Abstract
The rehabilitation of alveolar bone defects of moderate to severe size is often challenging. Currently, the therapeutic approaches used include, among others, the guided bone regeneration technique combined with various bone grafts. Although these techniques are widely applied, several limitations and complications have been reported such as morbidity, suboptimal graft/membrane resorption rate, low structural integrity, and dimensional stability. Thus, the development of biomimetic scaffolds with tailor-made characteristics that can modulate cell and tissue interaction may be a promising tool. This article presents a critical consideration in scaffold’s design and development while also providing information on various fabrication methods of these nanosystems. Their utilization as delivery systems will also be mentioned.
1. Introduction
One of the most significant areas of the human body, in terms of function and aesthetic, is the oral and maxillofacial region. Due to the anatomical complexity and the tissue variability, the restoration of alveolar and maxillofacial bone defects occurring from inflammation, periodontal disease, neoplastic pathology, or trauma is challenging to achieve [1,2]. Bone has a limited healing capacity which is inadequate to regenerate larger size defects [3,4]. Furthermore, the use of titanium dental implants is considered a predictable treatment option for partial and full edentulism, provided that there is an adequate bone amount at the recipient site for the successful placement of implants in the prosthodontically-driven ideal position [5]. Subsequently, the augmentation of the defected sites followed by the restoration with dental implants requires the advancement of bone tissue engineering (BTE). BTE is a rapidly growing field, which develops biofunctional tissues that can substitute the diseased or damaged ones [6]. Guided tissue regeneration (GTR) is a principle introduced in the mid-1980s. According to this principle, it is possible to achieve the regeneration of a certain type of tissue, when the defect is populated with cells capable of regenerating this particular type of lost tissue during the healing phase. Based on this principle, the guided bone regeneration (GBR) concept was developed [7,8,9]. Dahlin et al. (1988) introduced GBR as a therapeutic modality to achieve bone regeneration [10]. This concept utilizes barrier membranes (resorbable/non-resorbable) to prevent the ingrowth of certain cell types, including rapidly proliferating epithelium and connective tissue, hence promoting the growth of slower-growing cells that are responsible for bone formation [9,11,12,13,14]. In many instances, GBR is combined with bone grafting procedures/materials. Currently, the transplantation of autogenous bone from an intra-oral or extra-oral donor site is considered the gold standard method due to the low immunogenicity and disease transmission risk [15]. Even though anatomical areas, including the mandibular symphysis and maxillary tuberosity, may provide excellent autologous bone grafting sites, the harvesting capacity is limited, the risk of donor site morbidity and wound infection is high, and the surgical time is drastically prolonged, resulting in the patient’s discomfort [15,16,17]. Through this development, alternative sources of bone grafts have been explored [18]. Allografts originate from the same intraspecies, while xenografts are of bovine or porcine origin. Concerns have been reported that these alternatives have certain drawbacks, including pathogen transmission and immune rejection [15,19]. Another category of bone grafts is synthetic alloplasts, which are fabricated from ceramics, polymers, and metals [4,20]. Even though they are able to withstand increased mechanical load and stress, their utilization is limited. Additionally, their limited integration with the host’s tissue at the defect site, and the considerable risk of infection or failure due to fatigue during implantation, has been reported [15,19,21].
Due to the beforementioned drawbacks of bone grafting materials and the necessity to reconstruct the alveolar bone defects, novel approaches have been investigated. BTE and regenerative medicine (RM) have developed a new concept of utilizing scaffolding nanosystems either alone or combined with growth factors and cell or gene delivery. This concept, termed tissue engineered construct (TEC), may enhance bone repair and regeneration [15,19,20,21,22,23]. The development of a functional TEC from BTE/RM requires: (a) the presence of appropriate cells, (b) a scaffolding material that supports cell growth into an organized tissue, (c) the use of biological factors to promote cellular activity and the formation of bone tissue, and (d) the vascularization of the TEC, which will provide nutritional and oxygen supply for the implanted cells as well as eliminating catabolic end products [24,25]. Additionally, scaffolding materials can be utilized as drug delivery systems, to promote tissue healing and enhance the therapeutic effect through the release of therapeutic agents [26,27,28]. The release of antibacterial agents incorporated inside these scaffolds may suppress bacterial growth and inhibit postoperative infections which are critical in oral and maxillofacial surgery [12]. Thus, Donos et al. (2023) suggested that the mechanical and antibacterial properties of GBR scaffolds should be further explored [12].
According to Walmsley et al. (2015), conventional scaffolding nanomaterials possess poor physicochemical properties and mechanical strength, and low cellular differentiation, while being unable to synthesize the necessary extrinsic factors to positively influence osteogenesis. Several authors reported that the combined action of scaffolds with cells and growth factors may not regenerate adequately the bone defect [25]. This statement is based on the inability to control the degradation of the matrix and the delivery of drug and biological growth factors [24,29,30].
In addition to these biomaterials, the utilization of clinical strategies such as GBR has improved the clinical outcomes of those cases [31]. GBR has become the standard clinical approach technique to restore bone defects, promote bone regeneration, and augment alveolar ridge volume in the oral and maxillofacial region [9]. This approach is frequently used in dental implantology to ensure the long-term prognosis of osseo-integrated implants [2,32]. A variety of materials are utilized in those approaches including bone substitutes and membrane barriers. Membranes are used to selectively promote the adhesion, migration, and proliferation of osteoblasts, while excluding the infiltration of other rapidly proliferating connective and epithelial tissue which would arrest osteogenesis [2,32,33,34]. Bone regeneration is a complex process with various critical factors affecting its initiation such as a source of cells, a scaffold that facilitates bone matrix deposition, signaling molecules, mechanical stability and adequate blood supply [12].
Conventional/monophasic scaffolds were determined to be inadequate to mimic the complex morphology of bone. Thus, the development of multiphasic scaffolds with distinguishable compartments, different biomechanical composition, and tailored architecture that simulates the desired tissue characteristics, may be a promising tool to achieve bone regeneration [6,35]. These multifunctional scaffolds are osteoconductive, can act as barriers, release bioactive substances, and consequently promote bone regeneration, mineralization, and clinical bone repair [36,37]. The progression of technology will eventually address the current limitations in biomaterial fabrication, image acquisition, model development, and design. The advances in image acquisition may improve spatial resolution and accuracy, thus an accurate representation model of the native bone can be developed. The utilization of novel fabrication techniques and the optimization of methodology to control pore shape and size will result in advanced 3D scaffolds development. These systems may possess improved properties, complex architecture, drug/molecular loading capacity, and the ability to direct bone regeneration and healing [6,38,39].
This review aims to provide information on the scaffolding systems used to treat alveolar bone defects. Additionally, a variety of manufacturing methods to produce these systems will be described and their role in GBR and dental implant placement will be presented.
2. Methodology
This literature review was conducted through different official databases, including PubMed, Google Scholar, Elsevier, and ScienceDirect, to identify the relevant publications according to the topic. Various keywords have been used in those search engines such as “scaffolds”, “nanomaterials”, “alveolar bone defect”, “tissue engineering”, “regenerative medicine”, “alveolar bone regeneration”, “guided bone regeneration”, “dental implants” and combinations of those terms. No limitation regarding an article’s publication date was set, but articles published during the last 6 years were preferred.
3. Guided Bone Regeneration Technique and Its Role in Alveolar Bone Defects
Guided bone regeneration is a frequently applied and predictable technique to restore bone defects in the maxillofacial region. Depending on the defect’s morphology and severity of bone loss, concepts such as vertical and horizontal bone regeneration have been widely explored [40,41,42]. These two aspects strongly influence the type, extent, and prognosis of the rehabilitation procedure [43]. A variety of materials are utilized in those approaches to promote the adhesion, migration, and proliferation of osteoblasts [2]. Additionally, polymeric nanomaterials are used as a physical barrier to prevent the ingrowth of rapidly proliferating connective and epithelial tissue at the defect site, thus promoting bone regeneration [34]. The utilization of various grafting materials, including autografts, allografts, and xenografts, has become a common practice for clinicians.
A pivotal clinical approach to address dentition and bone defects is the combination of GBR with dental implants. Frequently, the insufficient alveolar bone volume occurring from local factors including periodontitis, trauma, and localized alveolar process resorption, may be challenging to restore [44]. To overcome this issue, various methods have been employed such as GBR combined with bone grafts and barrier membranes [45]. While autogenous bone grafting may have a limited capacity to restore larger bone defects, the utilization of allografts and xenografts could overcome the challenges in bone augmentation [46]. The barrier membranes’ role is to form a protective layer at the defect site, hindering the ingrowth of proliferating connecting and fibrous tissue. Moreover, they create a microenvironment that would enhance alveolar bone regeneration and provide the necessary bone volume for dental implants [47]. Alternatively, GBR combined with barrier membranes and bone grafts could be used to regenerate bone defects around dental implants (e.g., dehiscence or fenestration defects) [48]. Additionally, several authors have reported that the utilization of membranes are carriers for various growth factors such as bone morphogenetic protein-2 (BMP-2), insulin-like growth factor (IGF), and other factors that promote bone regeneration and development [49,50]. Membranes also provide blood clot stability, nutrient and oxygen transportation, and establishes microcirculation of the treated defect. Omar et al. (2019) stated that membranes are not only hosting but modulating the membrane-associated cellular activities and processes [50]. Thus, choosing the appropriate GBR membrane may have a substantial impact on the therapeutic outcome [44]. The healing of a critical-sized bone defected through GBR without the use of barrier membranes requires scaffolds that are able to support space maintenance, promote bone development, and inhibit fibrous soft tissue ingrowth. Wang et al. (2022) suggested the utilization of osteoconductive bone substitutes, collagen, and poly(lactic-co-glycolic acid) (PLGA), among other materials [2].
3.1. Biomaterials for Bone Regeneration
Bone grafting materials are frequently used by clinicians in larger defects to overcome bone’s self-healing limitations. According to the literature, the ideal bone grafting material should possess various key properties including [51,52,53,54]:
- Biocompatibility, a critical property that prevents an inflammatory response;
- Controlled biodegradability;
- Adequate pore size, minimum requirement is 100 μm, but larger than 300 μm is the optimal for vascularization and bone formation;
- Interconnected porosity, which allows the diffusion bone cells, nutrients and waste products;
- Appropriate surface, that allows cell attachment, migration and proliferation while promoting vascular ingrowth;
- Tolerable elasticity and mechanical compressive strength, supporting the adjacent tissue load.
Autografts have been considered the gold standard grafting material for bone repair due to their histocompatibility and non-immunogenic nature. Additionally, they have been characterized as osteo-inductive and osteoconductive, while simultaneously promoting osteogenesis [55]. Despite these benefits, concerns have been made regarding donor site injury, morbidity, and scarring. A patient’s autologous bone can be harvested from a healthy site, which increases the risk of bleeding, inflammation, pain, and infection at the operated sites [54].
Allografts are bone grafting materials harvested from individuals of the same species. Their benefits include the histocompatible nature and the availability in different forms, depending on surgical site requirements [56]. Compared to autografts, concerns have been reported regarding the increased risk of infection transmission and immunoreaction and the high long-term failure rate [54,57,58].
Xenografts are bone grafting materials harvested across different species. Concerns have been reported for disease transmission, increased immune response of the host, reduced osteoinductive properties, and the absence of viable cells [59,60]. Even though xenografts have been used in various regenerative approaches with positive clinical outcomes, a higher patient reactivity compared to the other bone grafts have been documented [61].
Alloplasts are synthetic grafting materials developed to overcome the disadvantages of the beforementioned categories, among others, limitations in bone harvesting capacity, immunological reactions, infection transmission, and long-term failure. Various products have been developed such as hydroxyapatite (HA), biphasic calcium sulfate (BCS), β-tricalcium phosphate (β-TCP), and biphasic calcium phosphate (BCP) [62]. These materials are biocompatible and osteoconductive with low production costs [34]. It has been observed that β-TCP has a similar degradation rate compared to new bone formation while providing the in-growth of vascular and cellular components [63,64]. Different research groups reported that the combination of BCS with β-TCP may offer enhanced healing while HA is similar to the inorganic bone matrix [65,66]. β-TCP has been coated with poly(lactic-co-glycolic acid) (PLGA) on alveolar ridge preservation, maintaining the necessary alveolar dimensions and stability requirements for dental implant placement [67].
3.2. Membranes (Resorbable/Non-Resorbable)
Guided bone regeneration membranes have been widely used in the field of bone regeneration to isolate alveolar bone defects with a resorbable or non-resorbable mat-like material. The membrane’s role is to function as a physical barrier obstructing gingival cell invasion and proliferating connective tissue ingrowth. These materials should possess a variety of characteristics to positively influence bone regeneration including [68,69,70]:
- Biocompatibility, to integrate with host’s tissues without initiating an inflammatory response;
- Biodegradability, with an appropriate degradation profile according to the host’s tissue;
- Biological activity;
- Competent physical and mechanical properties;
- Porosity and occlusive properties;
- Tolerable strength to withstand the forces of adjacent tissues, preventing membranes collapse;
- Exposure tolerance.
