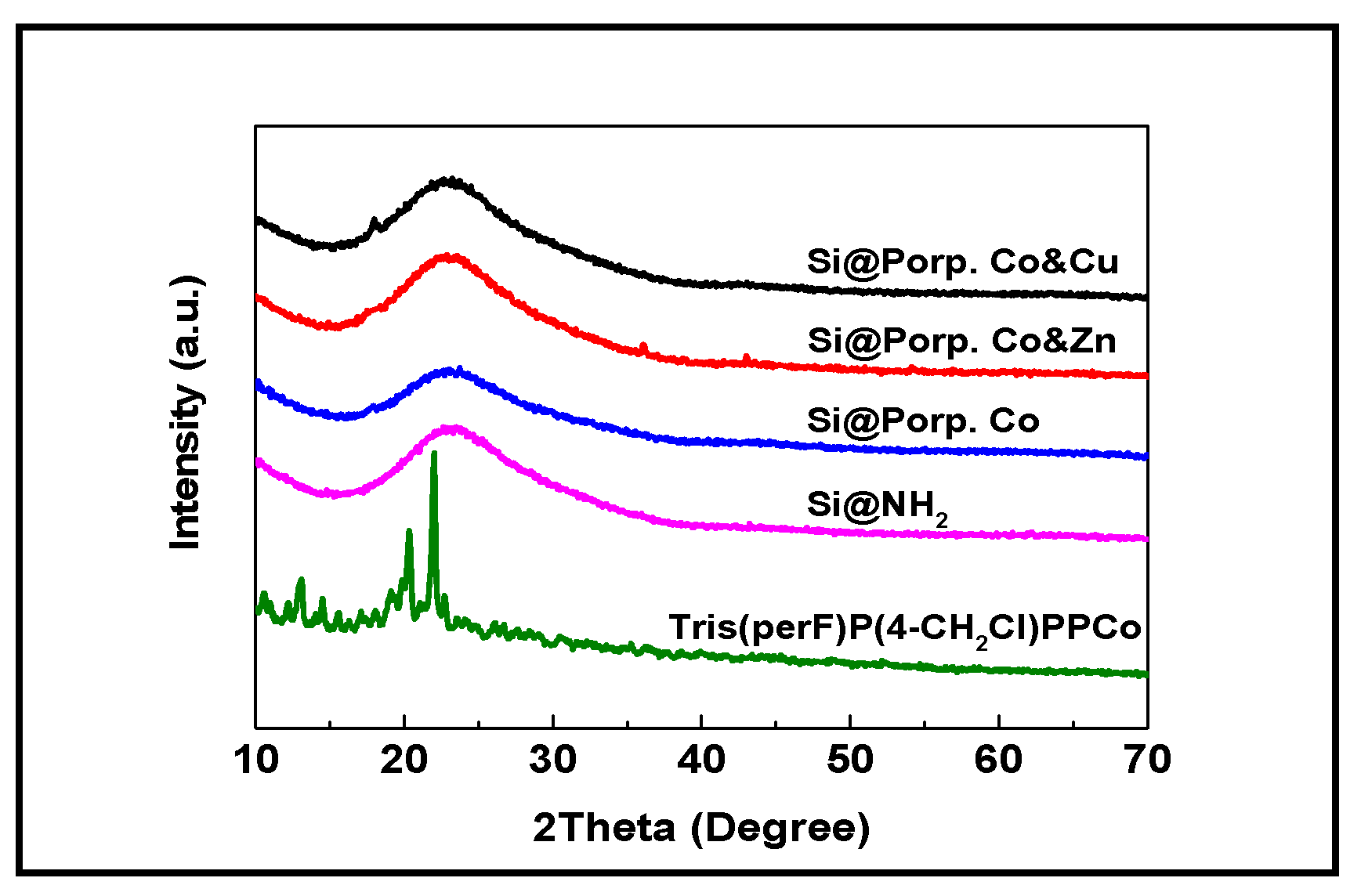
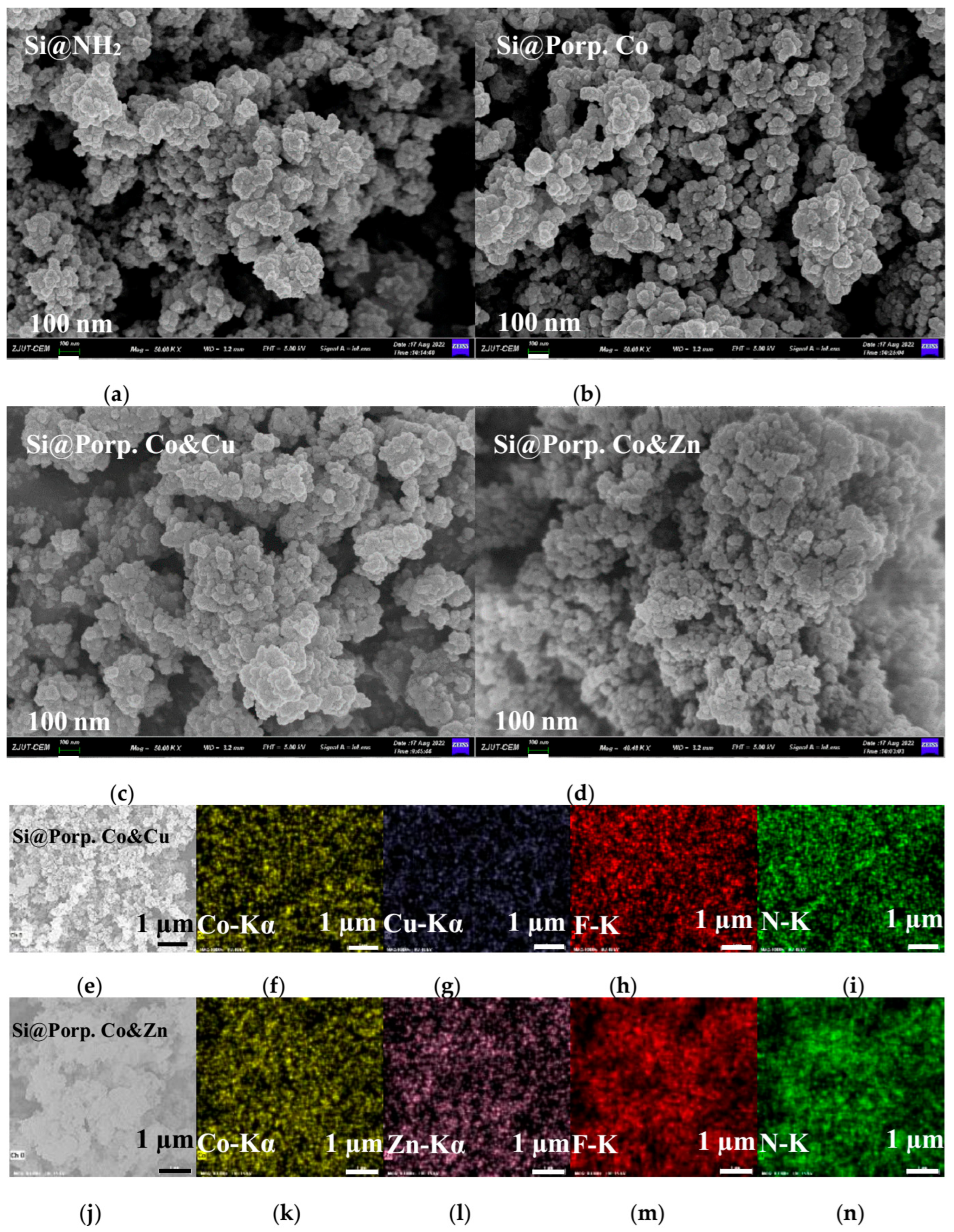
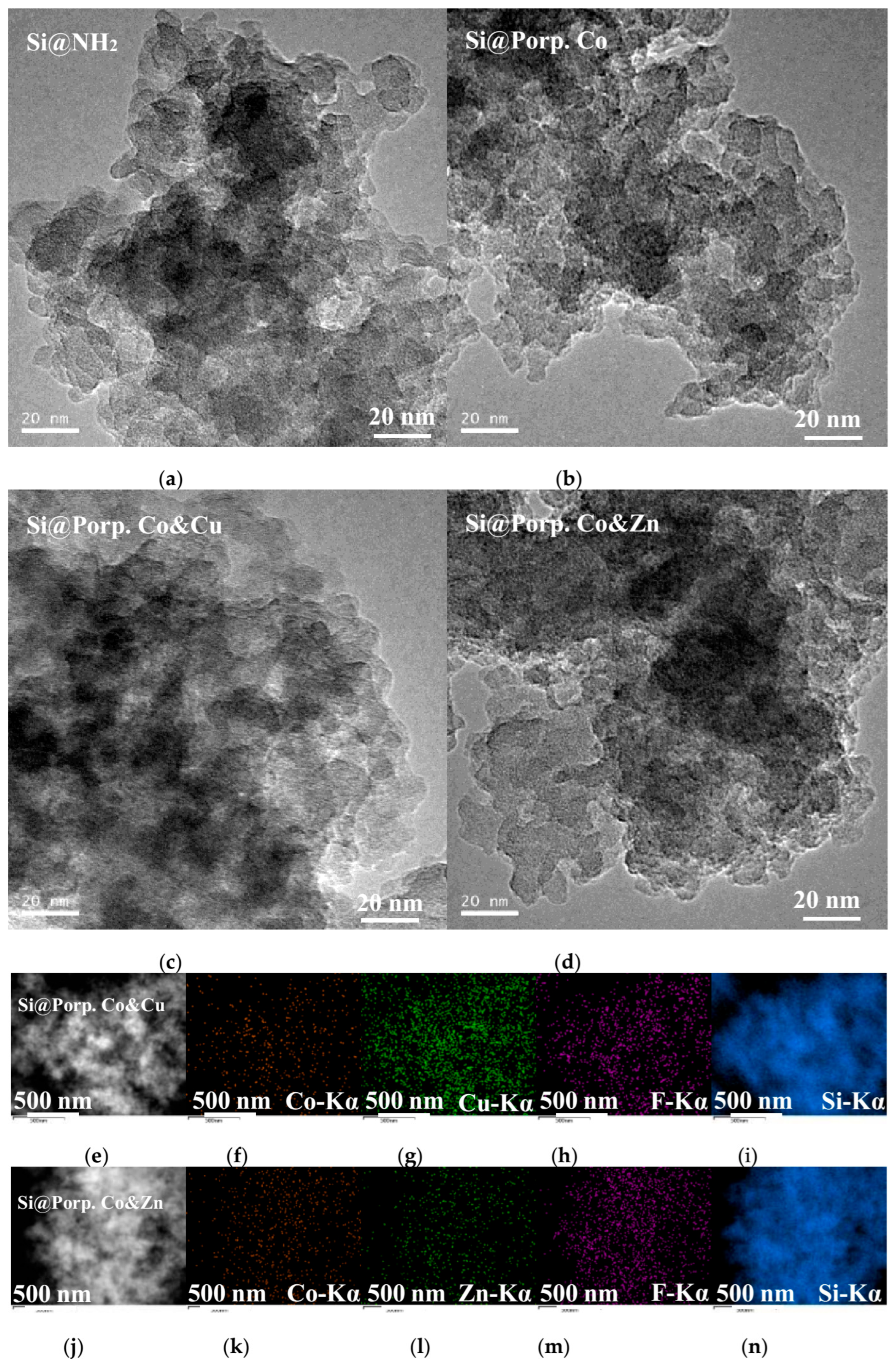
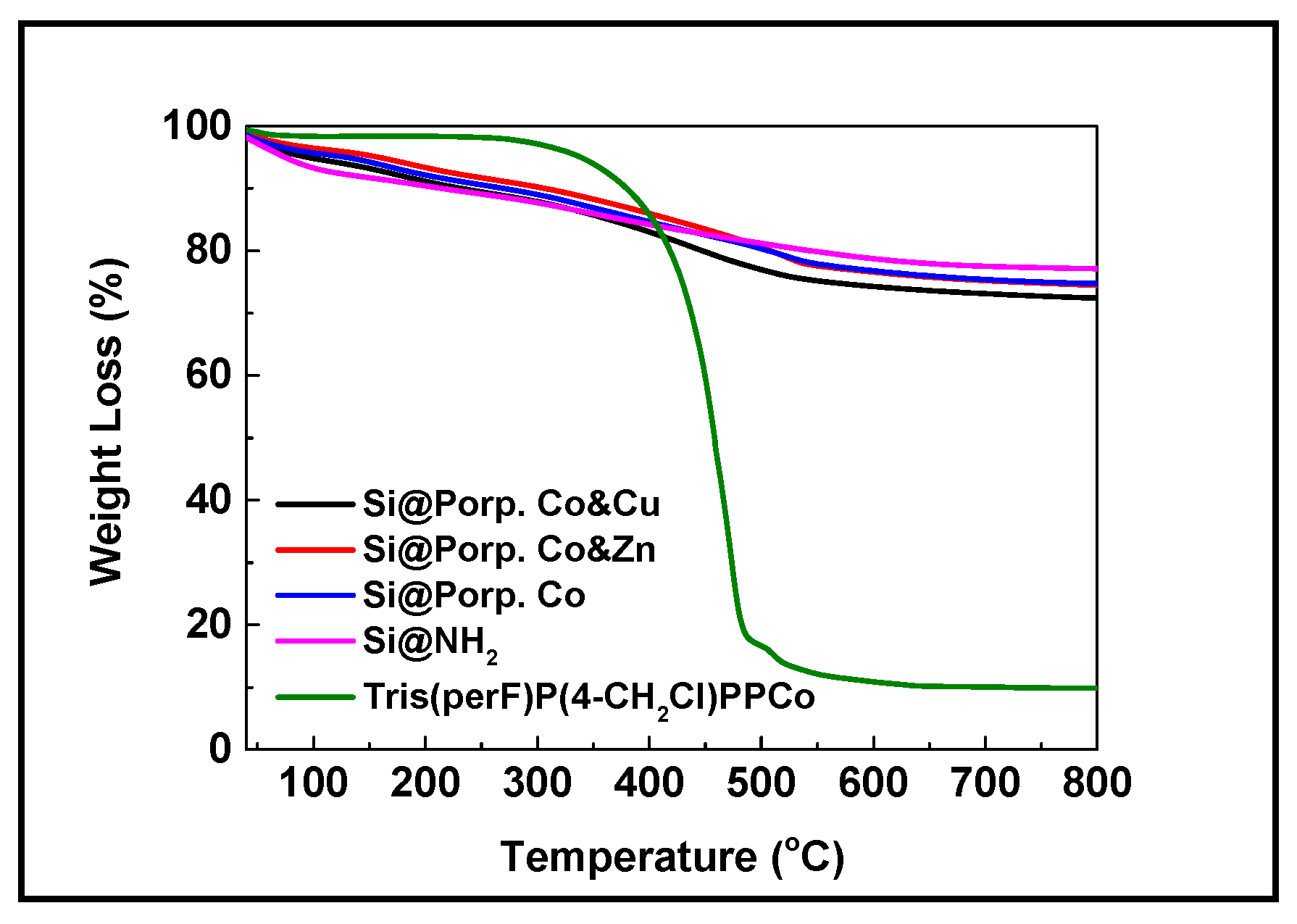
3.1. Characterization
3.2. Preliminary Exploration Experiments
3.3. Study on Catalyst Structure
3.3.1. Effect of Metal Centers
3.3.2. Effect of Porphyrin Ligands
3.3.3. Effect of Preparation Process
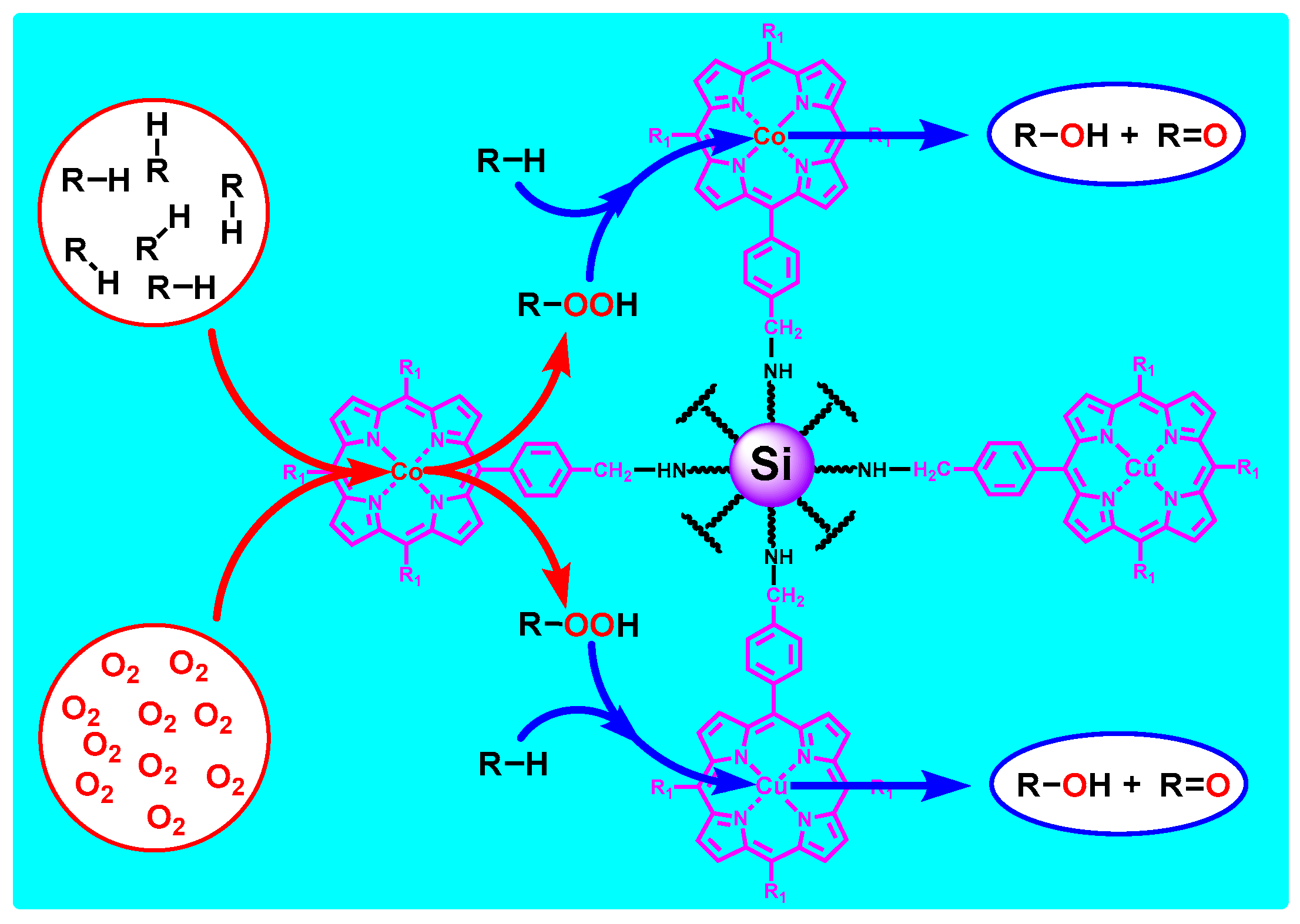
3.4. Relay Catalysis Study
3.5. Further Optimizations
3.6. Apparent Kinetic Study
3.7. Substrate Scope Study
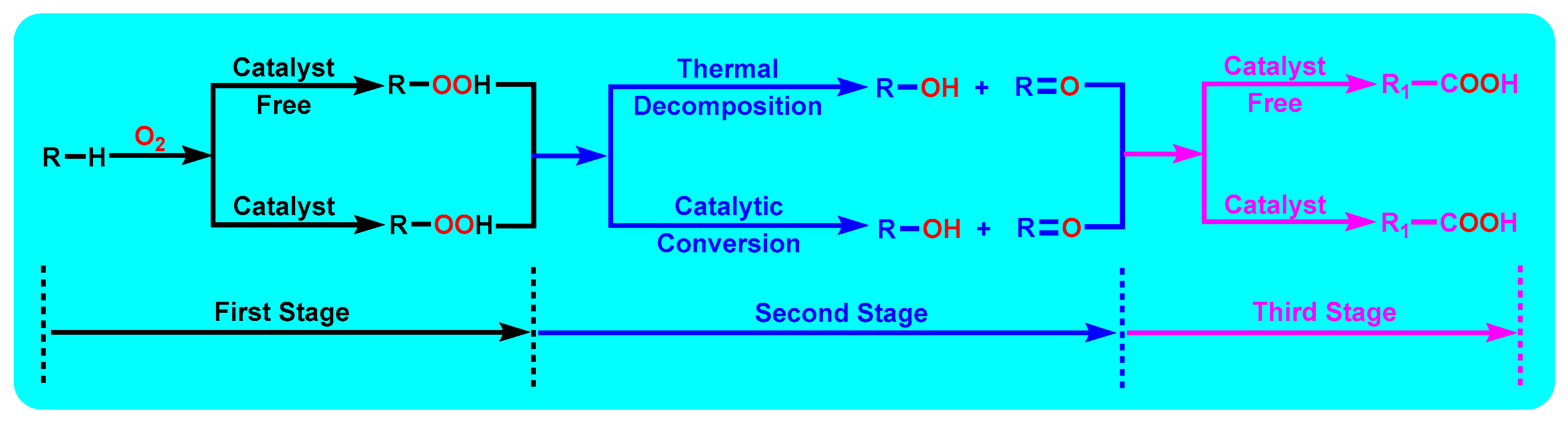
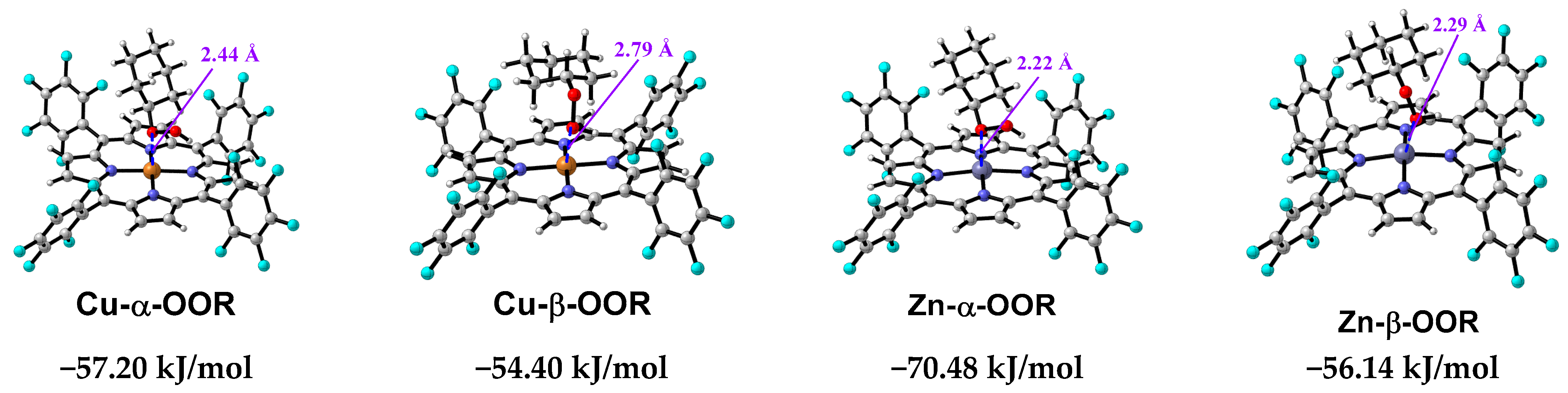
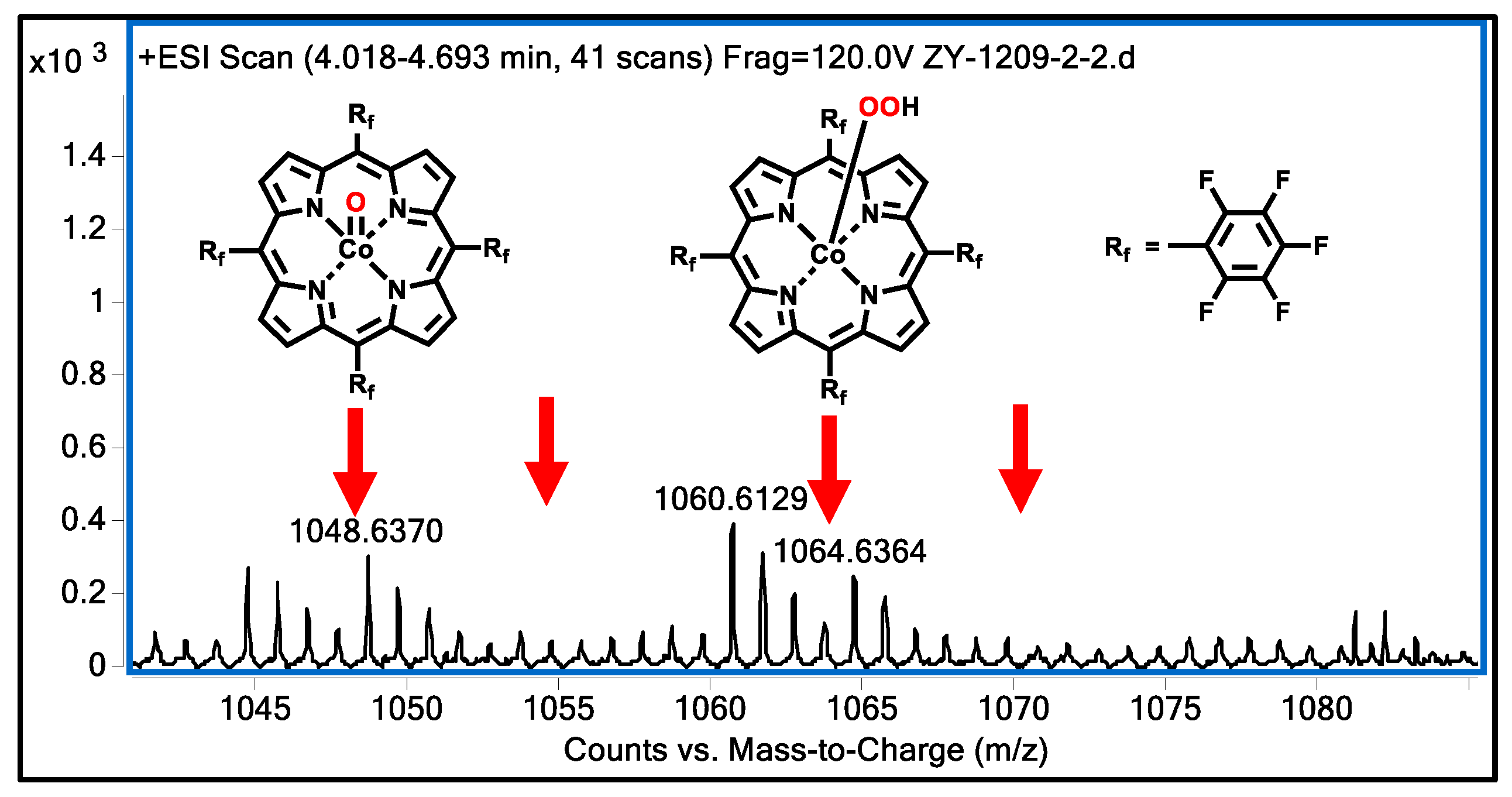
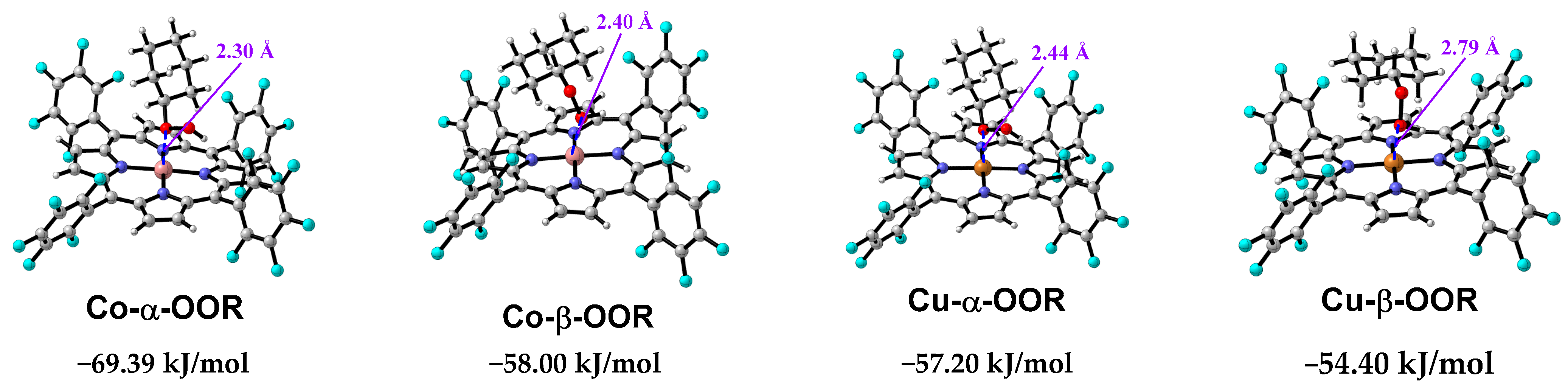
3.8. Mechanism Study
3.9. Literature Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.Y.; Hu, M.; Gui, Q.F.; Gu, J.; Xu, W.L.; Xiao, Q.B.; Huang, W. Highly enhanced direct catalytic oxidation of cyclohexane to adipic acid with molecular oxygen: Dynamic collaboration between zeolite channel micro-environment and Au clusters. Chem. Eng. J. 2023, 467, 143501. [Google Scholar] [CrossRef]
- Liu, Z.H.; Sun, S.Y.; Yang, F.; Liu, H.C.; Sun, Y.; Ta, N.; Zhang, G.; Che, S.; Li, Y.F. Synergistic effect of Fe/Cu-N-C dual single-atom catalyst for C-H bond oxidation. J. Colloid Interface Sci. 2023, 632, 237–248. [Google Scholar] [CrossRef]
- Buker, J.; Huang, X.B.; Bitzer, J.; Kleist, W.; Muhler, M.; Peng, B.X. Synthesis of Cu single atoms supported on mesoporous graphitic carbon nitride and their application in liquid-phase aerobic oxidation of cyclohexene. ACS Catal. 2021, 11, 7863–7875. [Google Scholar] [CrossRef]
- Biswas, R.; Kanti, D.; Bhaduri, S.N.; Bhaumik, A.; Biswas, P. AgNPs immobilized over functionalized 2D hexagonal SBA-15 for catalytic C-H oxidation of hydrocarbons with molecular oxygen under solvent-free conditions. ACS Sustain. Chem. Eng. 2020, 8, 5856–5867. [Google Scholar] [CrossRef]
- Deng, Y.C.; Chen, Z.C.; Huang, J.N.; Yang, G.X.; Zhang, Q.; Liu, Z.L.; Cao, Y.H.; Peng, F. MnO2 nanoparticles supported on CNTs for cumene oxidation: Synergistic effect and kinetic modelling. Chem. Eng. J. 2022, 444, 136666. [Google Scholar] [CrossRef]
- Yuan, E.X.; Zhou, M.X.; Jian, P.M.; Hou, X. Atomically dispersed Co/C3N4 for boosting aerobic cyclohexane oxidation. Appl. Surf. Sci. 2023, 613, 155886. [Google Scholar] [CrossRef]
- Chen, S.X.; Li, Y.W.; Wang, Z.C.; Jin, Y.; Liu, R.X.; Li, X.A. Poly(ionic liquid)s hollow spheres nanoreactor for enhanced cyclohexane catalytic oxidation. J. Catal. 2022, 411, 135–148. [Google Scholar] [CrossRef]
- Wang, H.B.; Zhang, Q.H.; Zhu, Z.W.; Yang, Y.Y.; Ye, Y.C.; Lv, Z.G.; Chen, C. High-efficient metal-free aerobic oxidation of aromatic hydrocarbons by N, N-dihydroxypyromellitimide and 1,4-diamino-2,3-dichloroanthraquinone. Mol. Catal. 2022, 518, 112078. [Google Scholar] [CrossRef]
- Shen, H.M.; Guo, A.B.; Zhang, Y.; Liu, Q.P.; Qin, J.W.; She, Y.B. Relay catalysis of hydrocarbon oxidation using O2 in the confining domain of 3D metalloporphyrin-based metal-organic frameworks with bimetallic catalytic centers. Chem. Eng. Sci. 2022, 260, 117825. [Google Scholar] [CrossRef]
- Li, K.X.; Li, H.; Wei, S.; Liu, Y.M.; Li, Y.W.; Zhang, R.R.; Liu, R.X. High performance BaCO3-CeO2 composite catalyst for solvent-free selective oxidation of cyclohexane with molecular oxygen. Mol. Catal. 2023, 535, 112851. [Google Scholar] [CrossRef]
- Arumugam, S.; Singh, V.; Tathod, A.P.; Daniel, S.; Viswanadham, N. CeO2-TiO2 nanoparticle-grafted gC3N4 sheets as an efficient catalyst for the oxidation of cyclohexane to KA oil. Ind. Eng. Chem. Res. 2022, 61, 18372–18381. [Google Scholar] [CrossRef]
- Wang, X.Y.; Feng, X.Y.; Liu, J.C.; Huang, Z.L.; Zong, S.; Liu, L.L.; Liu, J.R.; Fang, Y.X. Photo-thermo catalytic selective oxidation of cyclohexane by in-situ prepared nonstoichiometric molybdenum oxide and silver-palladium alloy composite. J. Colloid Interface Sci. 2022, 607, 954–966. [Google Scholar] [CrossRef]
- Graca, I.; Chadwick, D. NH4-exchanged zeolites: Unexpected catalysts for cyclohexane selective oxidation. Micropor. Mesopor. Mat. 2020, 294, 109873. [Google Scholar] [CrossRef]
- Guo, A.B.; Qin, J.W.; Wang, K.K.; Liu, Q.P.; Wu, H.K.; Wang, M.; Shen, H.M.; She, Y.B. Synergetic catalytic oxidation of C-H bonds in cycloalkanes and alkyl aromatics by dimetallic active sites in 3D metalloporphyrinic MOFs employing O2 as oxidant with increased conversion and unconsumed selectivity. Mol. Catal. 2023, 535, 112853. [Google Scholar] [CrossRef]
