3D Printing Materials Mimicking Human Tissues after Uptake of Iodinated Contrast Agents for Anthropomorphic Radiology Phantoms
Abstract
1. Introduction
2. Materials and Methods
2.1. Doping of Printing Polymers
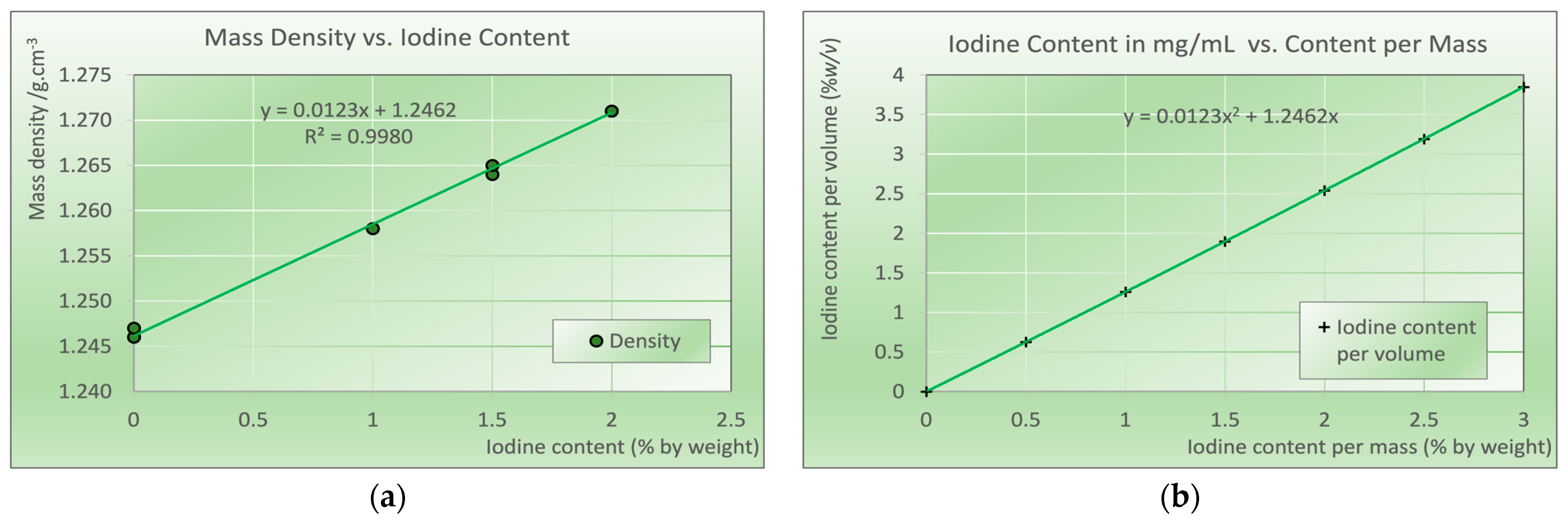
2.2. Determination of Mass Density of Printed Samples
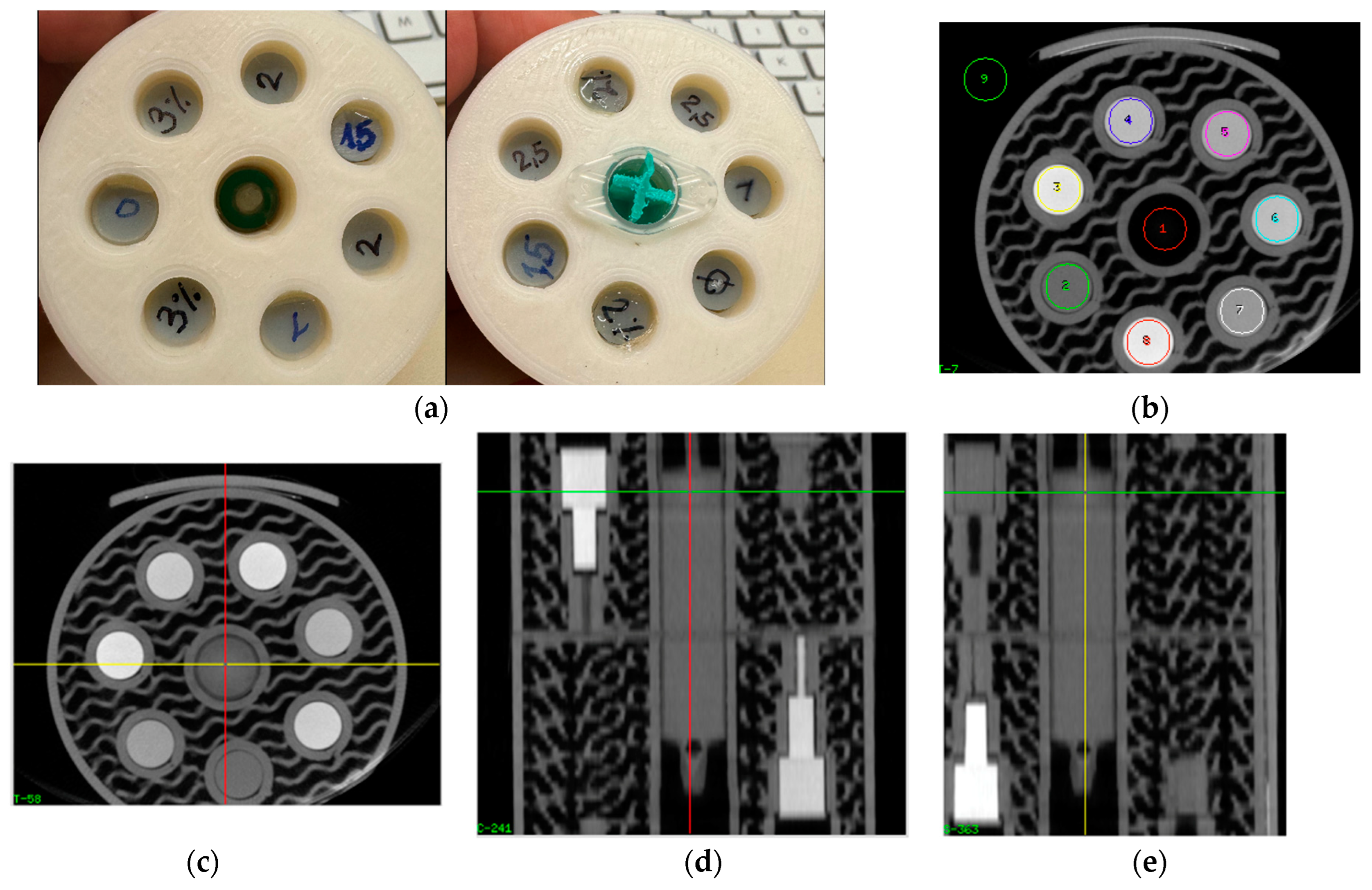
2.3. Samples for Measurement of Attenuation vs. Iodine Content
2.4. CT Scanning of Iodine Samples
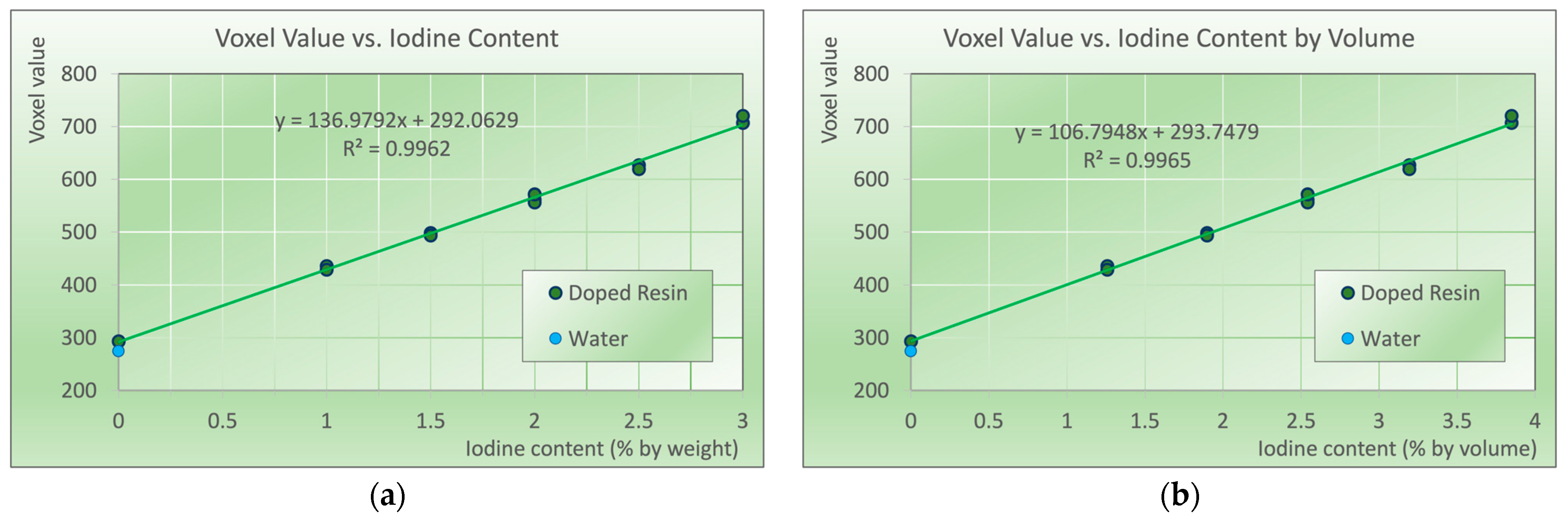
2.5. Data Analysis
3. Results
Mass Density of Printed Iodine Samples and Conversion to % w/v
4. Discussion
Limitations, Future Work, and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wegner, M.; Gargioni, E.; Krause, D. Classification of phantoms for medical imaging. Procedia CIRP 2023, 119, 1140–1145. [Google Scholar] [CrossRef]
- Petoussi-Henss, N.; Bolch, W.E.; Eckerman, K.F.; Endo, A.; Hertel, N.; Hunt, J.; Menzel, H.G.; Pelliccioni, M.; Schlattl, H.; Zankl, M. ICRP Publication 116—The first ICRP/ICRU application of the male and female adult reference computational phantoms. Phys. Med. Biol. 2014, 59, 5209–5224. [Google Scholar] [CrossRef] [PubMed]
- Bolch, W.; Lee, C.; Wayson, M.; Johnson, P. Hybrid computational phantoms for medical dose reconstruction. Radiat. Environ. Biophys. 2010, 49, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Tino, R.; Yeo, A.; Leary, M.; Brandt, M.; Kron, T. A Systematic Review on 3D-Printed Imaging and Dosimetry Phantoms in Radiation Therapy. Technol. Cancer Res. Treat. 2019, 18, 1533033819870208. [Google Scholar] [CrossRef] [PubMed]
- Filippou, V.; Tsoumpas, C. Recent advances on the development of phantoms using 3D printing for imaging with CT, MRI, PET, SPECT, and ultrasound. Med. Phys. 2018, 45, e740–e760. [Google Scholar] [CrossRef]
- Solomon, J.; Ba, A.; Bochud, F.; Samei, E. Comparison of low-contrast detectability between two CT reconstruction algorithms using voxel-based 3D printed textured phantoms. Med. Phys. 2016, 43, 6497. [Google Scholar] [CrossRef]
