Effect of Surface Morphology and Internal Structure on the Tribological Behaviors of Snake Scales from Dinodon rufozonatum
Abstract
1. Introduction
2. Materials and Methods
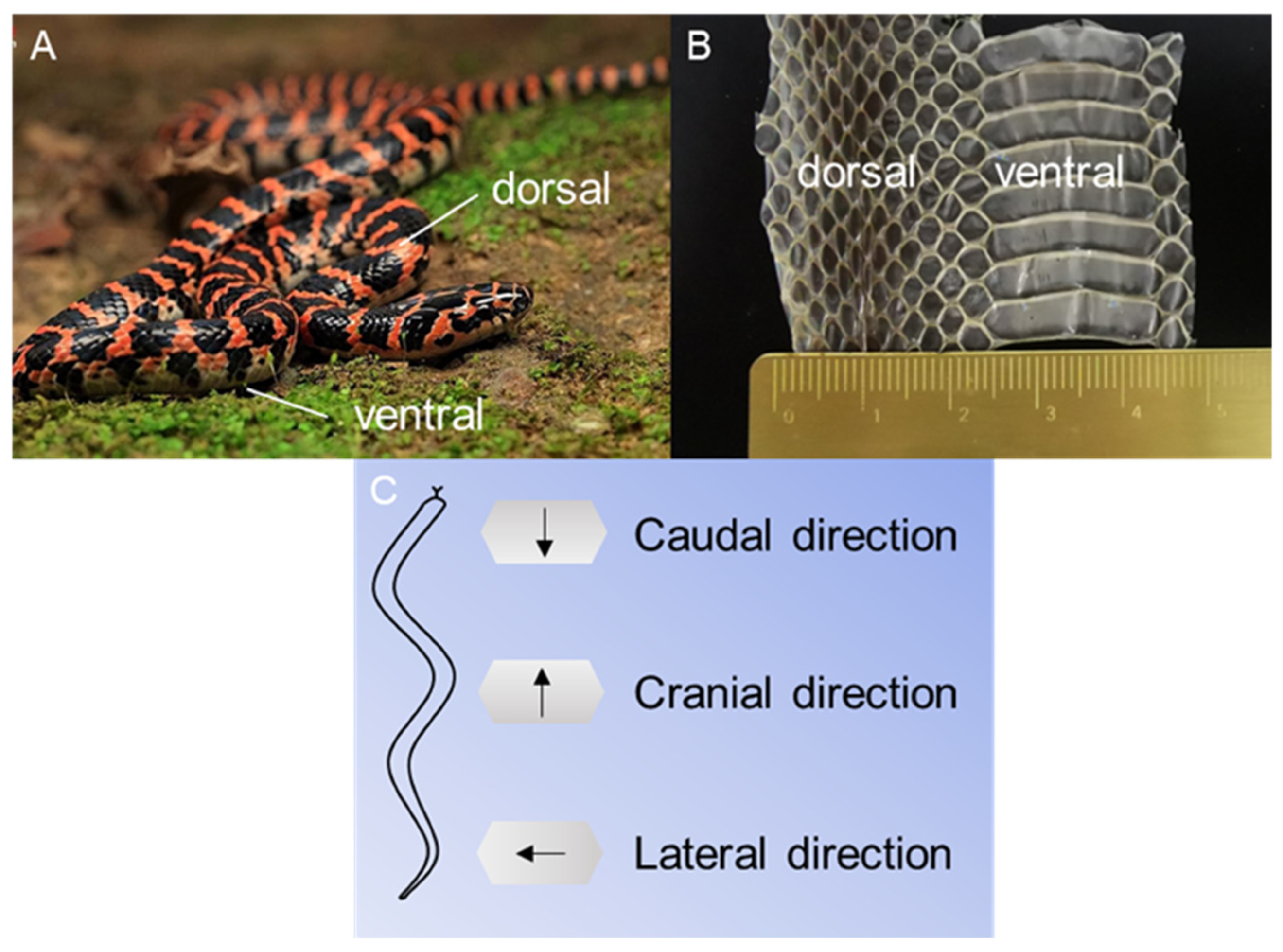
2.1. Animals and Preparations
2.2. Atomic Force Microscopy (AFM)
2.3. Nanoindentation Test Procedure
2.4. Scratch Test Procedure
2.5. Scanning Electron Microscopy (SEM)
2.6. Raman Spectroscopy (RS)
2.7. Tribological Testing of Biomimetic Surfaces
3. Results
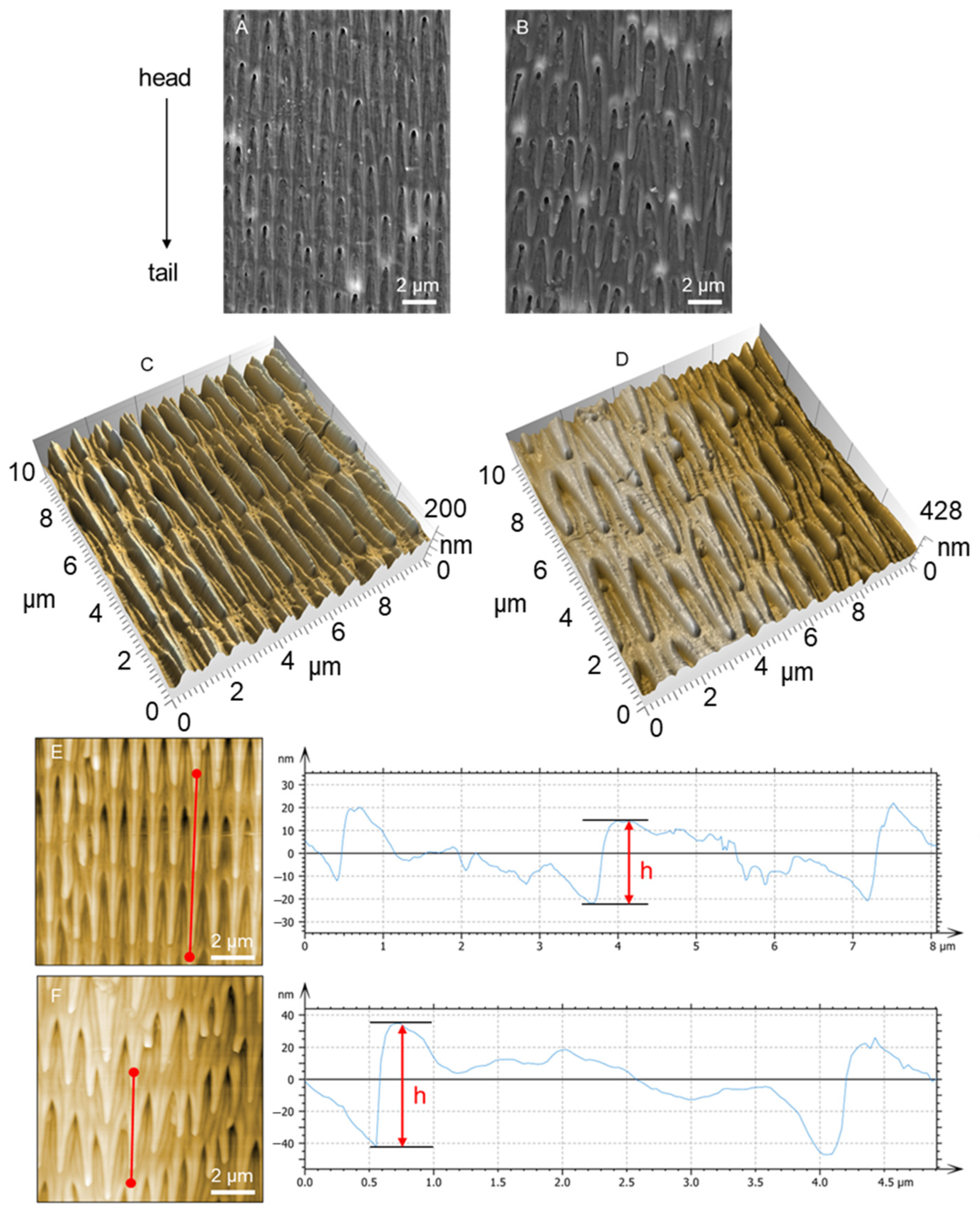
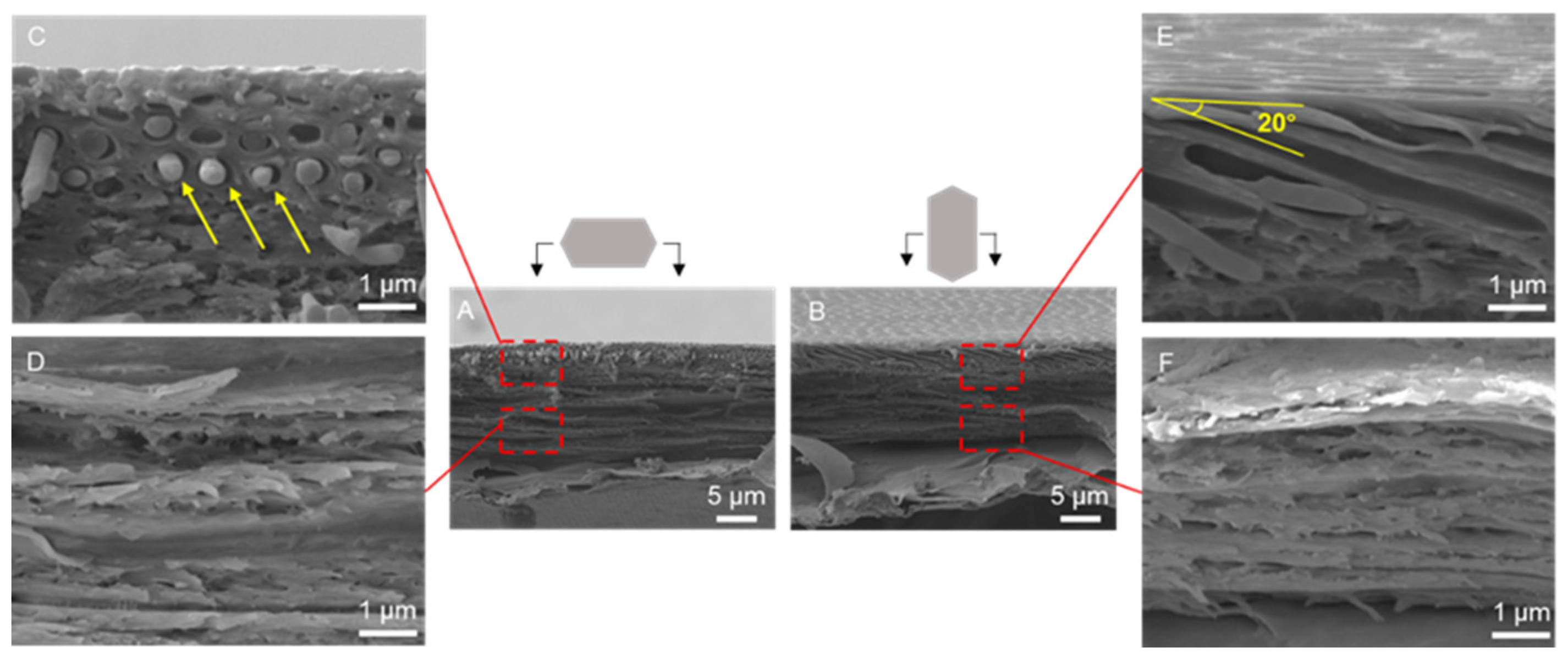
3.1. Morphology and Structure of Scales
3.2. Mechanical Properties of the Scales
3.3. Coefficient of Friction of the Scales
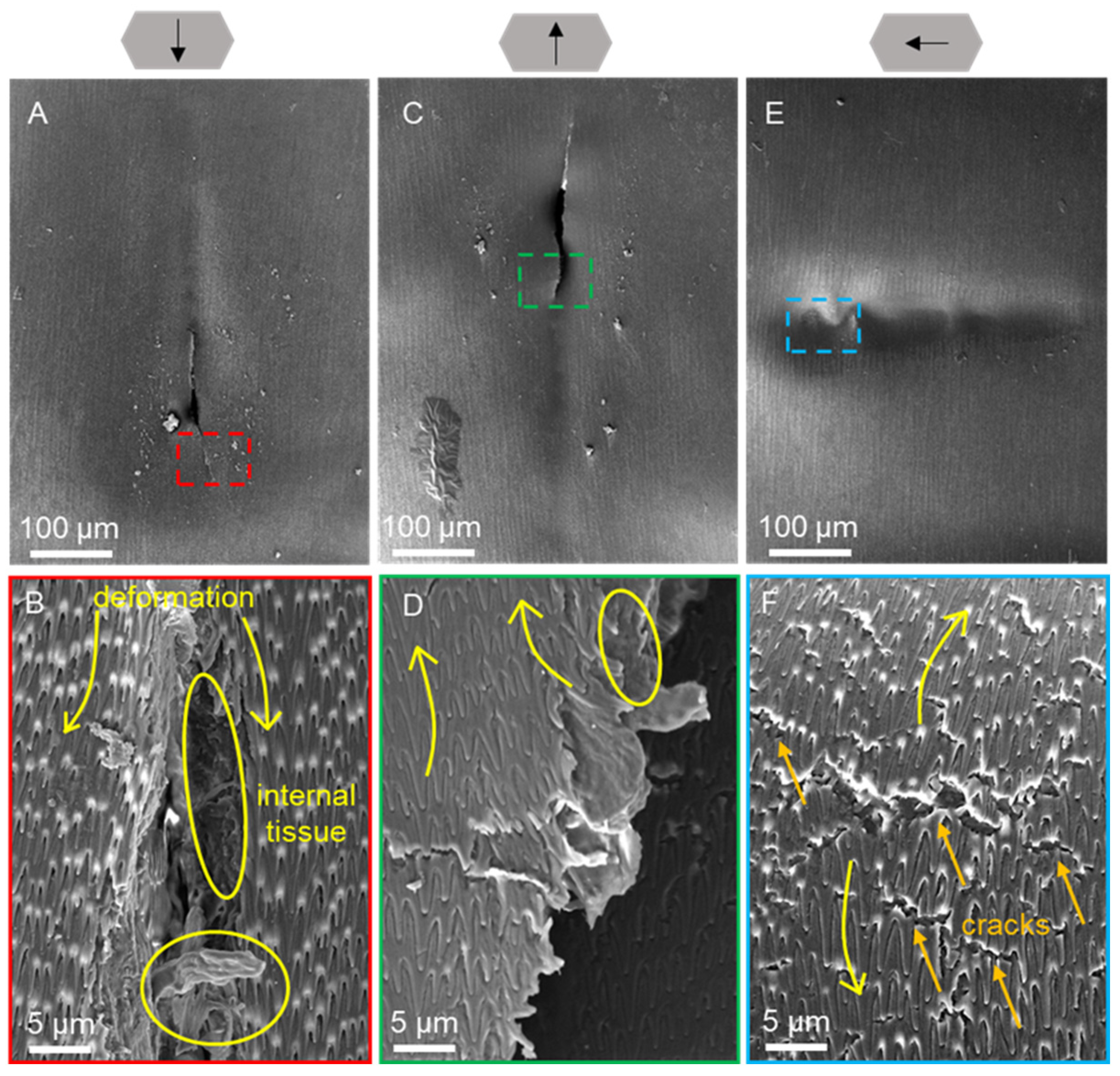
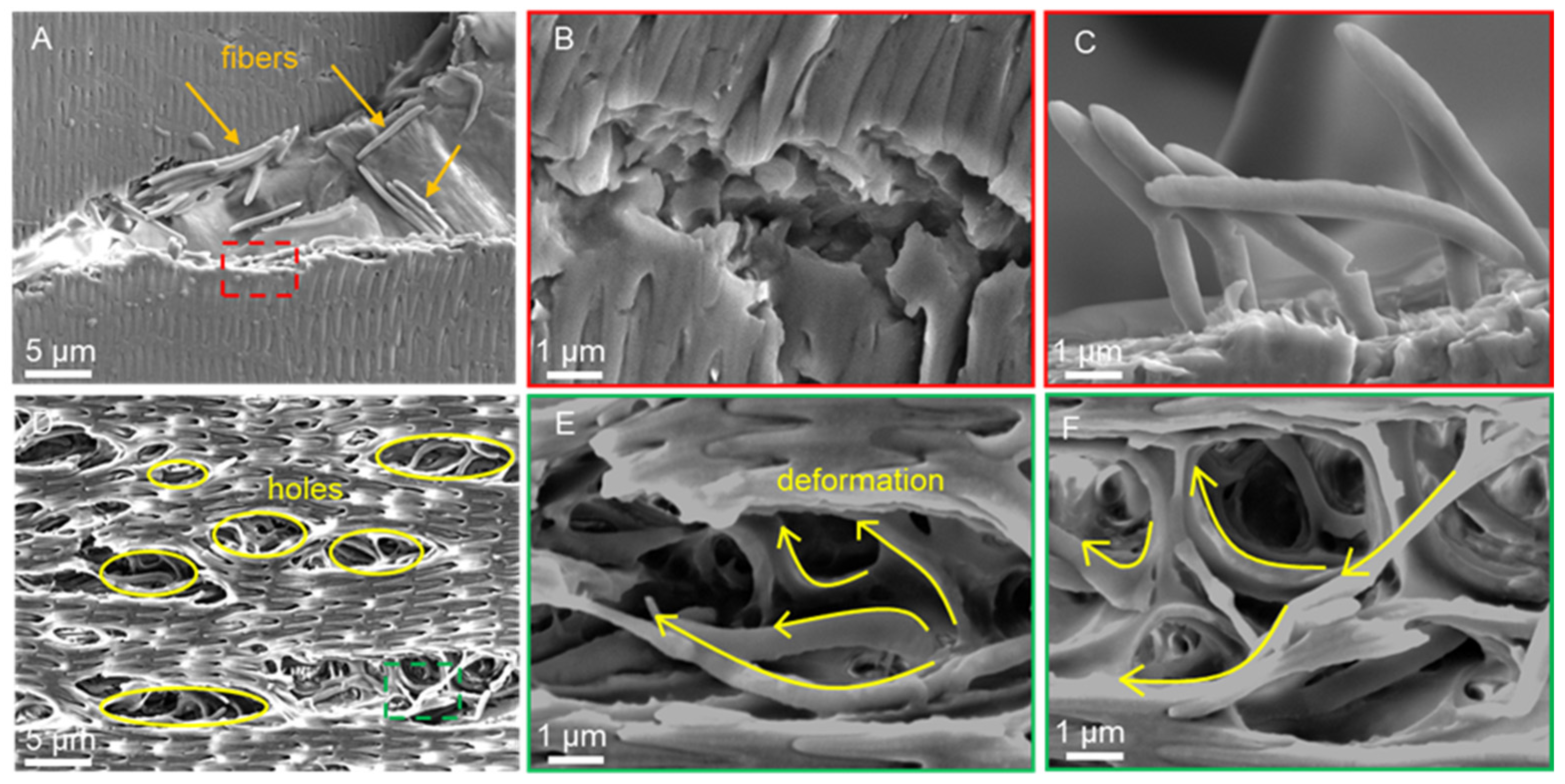
3.4. Scratch Morphology of Dry Scales
3.5. Scratch Morphology of Wet Scales
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nepal, D.; Kang, S.; Adstedt, K.M.; Kanhaiya, K.; Bockstaller, M.R.; Brinson, L.C.; Buehler, M.J.; Coveney, P.V.; Dayal, K.; El-Awady, J.A.; et al. Hierarchically Structured Bioinspired Nanocomposites. Nat. Mater. 2023, 22, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, H.A.; El Mansori, M. Tribological Analysis of the Ventral Scale Structure in a Python Regius in Relation to Laser Textured Surfaces. Surf. Topogr. Metrol. Prop. 2013, 1, 015001. [Google Scholar] [CrossRef]
- Zheng, L.; Zhong, Y.; Gao, Y.; Li, J.; Zhang, Z.; Liu, Z.; Ren, L. Coupling Effect of Morphology and Mechanical Properties Contributes to the Tribological Behaviors of Snake Scales. J. Bionic Eng. 2018, 15, 481–493. [Google Scholar] [CrossRef]
- Comanns, P.; Winands, K.; Pothen, M.; Bott, R.A.; Wagner, H.; Baumgartner, W. The Texas Horned Lizard as Model for Robust Capillary Structures for Passive Directional Transport of Cooling Lubricants. In Bioinspiration, Biomimetics, and Bioreplication 2016; Martín-Palma, R.J., Lakhtakia, A., Knez, M., Eds.; SPIE: Las Vegas, NV, USA, 2016; p. 979711. [Google Scholar]
- Liu, Z.; Yin, W.; Tao, D.; Tian, Y. A Glimpse of Superb Tribological Designs in Nature. Biotribology 2015, 1, 11–23. [Google Scholar] [CrossRef]
- Filippov, A.; Gorb, S.N. Frictional-Anisotropy-Based Systems in Biology: Structural Diversity and Numerical Model. Sci. Rep. 2013, 3, 1240. [Google Scholar] [CrossRef]