- Shen, H.M.; Wang, X.; Huang, H.; Liu, Q.P.; Lv, D.; She, Y.B. Staged oxidation of hydrocarbons with simultaneously enhanced conversion and selectivity employing O2 as oxygen source catalyzed by 2D metalloporphyrin-based MOFs possessing bimetallic active centers. Chem. Eng. J. 2022, 443, 136126. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Tang, S.L.Y.; Smith, R.L.; Poliakoff, M. Principles of green chemistry: PRODUCTIVELY. Green Chem. 2005, 7, 761–762. [Google Scholar] [CrossRef]
- Tang, S.Y.; Bourne, R.A.; Poliakoff, M.; Smith, R.L. The 24 principles of green engineering and green chemistry: “IMPROVEMENTS PRODUCTIVELY”. Green Chem. 2008, 10, 268–269. [Google Scholar] [CrossRef]
- Zimmerman, J.B.; Anastas, P.T.; Erythropel, H.C.; Leitner, W. Designing for a green chemistry future. Science 2020, 367, 397–400. [Google Scholar] [CrossRef]
- Wang, K.; Li, S.S.; Wang, J.L.; He, Z.H.; Wang, D.; Zhang, R.R.; Wang, W.T.; Yang, Y.; Liu, Z.T. Photothermal oxidation of cyclohexane over CoLaOx/WO3 Z-scheme composites with p-n heterojunction in solvent-free conditions. Catal. Today 2023, 409, 42–52. [Google Scholar] [CrossRef]
- Wei, S.; Li, H.; Li, K.X.; Zhang, R.R.; Wang, G.S.; Liu, R.X. Design of prussian blue analogue-derived magnetic binary Ce-Fe oxide catalysts for the selective oxidation of cyclohexane. Ind. Eng. Chem. Res. 2022, 61, 17842–17853. [Google Scholar] [CrossRef]
- Wu, S.T.; He, Y.R.; Wang, C.H.; Zhu, C.M.; Shi, J.; Chen, Z.Y.; Wan, Y.; Hao, F.; Xiong, W.; Liu, P.L.; et al. Selective Cl-decoration on nanocrystal facets of hematite for high-efficiency catalytic oxidation of cyclohexane: Identification of the newly formed Cl-O as active sites. ACS Appl. Mater. Inter. 2020, 12, 26733–26745. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Y.; Wu, C.; Xie, J.; Gu, X.; Yu, P.; Zong, M.; Gates, I.D.; Liu, H.; Rong, J. Electrophilic oxygen on defect-rich carbon nanotubes for selective oxidation of cyclohexane. Catal. Sci. Technol. 2020, 10, 332–336. [Google Scholar] [CrossRef]
- Muhumuza, E.; Wu, P.P.; Nan, T.; Zhao, L.M.; Bai, P.; Mintova, S.; Yan, Z.F. Perovskite-type LaCoO3 as an efficient and green catalyst for sustainable partial oxidation of cyclohexane. Ind. Eng. Chem. Res. 2020, 59, 21322–21332. [Google Scholar] [CrossRef]
- Huang, H.; Fan, H.H.; Ge, Y.Z.; Liu, Y.C.; Song, C.C.; Lin, H.; Zhang, X.T.; Li, B.; Nie, X.W.; Zhang, S.F.; et al. Solvent-free oxidation of toluene to benzaldehyde using electron-rich Au clusters confined in silicalite-1. Chem. Eng. J. 2023, 458, 141446. [Google Scholar] [CrossRef]
- Shen, H.M.; Wang, X.; Ning, L.; Guo, A.B.; Deng, J.H.; She, Y.B. Efficient oxidation of cycloalkanes with simultaneously increased conversion and selectivity using O2 catalyzed by metalloporphyrins and boosted by Zn(AcO)2: A practical strategy to inhibit the formation of aliphatic diacids. Appl. Catal. A-Gen. 2021, 609, 117904. [Google Scholar] [CrossRef]
- Ni, J.Y.; Cong, S.Z.; Ning, L.; Wang, M.; Shen, H.M.; She, Y.B. Binary catalytic systems constructed by porphyrin cobalts(II) with confining nano-region and Zn(OAc)2 for oxygenation of cycloalkanes with O2 in relay mode. Mol. Catal. 2022, 531, 112676. [Google Scholar] [CrossRef]
- Ni, J.Y.; He, B.; Huang, H.; Ning, L.; Liu, Q.P.; Wang, K.K.; Wu, H.K.; Shen, H.M.; She, Y.B. Cycloalkanes oxidation with O2 in high-efficiency and high-selectivity catalyzed by 3D MOFs with limiting domain and Zn(AcO)2 through synergistic mode. Mol. Catal. 2023, 540, 113027. [Google Scholar] [CrossRef]
- Shen, H.M.; Ye, H.L.; Ni, J.Y.; Wang, K.K.; Zhou, X.Y.; She, Y.B. Oxidation of a-C-H bonds in alkyl aromatics with O2 catalyzed by highly dispersed cobalt(II) coordinated in confined reaction channel of porphyrin-based POFs with simultaneously enhanced conversion and selectivity. Chem. Eng. Sci. 2023, 270, 118472. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.S.; Lee, T.R. The wettability of fluoropolymer surfaces: Influence of surface dipoles. Langmuir 2008, 24, 4817–4826. [Google Scholar] [CrossRef]
- Marquez, M.D.; Zenasni, O.; Rodriguez, D.; Yu, T.L.; Sakunkaewkasem, S.; Figueira, F.T.; Czader, A.; Baldelli, S.; Lee, T.R. Burying the inverted surface dipole: Self-assembled monolayers derived from alkyl-terminated partially fluorinated alkanethiols. Chem. Mater. 2020, 32, 953–968. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Bressler, A.H.; Castano, C.E.; Fergusson, C.P.; Mohammadi, R. Rational strategy for the atmospheric icing prevention based on chemically functionalized carbon soot coatings. Appl. Surf. Sci. 2016, 390, 452–460. [Google Scholar] [CrossRef]