- Irnstorfer, N.; Unger, E.; Hojreh, A.; Homolka, P. An anthropomorphic phantom representing a prematurely born neonate for digital x-ray imaging using 3D printing: Proof of concept and comparison of image quality from different systems. Sci. Rep. 2019, 9, 14357. [Google Scholar] [CrossRef]
- Schopphoven, S.; Cavael, P.; Bock, K.; Fiebich, M.; Mader, U. Breast phantoms for 2D digital mammography with realistic anatomical structures and attenuation characteristics based on clinical images using 3D printing. Phys. Med. Biol. 2019, 64, 215005. [Google Scholar] [CrossRef]
- Homolka, P.; Figl, M.; Wartak, A.; Glanzer, M.; Dunkelmeyer, M.; Hojreh, A.; Hummel, J. Design of a head phantom produced on a 3D rapid prototyping printer and comparison with a RANDO and 3M lucite head phantom in eye dosimetry applications. Phys. Med. Biol. 2017, 62, 3158–3174. [Google Scholar] [CrossRef]
- IAEA. Radiation Protection and Safety in Medical Uses of Ionizing Radiation; IAEA: Vienna, Austria, 2018; Vol. IAEA Specific Safety Guide No. 46. [Google Scholar]
- IAEA. Handbook of Basic Quality Control Tests for Diagnostic Radiology; IAEA: Vienna, Austria, 2023; Vol. IAEA Human Health Series No. 47. [Google Scholar]
- Abadi, E.; Segars, W.P.; Tsui, B.M.W.; Kinahan, P.E.; Bottenus, N.; Frangi, A.J.; Maidment, A.; Lo, J.Y.; Samei, E. Virtual clinical trials in medical imaging: A review. J. Med. Imaging 2020, 7, 042805. [Google Scholar] [CrossRef] [PubMed]
- Kiarashi, N.; Nolte, A.C.; Sturgeon, G.M.; Segars, W.P.; Ghate, S.V.; Nolte, L.W.; Samei, E.; Lo, J.Y. Development of realistic physical breast phantoms matched to virtual breast phantoms based on human subject data. Med. Phys. 2015, 42, 4116–4126. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Ozawa, S.; Miura, H.; Yamada, K.; Habara, K.; Hayata, M.; Kusaba, H.; Kawahara, D.; Miki, K.; Nakashima, T.; et al. Development of a CT number calibration audit phantom in photon radiation therapy: A pilot study. Med. Phys. 2020, 47, 1509–1522. