Advancement in Soft Hydrogel Grippers: Comprehensive Insights into Materials, Fabrication Strategies, Grasping Mechanism, and Applications
Abstract
1. Introduction
2. Materials of the Hydrogel Grippers
2.1. PNIPAM
2.2. PAA
2.3. PVA
2.4. PEGDA
2.5. P(MAAM-co-MAA)
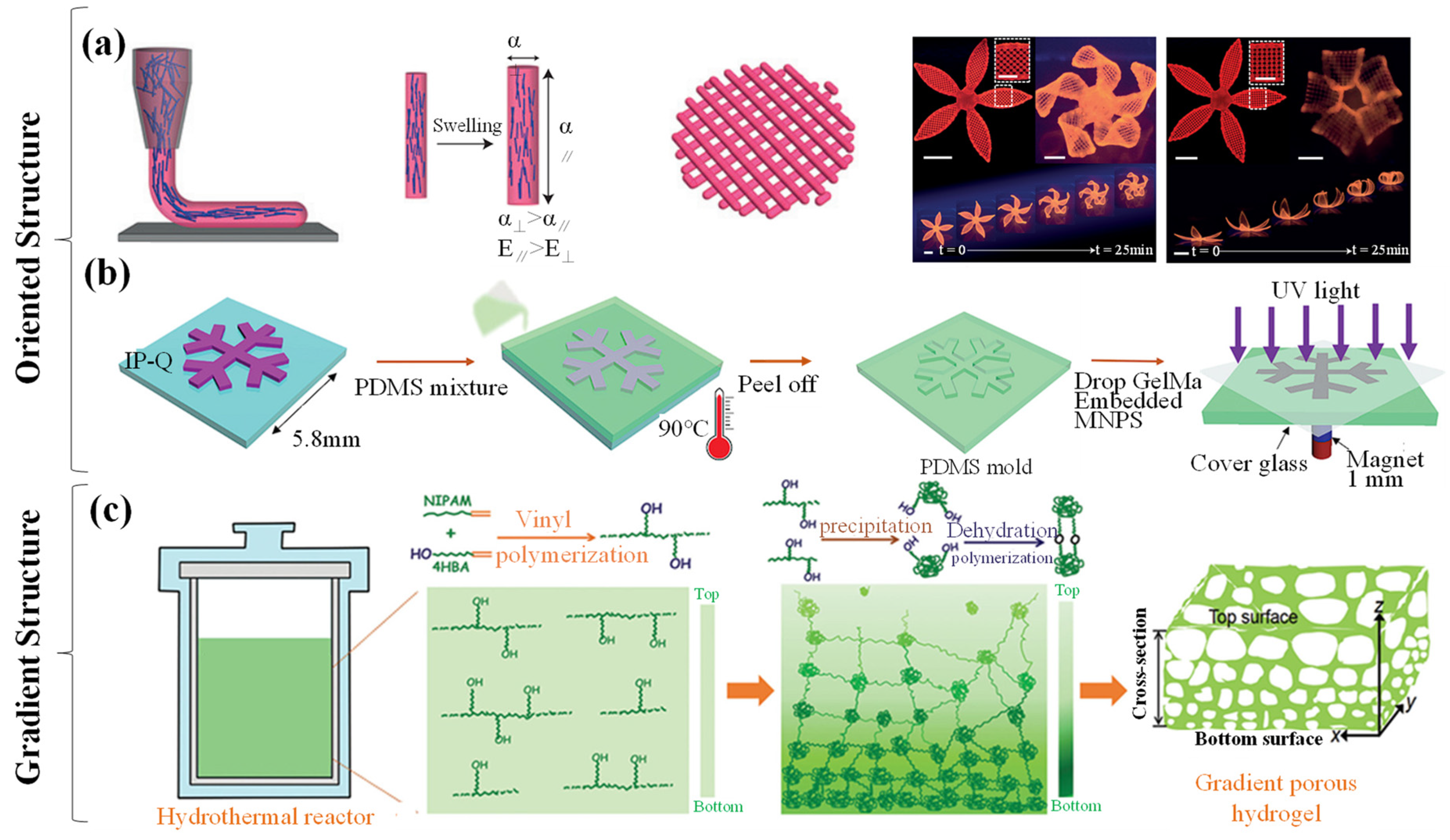
3. Manufacturing Strategies
3.1. One-Step Synthesis
3.2. Structure Modification
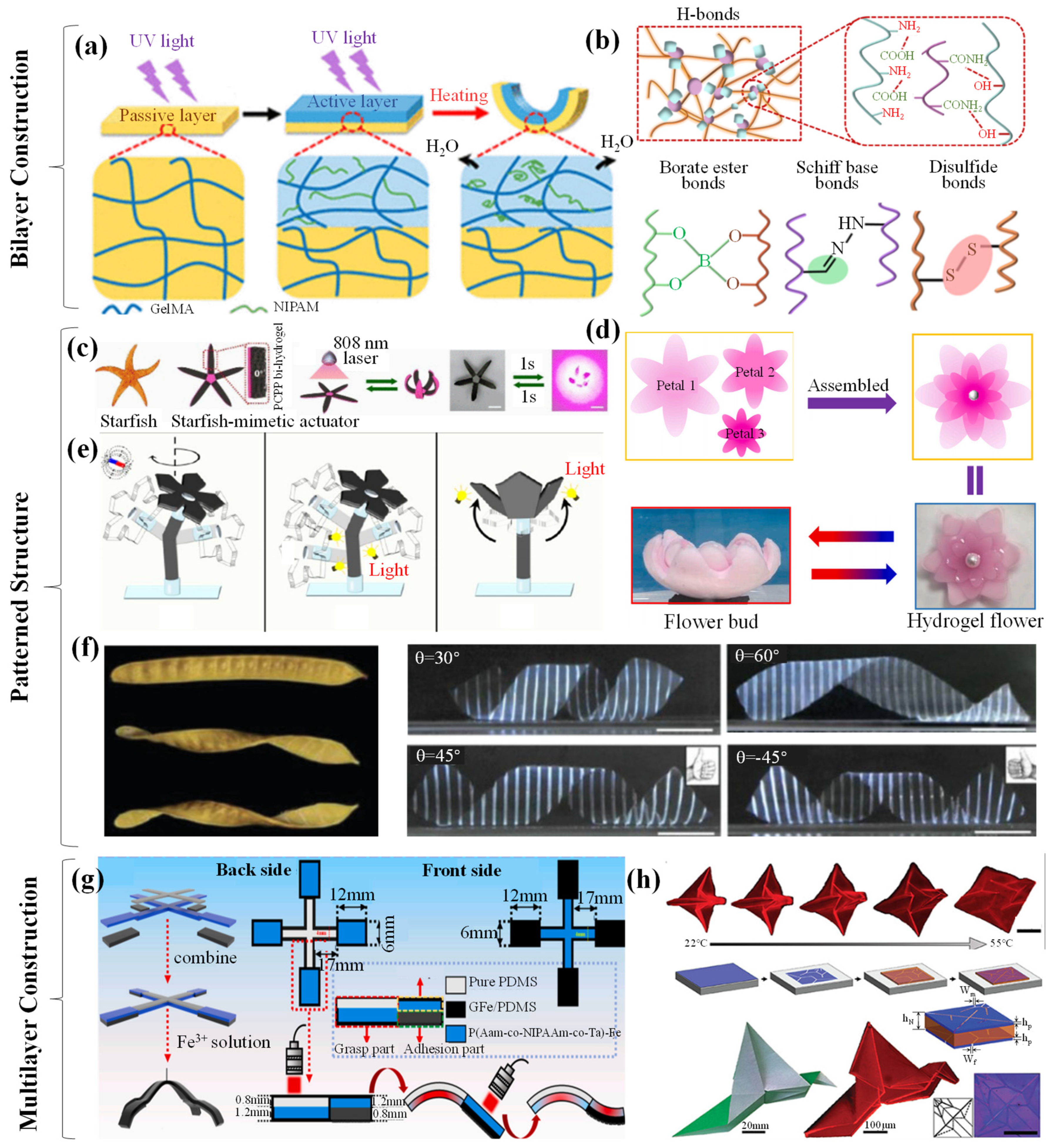
4. Stimuli-Responsive Hydrogel Grippers
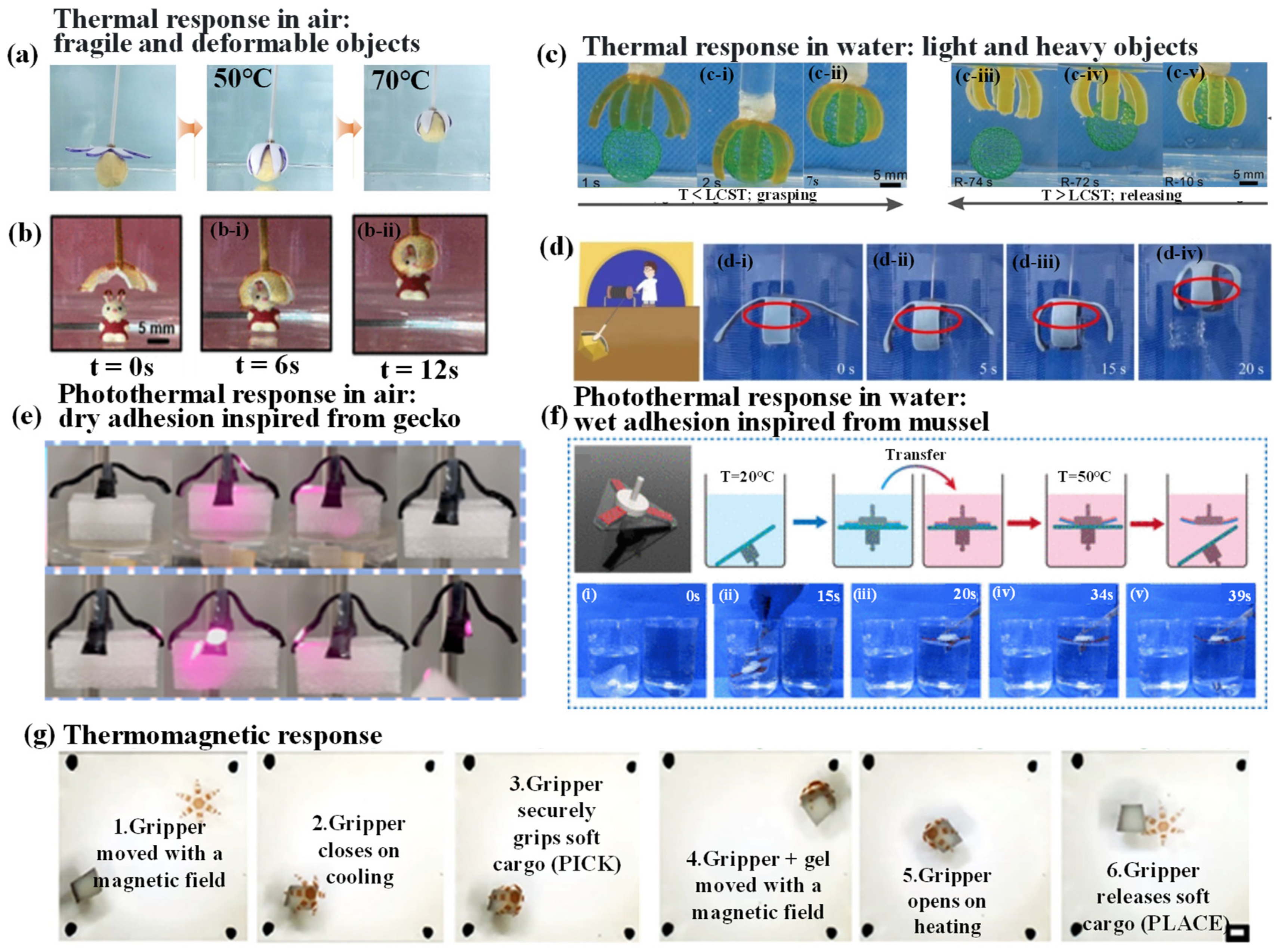
4.1. Thermal-Responsive Hydrogel Grippers
4.1.1. Thermal-Responsive Driving Mechanisms

4.1.2. Thermal-Responsive Grab and Release of Objects
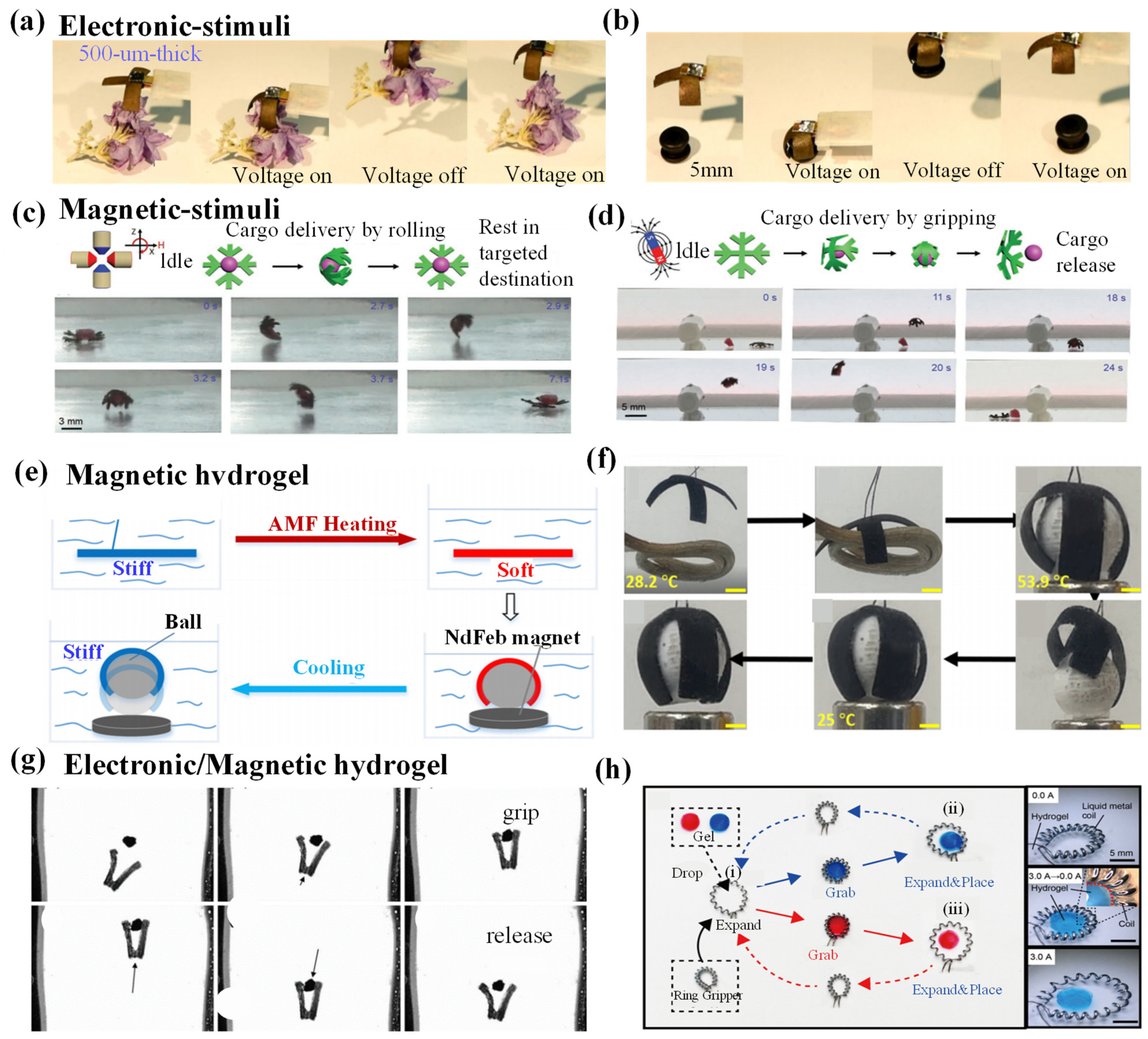
4.2. Electronic-/Magnetic-Responsive Hydrogel Grippers
4.2.1. Electronic-/Magnetic-Responsive Driving Mechanisms
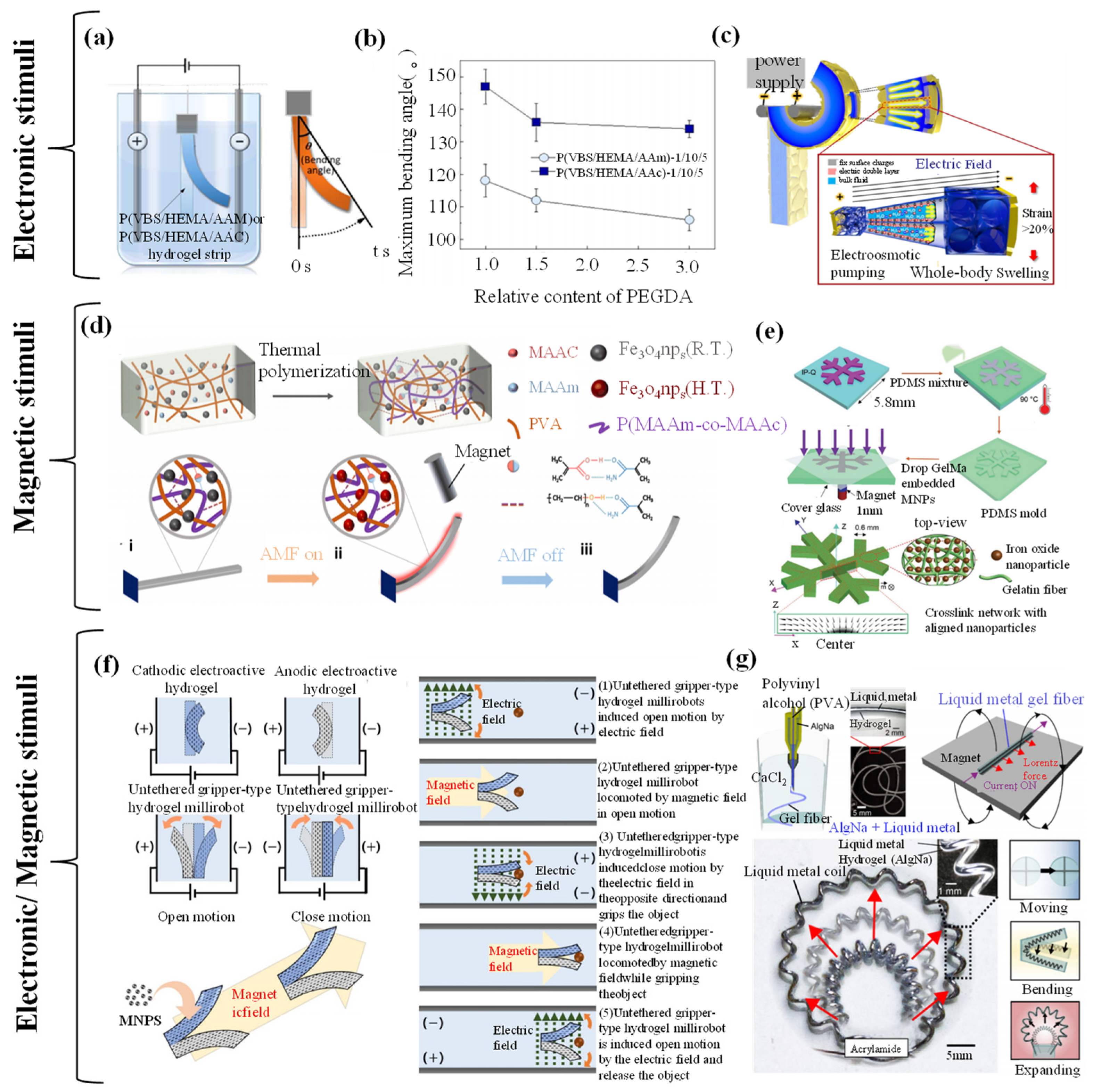
4.2.2. Electronic-/Magnetic-Responsive Grab and Release of Objects
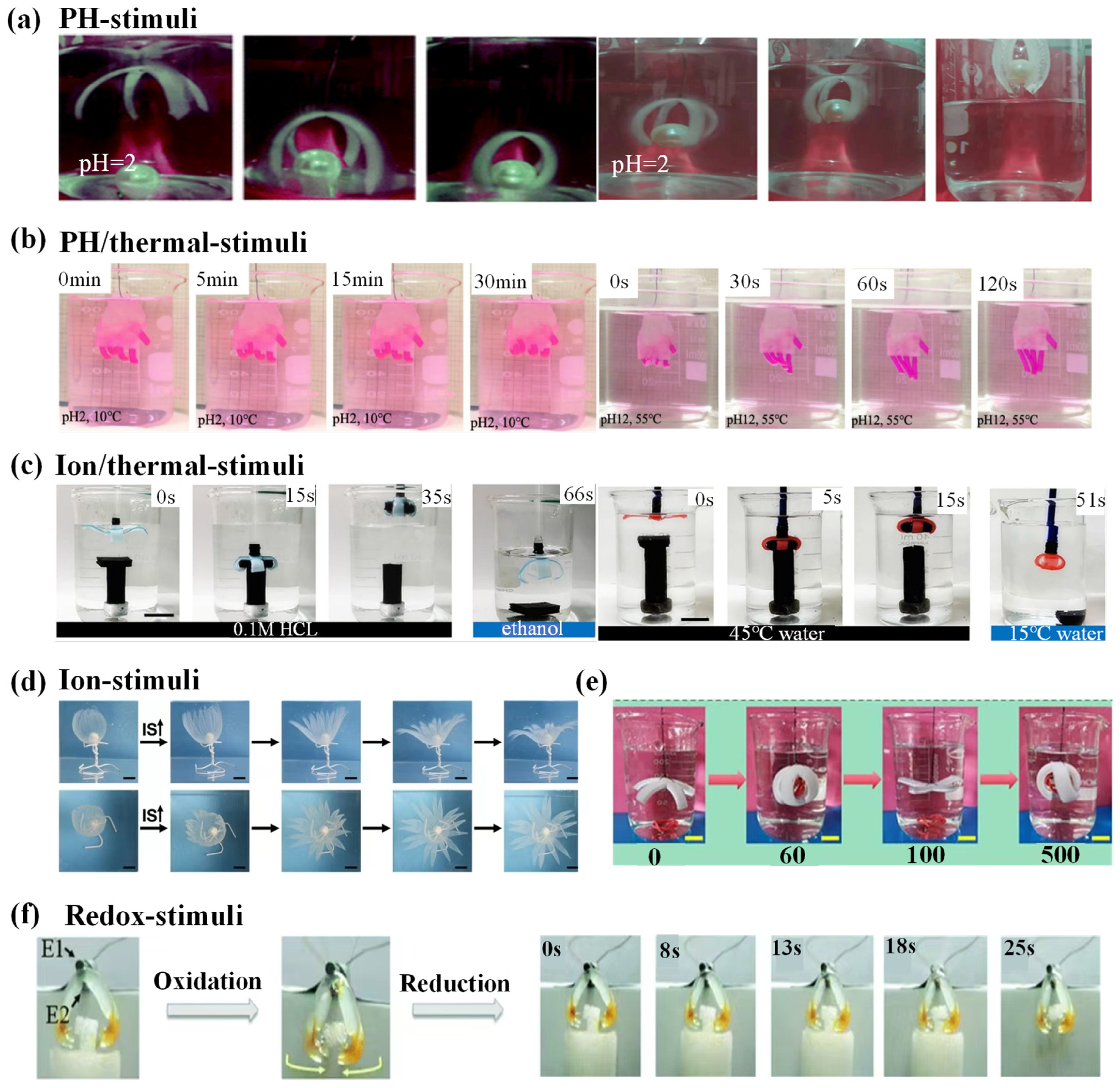
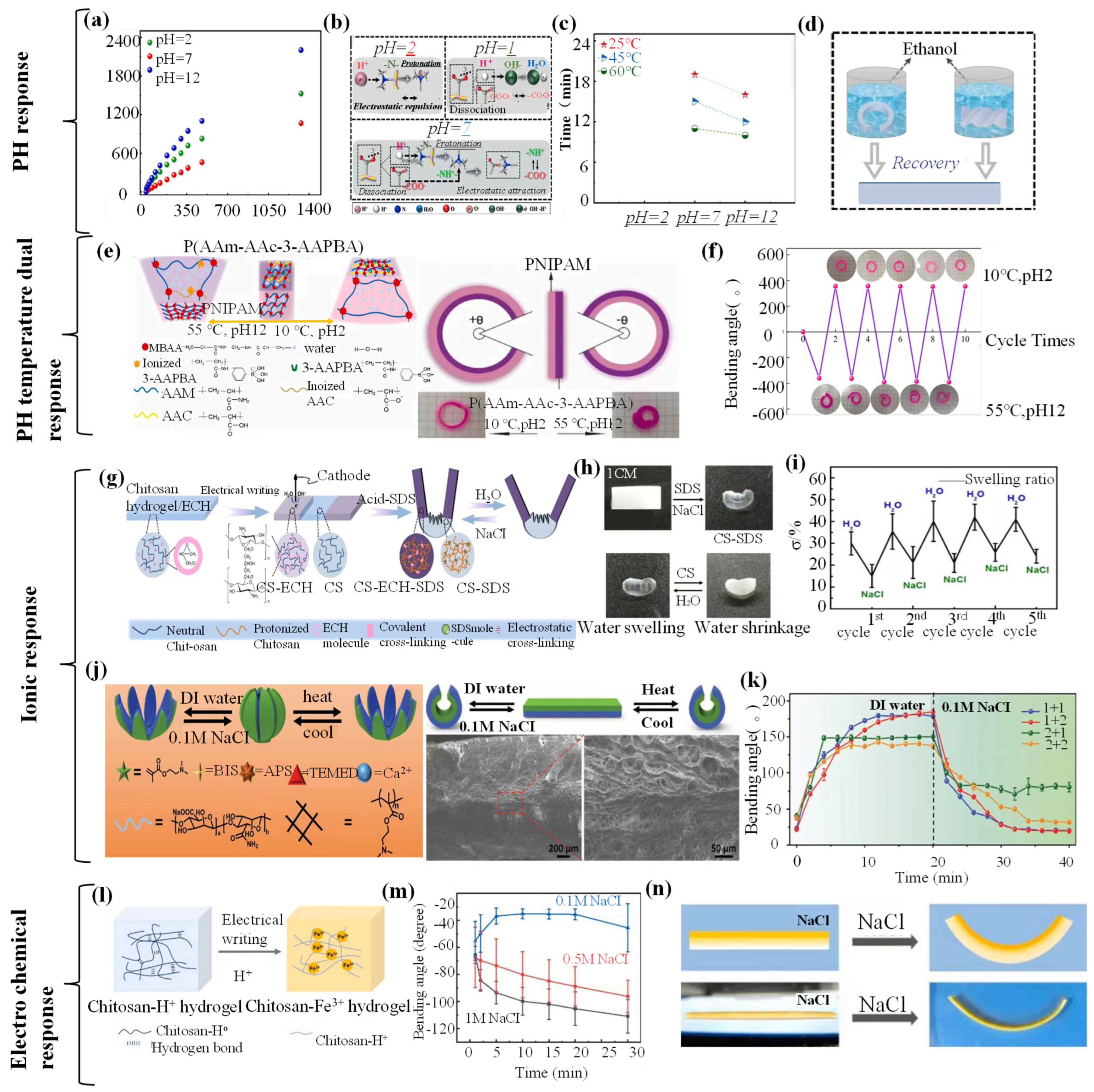
4.3. Chemical-Responsive Hydrogel Grippers
4.3.1. Chemical-Responsive Driving Mechanisms
4.3.2. Chemical-Responsive Grab and Release of Objects
4.4. Others
4.4.1. Other Driving Mechanisms
4.4.2. Other Grab and Release of Objects
| Response Type | Materials | Fabrication | Gripper Effect | Advantage | Disadvantages | Ref. |
|---|---|---|---|---|---|---|
| Thermal | PNIPAm, PAA-Ca(CH3COO)2, P(MAAm-co-MAAc) and PNI-PAM | One-step method | It can lift an object 500 times its own weight. | Self-locking ability, high grasping ability, and versatility. | Limited mechanical properties, temperature dependence, and complex preparation. | [103] |
| PNIPAm, PAA-Ca(CH3COO)2, P(MAAm-co-MAAc and PNI-PAM | One-step polymerization method | It can withstand a weight more than 47.6 times its own weight. | High load-bearing capacity, multiple bending states, and brush-like adhesion between hydrogels. | The preparation process is complex and relies on temperature control. There may be durability issues. | [94] | |
| NIPAM and TEMED | One-step polymerization method | Compared with traditional PNIPAM materials, the weight is reduced by 7.5 times. | Fast response, high power output, reusable, and no chemical modification required. | Sensitive to temperature, dependent on water environment, and has a complex structure. | [115] | |