- Greiner, C.; Schäfer, M. Bio-Inspired Scale-like Surface Textures and Their Tribological Properties. Bioinspir. Biomim. 2015, 10, 044001. [Google Scholar] [CrossRef] [PubMed]
- Fears, K.P.; Kolel-Veetil, M.K.; Barlow, D.E.; Bernstein, N.; So, C.R.; Wahl, K.J.; Li, X.; Kulp, J.L.; Latour, R.A.; Clark, T.D. High-Performance Nanomaterials Formed by Rigid yet Extensible Cyclic β-Peptide Polymers. Nat. Commun. 2018, 9, 4090. [Google Scholar] [CrossRef]
- Liu, Z.; Meyers, M.A.; Zhang, Z.; Ritchie, R.O. Functional Gradients and Heterogeneities in Biological Materials: Design Principles, Functions, and Bioinspired Applications. Prog. Mater. Sci. 2017, 88, 467–498. [Google Scholar] [CrossRef]
- Meyers, M.A.; McKittrick, J.; Chen, P.-Y. Structural Biological Materials: Critical Mechanics-Materials Connections. Science 2013, 339, 773–779. [Google Scholar] [CrossRef]
- Naleway, S.E.; Porter, M.M.; McKittrick, J.; Meyers, M.A. Structural Design Elements in Biological Materials: Application to Bioinspiration. Adv. Mater. 2015, 27, 5455–5476. [Google Scholar] [CrossRef]
- Vitt, L.J.; Caldwell, J.P. Herpetology: An Introductory Biology of Amphibians and Reptiles, 4th ed.; Academic Press: Boston, MA, USA, 2014; pp. 195–284. [Google Scholar]
- Abdel-Aal, H.A. Surface Structure and Tribology of Legless Squamate Reptiles. J. Mech. Behav. Biomed. Mater. 2018, 79, 354–398. [Google Scholar] [CrossRef] [PubMed]
- Baio, J.E.; Spinner, M.; Jaye, C.; Fischer, D.A.; Gorb, S.N.; Weidner, T. Evidence of a Molecular Boundary Lubricant at Snakeskin Surfaces. J. R. Soc. Interface 2015, 12, 20150817. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wu, J.; Zhang, S.; Sun, S.; Zhang, Z.; Liang, S.; Liu, Z.; Ren, L. Bionic Coupling of Hardness Gradient to Surface Texture for Improved Anti-Wear Properties. J. Bionic Eng. 2016, 13, 406–415. [Google Scholar] [CrossRef]
- Spinner, M.; Bleckmann, H.; Westhoff, G. Morphology and Frictional Properties of Scales of Pseudopus apodus (Anguidae, Reptilia). Zoology 2015, 118, 171–175. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, Y.; Ren, L. Particle Erosion Resistance of Bionic Samples Inspired from Skin Structure of Desert Lizard, Laudakin stoliczkana. J. Bionic Eng. 2012, 9, 465–469. [Google Scholar] [CrossRef]