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, C.; Harrington, J.C.; DeWerd, L.A. An electron density calibration phantom for CT-based treatment planning computers. Med. Phys. 1992, 19, 325–327. [Google Scholar] [CrossRef]
- Cockmartin, L.; Bosmans, H.; Marshall, N.W. Investigation of test methods for QC in dual-energy based contrast-enhanced digital mammography systems: I. Iodine signal testing. Phys. Med. Biol. 2023, 68. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yang, X.; Hintenlang, D.E.; White, R.D. Effects of Patient Size and Radiation Dose on Iodine Quantification in Dual-Source Dual-Energy CT. Acad. Radiol. 2021, 28, 96–105. [Google Scholar] [CrossRef]
- Euler, A.; Solomon, J.; Mazurowski, M.A.; Samei, E.; Nelson, R.C. How accurate and precise are CT based measurements of iodine concentration? A comparison of the minimum detectable concentration difference among single source and dual source dual energy CT in a phantom study. Eur. Radiol. 2019, 29, 2069–2078. [Google Scholar] [CrossRef]
- Leithner, R.; Knogler, T.; Homolka, P. Development and production of a prototype iodine contrast phantom for CEDEM. Phys. Med. Biol. 2013, 58, N25–N35. [Google Scholar] [CrossRef]
- McGarry, C.K.; Grattan, L.J.; Ivory, A.M.; Leek, F.; Liney, G.P.; Liu, Y.; Miloro, P.; Rai, R.; Robinson, A.P.; Shih, A.J.; et al. Tissue mimicking materials for imaging and therapy phantoms: A review. Phys. Med. Biol. 2020, 65. [Google Scholar] [CrossRef]
- ICRU. ICRU Report 44. Tissue Substitutes in Radiation Dosimetry and Mesurement; ICRU Report 44; International Commission on Radiation Units and Mesurement: Bethesda, MD, USA, 1989. [Google Scholar]
- Okkalidis, N. 3D printing methods for radiological anthropomorphic phantoms. Phys. Med. Biol. 2022, 67, 15TR04. [Google Scholar] [CrossRef]