- Shen, H.M.; Hu, M.Y.; Liu, L.; Qi, B.; Ye, H.L.; She, Y.B. Efficient and selective oxidation of tertiary benzylic C-H bonds with O2 catalyzed by metalloporphyrins under mild and solvent-free conditions. Appl. Catal. A-Gen. 2020, 599, 117599. [Google Scholar] [CrossRef]
- Ou, J.H.; Xiang, J.; Liu, J.X.; Sun, L.C. Surface-supported metal-organic framework thin-film-derived transparent CoS1.097@N-doped carbon film as an efficient counter electrode for bifacial dye-sensitized solar cells. ACS Appl. Mater. Inter. 2019, 11, 14862–14870. [Google Scholar] [CrossRef]
- Chen, L.Y.; Wang, Y.X.; Yu, F.Y.; Shen, X.S.; Duan, C.Y. A simple strategy for engineering heterostructures of Au nanoparticle-loaded metal-organic framework nanosheets to achieve plasmon-enhanced photocatalytic CO2 conversion under visible light. J. Mater. Chem. A 2019, 7, 11355–11361. [Google Scholar] [CrossRef]
- Xin, Z.F.; Wang, Y.R.; Chen, Y.F.; Li, W.L.; Dong, L.Z.; Lan, Y.Q. Metallocene implanted metalloporphyrin organic framework for highly selective CO2 electroreduction. Nano Energy 2020, 67, 104233. [Google Scholar] [CrossRef]
- Zhang, D.X.; Du, P.Y.; Chen, J.; Guo, H.X.; Lu, X.Q. Pyrazolate-based porphyrinic metal-organic frameworks as catechol oxidase mimic enzyme for fluorescent and colorimetric dual-mode detection of dopamine with high sensitivity and specificity. Sens. Actuat. B-Chem. 2021, 341, 130000. [Google Scholar] [CrossRef]
- Wang, L.; Jin, P.X.; Huang, J.W.; She, H.D.; Wang, Q.Z. Integration of copper(II)-porphyrin zirconium metal-organic framework and titanium dioxide to construct Z-scheme system for highly improved photocatalytic CO2 reduction. ACS Sustain. Chem. Eng. 2019, 7, 15660–15670. [Google Scholar] [CrossRef]
- Yan, T.T.; Guo, J.H.; Liu, Z.Q.; Sun, W.Y. Metalloporphyrin encapsulation for enhanced conversion of CO2 to C2H4. ACS Appl. Mater. Inter. 2021, 13, 25937–25945. [Google Scholar] [CrossRef]
- Ang, H.X.; Hong, L. Polycationic polymer-regulated assembling of 2D MOF nanosheets for high-performance nanofiltration. ACS Appl. Mater. Inter. 2017, 9, 28079–28088. [Google Scholar] [CrossRef]
- Cong, M.Y.; Chen, X.Y.; Xia, K.; Ding, X.; Zhang, L.L.; Jin, Y.; Gao, Y.; Zhang, L.X. Selective nitrogen reduction to ammonia on iron porphyrin-based single-site metal-organic frameworks. J. Mater. Chem. A 2021, 9, 4673–4678. [Google Scholar] [CrossRef]
- Huang, L.Y.; Hu, X.; Shan, H.Y.; Yu, L.; Gu, Y.X.; Wang, A.J.; Shan, D.; Yuan, P.X.; Feng, J.J. High-performance electrochemiluminescence emitter of metal organic framework linked with porphyrin and its application for ultrasensitive detection of biomarker mucin-1. Sens. Actuat. B-Chem. 2021, 344, 130300. [Google Scholar] [CrossRef]
- Lai, Y.M.; Zhu, X.Q.; Li, J.; Gou, Q.Z.; Li, M.; Xia, A.; Huang, Y.; Zhu, X.; Liao, Q. Recovery and regeneration of anode graphite from spent lithium-ion batteries through deep eutectic solvent treatment: Structural characteristics, electrochemical performance and regeneration mechanism. Chem. Eng. J. 2023, 457, 141196. [Google Scholar] [CrossRef]
- Zuo, J.Q.; Zhu, E.A.; Yin, W.J.; Yao, C.Y.; Liao, J.J.; Ping, X.N.; Zhu, Y.Q.; Cai, X.T.; Rao, Y.C.; Feng, H.; et al. Long-term spatiotemporal and highly specific imaging of the plasma membrane of diverse plant cells using a near-infrared AIE probe. Chem. Sci. 2023, 14, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.L.; Wen, Z.; Xiao, P.W.; Wang, P.M.; Luo, J.H.; Han, X.; Zhao, S.L. Emulsification mechanism of surfactants in different oil phases: A dissipative particle dynamics study. Colloid. Surface A 2023, 665, 131213. [Google Scholar] [CrossRef]
- Huang, S.R.; Cong, P.Q.; Liu, Z.J.; Wu, F.Y.; Gong, C.X.; Chen, L.; Chen, Y.W. Over 70% fill factor of all-polymer solar cells guided by the law of similarity and intermiscibility. Sol. RRL 2021, 5, 2100019. [Google Scholar] [CrossRef]
- Shen, H.M.; Zhang, L.; Deng, J.H.; Sun, J.; She, Y.B. Enhanced catalytic performance of porphyrin cobalt(II) in the solvent-free oxidation of cycloalkanes (C5~C8) with molecular oxygen promoted by porphyrin zinc(II). Catal. Commun. 2019, 132, 105809. [Google Scholar] [CrossRef]