The “gold standard” for non-resorbable barrier membranes are the high-density polytetrafluoroethylene (n-PTFE) and the titanium-reinforced version of high-density PTFE (tn-PTFE). These membranes have been used in GBR and/or GTR procedures due to their exceptional properties, among which is the exclusion of the undesired cells to interfere in the bone healing process, thus facilitating bone regeneration. Due to their non-biodegradable nature, an additional surgery is required for their removal, resulting in patients’ discomfort and pain [71]. Consequently, a variety of natural, synthetic, and composite materials have been developed to replace the non-resorbable membranes with degradable ones [72]. To create a resorbable product, distinctive material-processing techniques based on solvent casting and melting have been utilized to create polymer-based membranes. Additionally, solvent casting/particulate leaching and phase inversion were utilized to create pores on both membranes and 3D scaffolds [71]. Lastly, electrospinning is a promising technique for processing membranes to synthesize biomimetic nanomatrices. Another category of resorbable membranes is synthetic membranes. They are based on polyesters such as PGA, poly(lactic acid) (PLA), and polycaprolactone (PCL) and their co-polymers or tissue-derived collagens. Their favoring properties assisting in the regeneration of periodontal apparatus include biocompatibility and biodegradability (4–6 weeks) with superior handling compared to PTFE membranes [18,71].
4. Scaffolds in BTE/RM
The utilization of scaffolds in tissue engineering and regenerative medicine can provide a key element to enhance the regeneration of tissue defects. The biocompatible nature alongside its physicochemical characteristics may reproduce a native extracellular matrix and provide the environmental characteristics to promote cell adhesion, proliferation, and differentiation [25]. According to Saiz et al. (2013) and Hosseinpour et al. (2017), scaffolds are required to modulate the interaction between functions and materials, including cell encapsulation, control drug/chemical release, and scaffold’s engineered surface [24,30]. In order to design an ideal scaffold, it is important to consider the degradation kinetics and physicochemical properties as well as the stimulation of tissue ingrowth, maturation, and remodeling [21,24,73].
Various nanocarriers have been evaluated for bone regeneration including polymers of synthetic (PLA, PCL, PGA) or natural (chitosan, alginate, collagen, fibrin) origin, bioceramics/glass (HA, β-TCP), and composites (PLA-chitosan, PLGA-HA) [15]. The incorporation of growth factors and cells in those delivery systems may positively influence the regeneration process through the formation of a microenvironment that resembles the target tissue natural state. A plethora of stem cells such as adipose-derived (ADSCs), mesenchymal (MSCs), induced pluripotent (iPSCs), and bone-marrow stromal cells have been employed from various research groups. Additionally, cell-specific markers and transcription factors have been determined and analyzed including alkaline phosphatase (ALP), osteopontin, osteocalcin, osteonectin, and Runx2. These groups may help to assess the osteogenicity during stem cell differentiation [74].
4.1. The Critical Properties of Scaffolds
The regeneration of maxillofacial and oral osseous defects requires the development of a scaffold nanosystem that may mimic the structural, mechanical, chemical, and biological properties of the patient’s bone. Thus, scaffold’s development and design are based on critical considerations that influence the materials used, the fabrication techniques and the functionalization methods [15,24,25,30,55,75,76,77]:
- Biocompatible nanomaterial with non-toxic degradation;
- Bioactivity, which will promote the interaction of a material’s surface and the adjacent cells;
- Analogous physicochemical characteristics similar to extracellular matrix (ECM) of the targeted bone native state;
- Ability to withstand the conditions of oral microenvironment (pH, temperature)
- Shape maintenance after implantation;
- Sufficient porosity and adequate pore diameter, orientation, and distribution
- Allow the incorporation of molecules and cells;
- Allow surface modifications;
- Degradable;
- Controllable degradation and release of substances. Scaffold’s degradation should be similar to the tissue regenerated;
- Osteoinductive and osteoconductive properties to promote cell infiltration;
- Angiogenic;
These ideal properties are summarized in Figure 1.
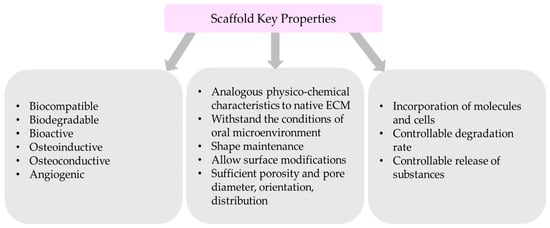
Figure 1.
Summary of scaffold key properties.
4.2. Scaffold Architecture
The biomimetic scaffold-based approach requires the inclusion of several characteristics to achieve adequate bone regeneration, replication of the missing tissue structure, biological, and mechanical properties. The bone substitutes incorporated into the scaffold should promote osteoinductivity, osteoconductivity, and osseointegration [78]. Osteoinduction is the process of stimulating pluripotent precursor cells to differentiate into osteoblasts [78,79]. Osteocondution promotes the development of the scaffold’s surface as well as within its pores and channels through cell adhesion, proliferation, and eventually forming a new extracellular matrix [80]. Overmann et al. (2020) stated that osseointegration is the establishment of a direct and stable connection between the scaffold and bone tissue without the ingrowth of fibrous tissue [81].
Scaffolds in regenerative strategies are required to be not only biocompatible but also biodegradable to promote the tissue’s innate healing [81,82]. Hence, the optimal system’s degradation rate should be equal to tissue’s regeneration rate. A variety of factors may influence the resorption rate including the local tissue environment, scaffold’s composition, and rate of disintegration. The two main mechanisms responsible for scaffolds resorption are passive hydrolysis (on natural polymeric scaffolds) and enzymatic cleavage (on synthetic polymeric scaffolds). Naturally, these polymers would eventually degrade, but the degradation rate is determined by factors such as molecular weight, comonomer ratio, residual monomer content, chain structure, annealing, crystallinity, and sterilization techniques. Hence, successful tissue regeneration relies on designing a scaffold based on the intricate interplay of those mechanisms while also synchronizing the rates of degradation and tissue regeneration [28,83,84]. Additionally, the capacity of intrinsic variability of patient’s regenerative mechanisms may significantly influence the tissue regrowth. Hence, the material of choice (natural or synthetic polymers, ceramics, and others) may vary in each individual case [85,86]. The plethora of materials utilized as scaffolds possess distinct characteristics regarding elasticity, stiffness, and compressive strength, thus influencing mechanical support and eventually regeneration [78,86,87].
Scaffold’s architecture is crucial, because it provides structural support and orientation to the endogenous and exogenous cells [88]. Moreover, it constitutes the appropriate microenvironment for scaffold-to-tissue integration and cell-to-cell interaction at the site of implantation. Thus, it is pivotal to develop 3D constructs with high porosity and enhanced interconnectivity [53,89]. A key element of bone repair and regeneration is facilitated shortly after implantation with the blood infiltration into the scaffold through the porous structure. Additionally, blood clots are stabilized, and an early microenvironment is formed [90,91]. The pores with a larger diameter ranging between 100 and 700 μm may promote the vascularization process, while smaller ones can inhibit cell growth due to a localized ischemia [53,92,93,94,95]. As mentioned earlier, high porosity is critical to support the transportation of gases, nutrients, and waste product removal. Consequently, the metabolic process and cellular growth is achieved [96,97,98,99].
Extracellular matrix (ECM) is a vital component in nature with an amorphous porous structure. Through bioactive molecules, mechanical stimuli, and spatial patterning, this natural scaffolding system modulates cellular recruitment, growth, and differentiation [100]. In that regard, the decellularized extracellular matrix has been used in tissue repair and regeneration to imitate a 3D microenvironment at the defected sites. Various sources of decellularized ECM are available for clinicians, such as human, bovine, and porcine dermis and human amniotic membrane [101]. Attempts have been made by different research groups to reproduce the hierarchical anatomy of periodontium with biomimetic scaffolds, periodontal progenitor cells, and decellularized ECM [102,103,104,105].
Scaffolds are the core of tissue-engineered constructs and may provide cells with the appropriate spatiotemporal guidance through their complex architecture [88]. These characteristics are dependent on a scaffold’s design, material selection, fabrication technique employed, and functionalization method. Figure 2 summarizes the various elements of this tissue engineering approach [76].
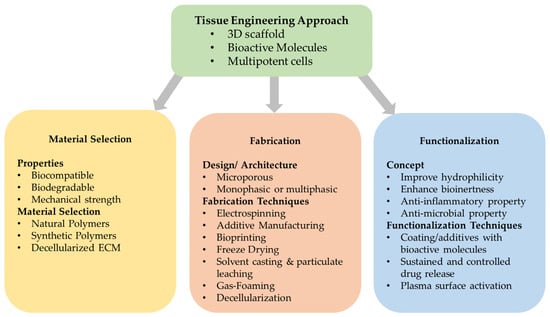
Figure 2.
Summary of the various elements influencing scaffold’s design and fabrication concept. In tissue engineering, bioactive molecules and multipotent cells are combined with 3D scaffolds. The scaffold’s characteristics are determined from the materials used, the fabrication techniques and design and the functionalization process. Adapted and modified from Yamada et al. (2022) [76].
4.3. Scaffold Fabrication Methods
Since their early development, a variety of biofabrication techniques have been used to manufacture biodegradable scaffolds and tissue-engineered constructs with highly customizable geometries. The most frequently used approaches are electrospinning and the additive manufacturing technique with the incorporation of relevant cells in a later stage of production. Electrospinning is a technique that can fabricate nanoscaled to microscaled fibrous scaffolds which can mimic the patient’s collagen fibrous network [106,107,108,109,110]. With this fabrication method it is possible to develop highly porous nanoscaffolds with various pore sizes and shapes similar to the native extracellular matrix [111]. Due to the low tunability of the pore sizes, shapes, orientation, and distribution that electrospinning offers, novel approaches that will be later described have been investigated. In that regard, 3D printing may fabricate a multiphasic nanosystem compared to the monophasic nanosystem that electrospinning offers [76,89]. Additive manufacturing techniques could be subdivided into stereolithography (SLA), fused deposition modelling (FDM), digital light processing (DLP), direct ink writing (DIW), and selective laser sintering (SLS) [6]. Gas foaming and salt leaching techniques both use gas and salt, respectively, as porogen additives, in comparison to freeze drying and phase separation techniques that use sublimation and volatilization of solvent and water into the polymer solution [76]. Bioprinting is a biomimetic approach to form tissue-engineered constructs which combines hydrogels and cells [112]. Bioassembly is another technique that has been reported but has a limited use for dento-alveolar regeneration [113,114]. The most popular biofabrication approaches to develop scaffolds for dentoalveolar regeneration are additive manufacturing, bioprinting, and electrospinning. These biofabrication approaches and their variations are illustrated in Figure 3.
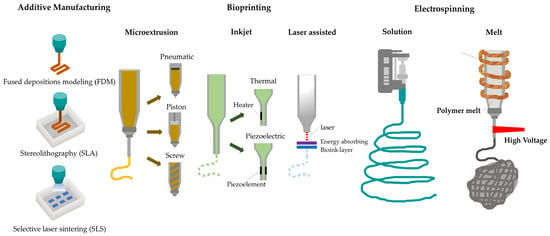
Figure 3.
Dentoalveolar scaffold biofabrication approaches. Additive manufacturing: (FDM, SLA, SLS). Bioprinting: microextrusion, inkjet (droplet-on-demand), laser-assisted. Electrospinning: solution-based, melt electrowriting.
4.3.1. Electrospinning
This technique applies high voltage to a polymeric solution to create a nanofibrous or microfibrous scaffold [115]. The high voltage overcomes the liquid’s surface tension resulting in the elongation of liquid droplets to nanofibers. An electrospinning apparatus is comprising [116,117,118]:
- High voltage power supply;
- Syringe pump;
- Metallic needle;
- Stationary or rotating metallic collector for fiber collection.
The scaffold is formed when the fiber collector and spinneret are connected to electrical terminals with opposite ends. The material is drawn out from the potential difference and deposited onto the collector fabricating the desired nanofibers [119]. Electrospinning may produce highly porous polymer structures of natural or synthetic origin with an increased surface area such as gelatin nanofibers, collagen and polycaprolactone (PCL) [120]. Several research groups have used electrospinning to fabricate scaffolds for alveolar bone regeneration, implant integration, gingival tissue and periodontal ligament regeneration [121,122,123,124,125,126,127]. This technique produces meshes with an increased surface area and high porosity promoting cell attachment. Small pore size and densification may hinder cell migration. The development of a novel solution-based electrospinning apparatus enhanced fiber deposition control, and consequently, the fabrication of microporous meshes and pre-designed struts was achieved [128]. Electrospun meshes have been used for controlled drug and molecular release. According to Rad et al. (2019), they are not only effective in promoting faster regeneration, but they can also suppress bacterial colonization through the incorporation of bioactive glass nanoparticles [129].
In comparison to solution electrospinning, melt electrospinning may adequately control fiber deposition. The pore size of the fabricated structure is above micron size. Recently, two research groups have reported the combination of FDM and electrospun melt meshes of PCL origin to produce a biphasic scaffold [130,131].