- Ma, X.; Figl, M.; Unger, E.; Buschmann, M.; Homolka, P. X-ray attenuation of bone, soft and adipose tissue in CT from 70 to 140 kV and comparison with 3D printable additive manufacturing materials. Sci. Rep. 2022, 12, 14580. [Google Scholar] [CrossRef]
- Ahmed, A.M.M.; Buschmann, M.; Breyer, L.; Kuntner, C.; Homolka, P. Tailoring the Mass Density of 3D Printing Materials for Accurate X-ray Imaging Simulation by Controlled Underfilling for Radiographic Phantoms. Polymers 2024, 16, 1116. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Buschmann, M.; Unger, E.; Homolka, P. Classification of X-Ray Attenuation Properties of Additive Manufacturing and 3D Printing Materials Using Computed Tomography From 70 to 140 kVp. Front. Bioeng. Biotechnol. 2021, 9, 763960. [Google Scholar] [CrossRef] [PubMed]
- Kalidindi, Y.; Ganapathy, A.K.; Nayak, Y.; Elumalai, A.; Chen, D.Z.; Bishop, G.; Sanchez, A.; Albers, B.; Shetty, A.S.; Ballard, D.H. Computed Tomography Attenuation of Three-Dimensional (3D) Printing Materials-Depository to Aid in Constructing 3D-Printed Phantoms. Micromachines 2023, 14, 1928. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, G.P.; Rezaeifar, B.; Lackner, N.; Haanen, B.; Reniers, B.; Verhaegen, F. Dual-energy CT evaluation of 3D printed materials for radiotherapy applications. Phys. Med. Biol. 2023, 68, 035005. [Google Scholar] [CrossRef] [PubMed]
- Bibb, R.; Thompson, D.; Winder, J. Computed tomography characterisation of additive manufacturing materials. Med. Eng. Phys. 2011, 33, 590–596. [Google Scholar] [CrossRef]
- Okkalidis, N.; Marinakis, G. Technical Note: Accurate replication of soft and bone tissues with 3D printing. Med. Phys. 2020, 47, 2206–2211. [Google Scholar] [CrossRef]
- Fredenberg, E.; Dance, D.R.; Willsher, P.; Moa, E.; von Tiedemann, M.; Young, K.C.; Wallis, M.G. Measurement of breast-tissue x-ray attenuation by spectral mammography: First results on cyst fluid. Phys. Med. Biol. 2013, 58, 8609–8620. [Google Scholar] [CrossRef]