| NIPAM, AA, PEGDA | One-step ultraviolet polymerization method | Response performance in water at 60 °C within just 9 s. | Ultrafast thermal response speed, high strength, and biomimicry. | The preparation process is relatively complex, the material cost is high, and it is limited by temperature. | [116] | |
| Photothermal | ANF/GNP and PEG | One-step method | Maintain a temperature above 90 °C when the light power density is 200 mW cm−2. | It has a high in-plane thermal conductivity, excellent photothermal conversion performance, and temperature-dependent flexibility and shape memory behavior. | The mechanical properties may be relatively low, and the preparation process is slightly complicated. | [117] |
| AAm, NIPAAm, TA, and PDMS | One-step method | It can be increased from 17.9 °C to 107 °C within 30 s. | Highly biomimetic, near-infrared responsive, reversible conversion, and good flexibility. | Dependence on near-infrared light, limited temperature range, and limited load-bearing capacity. | [98] | |
| Electric | VBS, APS, CaCl2, and TMEDA, AAm, HEMA, PBS, FBS, and CCK | Ultraviolet light irradiation process method | Even at a low voltage of 10 V, it exhibits a bending deflection of more than 100° within 1 min. | Rapid deformation at low voltage, good biocompatibility. | Limited mechanical strength and high energy consumption. | [124] |
| TREN, PAA, TREN solution | Ultraviolet polymerization | Capable of gripping and lifting objects weighing approximately 31 times the weight of the gripper. | High performance, low power consumption, high strain capacity, long life, high energy density, and multi-degree-of-freedom motion | Copper pole limitations affect bending performance, and its widespread use is dependent on external support. | [125] | |