- Shen, H.M.; Wang, X.; Guo, A.B.; Zhang, L.; She, Y.B. Catalytic oxidation of cycloalkanes by porphyrin cobalt(II) through efficient utilization of oxidation intermediates. J. Porphyr. Phthalocya 2020, 24, 1166–1173. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Fu, B.; Jin, W.D.; Wang, X.; Wang, K.K.; Wang, M.; She, Y.B.; Shen, H.M. Efficient and selective oxygenation of cycloalkanes and alkyl aromatics with oxygen through synergistic catalysis of bimetallic active centers in two-dimensional metal-organic frameworks based on metalloporphyrins. Biomimetics 2023, 8, 325. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Zhang, L.; Xu, Z.M.; Yuan, X.Q. Chemical Reaction Engineering, 2nd ed.; East China University of Science and Technology Press: Shanghai, China, 2007. [Google Scholar]
- Ding, L.H.; Sun, X.L.; Huang, C.P.; Li, J.W.; Chen, B.H. Insights into the mechanism of cumene catalytic oxidation using ionic liquid [Bmim]OH. Mol. Catal. 2023, 538, 113008. [Google Scholar] [CrossRef]
- Lu, Y.H.; Sheng, X.; Zhang, J.; Wang, Y.J.; Du, L.; Zhu, J.Q. Cumene autooxidation to cumene hydroperoxide based on a gas-liquid microdispersion strategy. Chem. Eng. Process. 2022, 174, 108861. [Google Scholar] [CrossRef]
- Deng, J.; Li, Y.H.; Cao, Y.H.; Wang, H.J.; Yu, H.; Zhang, Q.; Zuo, J.L.; Peng, F. Trace amounts of Cu(OAc)2 boost the efficiency of cumene oxidation catalyzed by carbon nanotubes washed with HCl. Catal. Sci. Technol. 2020, 10, 2523–2530. [Google Scholar] [CrossRef]
- Hong, Y.; Fang, Y.X.; Zhou, X.T.; Du, G.; Mai, J.J.; Sun, D.L.; Shao, Z.P. Ionic liquid-modified Co/ZSM-5 catalyzed the aerobic oxidation of cyclohexane: Toward improving the activity and selectivity. Ind. Eng. Chem. Res. 2019, 58, 19832–19838. [Google Scholar] [CrossRef]
- Pamin, K.; Tabor, E.; Gorecka, S.; Kubiak, W.W.; Rutkowska-Zbik, D.; Poltowicz, J. Three generations of cobalt porphyrins as catalysts in the oxidation of cycloalkanes. ChemSusChem 2019, 12, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Dey, A. Electron transfer control of reductase versus monooxygenase: Catalytic C-H bond hydroxylation and alkene epoxidation by molecular oxygen. ACS Central Sci. 2019, 5, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.J.; Xiong, Q.; Jiang, L.; Wang, Y.F.; Tang, Q.Y.; He, J.; Wang, J.Q. Carbon supported copper catalyst prepared in situ by one-pot pyrolysis of Bougainvillea glabra: An efficient and stable catalyst for selective oxidation of cyclohexane. Appl. Surf. Sci. 2022, 576, 151833. [Google Scholar] [CrossRef]
- Gutierrez-Tarrino, S.; Gaona-Miguelez, J.; Ona-Burgos, P. Tailoring the electron density of cobalt oxide clusters to provide highly selective superoxide and peroxide species for aerobic cyclohexane oxidation. Dalton Trans. 2021, 50, 15370–15379. [Google Scholar] [CrossRef]
- Xie, C.J.; Wang, W.; Yang, Y.P.; Jiang, L.; Chen, Y.J.; He, J.; Wang, J.Q. Enhanced stability and activity for solvent-free selective oxidation of cyclohexane over Cu2O/CuO fabricated by facile alkali etching method. Mol. Catal. 2020, 495, 111134. [Google Scholar] [CrossRef]
- Xu, S.N.; Draksharapu, A.; Rasheed, W.; Que, L. Acid pKa dependence in O-O bond heterolysis of a nonheme FeIII-OOH intermediate to form a potent FeV=O oxidant with herne compound I-like reactivity. J. Am. Chem. Soc. 2019, 141, 16093–16107. [Google Scholar] [CrossRef]
- Solomon, E.I.; Brunold, T.C.; Davis, M.I.; Kemsley, J.N.; Lee, S.K.; Lehnert, N.; Neese, F.; Skulan, A.J.; Yang, Y.S.; Zhou, J. Geometric and electronic structure/function correlations in non-heme iron enzymes. Chem. Rev. 2000, 100, 235–349. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Shi, H.T.; Lee, C.S.; Yiu, S.M.; Man, W.L.; Lau, T.C. Room temperature aerobic peroxidation of organic substrates catalyzed by cobalt(III) alkylperoxo complexes. J. Am. Chem. Soc. 2021, 143, 14445–14450. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.M.R.; Pombeiro, A.J.L.; Martins, L.M.D.R.S. Green oxidation of cyclohexane catalyzed by recyclable magnetic transition-metal silica coated nanoparticles. Catal. Commun. 2019, 125, 15–20. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Z.X.; Zou, X.Y.; Zhang, Z.J.; Fu, G.Y.; Li, L.; Zhang, X.; Luo, F. Enhancing catalytic aerobic oxidation performance of cyclohexane via size regulation of mixed-valence {V16} cluster-based metal-organic frameworks. New J. Chem. 2019, 43, 14527–14535. [Google Scholar] [CrossRef]
- Fu, S.J.; You, K.Y.; Ni, W.J.; Chen, Z.P.; Zhao, F.F.; Yan, D.J.; Zhang, X.W.; Luo, H. One-step highly selective catalytic oxidation of cyclohexane to KA-oil over functional CeMn0.5Co0.5Ox composite oxide: Synergistic effects between Mn and Co species with different valences and metal ion ratios. Chem. Eng. Sci. 2023, 277, 118878. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.W.; Wang, R.R.; Zhong, S.; Zhang, R.R.; Liu, R.X. Constructing defective Co3V2O8 hexagonal prism for solvent-free selective oxidation of cyclohexane: Strategy of ionic liquid mediation. Ind. Eng. Chem. Res. 2023, 62, 8754–8766. [Google Scholar] [CrossRef]