4.3.2. Additive Manufacturing
Additive manufacturing (AM), also known as 3D printing, incorporates a group of novel techniques used to fabricate three-dimensional (3D) tissue-engineering constructs that have been designed through computer-aided technologies in a layer-by-layer approach [132,133]. The bone defect is scanned with magnetic resonance imaging (MRI), or cone beam computed tomography (CBCT) and a scaffold model with volumetric shape that would fit into the defect is then designed [132,133,134,135]. The most abundantly used AM techniques in dentoalveolar settings are SLA, SLS, and FDM [46]. Selective laser sintering of ceramics, polymers and their combination, and selective laser melting (SLM) techniques have been used to form 3D constructs layer by layer. Various groups have applied these scaffolds in alveolar bone augmentation [136,137,138]. A patient-specific implant has been developed by Rasperini et al. (2015), utilizing PCL and SLS. According to the authors, limited regeneration was observed due to the polymer selected and scaffold’s design [137]. Through fused-deposition modeling, thermoplastic polymers or composite polymers can be processed with inorganic materials. The applicability of this technique has been reported in alveolar bone augmentation, periodontitis treatment, and whole tooth regeneration [127,130,137,139].
4.3.3. Bioprinting
Bioprinting could be classified as an additive manufacturing process due to their similarities, and Direct Ink Writing (DIW) can enable the development of complex 3D structures for biomedical applications [140]. Through this approach, developers have combined hydrogels and cells to fabricate biomimetic tissue-engineered constructs [112]. The most abundantly used bioprinting techniques are light or laser-based, extrusion-based and inkjet or droplet-on-demand [112]. These technologies utilize a variety of bioinks and particularly cells, hydrogels, or their combination [141]. The 3D printers utilized in the biomedical field may accurately fabricate scaffolds with a resolution of 10 μm or more [142,143,144]. The lowest acceptable limit of pore diameter that significantly promotes osteogenesis has been defined by Hulbert et al. (1970) at 100 μm [145]. The larger diameter pores (150–200 μm) have been reported to facilitate the highest degree of new bone formation, which is within the range of the Haversian bone system (100–200 μm) [146]. Additionally, smaller pore sizes (less than 100 μm) can promote chondrogenesis before osteogenesis, while low porosity with non-interconnected pores may prevent nutrient transportation. Hence, bone regeneration is hindered [147,148,149]. Different research groups have used extrusion-based bioprinters and combined them with bioinks to produce dental constructs [141,150,151]. Droplet-on-demand (DoD) is a bioprinting technique that can fabricate only small scaffolds. This limitation is related to the frequency used, droplet volume, and the actuation mechanism [152]. Laser-based bioprinting delivers small volumes of bioinks to the targeted platforms through a laser beam. Few authors have reported its use for the development of dento-alveolar constructs [153,154,155,156].
4.3.4. Freeze Drying
Freeze drying is a three-step process of drying polymeric solutions. It starts with solution preparation, followed by molding or casting of the solution, and later, freezing and drying under low pressure. At the last stage of fabrication, the ice and water are removed through sublimation and desorption, respectively. The scaffold’s pores may range between 15 and 200 μm with up to 90% porosity. Pore size can be modulated through temperature, polymer concentration, and freeze rate [157]. The utilization of a high-strong vacuum is mandatory to fabricate scaffolds with interconnectivity and increased porosity [6]. Natural or synthetic polymers and composites can be fabricated through this process including gelatin/hyaluronic acid and collagen/hyaluronic acid [6,158]. Shrestha et al. (2021) developed an artificial bone extracellular matrix substitute with favoring biological behavior and excellent osteoinductive properties. According to the authors, this system of multiwalled carbon nanotubes incorporating zein and chitosan into polyurethane may ensure bone cell regeneration and can be used as an artificial bone-grafting material [159].
4.3.5. Solvent-Casting and Particulate Leaching
This is a commonly applied technique to develop scaffolds when mixing water-soluble salt particles such as sodium citrate and sodium chloride into a biodegradable polymer solution. In order to remove the solvent, a process called lyophilization is applied to the mixture. Leaching out the salt particles will result in the development of a porous scaffold. This approach is simple and provides adequate control of the pore size and porosity. The variability of salt’s particle size and salt-to-polymer ratio will strongly influence the scaffold’s structure [160]. Through this fabrication technique a PLA/HA composite scaffold has been developed by Zimina et al. (2020) with 79% porosity, good suppression of tissue ingrowth, and improved adhesion of the mesenchymal stromal cells compared to the PLA alone. Additionally, due to the addition of HA, the system presented a limited inflammatory response and has been suggested for the restoration of maxillofacial defects [161].
4.3.6. Gas-Foaming Process
Gas-foaming process is a scaffold fabrication technique that can be classified into chemical foaming and physical foaming [162]. This characterization is based on the development of a blowing agent into the polymeric matrix. In tissue engineering and regenerative medicine, chemical foaming is prohibited due to the residues inside the polymeric matrix that may influence the scaffold’s biocompatibility [163]. Alternatively, physical foaming utilizes blowing agents, including N2 and CO2, at a high pressure to saturate the polymer disks [164]. This high pressure is later reduced, resulting in a thermodynamic instability and the formation of a 3D porous polymer structure is achieved [163,164]. Scaffolds with a pore size of approximately 100μm and up to 93% porosity but with poor interconnectivity could be fabricated through this method [164,165]. Sukpaita et al. (2021) investigated the mineralized tissue regenerative potential of a biocompatible, biodegradable, osteoconductive, and chitosan-based scaffold. Despite its advantages, the authors concluded that pure chitosan is not adequate to support regeneration due to its limitations such as rapid degradation rate, poor mechanical properties, and low osteoinductivity. Thus, chitosan should be combined with other biomaterials and/or bioactive molecules to improve the system’s characteristics [166].
4.3.7. Decellularization
Decellularization is the process of cell removal while preserving key properties of extracellular matrix such as architectural integrity and composition. Additionally, the decellularized ECM should be able to promote cell growth and differentiation as before. The plethora of processing techniques used to obtain the decellularized bone matrix include enzymatic methods, surfactants, hydrostatic pressure, thermal shock, and sonication [167]. Hydrostatic pressure is a promising technique that minimizes protein denaturation while also preventing the use of chemical agents, hence providing a higher quantity of ECM [168]. The last step of decellularization involves the incorporation of dehydrated alcohol and nucleases to eliminate cellular remains. In bone tissue engineering, decellularized bone matrix has been frequently employed as a scaffolding system to mimic native bone [6,169]. Santos et al. (2024) investigated the regenerative properties of an electrospun PCL/Chitosan nanofibrous scaffold loaded with bioactive cell-derived extracellular matrix. This nanosystem promoted cell proliferation and enhanced osteogenic differentiation, while also increasing bone-specific marker gene expression, calcium deposition, and alkaline phosphatase activity. Additionally, higher cell mineralization was observed and the scaffold’s use for alveolar bone regeneration was suggested [170].
The advantages and disadvantages of those techniques are summarized in the following table (Table 1), along with material examples fabricated through those methods.

Table 1.
Advantages and disadvantages of scaffold fabrication methods.
4.4. Biodegradable and Nonbiodegradable Scaffolds
The materials used for a scaffold may be either biodegradable or non-biodegradable. Biodegradable scaffolds allow tissue neogenesis through the replacement of the degraded biomaterial. These materials can be classified as bioactive, biotolerant, and bioinert, depending on the implanted tissue [188]. The novel scaffold fabrication approaches require modern biomaterials that can interact with the stem cells incorporated inside the scaffold and promote their differentiation. Both scaffold categories are able to direct and regulate stem cell differentiation into the desired somatic cells [189]. Biomaterials of natural origin (e.g., collagen, elastin, fibrin, alginate, chitosan) are biocompatible and biodegradable with a low immune response. Their limitations could be summarized as poor mechanical strength, inconsistent purity resulting in lot-to-lot variability, and difficulty in sterilization and purification [189,190,191].
Synthetic biomaterials are of non-natural origin and can be produced at a large scale. Additionally, they can have tunable characteristics such as high flexibility, controlled composition and degradation rate, and improved mechanical properties while allowing their functionalization [189]. The most frequently used biomaterials, among others, are polycaprolactone (PCL), poly(glycolic acid) (PGA), poly(lactic-co-glycolic acid) (PLGA), polyvinyl alcohol (PVA), and poly(ethylene glycol)diacrylate (PEGDA) [77,189].
4.5. Additional Scaffold Categories
4.5.1. Monophasic Scaffolds
The first development of monophasic scaffolds (MNPS) was inspired by the concepts of GBR/GTR. It was based on the utilization of biomaterials for space maintenance and to promote tissue neogenesis [192]. Consequently, monophasic tissue engineered constructs were fabricated following those principles. To overcome their impaired bioactivity, they were combined with biological additives and fillers. A well-documented approach to enhance the regenerative effect of those scaffolds was the encapsulation of cells in hydrogel systems or their placement directly into the scaffold, which was consecutively transplanted into the defect site. In this approach the MNPS were not only a nanoplatform for targeted cell delivery but also a barrier to maintain space for cell growth [35]. Alveolar bone regeneration facilitated by MNPS was determined to be not sufficient to restore the defect site at its primary condition. Consequently, the development of multiphasic scaffolds was apparent [193].
4.5.2. Multiphasic Scaffolds
The development of multiphasic tissue-engineered constructs was necessary to achieve periodontal regeneration. These scaffolds have distinguishable compartments with different biomechanical composition and architectural nature, such as pore size/shape and porosity. These constructs can even mimic the strict hierarchical organization of periodontium. Multiphasic scaffolds are a large group of tissue-engineered constructs consisting of biphasic and triphasic scaffolds [35]. Their distinct compartment design is illustrated in Figure 4 and is compared to monophasic scaffolds. A variety of groups have developed biphasic scaffolds to achieve periodontal ligament and alveolar bone regeneration, but their attempts failed to provide a new cementum layer at the interface of the tooth. Their approach was based on the ability of endogenous cells to promote new cementum apposition, or the utilization of in vitro differentiated cells. Thus, the development of multiphasic scaffolds incorporating a third layer, which will promote cementogenesis, is necessary [35]. Triphasic scaffolds have emerged to address the issue of cementum regeneration. The three-compartment design may promote the formation of cementum on the root surface, direct the insertion of the periodontal ligament, and provide adequate rigidity [194]. Designing such a complex biomaterial may be challenging, while also the presence of low interphase cohesion resulted in a diminished mechanical stability of the system. The utilization of various novel fabrication techniques including simultaneous multiphase crosslinking and additive fabrication may address this dilemma [195,196].
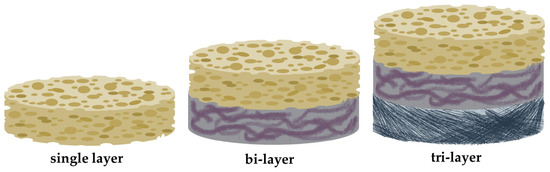
Figure 4.
Illustration of monophasic scaffolds (single layer) in comparison to the distinguishable compartments of multiphasic scaffolds (biphasic-bilayer and triphasic-tri-layer) that requires different materials, fabrication techniques, and functionalization approaches.
4.5.3. Hybrid Scaffolds
Hybrid scaffolds are technological products that are developed to regenerate multi-tissues (e.g., alveolar bone and periodontal ligament) through the combination of scaffolds with various geometrical scales [46,130,197]. Vaquette et al. (2021) combined FDM with melt electrospun scaffolds in a multidimensional hybrid alveolar bone augmentation approach [130]. The melted electrospun mesh was incorporated into the core of scaffolds and the latter were surrounded by FDM constructs. Poly-l-lactic acid was used to cover the scaffolds in all sides except one, which was in contact with the bone. According to the authors, at 8 weeks, a sufficient bone had been formed, and after the protective case’s removal, a dental implant was placed [130].
4.5.4. Smart Scaffolds
Various smart biomaterials have been developed to enhance tissue repair and regeneration. These materials possess intelligent characteristics and functions [198]. The development of a smart construct requires the incorporation of bioactive materials with tunable physicochemical characteristics [199,200].
Categories of smart scaffold constructs combined with stem cells for bone tissue engineering include the following:
- Biomimetic and bionic. Mittal et al. (2010) developed a porous biomimetic scaffold containing PLGA microspheres and peptides. This system was able to replicate the structure and composition of natural tissues [201].
- Immune sensitives. Zeng et al. (2017) coated a mesoporous bioactive glass scaffold with amino functional groups and reported its osteoimmunomodulatory efficacy on MSCs, macrophages, and bone marrow [202].
- Shape memory. Liu et al. (2014) loaded a shape-memory nanoporous scaffold with growth factors (BMP-2), attempting to repair a mandibular bone defect. The authors stated that the nanosystem could be applied in bone-regenerative medicine due to its potential [203].
- Electromechanical stimulus. Damaraju et al. (2017) developed flexible 3D fibrous scaffolds that are able to initiate the differentiation of MSCs and tissue formation [204]. A similar scaffold (Piezoelectric poly(vinylidene fluoride-trifluoroethylene)) incorporating zinc oxide nanoparticle enhanced the adhesion and proliferation of hMSCs while also improving blood vessel formation [205].
4.5.5. Personalized Scaffolds CAD/CAM
Through the progression of technology, it is now possible to achieve personalized fabrication of biomedical tools according to the anatomical defects. The combined action of precise image acquisition and 3D bioprinting equipment may develop individual-specific scaffolds with tailor-made characteristics [206]. This process starts with image acquisition, followed by image processing, 3D reconstruction, and 3D computer-aided design (CAD) modeling. In the last step of fabrication process, termed rapid prototyping, the customized tissue-engineered construct is bioprinted [207]. Then, the personalized bioprinted scaffold could be implanted at the defect site, as shown in Figure 5 [208].