| Magnetic | MAAm, Fe3O4 and KPS, MAAc, PVA, TMEDA, MBAA, NH4OH, MPS, TEOS | One-step polymerization method | Excellent mechanical properties, up to 19.7 MPa Young’s modulus, 14.6 MPa tensile breaking stress, and 390% strain at break. | Outstanding reversible drive deformation capability, its stiffness and shape can be precisely controlled by adjusting temperature and magnetic field strength. | Requires an alternating magnetic field as an external stimulus to drive the deformation of the hydrogel, which does not allow for fully autonomous actuation. | [129] |
| GelMa, LAP, SPIONs | Ultraviolet polymerization | Can lift up to 9.5 mg at a magnetic field strength of 5–25 mT. | Programmable 3D magnetic anisotropy, biodegradability, multifunctionality. | Limited load capacity, more complex manufacturing process, magnetic field dependence. | [48] | |
| Electric–magnetic | CAA, PEGA, DMC, AM, MBA, MeOH, PBS, TPO | Ultraviolet polymerization | Under lower electric fields (2 V cm−1 and 3 V cm−1), the time required for the application of an electric field to a hydrogel to reach the maximum bending angle is approximately 120 s. While under higher electric fields (4 V cm−1 and 5 V cm−1), it is around 80 s. | Capable of performing simultaneous gripping and moving of objects, it can be manipulated remotely and is biocompatible. | Electrode fragmentation issues, reliance on clean electrolytes, and optimization of electrode materials. | [132] |
| AlgNa, PVA, acrylamide gel, CaCl2 | Microfloppies control technology | An ultrafast response of 260.5 mm s−1 with high-frequency controllability (6 Hz) and a large deformation of 172% with hydrogel actuation are observed. | Ultrafast response, high-frequency control, handling of fragile objects | Moisture loss due to heat generated by current flow. | [133] | |
| pH | AA and DMAEMA | One-step method | The soft clip bends gradually in water, holding the copper block and lifting it by 2 mm (hydrogel clip 10.2039 g, a piece of copper 2.2921 g). | PAD4 hydrogel showed complex deformation under different pH conditions and recovered to its original shape in ethanol. High mechanical strength. | The tensile fracture strength decreases after expansion. The response speed slows down. The bending angle decreases. | [142] |