- Westrup, K.C.M.; da Silva, R.M.; Mantovani, K.M.; Bach, L.; Stival, J.F.; Zamora, P.G.P.; Wypych, F.; Machado, G.S.; Nakagaki, S. Light-assisted cyclohexane oxidation catalysis by a manganese(III) porphyrin immobilized onto zinc hydroxide salt and zinc oxide obtained by zinc hydroxide salt hydrothermal decomposition. Appl. Catal. A-Gen. 2020, 602, 117708. [Google Scholar] [CrossRef]
- Han, J.; Tan, L.M.; Wan, Y.J.; Li, G.; Anderson, S.N. C(sp3)-H oxidation and chlorination catalysed by a bioinspired pincer iron(III) complex. Dalton Trans. 2022, 51, 11620–11624. [Google Scholar] [PubMed]
- Kuznetsov, M.L.; Pombeiro, A.J.L. Metal-free and iron(II)-assisted oxidation of cyclohexane to adipic acid with ozone: A theoretical mechanistic study. J. Catal. 2021, 399, 52–66. [Google Scholar] [CrossRef]
- Chen, J.; Yao, J.P.; Li, X.X.; Wang, Y.; Song, W.X.; Cho, K.B.; Lee, Y.M.; Nam, W.; Wang, B. Bromoacetic acid-promoted nonheme manganese-catalyzed alkane hydroxylation inspired by α-ketoglutarate-dependent oxygenases. ACS Catal. 2022, 12, 6756–6769. [Google Scholar] [CrossRef]
- Wada, T.; Sugimoto, H.; Morimoto, Y.; Itoh, S. Alkane hydroxylation by m-chloroperbenzoic acid catalyzed by nickel(II) complexes of linear N-4-tetradentate ligands. Polyhedron 2022, 227, 116150. [Google Scholar]
- Sato, Y.; Okamura, M.; Hikichi, S. Heteroleptic and homoleptic Iron(III) complexes with a tris(N-heterocyclic carbene) borate ligand: Synthesis, characterization, and catalytic application. Eur. J. Inorg. Chem. 2023, 26, e202200728. [Google Scholar] [CrossRef]
- Morimoto, Y.; Hanada, S.; Kamada, R.; Fukatsu, A.; Sugimoto, H.; Itoh, S. Hydroxylation of unactivated C(sp3)-H bonds with m-chloroperbenzoic acid catalyzed by an iron(III) complex supported by a trianionic planar tetradentate ligand. Inorg. Chem. 2021, 60, 7641–7649. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.R.; Liu, J.C.; Zheng, J.; Feng, X.Y.; Zong, S.; Liu, L.L.; Fang, Y.X. Z-scheme WO3-Co3O4 heterojunction for the boosted photo-thermo catalytic oxidation of cyclohexane. Mol. Catal. 2023, 542, 113100. [Google Scholar] [CrossRef]
- Han, X.Y.; Liu, Y.F.; Qi, Z.Y.; Zhang, Q.L.; Zhao, P.F.; Wang, L.; Gao, L.F.; Zheng, G.X. Graphene supported CoO nanoparticles as an advanced catalyst for aerobic oxidation of cyclohexane. New J. Chem. 2022, 46, 2792–2797. [Google Scholar] [CrossRef]
- Qiu, Y.; Wu, C.C.; Gates, I.D.; Yang, Y.X.; Guo, S.H.; Men, Z.W.; Lou, B.; Yang, X.J.; Shi, N.; Wen, F.S.; et al. Isolated Co-Ti-Y trimetallic synergistic catalysis based on apparent anti-electronegative polarization. Adv. Funct. Mater. 2022, 32, 2207482. [Google Scholar] [CrossRef]
- Fu, S.J.; You, K.Y.; Chen, Z.P.; Liu, T.B.; Wang, Q.; Zhao, F.F.; Ai, Q.H.; Liu, P.L.; Luo, H.A. Ultrasound-assisted co-precipitation synthesis of mesoporous Co3O4-CeO2 composite oxides for highly selective catalytic oxidation of cyclohexane. Front. Chem. Sci. Eng. 2022, 16, 1211–1223. [Google Scholar] [CrossRef]
- Zhang, W.; Xue, H.N.; Wang, M.; Wu, Z.Y.; Ding, L.L.; Deng, Y.Y.; Wang, W.; Wei, X.J.; Zhang, Z.W.; Xiang, M. Direct synthesis of Cu-containing beta zeolite without template for selective oxidation of cyclohexane. J. Porous Mat. 2022, 29, 371–380. [Google Scholar] [CrossRef]
- Tan, M.Y.; Zhu, L.; Liu, H.; Fu, Y.J.; Yin, S.F.; Yang, W.J. Microporous cobaltporphyrin covalent polymer mediated Co3O4@PNC nanocomposites for efficient catalytic C-H bond activation. Appl. Catal. A-Gen. 2021, 614, 118035. [Google Scholar] [CrossRef]
- Wang, L.Y.; Zhang, Y.B.; Yuan, H.R.; Du, R.F.; Yao, J.; Li, H.R. Selective aerobic oxidation of secondary C (sp3)-H bonds with NHPI/CAN catalytic system. Catal. Lett. 2021, 151, 1663–1669. [Google Scholar] [CrossRef]
- Reisi, B.; Chermahini, A.N.; Rodriguez-Padron, D.; Munoz-Batista, M.J.; Luque, R. Synthesis and characterization of Pd-Ni catalysts supported on KIT-6 and their application in cyclohexane oxidation using molecular oxygen. J. Ind. Eng. Chem. 2021, 102, 103–111. [Google Scholar] [CrossRef]
- Hao, F.; Sun, Y.; Wang, Y.D.; Lv, Y.; Liu, P.L.; Xiong, W.; Luo, H.A. Nitrogen-doped graphene loaded non-noble Co catalysts for liquid-phase cyclohexane oxidation with molecular oxygen. Korean J. Chem. Eng. 2021, 38, 1608–1616. [Google Scholar] [CrossRef]