- Cockmartin, L.; Marshall, N.W.; Zhang, G.; Lemmens, K.; Shaheen, E.; Van Ongeval, C.; Fredenberg, E.; Dance, D.R.; Salvagnini, E.; Michielsen, K.; et al. Design and application of a structured phantom for detection performance comparison between breast tomosynthesis and digital mammography. Phys. Med. Biol. 2017, 62, 758–780. [Google Scholar] [CrossRef]
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef]
- Jochelson, M.S.; Lobbes, M.B.I. Contrast-enhanced Mammography: State of the Art. Radiology 2021, 299, 36–48. [Google Scholar] [CrossRef]
- Zopfs, D.; Graffe, J.; Reimer, R.P.; Schafer, S.; Persigehl, T.; Maintz, D.; Borggrefe, J.; Haneder, S.; Lennartz, S.; Grosse Hokamp, N. Quantitative distribution of iodinated contrast media in body computed tomography: Data from a large reference cohort. Eur. Radiol. 2021, 31, 2340–2348. [Google Scholar] [CrossRef] [PubMed]
- Braeutigam, M.; Huebner-Steiner, U.; Pietsch, H.; Speck, U. Dual Energy CT Imaging; Seidensticker, P.R., Hofmann, L.K., Eds.; Springer: Heidelberg, Gernamy, 2008. [Google Scholar]
- Knogler, T.; Homolka, P.; Hornig, M.; Leithner, R.; Langs, G.; Waitzbauer, M.; Pinker-Domenig, K.; Leitner, S.; Helbich, T.H. Contrast-enhanced dual energy mammography with a novel anode/filter combination and artifact reduction: A feasibility study. Eur. Radiol. 2016, 26, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Klausz, R.; Rouxel, M.; Mancardi, X.; Carton, A.K.; Jeunehomme Patoureaux, F. Introduction of a comprehensive phantom for the quality control of contrast enhanced spectral mammography. In Proceedings of the European Congress of Radiology, Vienna, Austria, 28 February–4 March 2018. [Google Scholar]
- Jeunehomme, F. Mammographie Numérique Avec Injection de Produit de Contraste; Université de Paris-Sud: Paris, France, 2005; Available online: https://theses.fr/2005PA112025 (accessed on 29 August 2024).