| pH and thermal dual-responsive | P(AAm-AAc-3-AAPBA)/PNIPAM | Ultraviolet polymerization | It takes about 70 s to change the bending angle of the bilayer structure from 355 (10 °C, pH 2) to 360 (55 °C, pH 12). | Very sensitive to the temperature of the surrounding environment. The bending angle is large. The bending speed is fast. It can be reused. | The preparation process is complicated. The mechanical strength is low. | [143] |
| CS hydrogel | One-step polymerization method | The flexibility is improved in sodium chloride solution (elongation at break is 43.40 ± 3.46% and Young’s modulus is 133.29 ± 24.61 kPa). | Fast response. High power output. Fast bending speed. | After swelling in deionized water, it becomes rigid and the elongation at break decreases. Dependent on the water environment. | [144] | |
| Ionic strength and thermal dual-responsive | Alg-PDMAEMA layer | Crosslinking synthesis method | Complex deformation from 2D to 3D can be realized. | Complex bending lines can be realized. | Dependence on water environment. Low mechanical strength. | [145] |
| Electrochemistry | Chitosan hydrogel | One-step method | The gripper can bend automatically in about 30 s, and its bending response is faster. | Fast reaction time. Shape memory effect. Reusable. | Dependence on electrolyte solution. Complex driving environment. | [146] |
| Light | MG-CMA | UV-light, PRLDA | The groups enable light to directly assemble gels and adjust mechanical and swelling properties without the use of small molecules or free radical polymerization. | Enables light to directly assemble gels and adjust mechanical and swelling properties without the use of small molecules or radical polymerization. | [164] | |
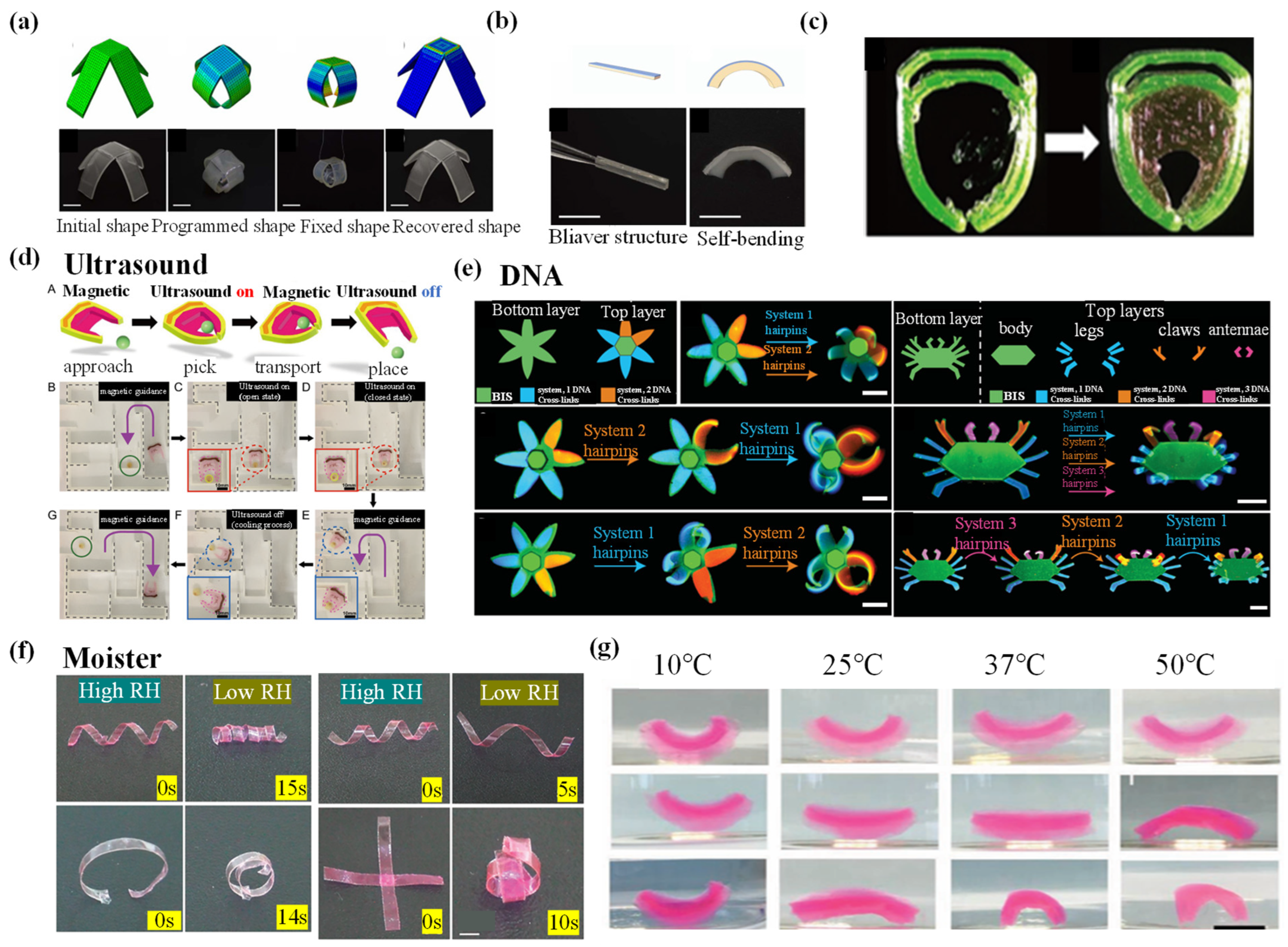
| DNA | DNA molecules | One-step method | The high degree of swelling of the DNA gel can lead to bending of structures that are a millimeter to a centimeter thick. First, the bilayer is 10 mm long × 7.23 mm thick, with a maximum expansion ratio of 3.72 ± 0.11, and should be folded into a complete circle after sequence-specific DNA trigger drive. | The gel is able to respond to specific DNA trigger signals, enabling complex and programmable shape changes. | Multistage, goal-oriented behavior that is not currently achievable. | [117] |