 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Temperature (°C) | Conversion (%) | Selectivity (%) | |||||
| C6-OOH | C6-OH | C6=O | C4-(COOH)2 | C3-(COOH)2 | Total b | |||
| 1 c | 110 | <0.20 | N. D. d | N. D. | N. D. | N. D. | N. D. | N. D. |
| 2 c | 115 | <0.20 | N. D. | N. D. | N. D. | N. D. | N. D. | N. D. |
| 3 c | 120 | <0.20 | N. D. | N. D. | N. D. | N. D. | N. D. | N. D. |
| 4 c | 125 | 0.22 | 86.4 | N. D. | 13.6 | N. D. | N. D. | >99 |
| 5 c | 130 | 0.44 | 57.8 | 31.0 | 11.2 | N. D. | N. D. | >99 |
| 6 e | 120 | 4.25 | 7.0 | 33.6 | 44.6 | 10.1 | 4.7 | 85.2 |
| 7 f | 120 | 0.53 | 84.6 | N. D. | 15.4 | N. D. | N. D. | >99 |
| 8 g | 120 | 3.87 | 7.4 | 31.9 | 45.5 | 13.3 | 1.9 | 84.8 |
| 9 h | 120 | 0.46 | 96.4 | N. D. | 3.6 | N. D. | N. D. | >99 |
| 10 i | 120 | 2.22 | 39.9 | 25.6 | 26.1 | 6.9 | 1.5 | 91.6 |
| 11 j | 120 | 0.42 | 79.5 | 0.8 | 19.7 | N. D. | N. D. | >99 |
| 12 k | 120 | 2.41 | 36.5 | 35.6 | 24.1 | 3.0 | 0.8 | 96.2 |
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Metal Centers | Conversion (%) | Selectivity (%) | |||||
| C6-OOH | C6-OH | C6=O | C4-(COOH)2 | C3-(COOH)2 | Total b | |||
| 1 | / | <0.20 | N. D. c | N. D. | N. D. | N. D. | N. D. | N. D. |
| 2 | Si@NH2 | 0.47 | 84.3 | N. D. | 15.7 | N. D. | N. D. | >99 |
| 3 | Co | 2.22 | 39.9 | 25.6 | 26.1 | 6.9 | 1.5 | 91.6 |
| 4 | Cu | 0.42 | 79.5 | 0.8 | 19.7 | N. D. | N. D. | >99 |
| 5 | Zn | 0.63 | 78.1 | 1.3 | 20.6 | N. D. | N. D. | >99 |
| 6 | Co and Cu | 2.41 | 36.5 | 35.6 | 24.1 | 3.0 | 0.8 | 96.2 |
| 7 | Co and Zn | 2.31 | 43.1 | 32.7 | 19.7 | 3.9 | 0.6 | 95.5 |
| 8 | Co and Ni | 1.44 | 79.4 | 0.2 | 17.5 | 2.8 | 0.1 | 97.1 |
| 9 | Cu and Zn | 0.39 | 78.8 | N. D. | 18.6 | 2.6 | N. D. | 97.4 |
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Substituent R1 | Conversion (%) | Selectivity (%) | |||||
| C6-OOH | C6-OH | C6=O | C4-(COOH)2 | C3-(COOH)2 | Total b | |||
| 1 |  | 2.20 | 27.7 | 38.2 | 29.0 | 4.0 | 1.1 | 94.9 |
| 2 |  | 1.93 | 44.9 | 30.6 | 20.3 | 2.4 | 1.8 | 95.8 |
| 3 |  | 2.17 | 40.8 | 37.6 | 17.1 | 3.6 | 0.9 | 95.5 |
| 4 | 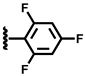 | 2.11 | 48.1 | 30.0 | 19.7 | 2.1 | 0.1 | 97.8 |
| 5 | 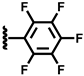 | 2.41 | 36.5 | 35.6 | 24.1 | 3.0 | 0.8 | 96.2 |
| 6 c |  | 4.89 | 15.5 | 38.9 | 34.6 | 7.7 | 3.3 | 89.0 |
| 7 d |  | 5.03 | 17.7 | 33.6 | 38.0 | 8.5 | 2.2 | 89.3 |
| 8 | 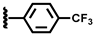 | 2.30 | 35.2 | 32.5 | 27.5 | 3.1 | 1.7 | 95.2 |
| 9 | 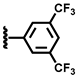 | 2.40 | 42.3 | 34.2 | 19.3 | 3.3 | 0.9 | 95.8 |
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Catalyst | Conversion (%) | Selectivity (%) | |||||
| C6-OOH | C6-OH | C6=O | C4-(COOH)2 | C3-(COOH)2 | Total b | |||
| 1 | Si@NH2 | 0.47 | 84.3 | N.D. c | 15.7 | N. D. | N. D. | >99 |
| 2 | Tris(perF)P(4-CH2Cl)PPCo | 3.87 | 7.4 | 31.9 | 45.5 | 13.3 | 1.9 | 84.8 |
| 3 | Tris(perF)P(4-CH2Cl)PPCu | 0.46 | 96.4 | N. D. | 3.6 | N. D. | N. D. | >99 |
| 4 d | Tris(perF)P(4-CH2Cl)PPCo& Tris(perF)P(4-CH2Cl)PPCu | 4.31 | 7.2 | 33.6 | 45.2 | 11.8 | 2.2 | 86.0 |
| 5 | Si@Porp. Co | 4.47 | 29.3 | 27.0 | 29.4 | 10.0 | 4.3 | 85.7 |
| 6 | Si@Porp. Cu | 0.66 | 76.3 | N. D. | 23.7 | N. D. | N. D. | >99 |
| 7 e | Si@Porp. Co& Si@Porp. Cu | 4.45 | 31.1 | 21.0 | 34.7 | 10.9 | 2.3 | 86.8 |
| 8 | Si@Porp. Co&Cu | 5.03 | 17.7 | 33.6 | 38.0 | 8.5 | 2.2 | 89.3 |
 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Conversion of C6H11OOH (%) | Conversion of C5H10 (×10−2%) | Selectivity (%) | |
| C5H9OH | C5H8O | ||||
| 1 | / | N. D. b | N. D. | N. D. | N. D. |
| 2 | Tris(perF)P(4-CH2Cl)PPCo | 38.8 | 1.33 | 73.9 | 26.1 |
| 3 | Tris(perF)P(4-CH2Cl)PPCu | 40.3 | 1.89 | 85.9 | 14.1 |
| 4 | Tris(perF)P(4-CH2Cl)PPZn | 30.1 | 1.36 | 80.3 | 19.7 |
| 5 c | Tris(perF)P(4-CH2Cl)PPCo& Tris(perF)P(4-CH2Cl)PPCu | 49.9 | 2.04 | 72.7 | 27.3 |
| 6 c | Tris(perF)P(4-CH2Cl)PPCo& Tris(perF)P(4-CH2Cl)PPZn | 45.3 | 1.82 | 75.7 | 24.3 |
| 7 | Si@Porp. Co | 40.7 | 1.77 | 79.9 | 20.1 |
| 8 | Si@Porp. Cu | 45.1 | 1.16 | 79.6 | 20.4 |
| 9 | Si@Porp. Zn | 29.4 | 0.88 | 80.1 | 19.9 |
| 10 | Si@Porp. Co&Cu | 56.8 | 2.29 | 74.9 | 25.1 |
| 11 | Si@Porp. Co&Zn | 48.3 | 2.07 | 77.8 | 22.2 |
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Catalyst Amount (%, g/mol) | Conversion (%) | Selectivity (%) | |||||
| C6-OOH | C6-OH | C6=O | C4-(COOH)2 | C3-(COOH)2 | Total b | |||
| 1 | 0.10 | 5.03 | 17.7 | 33.6 | 38.0 | 8.5 | 2.2 | 89.3 |
| 2 | 0.12 | 5.10 | 16.0 | 42.3 | 31.7 | 9.2 | 0.8 | 90.0 |
| 3 | 0.14 | 5.13 | 22.9 | 33.4 | 37.0 | 5.6 | 1.1 | 93.3 |