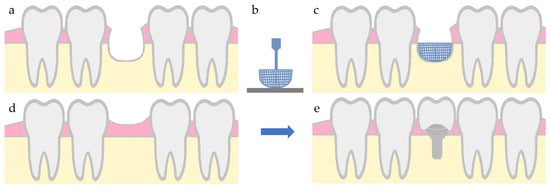
Figure 5.
Illustration of an alveolar bone defect regeneration with a printed scaffold, followed by dental implant placement. (a) defect in bone tissue, (b) additive manufacturing of a bone scaffold, (c) scaffold placement in the defect area, (d) bone regeneration, and (e) dental implant placement.
Magnetic resonance imaging (MRI) and computerized tomography (CT) are valuable tools to obtain precise data for the fabrication of a personalized scaffold. Scaffold’s production is achieved through computer-aided design and computer-aided manufacturing (CAD/CAM). Its design and architecture can be modified at a macro, micro, and even nanoscale [75]. The scaffold’s shape, internal porosity, and load-bearing capabilities are obtained based on a patient’s imaging data. When used as drug delivery systems, a microporous structure is necessary to support adequate drug delivery. Moreover, its mechanical properties should be similar to those of native tissues [207,209,210].
4.6. Scaffolds as Drug Delivery Systems
Another interesting utilization of scaffolds in regenerative medicine is the controlled drug/molecular release and immunomodulation. This approach may provide new aspects in bone regeneration due to the knowledge acquired at the cellular and gene level. Scaffolds are not only able to support tissue growth and targeted tissue response, but also to prevent undesirable cellular mechanisms. The advancements of scaffold design may improve cellular connection and activation, hence regulating cellular activity. In particular, the inhibition of cell attachment and the activation of cells may assist in the prevention of undesirable biological responses [211].
Zielińska et al. (2023) stated that drug/tissue delivery scaffolds should choose the appropriate scaffold type in order to influence the material’s architecture and structure (e.g., hydrogels, nanofibers, nanopatterns, microparticles, nanoparticles, or matrix) [212]. Additionally, the selection and incorporation of bioactive agents (among other cells, proteins, peptides, pharmaceutical molecules) into the scaffold may improve its characteristics. This process is called functionalization. Moreover, the scaffold’s surface may be further modified and the appropriate drug release profile (sustained, rapid or sequential) should be chosen [212]. The co-delivery of distinctive bioactive agents and drugs can reduce toxicity, minimize the drug dissociation rate, and eventually increase a drug’s effectiveness. Hence, the conceptualization of a scaffold incorporating bioactive agents may provide a universal drug delivery platform [212]. Therefore, they can be utilized as antimicrobial and anti-inflammatory agents when combined with the appropriate drug molecule.
4.6.1. Antimicrobial Effect
The unique structure of scaffolds suggested their use as drug delivery nanoplatforms against periopathogens. Several attempts have been made to ablate periodontal infection by loading antibacterial drugs into scaffolds and then implanting them at the defect site. Ferreira et al. (2021) reported the incorporation of metronidazole and tetracycline in polymeric scaffolds for the treatment of periodontal defects. The authors claimed that the development of a defect-specific antibiotic-laden scaffold was able to sustain periodontal reconstruction while ablating the infection present [213]. Ribeiro et al. (2020) developed a hybrid system of injectable hydrogels loaded with ciprofloxacin with a significant antimicrobial effect against E. faecalis [214].
4.6.2. Anti-Inflammatory Effect
Apart from bacterial growth inhibition, attempts have been made to modulate inflammation through scaffolds loaded with pharmacological agents (e.g., non-steroidal anti-inflammatory drugs) [215]. Scaffolds are implanted into the defect sites after the thorough elimination of dental plaque, calculus, irritants, and granulated tissue is performed, thus minimizing the inflammation modulation required after treatment [214]. During wound healing, a mild degree of inflammation at the treated site is expected. On the contrary, persistent inflammation will negatively influence the healing site and treatment outcome [216,217]. Yar et al. (2016) and Xu et al. (2019) reported the use of chitosan-based scaffolds loaded with meloxicam and aspirin, respectively, and both groups concluded that the sustained drug release reduced post-treatment inflammation [218,219]. Comparable results were reported by Batool et al. (2018), in a study determining the anti-inflammatory efficiency of PCL scaffolds loaded with ibuprofen [220].
5. Conclusions and Future Perspectives
Both conventional and novel state-of-the-art tissue engineering approaches have proven their potential in bone tissue engineering. The fabrication of scaffolds with characteristics similar to the target tissue such as biocompatibility, structural stability, osteoinductivity, osteoconductivity, physicochemical properties, porosity, adequate pore size, and pore interconnectivity is still a challenge to achieve. The advances of nanotechnology combined with key characteristics on fabrication methodology may assist in the development of novel biomaterials [221,222,223]. These biomaterials will be able to mimic the hierarchical organization and structure of native bone [6].
It has been reported that monophasic scaffolds are difficult to meet the requirements to achieve adequate bone regeneration due to the complexity of natural tissue. Scaffold’s architecture is crucial, since porosity, pore size, orientation, and interconnectivity may influence mechanical and biological properties of bone regeneration. Hence, the lack of control of those features during the manufacturing process will negatively impact the therapeutic outcome, especially in larger bone defects. It is apparent that scaffold fabrication techniques should provide tissue-engineering constructs that have a tailor-made structure and a defined pore shape and size. Thus, their advancements should focus on controlling these aspects [6]. Additionally, the ideal tissue-engineering construct should support long-term stability and space maintenance with controllable degradation rate. These characteristics may allow bone remodeling and eventually ensure implant longevity through the prevention of bone resorption upon implant placement [41]. Novel tissue-engineering approaches are utilizing various categories of stem cells, growth factors, genes, and biologic agents to achieve tissue regeneration. These groups may promote, among other bone regeneration, tissue vascularization and wound healing. Hence, it is crucial to understand their mechanisms and identify the appropriate group for each individual case.
The advances in imaging data acquisition (CT, MRI) and digital design and manufacturing (CAD/CAM) have provided a novel personalized approach in bone regeneration. The bioprinters used are able to produce accurate 3D scaffolds with complex architectures, even though limitations in hardware and materials have been reported. Even though extrusion-based additive manufacturing techniques are able to replicate complex geometries, their poor resolution and slow manufacturing process needs to be adjusted. On the other hand, techniques such as SLA could overcome these limitations, but novel materials with better characteristics should be developed [6]. The fabrication of layered scaffolds through conventional methods including electrospinning, solvent casting, gas-foaming, and freeze drying may have benefits regarding high porosity, good pore interconnectivity, and low production costs, but the complexity of alveolar bone and periodontium requires novel fabrication techniques [224].
Targeted drug delivery has gained more attention during the last decade. The development of advanced tissue engineering constructs—scaffolds—that ensure that proper transport of the incorporated substance and controlled drug release can be a promising tool. In that regard, researchers should enhance the stability and functionality of these systems in order to achieve an improved therapeutic outcome [212].
Author Contributions
Conceptualization, T.-F.V., X.D. and N.L.; methodology, T.-F.V., X.D. and N.L.; formal analysis, T.-F.V., X.D. and N.L.; investigation, T.-F.V.; resources, N.L.; writing—original draft preparation, T.-F.V., X.D. and N.L.; writing—review and editing, T.-F.V., X.D., H.K., M.G. and N.L.; visualization, N.L.; supervision, X.D. and N.L.; project administration, N.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Xia, D.; Yang, F.; Zheng, Y.; Liu, Y.; Zhou, Y. Research Status of Biodegradable Metals Designed for Oral and Maxillofacial Applications: A Review. Bioact. Mater. 2021, 6, 4186–4208. [Google Scholar] [CrossRef]
- Wang, B.; Feng, C.; Liu, Y.; Mi, F.; Dong, J. Recent Advances in Biofunctional Guided Bone Regeneration Materials for Repairing Defective Alveolar and Maxillofacial Bone: A Review. Jpn. Dent. Sci. Rev. 2022, 58, 233–248. [Google Scholar] [CrossRef]
- Armiento, A.R.; Hatt, L.P.; Sanchez Rosenberg, G.; Thompson, K.; Stoddart, M.J. Functional Biomaterials for Bone Regeneration: A Lesson in Complex Biology. Adv. Funct. Mater. 2020, 30, 1909874. [Google Scholar] [CrossRef]
- Lagopati, N.; Agathopoulos, S. Hydroxyapatite Scaffolds Produced from Cuttlefish Bone via Hydrothermal Transformation for Application in Tissue Engineering and Drug Delivery Systems. In Marine-Derived Biomaterials for Tissue Engineering Applications; Choi, A., Ben-Nissan, B., Eds.; Springer Series in Biomaterials Science and Engineering; Springer: Singapore, 2019; Volume 14. [Google Scholar] [CrossRef]
- Sailer, I.; Karasan, D.; Todorovic, A.; Ligoutsikou, M.; Pjetursson, B.E. Prosthetic failures in dental implant therapy. Periodontol. 2000 2022, 88, 130–144. [Google Scholar] [CrossRef]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Nyman, S.; Lindhe, J.; Karring, T.; Rylander, H. New attachment following surgical treatment of human periodontal disease. J. Clin. Periodontol. 1982, 9, 290–296. [Google Scholar] [CrossRef]
- Gottlow, J.; Nyman, S.; Karring, T.; Lindhe, J. New attachment formation as the result of controlled tissue regeneration. J. Clin. Periodontol. 1984, 11, 494–503. [Google Scholar] [CrossRef]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implants Res. 2010, 21, 567–576. [Google Scholar] [CrossRef]
- Dahlin, C.; Linde, A.; Gottlow, J.; Nyman, S. Healing of bone defects by guided tissue regeneration. Plast. Reconst. Surg. 1988, 81, 672–676. [Google Scholar] [CrossRef]
- Dahlin, C.; Sennerby, L.; Lekholm, U.; Linde, A.; Nyman, S. Generation of new bone around titanium implants using a membrane technique: An experimental study in rabbits. Int. J. Oral Maxillofac. Implants 1989, 4, 19–25. [Google Scholar]
- Donos, N.; Akcali, A.; Padhye, N.; Sculean, A.; Calciolari, E. Bone regeneration in implant dentistry: Which are the factors affecting the clinical outcome? Periodontol. 2000 2023, 93, 26–55. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Urban, I.; Monje, A.; Kunrath, M.F.; Dahlin, C. Guided bone regeneration in implant dentistry: Basic principle, progress over 35 years, and recent research activities. Periodontol. 2000 2023, 93, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Laney, W. Glossary of Oral and Maxillofacial Implants. Int. J. Oral Maxillofac. Implants 2017, 32, Gi-G200. [Google Scholar] [CrossRef] [PubMed]