| Ultrasound | NIPAM | 3D printed | The printability of AAm-based inks is between 0.21 mm and 0.41 mm nozzle diameters, and the corresponding printing pressure is between 15 and 45 kPa. In the case of NIPAM-based inks, a precise range of printing pressures (10–30 kPa) is observed in the range of nozzle diameters from 0.21 to 0.41 mm. | Compliant. | The attenuation coefficient is large. | [165] |
| Moisture | PAM | Crosslinking | At 60% ΔRH, the bending angle of the actuator with different PET thicknesses varies. The 22 μm thick PET actuator has a response time of 6 s and a recovery time of 9 s, with a maximum bending angle of approximately 297°. | With a small temperature change (3.9 °C), OS oscillation drives with a large oscillation amplitude (14.4 mm) can be realized. | Creeping actuators: much slower than light-responsive actuators. Moisture control electric switch: the frequency is relatively slow. Jump actuators: light-responsive jump actuators have a relatively low jump height. Mechanical gripper: the gripping time and weight ratio of the gripper cannot meet the requirements. | [162] |
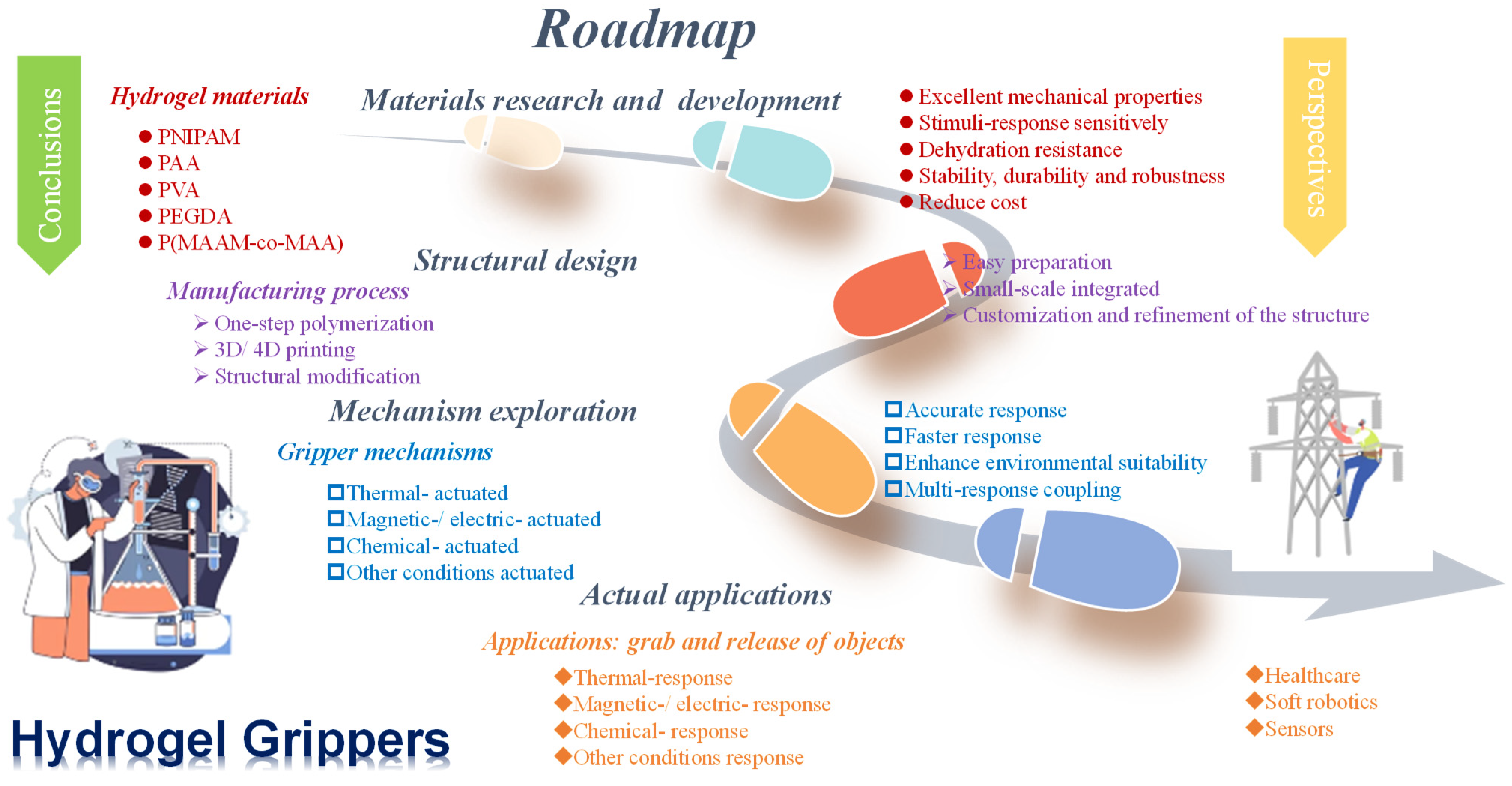
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takács, K.; Mason, A.; Christensen, L.B.; Haidegger, T. Robotic Grippers for Large and Soft Object Manipulation. In Proceedings of the 2020 IEEE 20th International Symposium on Computational Intelligence and Informatics (CINTI), Budapest, Hungary, 5–7 November 2020; pp. 133–138. [Google Scholar]
- Manti, M.; Hassan, T.; Passetti, G.; D’Elia, N.; Laschi, C.; Cianchetti, M. A Bioinspired Soft Robotic Gripper for Adaptable and Effective Grasping. Soft Robot. 2015, 2, 107–116. [Google Scholar] [CrossRef]
- Liu, F.; Sun, F.; Fang, B.; Li, X.; Sun, S.; Liu, H. Hybrid Robotic Grasping with a Soft Multimodal Gripper and a Deep Multistage Learning Scheme. IEEE Trans. Robot. 2023, 39, 2379–2399. [Google Scholar] [CrossRef]
- Wang, Y.; Gupta, U.; Parulekar, N.; Zhu, J. A Soft Gripper of Fast Speed and Low Energy Consumption. Sci. China Technol. Sci. 2019, 62, 31–38. [Google Scholar] [CrossRef]
- Lu, Z.; Li, W.; Zhang, L. Research Development of Soft Manipulator: A Review. Adv. Mech. Eng. 2020, 12, 168781402095009. [Google Scholar]