| 4 | 0.16 | 5.27 | 15.8 | 41.9 | 34.6 | 6.2 | 1.5 | 92.3 |
| 5 | 0.18 | 4.93 | 17.5 | 43.5 | 29.0 | 7.6 | 2.4 | 90.0 |
| 6 | 0.20 | 4.73 | 24.6 | 33.4 | 32.2 | 8.6 | 1.2 | 90.2 |
| 7 | 0.22 | 4.70 | 15.6 | 24.0 | 49.4 | 7.4 | 3.6 | 89.0 |
| Entry | Pressure (MPa) | Conversion (%) | Selectivity (%) | |||||
|---|---|---|---|---|---|---|---|---|
| C6-OOH | C6-OH | C6=O | C4-(COOH)2 | C3-(COOH)2 | Total b | |||
| 1 | 0.4 | 1.52 | 23.5 | 44.6 | 28.0 | 3.3 | 0.6 | 96.1 |
| 2 | 0.6 | 2.63 | 30.4 | 33.1 | 31.7 | 4.1 | 0.7 | 95.2 |
| 3 | 0.8 | 4.69 | 10.8 | 46.4 | 37.9 | 4.2 | 0.7 | 95.1 |
| 4 | 1.0 | 5.27 | 15.8 | 41.9 | 34.6 | 6.2 | 1.5 | 92.3 |
| 5 | 1.2 | 4.96 | 11.1 | 41.5 | 37.6 | 8.3 | 1.5 | 90.2 |
| Entry | Reaction Time (h) | Conversion (%) | Selectivity (%) | |||||
|---|---|---|---|---|---|---|---|---|
| C6-OOH | C6-OH | C6=O | C4-(COOH)2 | C3-(COOH)2 | Total b | |||
| 1 | 4.0 | 1.77 | 50.8 | 24.1 | 21.3 | 2.4 | 1.4 | 96.2 |
| 2 | 6.0 | 3.12 | 20.3 | 12.7 | 62.5 | 3.5 | 1.0 | 95.5 |
| 3 | 8.0 | 5.27 | 15.8 | 41.9 | 34.6 | 6.2 | 1.5 | 92.3 |
| 4 | 10.0 | 5.40 | 14.4 | 32.7 | 38.4 | 10.6 | 3.9 | 85.5 |
| 5 | 12.0 | 5.77 | 21.9 | 18.1 | 41.2 | 13.4 | 5.4 | 81.2 |
| Entry | Catalysts | Temperature (°C) | k (mol·L−1·h−1) | R2 | Ea (kJ/mol) |
|---|---|---|---|---|---|
| 1 | / | 130 | 0.0095 | 0.9919 | 162.4 |
| 2 | 135 | 0.0174 | 0.9894 | ||
| 3 | 140 | 0.0307 | 0.9992 | ||
| 4 | Si@Porp. Co | 110 | 0.0377 | 0.9901 | 79.1 |
| 5 | 115 | 0.0502 | 0.9946 | ||
| 6 | 120 | 0.0709 | 0.9872 | ||
| 7 | Si@Porp. Co&Cu | 110 | 0.0402 | 0.9870 | 71.8 |
| 8 | 115 | 0.0549 | 0.9991 | ||
| 9 | 120 | 0.0713 | 0.9949 |
| Entry | Substrate | Conversion (%) | Selectivity (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Cx-OOH | Cx-OH | Cx=O | Cx-2-(COOH)2 | Cx-3-(COOH)2 | Total b | |||
| 1 |  | 3.57 | 52.6 | N. D. c | 43.3 | 4.1 | N.D. | 95.9 |
| 2 |  | 5.27 | 15.8 | 41.9 | 34.6 | 6.2 | 1.5 | 92.3 |
| 3 |  | 10.9 | 35.1 | 15.7 | 42.3 | 4.4 | 2.5 | 93.1 |
| 4 | 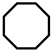 | 25.2 | 24.2 | 18.3 | 52.3 | 3.7 | 1.5 | 94.8 |
| 5 d | 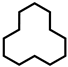 | 29.4 | 27.6 | 15.9 | 56.5 | N. D. | N. D. | >99 |
| Entry | Alkyl Aromatics | Products | Conversion (%) | Selectivity (%) |
|---|---|---|---|---|
| 1 a | 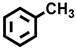 | 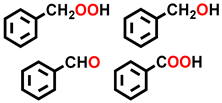 | 25.6 | 30.2, 5.6, 23.7, 40.5 |
| 2 a |  | 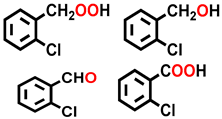 | 12.9 | 36.4, 6.4, 15.3, 41.9 |
| 3 a | 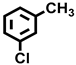 | 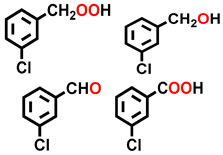 | 20.3 | 37.3, 11.4, 19.7, 31.6 |
| 4 a | 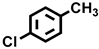 | 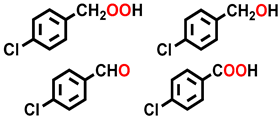 | 39.0 | 27.2, 8.3, 25.5, 39.0 |
| 5 a | 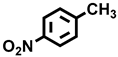 | 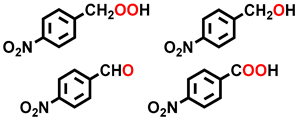 | 21.2 | 29.4, 9.9, 20.1, 40.6 |
| 6 a | 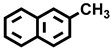 |  | 37.1 | 32.6, 6.0, 18.6, 42.8 |
| 7 b | 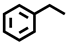 | 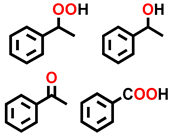 | 35.9 | 9.4, 29.6, 55.6, 5.4 |
| 8 c | 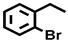 | 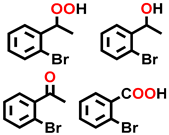 | 26.7 | 7.8, 30.0, 51.9, 10.3 |
| 9 c |  | 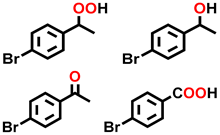 | 45.1 | 6.0, 6.3, 85.3, 2.4 |
| 10 c |  | 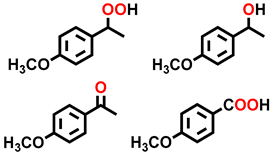 | 30.2 | 5.3, 20.2, 72.6, 1.9 |
| 11 c | 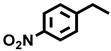 |  | 40.2 | 8.8, 8.9, 77.6, 4.7 |
| 12 b | 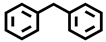 | 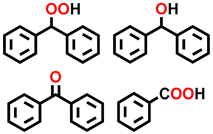 | 25.7 | 6.8, 12.9, 76.3, 4.0 |
| 13 c |  | 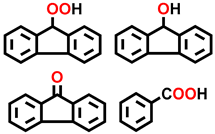 | 36.2 | 3.3, 4.7, 89.0, 3.0 |
| 14 d |  | 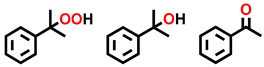 | 43.3 | 87.1, 3.4, 9.5 |
| 15 d | 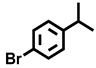 | 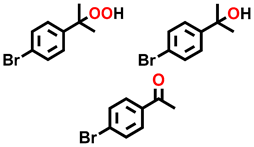 | 37.8 | 85.1, 6.6, 8.3 |
| 16 d | 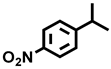 | 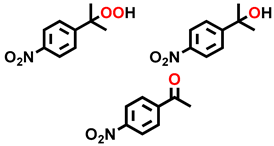 | 22.2 | 94.6, 1.5, 3.9 |
| Entry | Quenching Reagents | Conversion (%) | Selectivity (%) | |||||
|---|---|---|---|---|---|---|---|---|
| C6-OOH | C6-OH | C6=O | C4-(COOH)2 | C3-(COOH)2 | Total b | |||
| 1 | / | 5.27 | 15.8 | 41.9 | 34.6 | 6.2 | 1.5 | 92.3 |
| 2 | CBrCl3 | 1.25 | 94.8 | N. D. c | 5.2 | N. D. | N. D. | >99 |
| 3 | t-BuBr | 1.08 | 38.7 | N. D. | 61.3 | N. D. | N. D. | >99 |
| 4 | Ph2NH | 0.17 | 29.7 | 30.3 | 40.0 | N. D. | N. D. | >99 |
| Entry | Reaction Conditions | Quantitative Methods | Conversion (%) | Selectivity a (%) | Reference and Year |
|---|---|---|---|---|---|