- Mühlberger, M.; Rohn, M.; Danzberger, J.; Sonntag, E.; Rank, A.; Schumm, L.; Kirchner, R.; Forsich, C.; Gorb, S.; Einwögerer, B.; et al. UV-NIL Fabricated Bio-Inspired Inlays for Injection Molding to Influence the Friction Behavior of Ceramic Surfaces. Microelectron. Eng. 2015, 141, 140–144. [Google Scholar] [CrossRef]
- Baumgartner, W.; Saxe, F.; Weth, A.; Hajas, D.; Sigumonrong, D.; Emmerlich, J.; Singheiser, M.; Böhme, W.; Schneider, J.M. The Sandfish’s Skin: Morphology, Chemistry and Reconstruction. J. Bionic Eng. 2007, 4, 1–9. [Google Scholar] [CrossRef]
- Cuervo, P.; López, D.A.; Cano, J.P.; Sánchez, J.C.; Rudas, S.; Estupiñán, H.; Toro, A.; Abdel-Aal, H.A. Development of Low Friction Snake-Inspired Deterministic Textured Surfaces. Surf. Topogr. Metrol. Prop. 2016, 4, 024013. [Google Scholar] [CrossRef][Green Version]
- Abdel-Aal, H.A. Functional Surfaces for Tribological Applications: Inspiration and Design. Surf. Topogr. Metrol. Prop. 2016, 4, 043001. [Google Scholar] [CrossRef]
- Baum, M.J.; Heepe, L.; Fadeeva, E.; Gorb, S.N. Dry Friction of Microstructured Polymer Surfaces Inspired by Snake Skin. Beilstein J. Nanotechnol. 2014, 5, 1091–1103. [Google Scholar] [CrossRef]
- Baum, M.J.; Heepe, L.; Gorb, S.N. Friction Behavior of a Microstructured Polymer Surface Inspired by Snake Skin. Beilstein J. Nanotechnol. 2014, 5, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.-C.G.; Gorb, S.N. Ultrastructure and Wear Patterns of the Ventral Epidermis of Four Snake Species (Squamata, Serpentes). Zoology 2014, 117, 295–314. [Google Scholar] [CrossRef] [PubMed]
- Kovalev, A.; Filippov, A.; Gorb, S.N. Correlation Analysis of Symmetry Breaking in the Surface Nanostructure Ordering: Case Study of the Ventral Scale of the Snake Morelia viridis. Appl. Phys. A 2016, 122, 253. [Google Scholar] [CrossRef]
- Filippov, A.E.; Gorb, S.N. Modelling of the Frictional Behaviour of the Snake Skin Covered by Anisotropic Surface Nanostructures. Sci. Rep. 2016, 6, 23539. [Google Scholar] [CrossRef]
- Krey, K.; Farajallah, A. Skin Histology and Microtopography of Papuan White Snake (Micropechis ikaheka) in Relation to Their Zoogeographical Distribution. HAYATI J. Biosci. 2013, 20, 7–14. [Google Scholar] [CrossRef][Green Version]