- Pilipchuk, S.P.; Plonka, A.B.; Monje, A.; Taut, A.D.; Lanis, A.; Kang, B.; Giannobile, W.V. Tissue Engineering for Bone Regeneration and Osseointegration in the Oral Cavity. Dent. Mater. 2015, 31, 317–338. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-M.; Jin, Y. Periodontal Tissue Engineering and Regeneration: Current Approaches and Expanding Opportunities. Tissue Eng. Part B Rev. 2010, 16, 219–255. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.M.S.; Cortez, A.L.V.; Moreira, R.W.F.; Mazzonetto, R. Complications of Intraoral Donor Site for Bone Grafting Prior to Implant Placement. Implant. Dent. 2006, 15, 420–426. [Google Scholar] [CrossRef]
- Sculean, A.; Nikolidakis, D.; Schwarz, F. Regeneration of Periodontal Tissues: Combinations of Barrier Membranes and Grafting Materials—Biological Foundation and Preclinical Evidence: A Systematic Review. J. Clin. Periodontol. 2008, 35, 106–116. [Google Scholar] [CrossRef]
- Mudda, J.; Bajaj, M. Stem Cell Therapy: A Challenge to Periodontist. Indian J. Dent. Res. 2011, 22, 132–139. [Google Scholar] [CrossRef]
- Gatou, M.-A.; Vagena, I.-A.; Lagopati, N.; Pippa, N.; Gazouli, M.; Pavlatou, E.A. Functional MOF-Based Materials for Environmental and Biomedical Applications: A Critical Review. Nanomaterials 2023, 13, 2224. [Google Scholar] [CrossRef]
- Funda, G.; Taschieri, S.; Bruno, G.A.; Grecchi, E.; Paolo, S.; Girolamo, D.; Del Fabbro, M. Nanotechnology Scaffolds for Alveolar Bone Regeneration. Materials 2020, 13, 201. [Google Scholar] [CrossRef]
- Rios, H.F.; Lin, Z.; Oh, B.; Park, C.H.; Giannobile, W.V. Cell- and Gene-Based Therapeutic Strategies for Periodontal Regenerative Medicine. J. Periodontol. 2011, 82, 1223–1237. [Google Scholar] [CrossRef]
- Göker, F.; Ersanlı, S.; Arısan, V.; Cevher, E.; Güzel, E.E.; İşsever, H.; Ömer, B.; Durmuş Altun, G.; Morina, D.; Ekiz Yılmaz, T.; et al. Combined Effect of Parathyroid Hormone and Strontium Ranelate on Bone Healing in Ovariectomized Rats. Oral. Dis. 2018, 24, 1255–1269. [Google Scholar] [CrossRef]
- Saiz, E.; Zimmermann, E.A.; Lee, J.S.; Wegst, U.G.K.; Tomsia, A.P. Perspectives on the Role of Nanotechnology in Bone Tissue Engineering. Dent. Mater. 2013, 29, 103–115. [Google Scholar] [CrossRef]
- Walmsley, G.G.; McArdle, A.; Tevlin, R.; Momeni, A.; Atashroo, D.; Hu, M.S.; Feroze, A.H.; Wong, V.W.; Lorenz, P.H.; Longaker, M.T.; et al. Nanotechnology in Bone Tissue Engineering. Nanomedicine 2015, 11, 1253–1263. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Fu, X.; Li, Y.; Li, R. Aligned carbon nanofibers for nanofibers-guided bone regeneration and orthopedic applications: A pilot study. Arab. J. Chem. 2023, 16, 105075. [Google Scholar] [CrossRef]
- Alavi, S.E.; Cabot, P.J.; Raza, A.; Moyle, P.M. Developing GLP-1 conjugated self-assembling nanofibers using copper-catalyzed alkyne–azide cycloaddition and evaluation of their biological activity. Bioconjug. Chem. 2021, 32, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.E.; Gholami, M.; Shahmabadi, H.E.; Reher, P. Resorbable GBR Scaffolds in Oral and Maxillofacial Tissue Engineering: Design, Fabrication, and Applications. J. Clin. Med. 2023, 12, 6962. [Google Scholar] [CrossRef] [PubMed]
- Carbone, E.J.; Jiang, T.; Nelson, C.; Henry, N.; Lo, K.W.-H. Small Molecule Delivery through Nanofibrous Scaffolds for Musculoskeletal Regenerative Engineering. Nanomedicine 2014, 10, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour, S.; Ghazizadeh Ahsaie, M.; Rezai Rad, M.; Baghani, M.T.; Motamedian, S.R.; Khojasteh, A. Application of Selected Scaffolds for Bone Tissue Engineering: A Systematic Review. Oral Maxillofac. Surg. 2017, 21, 109–129. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Su, Y.; Kucine, A.J.; Cheng, K.; Zhu, D. Guided Bone Regeneration Using Barrier Membrane in Dental Applications. ACS Biomater. Sci. Eng. 2023, 9, 5457–5478. [Google Scholar] [CrossRef] [PubMed]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Patil, S.; Bhandi, S.; Bakri, M.M.H.; Albar, D.H.; Alzahrani, K.J.; Al-Ghamdi, M.S.; Alnfiai, M.M.; Tovani-Palone, M.R. Evaluation of Efficacy of Non-Resorbable Membranes Compared to Resorbable Membranes in Patients Undergoing Guided Bone Regeneration. Heliyon 2023, 9, e13488. [Google Scholar] [CrossRef]
- Deng, Y.; Liang, Y.; Liu, X. Biomaterials for Periodontal Regeneration. Dent. Clin. N. Am. 2022, 66, 659–672. [Google Scholar] [CrossRef]
- Hollister, S.J.; Pilipchuk, S.P.; Bartold, P.M.; Hutmacher, D.W.; Giannobile, W.V.; Ivanovski, S. Tissue Engineered Constructs for Periodontal Regeneration: Current Status and Future Perspectives. Adv. Healthc. Mater. 2018, 7, 1800457. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Y.; Xue, Y.; Shi, J.; Zhang, X.; Liu, Y.; Midgley, A.C.; Wang, S. Multifunctional triple-layered composite scaffolds combining platelet-rich fibrin promote bone regeneration. ACS Biomater. Sci. Eng. 2019, 5, 6691–6702. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, M.; He, J. A review of biomimetic scaffolds for bone regeneration: Toward a cell-free strategy. Bioeng. Transl. Med. 2020, 6, e10206. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, S.; Vaquette, C.; Gronthos, S.; Hutmacher, D.W.; Bartold, P.M. Multiphasic Scaffolds for Periodontal Tissue Engineering. J. Dent. Res. 2014, 93, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, S. Periodontal Regeneration. Aust. Dent. J. 2009, 54, S118–S128. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zeng, X.; Zou, S.; Xu, Y.; Duan, P. Recent Advances in Horizontal Alveolar Bone Regeneration. Biomed. Mater. 2023, 18, 052004. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Mitchell, J.; Ivanovski, S. Recent Advances in Vertical Alveolar Bone Augmentation Using Additive Manufacturing Technologies. Front. Bioeng. Biotechnol. 2022, 9, 798393. [Google Scholar] [CrossRef]
- Urban, I.A.; Montero, E.; Amerio, E.; Palombo, D.; Monje, A. Techniques on Vertical Ridge Augmentation: Indications and Effectiveness. Periodontol. 2000 2023, 93, 153–182. [Google Scholar] [CrossRef]
- Benic, G.I.; Hämmerle, C.H. Horizontal bone augmentation by means of guided bone regeneration. Periodontol. 2000 2014, 66, 13–40. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, L.; Huang, C.; Yin, X.; Zhang, X.; Li, P.; Gu, X.; Fan, Y. Recent Advances in the Development of Magnesium-Based Alloy Guided Bone Regeneration (GBR) Membrane. Metals 2022, 12, 2074. [Google Scholar] [CrossRef]
- Fok, M.R.; Pelekos, G.; Tonetti, M.S. Feasibility and Needs for Simultaneous or Staged Bone Augmentation to Place Prosthetically Guided Dental Implants after Extraction or Exfoliation of First Molars Due to Severe Periodontitis. J. Clin. Periodontol. 2020, 47, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- EzEldeen, M.; Moroni, L.; Nejad, Z.M.; Jacobs, R.; Mota, C. Biofabrication of Engineered Dento-Alveolar Tissue. Biomater. Adv. 2023, 148, 213371. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yu, Y.; Han, L.; Ma, S.; Zhao, J.; Chen, H.; Yang, Z.; Zhang, F.; Xia, Y.; Zhou, Y. Biocompatibility and Osteogenic Activity of Guided Bone Regeneration Membrane Based on Chitosan-Coated Magnesium Alloy. Mater. Sci. Eng. C. 2019, 100, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Ku, J.-K. Guided Bone Regeneration. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M.S. Chitosan as a Vehicle for Growth Factor Delivery: Various Preparations and Their Applications in Bone Tissue Regeneration. Int. J. Biol. Macromol. 2017, 104, 1383–1397. [Google Scholar] [CrossRef] [PubMed]
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier Membranes: More than the Barrier Effect? J. Clin. Periodontol. 2019, 46, 103–123. [Google Scholar] [CrossRef]
- Saito, E.; Saito, A.; Kuboki, Y.; Kimura, M.; Honma, Y.; Takahashi, T.; Kawanami, M. Periodontal Repair Following Implantation of Beta-Tricalcium Phosphate with Different Pore Structures in Class III Furcation Defects in Dogs. Dent. Mater. J. 2012, 31, 681–688. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The Effect of Mean Pore Size on Cell Attachment, Proliferation and Migration in Collagen–Glycosaminoglycan Scaffolds for Bone Tissue Engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone Grafts: Which Is the Ideal Biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Finkemeier, C.G. Bone-Grafting and bone-graft subtitutes. J. Bone Jt. Surg. Am. 2002, 84, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Winkler, T.; Sass, F.A.; Duda, G.N.; Schmidt-Bleek, K. A Review of Biomaterials in Bone Defect Healing, Remaining Shortcomings and Future Opportunities for Bone Tissue Engineering. Bone Jt. Res. 2018, 7, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone Substitutes: A Review of Their Characteristics, Clinical Use, and Perspectives for Large Bone Defects Management. J. Tissue Eng. 2018, 9, 204173141877681. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.E.; Mosheiff, R. Tissue Engineering Approaches for Bone Repair: Concepts and Evidence. Injury 2011, 42, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G.; Moghaddam, A. Allograft Bone Matrix versus Synthetic Bone Graft Substitutes. Injury 2011, 42, S16–S21. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Sánchez, I.; Ortiz-Vigón, A.; Sanz-Martín, I.; Figuero, E.; Sanz, M. Effectiveness of Lateral Bone Augmentation on the Alveolar Crest Dimension. J. Dent. Res. 2015, 94, 128S–142S. [Google Scholar] [CrossRef] [PubMed]
- Fukuba, S.; Okada, M.; Nohara, K.; Iwata, T. Alloplastic Bone Substitutes for Periodontal and Bone Regeneration in Dentistry: Current Status and Prospects. Materials 2021, 14, 1096. [Google Scholar] [CrossRef]
- Yip, I.; Ma, L.; Mattheos, N.; Dard, M.; Lang, N.P. Defect healing with various bone substitutes. Clin. Oral Implants Res. 2015, 26, 606–614. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef] [PubMed]
- Mayer, Y.; Zigdon-Giladi, H.; Machtei, E.E. Ridge Preservation Using Composite Alloplastic Materials: A Randomized Control Clinical and Histological Study in Humans. Clin. Implant Dent. Relat. Res. 2016, 18, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Rajula, M.P.B.; Narayanan, V.; Venkatasubbu, G.D.; Mani, R.C.; Sujana, A. Nano-hydroxyapatite: A Driving Force for Bone Tissue Engineering. J. Pharm. Bioallied Sci. 2021, 13 (Suppl. S1), S11–S14. [Google Scholar] [CrossRef]
- Saito, H.; Couso-Queiruga, E.; Shiau, H.J.; Stuhr, S.; Prasad, H.; Allareddy, T.V.; Reynolds, M.A.; Avila-Ortiz, G. Evaluation of poly lactic-co-glycolic acid-coated β-tricalcium phosphate for alveolar ridge preservation: A multicenter randomized controlled trial. J. Periodontol. 2021, 92, 524–535. [Google Scholar] [CrossRef]
- Sanz, M.; Dahlin, C.; Apatzidou, D.; Artzi, Z.; Bozic, D.; Calciolari, E.; De Bruyn, H.; Dommisch, H.; Donos, N.; Eickholz, P.; et al. Biomaterials and Regenerative Technologies Used in Bone Regeneration in the Craniomaxillofacial Region: Consensus Report of Group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46, 82–91. [Google Scholar] [CrossRef]
- Mizraji, G.; Davidzohn, A.; Gursoy, M.; Gursoy, U.K.; Shapira, L.; Wilensky, A. Membrane Barriers for Guided Bone Regeneration: An Overview of Available Biomaterials. Periodontol. 2000 2023, 93, 56–76. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, D. Recent Advances in GTR Scaffolds. Bioinformation 2022, 18, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.M.G.; Kowolik, M.J.; Janowski, G.M. Recent Advances in the Development of GTR/GBR Membranes for Periodontal Regeneration—A Materials Perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Reise, M.; Wyrwa, R.; Müller, U.; Zylinski, M.; Völpel, A.; Schnabelrauch, M.; Berg, A.; Jandt, K.D.; Watts, D.C.; Sigusch, B.W. Release of Metronidazole from Electrospun Poly(l-Lactide-Co-d/l-Lactide) Fibers for Local Periodontitis Treatment. Dent. Mater. 2012, 28, 179–188. [Google Scholar] [CrossRef]