| 1 | Atomically-dispersed Co on graphitic carbon nitrogen as catalyst, solvent-free, 130 °C, 0.80 MPa O2, 6.0 h | GC + Chemical titration | 9.1 | 62 | [6] 2023 |
| 2 | Co8LaOx/WO3 Z-scheme nanocomposites as catalyst, visible light, solvent-free, 120 °C, 1.50 MPa O2, 3.0 h | GC + Chemical titration | 10.4 | 81 | [20] 2023 |
| 3 | CeMn0.5Co0.5Ox composite oxide as catalyst, solvent-free, 150 °C, 0.50 MPa O2, 2.0 h | GC + Chemical titration | 4.6 | 93 | [66] 2023 |
| 4 | Ionic liquid modified defective Co3V2O8 as catalyst, solvent-free, 150 °C, 1.00 MPa O2, 2.0 h | GC + Derivatization GC | 12.7 | 93 | [67] 2023 |
| 5 | BaCO3-CeO2 composite as catalyst, solvent-free, 150 °C, 1.00 MPa O2, 2.0 h | GC + Derivatization GC | 10.5 | 85 | [10] 2023 |
| 6 | Dimetallic MOF PCN-224(Co&Cu) as catalyst, solvent-free, 130 °C, 1.00 MPa O2, 4.0 h | GC + HPLC | 5.7 | 88 | [14] 2023 |
| 7 | Z-scheme WO3-Co3O4 as catalyst, 300 W Xe light, acetone as solvent, 120 °C, 1.50 MPa air, 8.0 h | GC | 11.5 | 99 | [75] 2023 |
| 8 | Graphene supported CoO as catalyst, NHPI as co-catalyst, acetone as solvent, 120 °C, 0.40 MPa O2, 6.0 h | GC | 32.4 | 75 | [76] 2022 |
| 9 | Co-Ti-Y trimetals on APO-5 as catalyst, BuOOH as co-catalyst, 120 °C, 3.00 MPa O2, 5.0 h | GC-MS | 41.7 | 65 | [77] 2022 |
| 10 | Dimetallic MOF Co-TCPPCu as catalyst, solvent-free, 120 °C, 1.00 MPa O2, 8.0 h | GC + HPLC | 5.3 | 95 | [15] 2022 |
| 11 | Dimetallic MOF PCN-224(Co&Ni) as catalyst, solvent-free, 130 °C, 0.80 MPa O2, 8.0 h | GC + HPLC | 7.3 | 85 | [9] 2022 |
| 12 | Mesoporous Co3O4-CeO2 as catalyst, solvent-free, 150 °C, 0.60 MPa O2, 1.5 h | GC + Chemical titration | 5.8 | 90 | [78] 2022 |
| 13 | Ce-Fe oxides derived from Prussian blue analogues as catalyst, solvent-free, 150 °C, 1.00 MPa O2, 3.0 h | GC + Derivatization GC | 9.3 | 75 | [21] 2022 |
| 14 | CoCl2 in ionic liquid nanoreactor as catalyst, solvent-free, 130 °C, 1.50 MPa O2, 1.0 h | GC + Derivatization GC | 7.8 | 74 | [7] 2022 |
| 15 | MoO3-x-Ag20Pd20 as catalyst, 300 W Xe light, acetone as solvent, 120 °C, 1.50 MPa air, 8.0 h | GC | 11.3 | 99 | [12] 2022 |
| 16 | Cu-containing beta zeolite as catalyst, solvent-free, 140 °C, 1.00 MPa O2, 2.0 h | GC | 9.7 | 94 | [79] 2022 |
| 17 | T(4-COOCH3)PPCo&Zn(AcO)2 as catalyst, solvent-free, 120 °C, 1.00 MPa O2, 8.0 h | GC + HPLC | 6.5 | 96 | [26] 2021 |
| 18 | Co3O4 in nitrogen-carbon as catalyst, acetonitrile as solvent, 110 °C, 1.00 MPa O2, 6.0 h | GC | 16.5 | 91 | [80] 2021 |
| 19 | Cerium ammonium nitrate, NHPI as catalysts, dichloroethane as solvent, 45 °C, 0.10 MPa O2, 24.0 h | GC | 9.0 | 79 | [81] 2021 |
| 20 | Pd-Ni supported on KIT-6 as catalyst, acetonitrile as solvent, 140 °C, 1.00 MPa O2, 8.0 h | GC | 10.8 | 95 | [82] 2021 |
| 21 | Co on nitrogen doped graphene as catalyst, solvent-free, 155 °C, 0.80 MPa O2, 1.5 h | GC + Chemical titration | 8.9 | 86 | [83] 2021 |
| 22 | Si@Porp. Co&Cu as catalyst, solvent-free, 120 °C, 1.00 MPa O2, 8.0 h | GC + HPLC | 5.3 | 92 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Feng, X.-L.; Ni, J.-Y.; Fu, B.; Shen, H.-M.; She, Y.-B. Efficient Inhibition of Deep Conversion of Partial Oxidation Products in C-H Bonds’ Functionalization Utilizing O2 via Relay Catalysis of Dual Metalloporphyrins on Surface of Hybrid Silica Possessing Capacity for Product Exclusion. Biomimetics 2024, 9, 272. https://doi.org/10.3390/biomimetics9050272
Zhang Y, Feng X-L, Ni J-Y, Fu B, Shen H-M, She Y-B. Efficient Inhibition of Deep Conversion of Partial Oxidation Products in C-H Bonds’ Functionalization Utilizing O2 via Relay Catalysis of Dual Metalloporphyrins on Surface of Hybrid Silica Possessing Capacity for Product Exclusion. Biomimetics. 2024; 9(5):272. https://doi.org/10.3390/biomimetics9050272
Chicago/Turabian StyleZhang, Yu, Xiao-Ling Feng, Jia-Ye Ni, Bo Fu, Hai-Min Shen, and Yuan-Bin She. 2024. "Efficient Inhibition of Deep Conversion of Partial Oxidation Products in C-H Bonds’ Functionalization Utilizing O2 via Relay Catalysis of Dual Metalloporphyrins on Surface of Hybrid Silica Possessing Capacity for Product Exclusion" Biomimetics 9, no. 5: 272. https://doi.org/10.3390/biomimetics9050272
APA StyleZhang, Y., Feng, X.-L., Ni, J.-Y., Fu, B., Shen, H.-M., & She, Y.-B. (2024). Efficient Inhibition of Deep Conversion of Partial Oxidation Products in C-H Bonds’ Functionalization Utilizing O2 via Relay Catalysis of Dual Metalloporphyrins on Surface of Hybrid Silica Possessing Capacity for Product Exclusion. Biomimetics, 9(5), 272. https://doi.org/10.3390/biomimetics9050272