- Arola, D.; Murcia, S.; Stossel, M.; Pahuja, R.; Linley, T.; Devaraj, A.; Ramulu, M.; Ossa, E.A.; Wang, J. The Limiting Layer of Fish Scales: Structure and Properties. Acta Biomater. 2018, 67, 319–330. [Google Scholar] [CrossRef]
- Murcia, S.; Lavoie, E.; Linley, T.; Devaraj, A.; Ossa, E.A.; Arola, D. The Natural Armors of Fish: A Comparison of the Lamination Pattern and Structure of Scales. J. Mech. Behav. Biomed. Mater. 2017, 73, 17–27. [Google Scholar] [CrossRef]
- Murcia, S.; Miyamoto, Y.; Varma, M.P.; Ossa, A.; Arola, D. Contributions of the Layer Topology and Mineral Content to the Elastic Modulus and Strength of Fish Scales. J. Mech. Behav. Biomed. Mater. 2018, 78, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Toni, M.; Alibardi, L. Alpha- and Beta-Keratins of the Snake Epidermis. Zoology 2007, 110, 41–47. [Google Scholar] [CrossRef]
- Drelich, A.J.; Monteiro, S.N.; Brookins, J.; Drelich, J.W. Fish Skin: A Natural Inspiration for Innovation. Adv. Biosyst. 2018, 2, 1800055. [Google Scholar] [CrossRef]
- Murcia, S.; Li, G.; Yahyazadehfar, M.; Sasser, M.; Ossa, A.; Arola, D. Effects of Polar Solvents on the Mechanical Behavior of Fish Scales. Mater. Sci. Eng. C 2016, 61, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Arola, D.; Ghods, S.; Son, C.; Murcia, S.; Ossa, E.A. Interfibril Hydrogen Bonding Improves the Strain-Rate Response of Natural Armour. J. R. Soc. Interface 2019, 16, 20180775. [Google Scholar] [CrossRef] [PubMed]
- Allam, A.A.; Abo-Eleneen, R.E. Scales Microstructure of Snakes from the Egyptian Area. Zool. Sci. 2012, 29, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Ghods, S.; Murcia, S.; Ossa, E.A.; Arola, D. Designed for Resistance to Puncture: The Dynamic Response of Fish Scales. J. Mech. Behav. Biomed. Mater. 2019, 90, 451–459. [Google Scholar] [CrossRef]
- Szewciw, L.; Barthelat, F. Mechanical Properties of Striped Bass Fish Skin: Evidence of an Exotendon Function of the Stratum Compactum. J. Mech. Behav. Biomed. Mater. 2017, 73, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Gil-Duran, S.; Arola, D.; Ossa, E.A. Effect of Chemical Composition and Microstructure on the Mechanical Behavior of Fish Scales from Megalops atlanticus. J. Mech. Behav. Biomed. Mater. 2016, 56, 134–145. [Google Scholar] [CrossRef]