- Ferracane, J.L.; Giannobile, W.V. Novel Biomaterials and Technologies for the Dental, Oral, and Craniofacial Structures. J. Dent. Res. 2014, 93, 1185–1186. [Google Scholar] [CrossRef]
- Fisher, S.; Franz-Odendaal, T. Evolution of the Bone Gene Regulatory Network. Curr. Opin. Genet. Dev. 2012, 22, 390–397. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous Scaffold Design for Tissue Engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Yamada, S.; Shanbhag, S.; Mustafa, K. Scaffolds in Periodontal Regenerative Treatment. Dent. Clin. N. Am. 2022, 66, 111–130. [Google Scholar] [CrossRef]
- Marin, E.; Boschetto, F.; Pezzotti, G. Biomaterials and Biocompatibility: An Historical Overview. J. Biomed. Mater. Res. A 2020, 108, 1617–1633. [Google Scholar] [CrossRef] [PubMed]
- Schulze, F.; Lang, A.; Schoon, J.; Wassilew, G.I.; Reichert, J. Scaffold Guided Bone Regeneration for the Treatment of Large Segmental Defects in Long Bones. Biomedicines 2023, 11, 325. [Google Scholar] [CrossRef]
- Mitra, D.; Whitehead, J.; Yasui, O.W.; Leach, J.K. Bioreactor Culture Duration of Engineered Constructs Influences Bone Formation by Mesenchymal Stem Cells. Biomaterials 2017, 146, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, P.; Przekora, A. Osteoconductive and Osteoinductive Surface Modifications of Biomaterials for Bone Regeneration: A Concise Review. Coatings 2020, 10, 971. [Google Scholar] [CrossRef]
- Overmann, A.L.; Aparicio, C.; Richards, J.T.; Mutreja, I.; Fischer, N.G.; Wade, S.M.; Potter, B.K.; Davis, T.A.; Bechtold, J.E.; Forsberg, J.A.; et al. Orthopaedic Osseointegration: Implantology and Future Directions. J. Orthop. Res. 2020, 38, 1445–1454. [Google Scholar] [CrossRef]
- Pawelec, K.M.; Planell, J.A. Bone Repair Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780081024515. [Google Scholar]
- Yannas, I.V. Regeneration of Skin. In Tissue and Organ Regeneration in Adults; Springer: New York, NY, USA, 2015; pp. 89–136. [Google Scholar]
- Latimer, J.M.; Maekawa, S.; Yao, Y.; Wu, D.T.; Chen, M.; Giannobile, W.V. Regenerative Medicine Technologies to Treat Dental, Oral, and Craniofacial Defects. Front. Bioeng. Biotechnol. 2021, 9, 704048. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The Development of Collagen Based Composite Scaffolds for Bone Regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Scheinpflug, J.; Pfeiffenberger, M.; Damerau, A.; Schwarz, F.; Textor, M.; Lang, A.; Schulze, F. Journey into Bone Models: A Review. Genes 2018, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & Scaffolds for Tissue Engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.A. Blood Clots and Tissue Regeneration of 3D Printed Dual Scale Porous Polymeric Scaffolds. Mater. Lett. 2021, 285, 129184. [Google Scholar] [CrossRef]
- Li, Z.; Lv, X.; Chen, S.; Wang, B.; Feng, C.; Xu, Y.; Wang, H. Improved Cell Infiltration and Vascularization of Three-Dimensional Bacterial Cellulose Nanofibrous Scaffolds by Template Biosynthesis. RSC Adv. 2016, 6, 42229–42239. [Google Scholar] [CrossRef]
- Hong, W.X.; Hu, M.S.; Esquivel, M.; Liang, G.Y.; Rennert, R.C.; McArdle, A.; Paik, K.J.; Duscher, D.; Gurtner, G.C.; Lorenz, H.P.; et al. The Role of Hypoxia-Inducible Factor in Wound Healing. Adv. Wound Care 2014, 3, 390–399. [Google Scholar] [CrossRef]
- Darby, I.A.; Hewitson, T.D. Hypoxia in Tissue Repair and Fibrosis. Cell Tissue Res. 2016, 365, 553–562. [Google Scholar] [CrossRef]
- Klenke, F.M.; Liu, Y.; Yuan, H.; Hunziker, E.B.; Siebenrock, K.A.; Hofstetter, W. Impact of Pore Size on the Vascularization and Osseointegration of Ceramic Bone Substitutes in Vivo. J. Biomed. Mater. Res. A 2008, 85A, 777–786. [Google Scholar] [CrossRef]
- Murphy, C.M.; O’Brien, F.J. Understanding the Effect of Mean Pore Size on Cell Activity in Collagen-Glycosaminoglycan Scaffolds. Cell Adh. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef]
- Jin, H.; Zhuo, Y.; Sun, Y.; Fu, H.; Han, Z. Microstructure Design and Degradation Performance in Vitro of Three-Dimensional Printed Bioscaffold for Bone Tissue Engineering. Adv. Mech. Eng. 2019, 11, 168781401988378. [Google Scholar] [CrossRef]
- Botchwey, E.A.; Dupree, M.A.; Pollack, S.R.; Levine, E.M.; Laurencin, C.T. Tissue Engineered Bone: Measurement of Nutrient Transport in Three-dimensional Matrices. J. Biomed. Mater. Res. A 2003, 67A, 357–367. [Google Scholar] [CrossRef]
- Mastrullo, V.; Cathery, W.; Velliou, E.; Madeddu, P.; Campagnolo, P. Angiogenesis in Tissue Engineering: As Nature Intended? Front. Bioeng. Biotechnol. 2020, 8, 188. [Google Scholar] [CrossRef]
- Dutta, R.C.; Dey, M.; Dutta, A.K.; Basu, B. Competent Processing Techniques for Scaffolds in Tissue Engineering. Biotechnol. Adv. 2017, 35, 240–250. [Google Scholar] [CrossRef]
- Nakayama, K.H.; Hou, L.; Huang, N.F. Role of Extracellular Matrix Signaling Cues in Modulating Cell Fate Commitment for Cardiovascular Tissue Engineering. Adv. Healthc. Mater. 2014, 3, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.; Giannobile, W.V. Extracellular Matrix-based Scaffolding Technologies for Periodontal and Peri-implant Soft Tissue Regeneration. J. Periodontol. 2020, 91, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.; Vaquette, C.; Theodoropoulos, C.; Hamlet, S.M.; Hutmacher, D.W.; Ivanovski, S. Decellularized Periodontal Ligament Cell Sheets with Recellularization Potential. J. Dent. Res. 2014, 93, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.; Vaquette, C.; Hutmacher, D.W.; Bartold, P.M.; Ivanovski, S. Fabrication and Characterization of Decellularized Periodontal Ligament Cell Sheet Constructs. Methods Mol. Biol. 2017, 1537, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.; Vaquette, C.; Hutmacher, D.W.; Bartold, P.M.; Ivanovski, S. Fabrication and Characterization of Decellularized Periodontal Ligament Cell Sheet Constructs. Methods Mol. Biol. 2023, 2588, 429–438. [Google Scholar] [CrossRef]
- Son, H.; Jeon, M.; Choi, H.-J.; Lee, H.-S.; Kim, I.-H.; Kang, C.-M.; Song, J.S. Decellularized Human Periodontal Ligament for Periodontium Regeneration. PLoS ONE 2019, 14, e0221236. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, E.; Sun, Y.; Jacobs, R.; Politis, C. Three-Dimensional Printed Final Occlusal Splint for Orthognathic Surgery: Design and Validation. Int. J. Oral Maxillofac. Surg. 2017, 46, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, N.; Endo, F.; Maeda, T.; Hotta, A. Electrospinning and Surface Modification Methods for Functionalized Cell Scaffolds. In Nanostructures for Novel Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 201–225. [Google Scholar]
- Lannutti, J.; Reneker, D.; Ma, T.; Tomasko, D.; Farson, D. Electrospinning for Tissue Engineering Scaffolds. Mater. Sci. Eng. C 2007, 27, 504–509. [Google Scholar] [CrossRef]
- Farag, A.; Hashimi, S.M.; Vaquette, C.; Bartold, P.M.; Hutmacher, D.W.; Ivanovski, S. The Effect of Decellularized Tissue Engineered Constructs on Periodontal Regeneration. J. Clin. Periodontol. 2018, 45, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Cortez Tornello, P.R.; Caracciolo, P.C.; Igartúa Roselló, J.I.; Abraham, G.A. Electrospun Scaffolds with Enlarged Pore Size: Porosimetry Analysis. Mater. Lett. 2018, 227, 191–193. [Google Scholar] [CrossRef]
- Jeon, J.E.; Vaquette, C.; Klein, T.J.; Hutmacher, D.W. Perspectives in Multiphasic Osteochondral Tissue Engineering. Anat. Rec. 2014, 297, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.; Camarero-Espinosa, S.; Baker, M.B.; Wieringa, P.; Moroni, L. Bioprinting: From Tissue and Organ Development to in Vitro Models. Chem. Rev. 2020, 120, 10547–10607. [Google Scholar] [CrossRef] [PubMed]
- Washio, K.; Tsutsumi, Y.; Tsumanuma, Y.; Yano, K.; Srithanyarat, S.S.; Takagi, R.; Ichinose, S.; Meinzer, W.; Yamato, M.; Okano, T.; et al. In Vivo Periodontium Formation Around Titanium Implants Using Periodontal Ligament Cell Sheet. Tissue Eng. Part. A 2018, 24, 1273–1282. [Google Scholar] [CrossRef]
- Monteiro, N.; Smith, E.E.; Angstadt, S.; Zhang, W.; Khademhosseini, A.; Yelick, P.C. Dental Cell Sheet Biomimetic Tooth Bud Model. Biomaterials 2016, 106, 167–179. [Google Scholar] [CrossRef]
- White, J.; Foley, M.; Rowley, A. A Novel Approach to 3D-Printed Fabrics and Garments. 3D Print. Addit. Manuf. 2015, 2, 145–149. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Rao Kummara, M.; Kamal, T.; Alghyamah, A.-A.A.; Jan Iftikhar, F.; Bano, B.; Khan, N.; Amjid Afridi, M.; Soo Han, S.; et al. Advances in the Scaffolds Fabrication Techniques Using Biocompatible Polymers and Their Biomedical Application: A Technical and Statistical Review. J. Saudi Chem. Soc. 2020, 24, 186–215. [Google Scholar] [CrossRef]
- Farag, M.M. Recent Trends on Biomaterials for Tissue Regeneration Applications: Review. J. Mater. Sci. 2023, 58, 527–558. [Google Scholar] [CrossRef]
- Li, Z.; Xie, M.-B.; Li, Y.; Ma, Y.; Li, J.-S.; Dai, F.-Y. Recent Progress in Tissue Engineering and Regenerative Medicine. J. Biomater. Tissue Eng. 2016, 6, 755–766. [Google Scholar] [CrossRef]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludağ, H. Current State of Fabrication Technologies and Materials for Bone Tissue Engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Arslan, Y.E.; Sezgin Arslan, T.; Derkus, B.; Emregul, E.; Emregul, K.C. Fabrication of Human Hair Keratin/Jellyfish Collagen/Eggshell-Derived Hydroxyapatite Osteoinductive Biocomposite Scaffolds for Bone Tissue Engineering: From Waste to Regenerative Medicine Products. Colloids Surf. B Biointerfaces 2017, 154, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Canciani, E.; Gagliano, N.; Paino, F.; Amler, E.; Divin, R.; Denti, L.; Henin, D.; Fiorati, A.; Dellavia, C. Polyblend Nanofibers to Regenerate Gingival Tissue: A Preliminary In Vitro Study. Front. Mater. 2021, 8, 670010. [Google Scholar] [CrossRef]
- Ghavimi, M.A.; Bani Shahabadi, A.; Jarolmasjed, S.; Memar, M.Y.; Maleki Dizaj, S.; Sharifi, S. Nanofibrous Asymmetric Collagen/Curcumin Membrane Containing Aspirin-Loaded PLGA Nanoparticles for Guided Bone Regeneration. Sci. Rep. 2020, 10, 18200. [Google Scholar] [CrossRef]
- Lim, J.; Jang, K.-J.; Son, H.; Park, S.; Kim, J.; Kim, H.; Seonwoo, H.; Choung, Y.-H.; Lee, M.; Chung, J. Aligned Nanofiber-Guided Bone Regeneration Barrier Incorporated with Equine Bone-Derived Hydroxyapatite for Alveolar Bone Regeneration. Polymers 2020, 13, 60. [Google Scholar] [CrossRef]
- Boda, S.K.; Almoshari, Y.; Wang, H.; Wang, X.; Reinhardt, R.A.; Duan, B.; Wang, D.; Xie, J. Mineralized Nanofiber Segments Coupled with Calcium-Binding BMP-2 Peptides for Alveolar Bone Regeneration. Acta Biomater. 2019, 85, 282–293. [Google Scholar] [CrossRef]
- Pouponneau, P.; Perrey, O.; Brunon, C.; Grossiord, C.; Courtois, N.; Salles, V.; Alves, A. Electrospun Bioresorbable Membrane Eluting Chlorhexidine for Dental Implants. Polymers 2020, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Watcharajittanont, N.; Putson, C.; Pripatnanont, P.; Meesane, J. Electrospun Polyurethane Fibrous Membranes of Mimicked Extracellular Matrix for Periodontal Ligament: Molecular Behavior, Mechanical Properties, Morphology, and Osseointegration. J. Biomater. Appl. 2020, 34, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Fan, W.; Xiao, Y.; Hamlet, S.; Hutmacher, D.W.; Ivanovski, S. A Biphasic Scaffold Design Combined with Cell Sheet Technology for Simultaneous Regeneration of Alveolar Bone/Periodontal Ligament Complex. Biomaterials 2012, 33, 5560–5573. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; de Malheiro, A.B.F.B.; van Blitterswijk, C.; Mota, C.; Wieringa, P.A.; Moroni, L. Direct Writing Electrospinning of Scaffolds with Multidimensional Fiber Architecture for Hierarchical Tissue Engineering. ACS Appl. Mater. Interfaces 2017, 9, 38187–38200. [Google Scholar] [CrossRef]