- Calderón, A.A.; Ugalde, J.C.; Zagal, J.C.; Pérez-Arancibia, N.O. Design, Fabrication and Control of a Multi-Material-Multi-Actuator Soft Robot Inspired by Burrowing Worms. In Proceedings of the 2016 IEEE International Conference on Robotics and Biomimetics (ROBIO), Qingdao, China, 3–7 December 2016; pp. 31–38. [Google Scholar]
- Wang, X.; Yang, B.; Tan, D.; Li, Q.; Song, B.; Wu, Z.-S.; del Campo, A.; Kappl, M.; Wang, Z.; Gorb, S.N.; et al. Bioinspired Footed Soft Robot with Unidirectional All-Terrain Mobility. Mater. Today 2020, 35, 42–49. [Google Scholar] [CrossRef]
- Umedachi, T.; Vikas, V.; Trimmer, B.A. Softworms: The Design and Control of Non-Pneumatic, 3D-Printed, Deformable Robots. Bioinspir. Biomim. 2016, 11, 025001. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, J.; Kim, K.K.; Won, P.; Hong, S.; Ko, S.H. Biomimetic Chameleon Soft Robot with Artificial Crypsis and Disruptive Coloration Skin. Nat. Commun. 2021, 12, 4658. [Google Scholar] [CrossRef]
- Onal, C.D.; Rus, D. Autonomous Undulatory Serpentine Locomotion Utilizing Body Dynamics of a Fluidic Soft Robot. Bioinspir. Biomim. 2013, 8, 026003. [Google Scholar] [CrossRef] [PubMed]
- Appiah, C.; Arndt, C.; Siemsen, K.; Heitmann, A.; Staubitz, A.; Selhuber-Unkel, C. Living Materials Herald a New Era in Soft Robotics. Adv. Mater. 2019, 31, 1807747. [Google Scholar] [CrossRef]
- Marchese, A.D.; Katzschmann, R.K.; Rus, D. A Recipe for Soft Fluidic Elastomer Robots. Soft Robot. 2015, 2, 7–25. [Google Scholar] [CrossRef]
- Wöhl, S.; Schuster, S. The Predictive Start of Hunting Archer Fish: A Flexible and Precise Motor Pattern Performed with the Kinematics of an Escape C-Start. J. Exp. Biol. 2007, 210, 311–324. [Google Scholar] [CrossRef]
- Tytell, E.D.; Lauder, G.V. The C-Start Escape Response of Polypterus Senegalus: Bilateral Muscle Activity and Variation during Stage 1 and 2. J. Exp. Biol. 2002, 205, 2591–2603. [Google Scholar] [CrossRef] [PubMed]
- Cianchetti, M.; Calisti, M.; Margheri, L.; Kuba, M.; Laschi, C. Bioinspired Locomotion and Grasping in Water: The Soft Eight-Arm OCTOPUS Robot. Bioinspir. Biomim. 2015, 10, 035003. [Google Scholar] [CrossRef] [PubMed]