- Abo-Eleneen, R.E.; Allam, A.A. Comparative Morphology of the Skin of Natrix tessellata (Family: Colubridae) and Cerastes vipera (Family: Viperidae). Zool. Sci. 2011, 28, 743–748. [Google Scholar] [CrossRef]
- Baum, M.J.; Kovalev, A.E.; Michels, J.; Gorb, S.N. Anisotropic Friction of the Ventral Scales in the Snake Lampropeltis getula californiae. Tribol. Lett. 2014, 54, 139–150. [Google Scholar] [CrossRef]
- Berthé, R.A.; Westhoff, G.; Bleckmann, H.; Gorb, S.N. Surface Structure and Frictional Properties of the Skin of the Amazon Tree Boa Corallus hortulanus (Squamata, Boidae). J. Comp. Physiol. A 2009, 195, 311–318. [Google Scholar] [CrossRef]
- Darbois Texier, B.; Ibarra, A.; Melo, F. Optimal Propulsion of an Undulating Slender Body with Anisotropic Friction. Soft Matter 2018, 14, 635–642. [Google Scholar] [CrossRef]
- Abdel-Aal, H.A.; Vargiolu, R.; Zahouani, H.; El Mansori, M. Preliminary Investigation of the Frictional Response of Reptilian Shed Skin. Wear 2012, 290, 51–60. [Google Scholar] [CrossRef][Green Version]
- Gray, J. The Mechanism of Locomotion in Snakes. J. Exp. Biol. 1946, 23, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Rieser, J.M.; Li, T.-D.; Tingle, J.L.; Goldman, D.I.; Mendelson, J.R. Functional Consequences of Convergently Evolved Microscopic Skin Features on Snake Locomotion. Proc. Natl. Acad. Sci. USA 2021, 118, e2018264118. [Google Scholar] [CrossRef] [PubMed]
- Jayne, B.C. What Defines Different Modes of Snake Locomotion? Integr. Comp. Biol. 2020, 60, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.L.; Nirody, J.; Scott, T.; Shelley, M.J. The Mechanics of Slithering Locomotion. Proc. Natl. Acad. Sci. USA 2009, 106, 10081–10085. [Google Scholar] [CrossRef]
- Allen, W.L.; Baddeley, R.; Scott-Samuel, N.E.; Cuthill, I.C. The Evolution and Function of Pattern Diversity in Snakes. Behav. Ecol. 2013, 24, 1237–1250. [Google Scholar] [CrossRef]
- Valkonen, J.; Niskanen, M.; Björklund, M.; Mappes, J. Disruption or Aposematism? Significance of Dorsal Zigzag Pattern of European Vipers. Evol. Ecol. 2011, 25, 1047–1063. [Google Scholar] [CrossRef]
- Aleksiuk, M. Metabolic and Behavioural Adjustments to Temperature Change in the Red-Sided Garter Snake (Thamnophis sirtalis Parietalis): An Integrated Approach. J. Therm. Biol. 1976, 1, 153–156. [Google Scholar] [CrossRef]