- Moonesi Rad, R.; Atila, D.; Evis, Z.; Keskin, D.; Tezcaner, A. Development of a Novel Functionally Graded Membrane Containing Boron-modified Bioactive Glass Nanoparticles for Guided Bone Regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 1331–1345. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Mitchell, J.; Fernandez-Medina, T.; Kumar, S.; Ivanovski, S. Resorbable Additively Manufactured Scaffold Imparts Dimensional Stability to Extraskeletally Regenerated Bone. Biomaterials 2021, 269, 120671. [Google Scholar] [CrossRef] [PubMed]
- Sudheesh Kumar, P.T.; Hashimi, S.; Saifzadeh, S.; Ivanovski, S.; Vaquette, C. Additively Manufactured Biphasic Construct Loaded with BMP-2 for Vertical Bone Regeneration: A Pilot Study in Rabbit. Mater. Sci. Eng. C 2018, 92, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.; Puppi, D.; Chiellini, F.; Chiellini, E. Additive Manufacturing Techniques for the Production of Tissue Engineering Constructs. J. Tissue Eng. Regen. Med. 2015, 9, 174–190. [Google Scholar] [CrossRef]
- Kaliampakou, C.; Lagopati, N.; Charitidis, C.A. Direct Ink Writing of Alginate–Gelatin Hydrogel: An Optimization of Ink Property Design and Printing Process Efficacy. Appl. Sci. 2023, 13, 8261. [Google Scholar] [CrossRef]
- Verykokou, S.; Ioannidis, C.; Angelopoulos, C. CBCT-Based Design of Patient-Specific 3D Bone Grafts for Periodontal Regeneration. J. Clin. Med. 2023, 12, 5023. [Google Scholar] [CrossRef]
- Yang, W.; Chen, D.; Wang, C.; Apicella, D.; Apicella, A.; Huang, Y.; Li, L.; Zheng, L.; Ji, P.; Wang, L.; et al. The Effect of Bone Defect Size on the 3D Accuracy of Alveolar Bone Augmentation Performed with Additively Manufactured Patient-Specific Titanium Mesh. BMC Oral Health 2022, 22, 557. [Google Scholar] [CrossRef]
- Chi, C.-Y.; Chen, C.-Y.; Huang, J.-Y.; Kuan, C.-Y.; Lin, Y.-Y.; Li, C.-H.; Yang, C.-C.; Lin, F.-H. Preparation and In-Vitro Evaluation of Fe2O3-Doped DP-Bioglass in Combination with 3D-Printing and Selective Laser Sintering Process (3DP-SLS) for Alveolar Bone Augmentation. Ceram. Int. 2021, 47, 12725–12734. [Google Scholar] [CrossRef]
- Rasperini, G.; Pilipchuk, S.P.; Flanagan, C.L.; Park, C.H.; Pagni, G.; Hollister, S.J.; Giannobile, W.V. 3D-Printed Bioresorbable Scaffold for Periodontal Repair. J. Dent. Res. 2015, 94, 153S–157S. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, C.; Li, X.; Fu, G.; Chen, D.; Huang, Y. Research on the Dimensional Accuracy of Customized Bone Augmentation Combined with 3D-printing Individualized Titanium Mesh: A Retrospective Case Series Study. Clin. Implant Dent. Relat. Res. 2021, 23, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, C.H.; Kim, B.K.; Mao, J.J. Anatomically Shaped Tooth and Periodontal Regeneration by Cell Homing. J. Dent. Res. 2010, 89, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Kaliampakou, C.; Lagopati, N.; Pavlatou, E.A.; Charitidis, C.A. Alginate–Gelatin Hydrogel Scaffolds; An Optimization of Post-Printing Treatment for Enhanced Degradation and Swelling Behavior. Gels 2023, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Gillispie, G.J.; Copus, J.S.; Zhang, W.; Atala, A.; Yoo, J.J.; Yelick, P.C.; Lee, S.J. The Effect of BMP-Mimetic Peptide Tethering Bioinks on the Differentiation of Dental Pulp Stem Cells (DPSCs) in 3D Bioprinted Dental Constructs. Biofabrication 2020, 12, 035029. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, X. Synthetic Polymers for Organ 3D Printing. Polymers 2020, 12, 1765. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Kundu, S.; Nag, A.; Xu, Y. 3D Printed Sensors for Biomedical Applications: A Review. Sensors 2019, 19, 1706. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, S.F.; Young, F.A.; Mathews, R.S.; Klawitter, J.J.; Talbert, C.D.; Stelling, F.H. Potential of ceramic materials as permanently implantable skeletal prostheses. J. Biomed. Mater. Res. 1970, 4, 433–456. [Google Scholar] [CrossRef]
- Iviglia, G.; Kargozar, S.; Baino, F. Biomaterials, Current Strategies, and Novel Nano-Technological Approaches for Periodontal Regeneration. J. Funct. Biomater. 2019, 10, 3. [Google Scholar] [CrossRef]
- Jin, Q.M.; Takita, H.; Kohgo, T.; Atsumi, K.; Itoh, H.; Kuboki, Y. Effects of geometry of hydroxyapatite as a cell substratum in BMP-induced ectopic bone formation. J. Biomed. Mater. Res. 2000, 52, 491–499. [Google Scholar] [CrossRef]
- Kuboki, Y.; Jin, Q.; Kikuchi, M.; Mamood, J.; Takita, H. Geometry of artificial ECM: Sizes of pores controlling phenotype expression in BMP-induced osteogenesis and chondrogenesis. Connect. Tissue Res. 2002, 43, 529–534. [Google Scholar] [CrossRef]
- Kuboki, Y.; Jin, Q.; Takita, H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J. Bone Jt. Surg. Am. 2001, 83, S105–S115. [Google Scholar] [CrossRef]
- Groll, J.; Burdick, J.A.; Cho, D.-W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Jüngst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A Definition of Bioinks and Their Distinction from Biomaterial Inks. Biofabrication 2018, 11, 013001. [Google Scholar] [CrossRef] [PubMed]
- Thattaruparambil Raveendran, N.; Vaquette, C.; Meinert, C.; Samuel Ipe, D.; Ivanovski, S. Optimization of 3D Bioprinting of Periodontal Ligament Cells. Dent. Mater. 2019, 35, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.; Moroni, L. High Throughput Screening with Biofabrication Platforms. In Essentials of 3D Biofabrication and Translation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 187–213. [Google Scholar]
- Narazaki, A.; Oyane, A.; Miyaji, H. Laser-Induced Forward Transfer with Optical Stamp of a Protein-Immobilized Calcium Phosphate Film Prepared by Biomimetic Process to a Human Dentin. Appl. Sci. 2020, 10, 7984. [Google Scholar] [CrossRef]
- Catros, S.; Fricain, J.-C.; Guillotin, B.; Pippenger, B.; Bareille, R.; Remy, M.; Lebraud, E.; Desbat, B.; Amédée, J.; Guillemot, F. Laser-Assisted Bioprinting for Creating on-Demand Patterns of Human Osteoprogenitor Cells and Nano-Hydroxyapatite. Biofabrication 2011, 3, 025001. [Google Scholar] [CrossRef]
- Ventura, R.D. An Overview of Laser-Assisted Bioprinting (LAB) in Tissue Engineering Applications. Med. Lasers 2021, 10, 76–81. [Google Scholar] [CrossRef]
- Kérourédan, O.; Ribot, E.J.; Fricain, J.-C.; Devillard, R.; Miraux, S. Magnetic Resonance Imaging for Tracking Cellular Patterns Obtained by Laser-Assisted Bioprinting. Sci. Rep. 2018, 8, 15777. [Google Scholar] [CrossRef]
- Preethi Soundarya, S.; Haritha Menon, A.; Viji Chandran, S.; Selvamurugan, N. Bone Tissue Engineering: Scaffold Preparation Using Chitosan and Other Biomaterials with Different Design and Fabrication Techniques. Int. J. Biol. Macromol. 2018, 119, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Lagopati, N.; Pippa, N.; Gatou, M.-A.; Papadopoulou-Fermeli, N.; Gorgoulis, V.G.; Gazouli, M.; Pavlatou, E.A. Marine-Originated Materials and Their Potential Use in Biomedicine. Appl. Sci. 2023, 13, 9172. [Google Scholar] [CrossRef]
- Shrestha, S.; Shrestha, B.K.; Ko, S.W.; Kandel, R.; Park, C.H.; Kim, C.S. Engineered cellular microenvironments from functionalized multiwalled carbon nanotubes integrating Zein/Chitosan @Polyurethane for bone cell regeneration. Carbohydr. Polym. 2021, 251, 117035. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X.; Langer, R. Fabrication of Biodegradable Polymer Foams for Cell Transplantation and Tissue Engineering. In Tissue Engineering; Humana Press: Totowa, NJ, USA, 1999; pp. 47–56. [Google Scholar]
- Zimina, A.; Senatov, F.; Choudhary, R.; Kolesnikov, E.; Anisimova, N.; Kiselevskiy, M.; Orlova, P.; Strukova, N.; Generalova, M.; Manskikh, V.; et al. Biocompatibility and Physico-Chemical Properties of Highly Porous PLA/HA Scaffolds for Bone Reconstruction. Polymers 2020, 12, 2938. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-T.; Ramesh, N.-S. Polymeric Foams; Lee, S.-T., Ramesh, N.S., Eds.; CRC Press: Boca Raton, FL, USA, 2004; ISBN 9780203506141. [Google Scholar]
- Fanovich, M.A.; Di Maio, E.; Salerno, A. Current Trend and New Opportunities for Multifunctional Bio-Scaffold Fabrication via High-Pressure Foaming. J. Funct. Biomater. 2023, 14, 480. [Google Scholar] [CrossRef] [PubMed]
- Chinnasami, H.; Dey, M.K.; Devireddy, R. Three-Dimensional Scaffolds for Bone Tissue Engineering. Bioengineering 2023, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.I. Polymer Synthesis and Processing Using Supercritical Carbon Dioxide. J. Mater. Chem. 2000, 10, 207–234. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Pimkhaokham, A.; Ampornaramveth, R.S. Chitosan-Based Scaffold for Mineralized Tissues Regeneration. Mar. Drugs 2021, 19, 551. [Google Scholar] [CrossRef]
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. BioMed Res. Int. 2017, 2017, 9831534. [Google Scholar] [CrossRef]
- Santoso, E.G.; Yoshida, K.; Hirota, Y.; Aizawa, M.; Yoshino, O.; Kishida, A.; Osuga, Y.; Saito, S.; Ushida, T.; Furukawa, K.S. Application of Detergents or High Hydrostatic Pressure as Decellularization Processes in Uterine Tissues and Their Subsequent Effects on In Vivo Uterine Regeneration in Murine Models. PLoS ONE 2014, 9, e103201. [Google Scholar] [CrossRef] [PubMed]
- Smoak, M.M.; Han, A.; Watson, E.; Kishan, A.; Grande-Allen, K.J.; Cosgriff-Hernandez, E.; Mikos, A.G. Fabrication and Characterization of Electrospun Decellularized Muscle-Derived Scaffolds. Tissue Eng. Part C Methods 2019, 25, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.S.; Cordeiro, R.; Moura, C.S.; Cabral, J.M.S.; Castelo Ferreira, F.; Silva, J.C.; Carvalho, M.S. Bioactive Nanofibrous Scaffolds Incorporating Decellularized Cell-Derived Extracellular Matrix for Periodontal Tissue Engineering. ACS Appl. Nano Mater. 2024, 7, 4501–4517. [Google Scholar] [CrossRef]
- Leong, K.F.; Cheah, C.M.; Chua, C.K. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 2003, 24, 2363–2378. [Google Scholar] [CrossRef]
- Yuan, B.; Zhou, S.Y.; Chen, X.S. Rapid prototyping technology and its application in bone tissue engineering. J. Zhejiang Univ. Sci. B. 2017, 18, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Rider, P.; Kačarević, Ž.P.; Alkildani, S.; Retnasingh, S.; Schnettler, R.; Barbeck, M. Additive Manufacturing for Guided Bone Regeneration: A Perspective for Alveolar Ridge Augmentation. Int. J. Mol. Sci. 2018, 19, 3308. [Google Scholar] [CrossRef] [PubMed]
- Tom, T.; Sreenilayam, S.P.; Brabazon, D.; Jose, J.P.; Joseph, B.; Madanan, K.; Thomas, S. Additive manufacturing in the biomedical field-recent research developments. Results Eng. 2022, 16, 100661. [Google Scholar] [CrossRef]
- Alqahtani, A.M. Guided Tissue and Bone Regeneration Membranes: A Review of Biomaterials and Techniques for Periodont Treatments. Polymers 2023, 15, 3355. [Google Scholar] [CrossRef]
- Stafin, K.; Śliwa, P.; Piątkowski, M. Towards Polycaprolactone-Based Scaffolds for Alveolar Bone Tissue Engineering: A Biomimetic Approach in a 3D Printing Technique. Int. J. Mol. Sci. 2023, 24, 16180. [Google Scholar] [CrossRef]
- Khalaf, A.T.; Wei, Y.; Wan, J.; Zhu, J.; Peng, Y.; Abdul Kadir, S.Y.; Zainol, J.; Oglah, Z.; Cheng, L.; Shi, Z. Bone Tissue Engineering through 3D Bioprinting of Bioceramic Scaffolds: A Review and Update. Life 2022, 12, 903. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 3429527, 2019. [Google Scholar] [CrossRef]