- Baldelli, P.; Bravin, A.; Di Maggio, C.; Gennaro, G.; Sarnelli, A.; Taibi, A.; Gambaccini, M. Evaluation of the minimum iodine concentration for contrast-enhanced subtraction mammography. Phys. Med. Biol. 2006, 51, 4233–4251. [Google Scholar] [CrossRef] [PubMed]
- Jong, R.A.; Yaffe, M.J.; Skarpathiotakis, M.; Shumak, R.S.; Danjoux, N.M.; Gunesekara, A.; Plewes, D.B. Contrast-enhanced digital mammography: Initial clinical experience. Radiology 2003, 228, 842–850. [Google Scholar] [CrossRef]
- Palma, B.A.; Rosado-Méndez, I.; Villaseñor, Y.; Brandan, M.E. Phantom study to evaluate contrast-medium-enhanced digital subtraction mammography with a full-field indirect-detection system. Med. Phys. 2010, 37, 577. [Google Scholar] [CrossRef]
- Takanami, K.; Higano, S.; Takase, K.; Kaneta, T.; Yamada, T.; Ishiya, H.; Mori, I.; Takahashi, S. Validation of the use of calibration factors between the iodine concentration and the computed tomography number measured outside the objects for estimation of iodine concentration inside the objects: Phantom experiment. Radiat. Med. 2008, 26, 237–243. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Speller, R. Quantitative contrast-enhanced mammography for contrast medium kinetics studies. Phys. Med. Biol. 2009, 54, 6041–6064. [Google Scholar] [CrossRef]
- Hill, M.L.; Mainprize, J.G.; Mawdsley, G.E.; Yaffe, M.J. A solid iodinated phantom material for use in tomographic x-ray imaging. Med. Phys. 2009, 36, 4409. [Google Scholar] [CrossRef]
- Shikhaliev, P.M. Dedicated phantom materials for spectral radiography and CT. Phys. Med. Biol. 2012, 57, 1575–1593. [Google Scholar] [CrossRef]
- White, D.R.; Martin, R.J.; Darlison, R. Epoxy resin based tissue substitutes. Br. J. Radiol. 1977, 50, 814–821. [Google Scholar] [CrossRef]
- X3msnake. Photonsters Validation Matrix v2. Available online: https://www.thingiverse.com/thing:4707289 (accessed on 25 September 2024).