- Porter, W.P. Solar Radiation through the Living Body Walls of Vertebrates with Emphasis on Desert Reptiles. Ecol. Monogr. 1967, 37, 273–296. [Google Scholar] [CrossRef]
- Farallo, V.R.; Forstner, M.R.J. Predation and the Maintenance of Color Polymorphism in a Habitat Specialist Squamate. PLoS ONE 2012, 7, e30316. [Google Scholar] [CrossRef]
- Isaac, L.A.; Gregory, P.T. Can Snakes Hide in Plain View? Chromatic and Achromatic Crypsis of Two Colour Forms of the Western Terrestrial Garter Snake (Thamnophis elegans): Differential Crypsis of Garter Snakes. Biol. J. Linn. Soc. 2013, 108, 756–772. [Google Scholar] [CrossRef][Green Version]
- Klein, M.-C.G.; Deuschle, J.K.; Gorb, S.N. Material Properties of the Skin of the Kenyan Sand Boa Gongylophis colubrinus (Squamata, Boidae). J. Comp. Physiol. A 2010, 196, 659–668. [Google Scholar] [CrossRef]
- Abdel-Aal, H.A.; El Mansori, M.; Zahouani, H. A Comparative Study of Frictional Response of Shed Snakeskin and Human Skin. Wear 2017, 376, 281–294. [Google Scholar] [CrossRef]
- Klein, M.-C.G.; Gorb, S.N. Epidermis Architecture and Material Properties of the Skin of Four Snake Species. J. R. Soc. Interface 2012, 9, 3140–3155. [Google Scholar] [CrossRef]
- Klein, M.-C.G.; Gorb, S.N. Scratch Resistance of the Ventral Skin Surface in Four Snake Species (Squamata, Serpentes). Zoology 2016, 119, 81–96. [Google Scholar] [CrossRef]
- Irish, F.J.; Williams, E.E.; Seling, E. Scanning Electron Microscopy of Changes in Epidermal Structure Occurring during the Shedding Cycle in Squamate Reptiles. J. Morphol. 1988, 197, 105–126. [Google Scholar] [CrossRef]
- Bhushan, B.; Kulkarni, A.V.; Bonin, W.; Wyrobek, J.T. Nanoindentation and Picoindentation Measurements Using a Capacitive Transducer System in Atomic Force Microscopy. Philos. Mag. A 1996, 74, 1117–1128. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Bose, S.; Li, S.; Mele, E.; Silberschmidt, V.V. Fracture Behaviour and Toughening Mechanisms of Dry and Wet Collagen. Acta Biomater. 2022, 142, 174–184. [Google Scholar] [CrossRef]
- Yang, W.; Gludovatz, B.; Zimmermann, E.A.; Bale, H.A.; Ritchie, R.O.; Meyers, M.A. Structure and Fracture Resistance of Alligator Gar (Atractosteus spatula) Armored Fish Scales. Acta Biomater. 2013, 9, 5876–5889. [Google Scholar] [CrossRef]