- Mazzolai, B.; Margheri, L.; Cianchetti, M.; Dario, P.; Laschi, C. Soft-Robotic Arm Inspired by the Octopus: II. From artificial requirements to innovative technological solutions. Bioinspir. Biomim. 2012, 7, 025005. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Luo, X.; Zhao, H.; Qiao, C.; Li, J.; Yi, J.; Yang, L.; Oropeza, F.J.; Hu, T.S.; Xu, Q.; et al. Recent Advances in Biomimetic Soft Robotics: Fabrication Approaches, Driven Strategies and Applications. Soft Matter 2022, 18, 7699–7734. [Google Scholar] [CrossRef] [PubMed]
- Majidi, C. Soft Robotics: A Perspective—Current Trends and Prospects for the Future. Soft Robot. 2014, 1, 5–11. [Google Scholar] [CrossRef]
- Rajagopalan, P.; Muthu, M.; Liu, Y.; Luo, J.; Wang, X.; Wan, C. Advancement of Electroadhesion Technology for Intelligent and Self-Reliant Robotic Applications. Adv. Intell. Syst. 2022, 4, 2200064. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, P.; Lin, Y.; Tang, W.; Jiao, Z.; Yang, H.; Zou, J. Fluid-Driven Artificial Muscles: Bio-Design, Manufacturing, Sensing, Control, and Applications. Bio-Des. Manuf. 2021, 4, 123–145. [Google Scholar] [CrossRef]
- AboZaid, Y.A.; Aboelrayat, M.T.; Fahim, I.S.; Radwan, A.G. Soft Robotic Grippers: A Review on Technologies, Materials, and Applications. Sens. Actuators A Phys. 2024, 372, 115380. [Google Scholar] [CrossRef]
- Li, S.; Bai, H.; Shepherd, R.F.; Zhao, H. Bio-Inspired Design and Additive Manufacturing of Soft Materials, Machines, Robots, and Haptic Interfaces. Angew. Chem. Int. Ed. 2019, 58, 11182–11204. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lyu, L.; Xu, Y.; Liang, H.; Zhang, X.; Ding, H.; Wu, Z. Intelligent Soft Surgical Robots for Next-Generation Minimally Invasive Surgery. Adv. Intell. Syst. 2021, 3, 2100011. [Google Scholar] [CrossRef]
- Pinelli, F.; Magagnin, L.; Rossi, F. Progress in Hydrogels for Sensing Applications: A Review. Mater. Today Chem. 2020, 17, 100317. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Li, T.; Zhang, J.; Tian, H. Stimuli-Responsive Hydrogels: Fabrication and Biomedical Applications. View 2022, 3, 20200112. [Google Scholar] [CrossRef]