- Robb, R.A.; Hanson, D.P.; Karwoski, R.A.; Larson, A.G.; Workman, E.L.; Stacy, M.C. Analyze: A comprehensive, operator-interactive software package for multidimensional medical image display and analysis. Comput. Med. Imaging Graph. 1989, 13, 433–454. [Google Scholar] [CrossRef] [PubMed]
- Lappchen, T.; Meier, L.P.; Furstner, M.; Prenosil, G.A.; Krause, T.; Rominger, A.; Klaeser, B.; Hentschel, M. 3D printing of radioactive phantoms for nuclear medicine imaging. EJNMMI Phys. 2020, 7, 22. [Google Scholar] [CrossRef]
- Gillett, D.; Marsden, D.; Ballout, S.; Attili, B.; Bird, N.; Heard, S.; Gurnell, M.; Mendichovszky, I.A.; Aloj, L. 3D printing (18)F radioactive phantoms for PET imaging. EJNMMI Phys. 2021, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Ceh, J.; Youd, T.; Mastrovich, Z.; Peterson, C.; Khan, S.; Sasser, T.A.; Sander, I.M.; Doney, J.; Turner, C.; Leevy, W.M. Bismuth Infusion of ABS Enables Additive Manufacturing of Complex Radiological Phantoms and Shielding Equipment. Sensors 2017, 17, 459. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, P.; Schwarz, F.B.; Ziegert, M.; Almasi, T.; Abdelhadi, O.; Nunninger, M.; Hamm, B.; Scheel, M. A radiopaque 3D printed, anthropomorphic phantom for simulation of CT-guided procedures. Eur. Radiol. 2018, 28, 4818–4823. [Google Scholar] [CrossRef] [PubMed]
- Mirón, V.; Ferrándiz, S.; Juárez, D.; Mengual, A. Manufacturing and characterization of 3D printer filament using tailoring materials. Procedia Manuf. 2017, 13, 888–894. [Google Scholar] [CrossRef]
- Homolka, P.; Gahleitner, A.; Nowotny, R. Temperature dependence of HU values for various water equivalent phantom materials. Phys. Med. Biol. 2002, 47, 2917–2923. [Google Scholar] [CrossRef]
- Hatamikia, S.; Oberoi, G.; Unger, E.; Kronreif, G.; Kettenbach, J.; Buschmann, M.; Figl, M.; Knausl, B.; Moscato, F.; Birkfellner, W. Additively Manufactured Patient-Specific Anthropomorphic Thorax Phantom With Realistic Radiation Attenuation Properties. Front. Bioeng. Biotechnol. 2020, 8, 385. [Google Scholar] [CrossRef]
- Homolka, P.; Nowotny, R. Production of phantom materials using polymer powder sintering under vacuum. Phys. Med. Biol. 2002, 47, N47–N52. [Google Scholar] [CrossRef]
- Homolka, P.; Gahleitner, A.; Prokop, M.; Nowotny, R. Optimization of the composition of phantom materials for computed tomography. Phys. Med. Biol. 2002, 47, 2907–2916. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Homolka, P.; Breyer, L.; Semturs, F. 3D Printing Materials Mimicking Human Tissues after Uptake of Iodinated Contrast Agents for Anthropomorphic Radiology Phantoms. Biomimetics 2024, 9, 606. https://doi.org/10.3390/biomimetics9100606
Homolka P, Breyer L, Semturs F. 3D Printing Materials Mimicking Human Tissues after Uptake of Iodinated Contrast Agents for Anthropomorphic Radiology Phantoms. Biomimetics. 2024; 9(10):606. https://doi.org/10.3390/biomimetics9100606
Chicago/Turabian StyleHomolka, Peter, Lara Breyer, and Friedrich Semturs. 2024. "3D Printing Materials Mimicking Human Tissues after Uptake of Iodinated Contrast Agents for Anthropomorphic Radiology Phantoms" Biomimetics 9, no. 10: 606. https://doi.org/10.3390/biomimetics9100606
APA StyleHomolka, P., Breyer, L., & Semturs, F. (2024). 3D Printing Materials Mimicking Human Tissues after Uptake of Iodinated Contrast Agents for Anthropomorphic Radiology Phantoms. Biomimetics, 9(10), 606. https://doi.org/10.3390/biomimetics9100606