- Chin, P.; McCullough, R.L.; Wu, W.-L. An Improved Procedure for Determining the Work of Adhesion for Polymer-Solid Contact. J. Adhes. 1997, 64, 145–160. [Google Scholar] [CrossRef]
- Hartmann, U. Intermolecular and Surface Forces in Noncontact Scanning Force Microscopy. Ultramicroscopy 1992, 42, 59–65. [Google Scholar] [CrossRef]
- Sharpe, S.S.; Koehler, S.A.; Kuckuk, R.M.; Serrano, M.; Vela, P.A.; Mendelson, J.; Goldman, D.I. Locomotor Benefits of Being a Slender and Slick Sand-Swimmer. J. Exp. Biol. 2015, 218, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Schiebel, P.E.; Astley, H.C.; Rieser, J.M.; Agarwal, S.; Hubicki, C.; Hubbard, A.M.; Diaz, K.; Mendelson Iii, J.R.; Kamrin, K.; Goldman, D.I. Mitigating Memory Effects during Undulatory Locomotion on Hysteretic Materials. eLife 2020, 9, e51412. [Google Scholar] [CrossRef]
- Yang, W.; Sherman, V.R.; Gludovatz, B.; Schaible, E.; Stewart, P.; Ritchie, R.O.; Meyers, M.A. On the Tear Resistance of Skin. Nat. Commun. 2015, 6, 6649. [Google Scholar] [CrossRef]
- Bose, S.; Li, S.; Mele, E.; Silberschmidt, V.V. Dry vs. Wet: Properties and Performance of Collagen Films. Part I. Mechanical Behaviour and Strain-Rate Effect. J. Mech. Behav. Biomed. Mater. 2020, 111, 103983. [Google Scholar] [CrossRef]
- Zhu, D.; Ortega, C.F.; Motamedi, R.; Szewciw, L.; Vernerey, F.; Barthelat, F. Structure and Mechanical Performance of a “Modern” Fish Scale. Adv. Eng. Mater. 2012, 14, B185–B194. [Google Scholar] [CrossRef]
- Zimmermann, E.A.; Gludovatz, B.; Schaible, E.; Dave, N.K.N.; Yang, W.; Meyers, M.A.; Ritchie, R.O. Mechanical Adaptability of the Bouligand-Type Structure in Natural Dermal Armour. Nat. Commun. 2013, 4, 2634. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, D.; Wang, J.; Bui, T.Q. Structure, Mechanical Behavior and Puncture Resistance of Grass Carp Scales. J. Bionic Eng. 2017, 14, 356–368. [Google Scholar] [CrossRef]
- Sasaki, N.; Nakayama, Y.; Yoshikawa, M.; Enyo, A. Stress Relaxation Function of Bone and Bone Collagen. J. Biomech. 1993, 26, 1369–1376. [Google Scholar] [CrossRef]
- Ji, B.; Gao, H. Mechanical Properties of Nanostructure of Biological Materials. J. Mech. Phys. Solids 2004, 52, 1963–1990. [Google Scholar] [CrossRef]