- Anjum, S.; Rahman, F.; Pandey, P.; Arya, D.K.; Alam, M.; Rajinikanth, P.S.; Ao, Q. Electrospun Biomimetic Nanofibrous Scaffolds: A Promising Prospect for Bone Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 9206. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, S.G.; James, R.; Nukavarapu, S.P.; Laurencin, C.T. Electrospun nanofiber scaffolds: Engineering soft tissues. Biomed. Mater. 2008, 3, 034002. [Google Scholar] [CrossRef]
- Khoramgah, M.S.; Ranjbari, J.; Abbaszadeh, H.A.; Tabatabaei Mirakabad, F.S.; Hatami, S.; Hosseinzadeh, S.; Ghanbarian, H. Freeze-dried multiscale porous nanofibrous three dimensional scaffolds for bone regenerations. Bioimpacts 2020, 10, 73–85. [Google Scholar] [CrossRef]
- Biscaia, S.; Branquinho, M.V.; Alvites, R.D.; Fonseca, R.; Sousa, A.C.; Pedrosa, S.S.; Caseiro, A.R.; Guedes, F.; Patrício, T.; Viana, T.; et al. 3D Printed Poly(ε-caprolactone)/Hydroxyapatite Scaffolds for Bone Tissue Engineering: A Comparative Study on a Composite Preparation by Melt Blending or Solvent Casting Techniques and the Influence of Bioceramic Content on Scaffold Properties. Int. J. Mol. Sci. 2022, 23, 2318. [Google Scholar] [CrossRef] [PubMed]
- Naghieh, S.; Sarker, M.; Izadifar, M.; Chen, X. Dispensing-based bioprinting of mechanically-functional hybrid scaffolds with vessel-like channels for tissue engineering applications—A brief review. J. Mech. Behav. Biomed. Mater. 2018, 78, 298–314. [Google Scholar] [CrossRef]
- Kim, D.; Lee, H.; Lee, G.-H.; Hoang, T.-H.; Kim, H.-R.; Kim, G.H. Fabrication of bone-derived decellularized extracellular matrix/ceramic-based biocomposites and their osteo/odontogenic differentiation ability for dentin regeneration. Bioeng. Transl. Med. 2022, 7, e10317. [Google Scholar] [CrossRef]
- Ostrovidov, S.; Ramalingam, M.; Bae, H.; Orive, G.; Fujie, T.; Shi, X.; Kaji, H. Bioprinting and biomaterials for dental alveolar tissue regeneration. Front. Bioeng. Biotechnol. 2023, 11, 991821. [Google Scholar] [CrossRef]
- Ananth, K.P.; Jayram, N.D. A comprehensive review of 3D printing techniques for biomaterial-based scaffold fabrication in bone tissue engineering. Ann. 3D Print. Med. 2024, 13, 100141. [Google Scholar] [CrossRef]
- Logeshwaran, A.; Elsen, R.; Nayak, S. Artificial Intelligence-Based 3D Printing Strategies for Bone Scaffold Fabrication and Its Application in Preclinical and Clinical Investigations. ACS Biomater. Sci. Eng. 2024, 10, 677–696. [Google Scholar] [CrossRef]
- Rahmati, M.; Pennisi, C.P.; Budd, E.; Mobasheri, A.; Mozafari, M. Biomaterials for Regenerative Medicine: Historical Perspectives and Current Trends. In Cell Biology and Translational Medicine; Springer: Cham, Switzerland, 2018; pp. 1–19. [Google Scholar]
- Geevarghese, R.; Sajjadi, S.S.; Hudecki, A.; Sajjadi, S.; Jalal, N.R.; Madrakian, T.; Ahmadi, M.; Włodarczyk-Biegun, M.K.; Ghavami, S.; Likus, W.; et al. Biodegradable and Non-Biodegradable Biomaterials and Their Effect on Cell Differentiation. Int. J. Mol. Sci. 2022, 23, 16185. [Google Scholar] [CrossRef]
- Singh, A.; Elisseeff, J. Biomaterials for Stem Cell Differentiation. J. Mater. Chem. 2010, 20, 8832. [Google Scholar] [CrossRef]
- Dawson, E.; Mapili, G.; Erickson, K.; Taqvi, S.; Roy, K. Biomaterials for Stem Cell Differentiation. Adv. Drug Deliv. Rev. 2008, 60, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Prato, G.P.; Cortellini, P. Factors Affecting the Healing Response of Intrabony Defects Following Guided Tissue Regeneration and Access Flap Surgery. J. Clin. Periodontol. 1996, 23, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Sufaru, I.-G.; Macovei, G.; Stoleriu, S.; Martu, M.-A.; Luchian, I.; Kappenberg-Nitescu, D.-C.; Solomon, S.M. 3D Printed and Bioprinted Membranes and Scaffolds for the Periodontal Tissue Regeneration: A Narrative Review. Membranes 2022, 12, 902. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.-S.D.; Costa, P.F.; Vaquette, C.; Ivanovski, S.; Hutmacher, D.W.; Malda, J. Additive Biomanufacturing: An Advanced Approach for Periodontal Tissue Regeneration. Ann. Biomed. Eng. 2017, 45, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Daghrery, A.; de Souza Araújo, I.J.; Castilho, M.; Malda, J.; Bottino, M.C. Unveiling the Potential of Melt Electrowriting in Regenerative Dental Medicine. Acta Biomater. 2023, 156, 88–109. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Tai, W.; Ho, M.; Chang, P. Combination of a Biomolecule-aided Biphasic Cryogel Scaffold with a Barrier Membrane Adhering PDGF-encapsulated Nanofibers to Promote Periodontal Regeneration. J. Periodontal Res. 2020, 55, 529–538. [Google Scholar] [CrossRef]
- Costa, P.F.; Vaquette, C.; Zhang, Q.; Reis, R.L.; Ivanovski, S.; Hutmacher, D.W. Advanced Tissue Engineering Scaffold Design for Regeneration of the Complex Hierarchical Periodontal Structure. J. Clin. Periodontol. 2014, 41, 283–294. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, S.; Zhou, C.; Cheng, L.; Gao, X.; Xie, X.; Sun, J.; Wang, H.; Weir, M.D.; Reynolds, M.A.; et al. Advanced Smart Biomaterials and Constructs for Hard Tissue Engineering and Regeneration. Bone Res. 2018, 6, 31. [Google Scholar] [CrossRef]
- Motamedian, S.R.; Hosseinpour, S.; Ahsaie, M.G.; Khojasteh, A. Smart Scaffolds in Bone Tissue Engineering: A Systematic Review of Literature. World J. Stem Cells 2015, 7, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Tanaka, M. Designing Smart Biomaterials for Tissue Engineering. Int. J. Mol. Sci. 2017, 19, 17. [Google Scholar] [CrossRef]
- Mittal, A.; Negi, P.; Garkhal, K.; Verma, S.; Kumar, N. Integration of Porosity and Bio-Functionalization to Form a 3D Scaffold: Cell Culture Studies and in Vitro Degradation. Biomed. Mater. 2010, 5, 045001. [Google Scholar] [CrossRef]
- Zeng, D.; Zhang, X.; Wang, X.; Huang, Q.; Wen, J.; Miao, X.; Peng, L.; Li, Y.; Jiang, X. The Osteoimmunomodulatory Properties of MBG Scaffold Coated with Amino Functional Groups. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, K.; Gong, T.; Song, J.; Bao, C.; Luo, E.; Weng, J.; Zhou, S. Delivery of Growth Factors Using a Smart Porous Nanocomposite Scaffold to Repair a Mandibular Bone Defect. Biomacromolecules 2014, 15, 1019–1030. [Google Scholar] [CrossRef]
- Damaraju, S.M.; Shen, Y.; Elele, E.; Khusid, B.; Eshghinejad, A.; Li, J.; Jaffe, M.; Arinzeh, T.L. Three-Dimensional Piezoelectric Fibrous Scaffolds Selectively Promote Mesenchymal Stem Cell Differentiation. Biomaterials 2017, 149, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Dan, P.; Sosnik, A.; Kalarikkal, N.; Tran, N.; Vincent, B.; Thomas, S.; Menu, P.; Rouxel, D. Electrospun Poly(Vinylidene Fluoride-Trifluoroethylene)/Zinc Oxide Nanocomposite Tissue Engineering Scaffolds with Enhanced Cell Adhesion and Blood Vessel Formation. Nano Res. 2017, 10, 3358–3376. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent Advances in 3D Printing of Biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef]
- Yu, N.; Nguyen, T.; Cho, Y.D.; Kavanagh, N.M.; Ghassib, I.; Giannobile, W.V. Personalized Scaffolding Technologies for Alveolar Bone Regenerative Medicine. Orthod. Craniofac. Res. 2019, 22, 69–75. [Google Scholar] [CrossRef]
- Yazdanian, M.; Arefi, A.H.; Alam, M.; Abbasi, K.; Tebyaniyan, H.; Tahmasebi, E.; Ranjbar, R.; Seifalian, A.; Rahbar, M. Decellularized and biological scaffolds in dental and craniofacial tissue engineering: A comprehensive overview. J. Mater. Res. Technol. 2021, 15, 1217–1251. [Google Scholar] [CrossRef]
- Park, S.-H.; Kang, B.-K.; Lee, J.E.; Chun, S.W.; Jang, K.; Kim, Y.H.; Jeong, M.A.; Kim, Y.; Kang, K.; Lee, N.K.; et al. Design and Fabrication of a Thin-Walled Free-Form Scaffold on the Basis of Medical Image Data and a 3D Printed Template: Its Potential Use in Bile Duct Regeneration. ACS Appl. Mater. Interfaces 2017, 9, 12290–12298. [Google Scholar] [CrossRef]
- Hollister, S.J.; Lin, C.Y.; Saito, E.; Lin, C.Y.; Schek, R.D.; Taboas, J.M.; Williams, J.M.; Partee, B.; Flanagan, C.L.; Diggs, A.; et al. Engineering Craniofacial Scaffolds. Orthod. Craniofac. Res. 2005, 8, 162–173. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Zielińska, A.; Karczewski, J.; Eder, P.; Kolanowski, T.; Szalata, M.; Wielgus, K.; Szalata, M.; Kim, D.; Shin, S.R.; Słomski, R.; et al. Scaffolds for Drug Delivery and Tissue Engineering: The Role of Genetics. J. Control. Release 2023, 359, 207–223. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Kantorski, K.Z.; Dubey, N.; Daghrery, A.; Fenno, J.C.; Mishina, Y.; Chan, H.-L.; Mendonça, G.; Bottino, M.C. Personalized and Defect-Specific Antibiotic-Laden Scaffolds for Periodontal Infection Ablation. ACS Appl. Mater. Interfaces 2021, 13, 49642–49657. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Daghrery, A.; Dubey, N.; Li, C.; Mei, L.; Fenno, J.C.; Schwendeman, A.; Aytac, Z.; Bottino, M.C. Hybrid Antimicrobial Hydrogel as Injectable Therapeutics for Oral Infection Ablation. Biomacromolecules 2020, 21, 3945–3956. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, T.E. The Management of Inflammation in Periodontal Disease. J. Periodontol. 2008, 79, 1601–1608. [Google Scholar] [CrossRef]
- Hu, Z.; Ma, C.; Rong, X.; Zou, S.; Liu, X. Immunomodulatory ECM-like Microspheres for Accelerated Bone Regeneration in Diabetes Mellitus. ACS Appl. Mater. Interfaces 2018, 10, 2377–2390. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Luan, X.; Liu, X. Recent Advances in Periodontal Regeneration: A Biomaterial Perspective. Bioact. Mater. 2020, 5, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Yar, M.; Farooq, A.; Shahzadi, L.; Khan, A.S.; Mahmood, N.; Rauf, A.; Chaudhry, A.A.; ur Rehman, I. Novel Meloxicam Releasing Electrospun Polymer/Ceramic Reinforced Biodegradable Membranes for Periodontal Regeneration Applications. Mater. Sci. Eng. C 2016, 64, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gu, Z.; Chen, X.; Shi, C.; Liu, C.; Liu, M.; Wang, L.; Sun, M.; Zhang, K.; Liu, Q.; et al. An Injectable and Thermosensitive Hydrogel: Promoting Periodontal Regeneration by Controlled-Release of Aspirin and Erythropoietin. Acta Biomater. 2019, 86, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Batool, F.; Morand, D.-N.; Thomas, L.; Bugueno, I.; Aragon, J.; Irusta, S.; Keller, L.; Benkirane-Jessel, N.; Tenenbaum, H.; Huck, O. Synthesis of a Novel Electrospun Polycaprolactone Scaffold Functionalized with Ibuprofen for Periodontal Regeneration: An In Vitro and In Vivo Study. Materials 2018, 11, 580. [Google Scholar] [CrossRef] [PubMed]
- Lagopati, N.; Kotsinas, A.; Veroutis, D.; Evangelou, K.; Papaspyropoulos, A.; Arfanis, M.; Falaras, P.; Kitsiou, P.V.; Pateras, I.; Bergonzini, A.; et al. Effect of Silver-modified Nanostructured Titanium Dioxide in Cancer. Cancer Genom. Proteom. 2021, 18 (Suppl. S3), 425–439. [Google Scholar] [CrossRef] [PubMed]
- Pantelis, P.; Theocharous, G.; Lagopati, N.; Veroutis, D.; Thanos, D.-F.; Lampoglou, G.-P.; Pippa, N.; Gatou, M.-A.; Tremi, I.; Papaspyropoulos, A.; et al. The Dual Role of Oxidative-Stress-Induced Autophagy in Cellular Senescence: Comprehension and Therapeutic Approaches. Antioxidants 2023, 12, 169. [Google Scholar] [CrossRef]
- Lagopati, N.; Valamvanos, T.-F.; Proutsou, V.; Karachalios, K.; Pippa, N.; Gatou, M.-A.; Vagena, I.-A.; Cela, S.; Pavlatou, E.A.; Gazouli, M.; et al. The Role of Nano-Sensors in Breath Analysis for Early and Non-Invasive Disease Diagnosis. Chemosensors 2023, 11, 317. [Google Scholar] [CrossRef]
- Abedi, N.; Rajabi, N.; Kharaziha, M.; Nejatidanesh, F.; Tayebi, L. Layered Scaffolds in Periodontal Regeneration. J. Oral Biol. Craniofac. Res. 2022, 12, 782–797. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).