- Vaquero, T.S.; Daddi, G.; Thakker, R.; Paton, M.; Jasour, A.; Strub, M.P.; Swan, R.M.; Royce, R.; Gildner, M.; Tosi, P.; et al. EELS: Autonomous Snake-like Robot with Task and Motion Planning Capabilities for Ice World Exploration. Sci. Robot. 2024, 9, eadh8332. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, Y.; Qian, M. Motion Control and Optimal Design of a Biomimetic Manipulator Based on Snake Coiling and Stretching. J. Bionic Eng. 2023, 20, 1514–1531. [Google Scholar] [CrossRef]
- Yenmiş, M.; Bayrakcı, Y.; Ayaz, D. Hierarchical Microstructure of the Scales in Grass Snake (Natrix natrix) and Dice Snake (Natrix tessellata). Biologia 2022, 77, 765–774. [Google Scholar] [CrossRef]

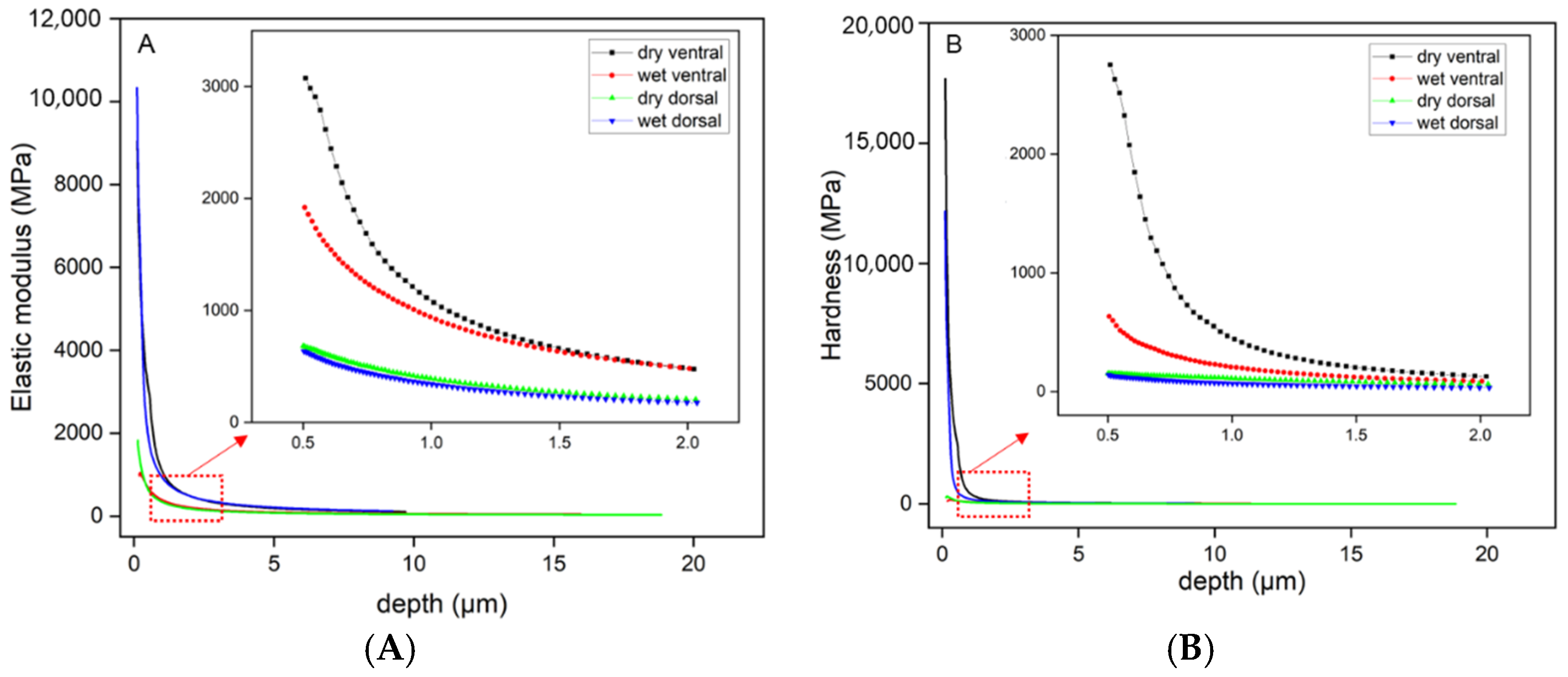
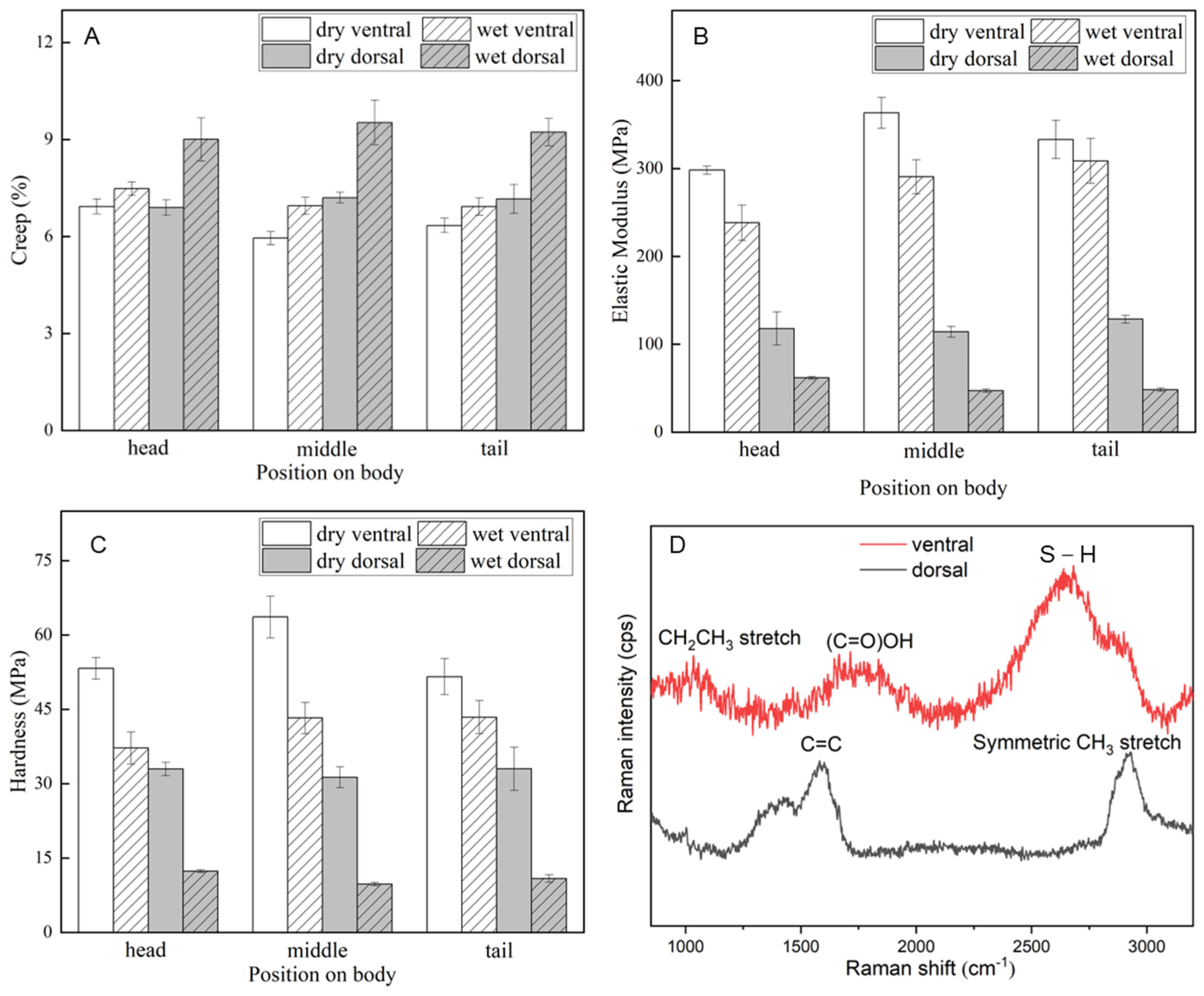
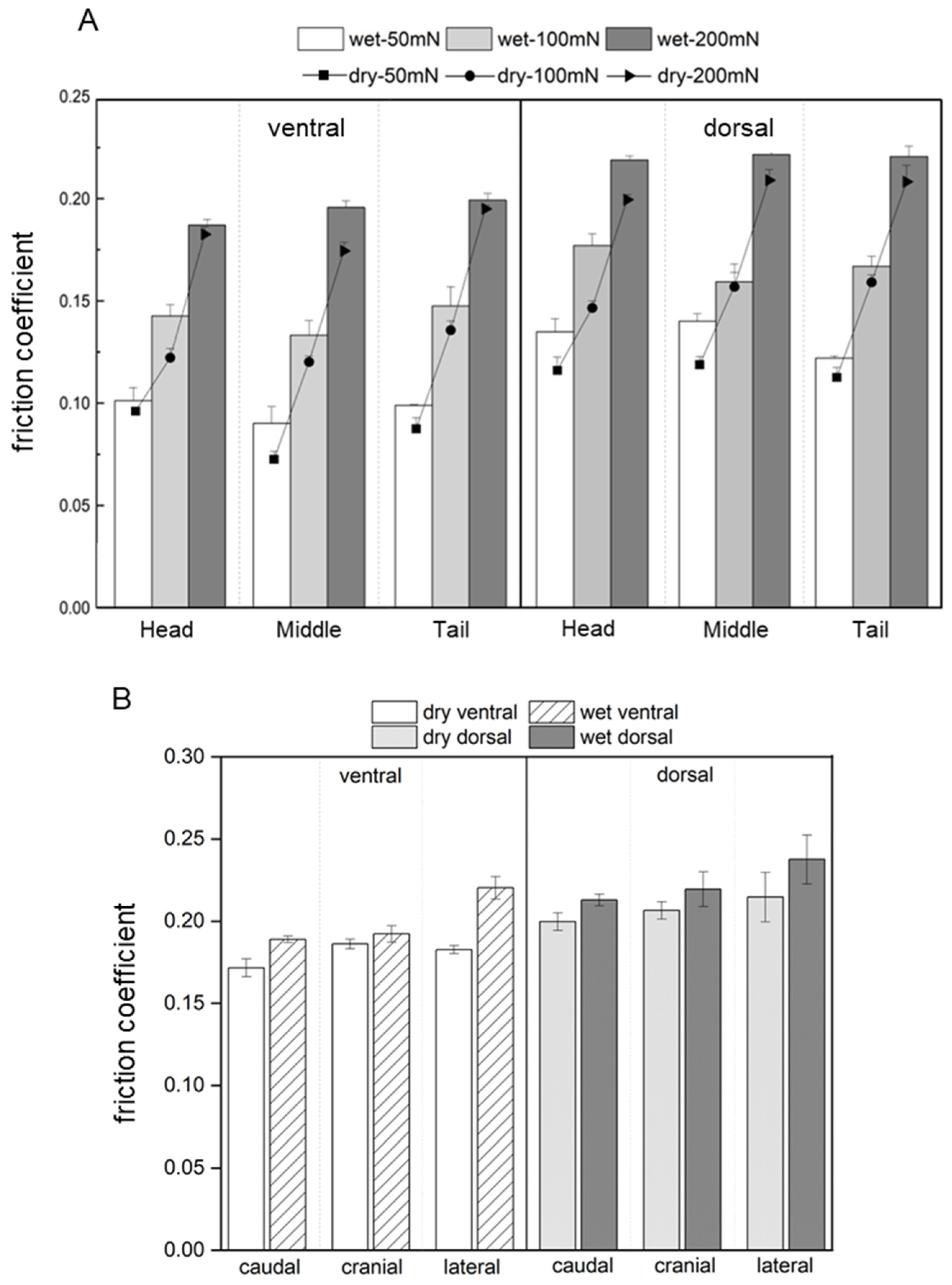
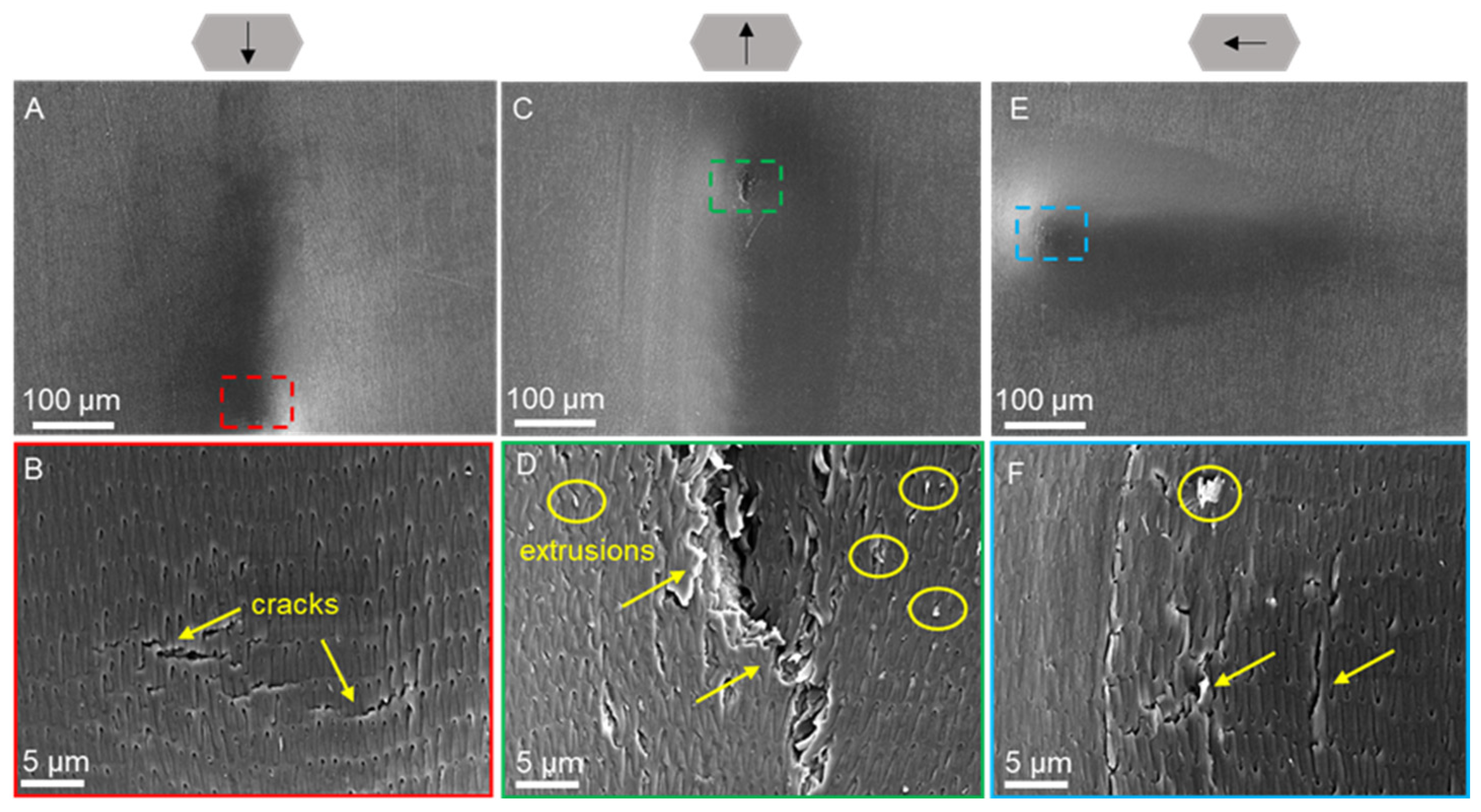

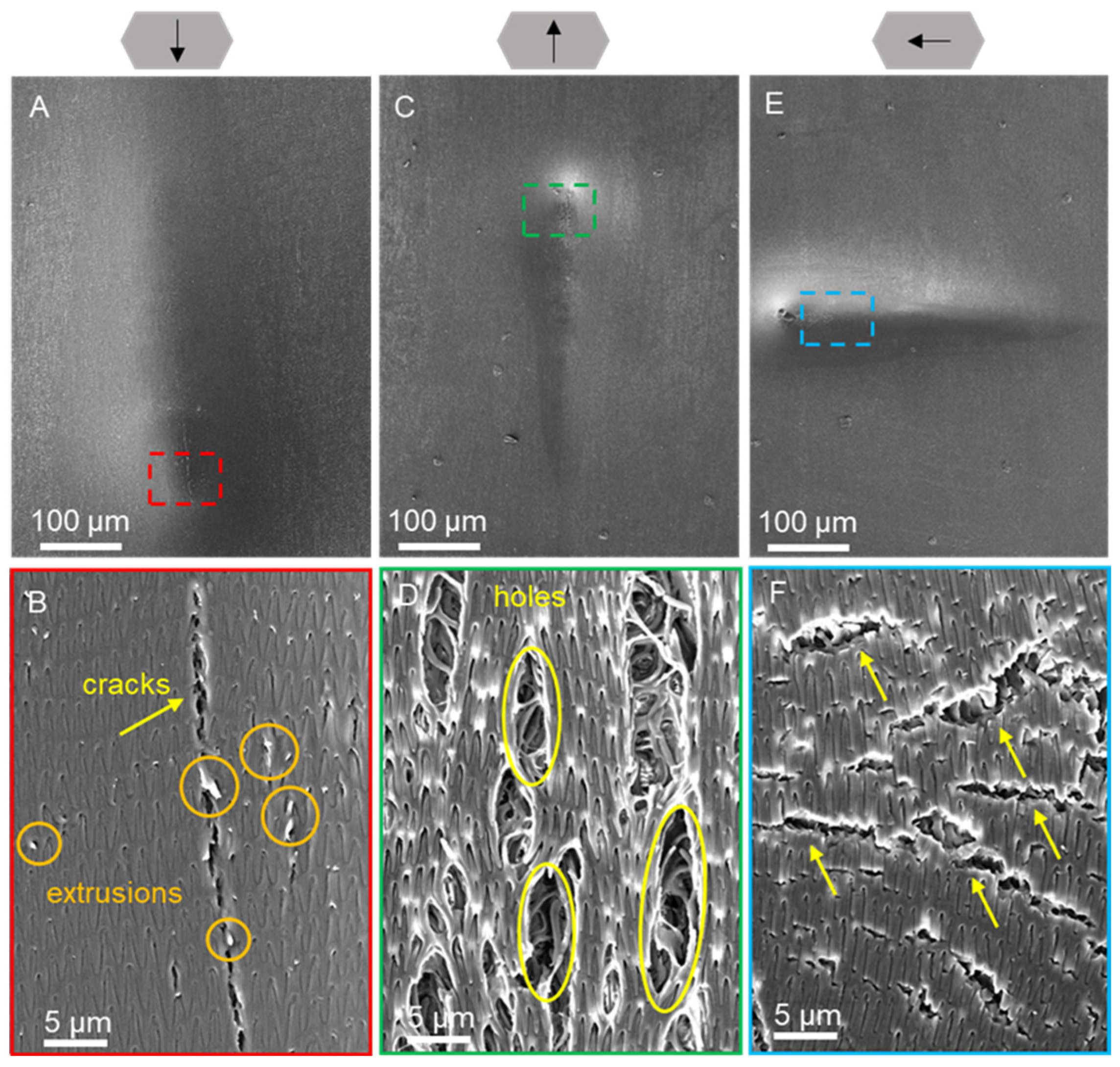

| Condition | Creep (%) | Elastic Modulus (MPa) | Hardness (MPa) |
|---|---|---|---|
| dry ventral | 6.41 ± 0.22 a | 331.58 ± 14.70 a | 56.16 ± 3.34 a |
| wet ventral | 7.12 ± 0.25 a | 279.13 ± 21.65 b | 41.28 ± 3.25 b |
| dry dorsal | 7.08 ± 0.29 a | 120.16 ± 9.85 c | 32.42 ± 2.61 c |
| wet dorsal | 9.25 ± 0.59 b | 52.43 ± 1.77 d | 10.98 ± 0.46 d |
| p value | <0.05 | <0.05 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, G.; Wang, J.; Dong, Y.; Hu, S.; Zheng, L.; Ren, L. Effect of Surface Morphology and Internal Structure on the Tribological Behaviors of Snake Scales from Dinodon rufozonatum. Biomimetics 2024, 9, 617. https://doi.org/10.3390/biomimetics9100617
Shi G, Wang J, Dong Y, Hu S, Zheng L, Ren L. Effect of Surface Morphology and Internal Structure on the Tribological Behaviors of Snake Scales from Dinodon rufozonatum. Biomimetics. 2024; 9(10):617. https://doi.org/10.3390/biomimetics9100617
Chicago/Turabian StyleShi, Ge, Jinhao Wang, Yuehua Dong, Song Hu, Long Zheng, and Luquan Ren. 2024. "Effect of Surface Morphology and Internal Structure on the Tribological Behaviors of Snake Scales from Dinodon rufozonatum" Biomimetics 9, no. 10: 617. https://doi.org/10.3390/biomimetics9100617
APA StyleShi, G., Wang, J., Dong, Y., Hu, S., Zheng, L., & Ren, L. (2024). Effect of Surface Morphology and Internal Structure on the Tribological Behaviors of Snake Scales from Dinodon rufozonatum. Biomimetics, 9(10), 617. https://doi.org/10.3390/biomimetics9100617