- Li, G.; Li, C.; Li, G.; Yu, D.; Song, Z.; Wang, H.; Liu, X.; Liu, H.; Liu, W. Development of Conductive Hydrogels for Fabricating Flexible Strain Sensors. Small 2022, 18, e2101518. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, Processing and Application of Hydrogels: A Review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Kloxin, C.J.; Scott, T.F.; Adzima, B.J.; Bowman, C.N. Covalent Adaptable Networks (CANs): A Unique Paradigm in Cross-Linked Polymers. Macromolecules 2010, 43, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Appel, E.A.; del Barrio, J.; Loh, X.J.; Scherman, O.A. Supramolecular Polymeric Hydrogels. Chem. Soc. Rev. 2012, 41, 6195–6214. [Google Scholar] [CrossRef] [PubMed]
- Guiseppi-Elie, A. Electroconductive Hydrogels: Synthesis, Characterization and Biomedical Applications. Biomaterials 2010, 31, 2701–2716. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, Y.; Duan, G.; Liang, Z.; Huang, Y.; Zhang, C.; Han, X.; Ma, C.; He, S.; Jiang, S. Recent Advances of Biomass-Based Smart Hydrogel Actuators: A Review. Chem. Eng. J. 2024, 498, 155157. [Google Scholar] [CrossRef]
- Chen, J.; Yan, X.; Zhang, T.; Yuan, X.; Zhang, Q. Advances of Liquid Metal Hydrogel Composites in Biomedical Applications. Biomed. Mater. 2023, 19, 012001. [Google Scholar] [CrossRef]
- Xu, C.; Yang, K.; Zhu, G.; Ou, C.; Jiang, J.; Zhuravlev, E.; Zhang, Y. Anti-Freezing Multifunctional Conductive Hydrogels: From Structure Design to Flexible Electronic Devices. Mater. Chem. Front. 2024, 8, 381–403. [Google Scholar] [CrossRef]
- Ding, M.; Jing, L.; Yang, H.; Machnicki, C.E.; Fu, X.; Li, K.; Wong, I.Y.; Chen, P.-Y. Multifunctional Soft Machines Based on Stimuli-Responsive Hydrogels: From Freestanding Hydrogels to Smart Integrated Systems. Mater. Today Adv. 2020, 8, 100088. [Google Scholar] [CrossRef]
- Apsite, I.; Salehi, S.; Ionov, L. Materials for Smart Soft Actuator Systems. Chem. Rev. 2022, 122, 1349–1415. [Google Scholar] [CrossRef] [PubMed]
- Shafranek, R.T.; Millik, S.C.; Smith, P.T.; Lee, C.-U.; Boydston, A.J.; Nelson, A. Stimuli-Responsive Materials in Additive Manufacturing. Prog. Polym. Sci. 2019, 93, 36–67. [Google Scholar] [CrossRef]
- Xin, Y.; Zhou, X.; Bark, H.; Lee, P.S. The Role of 3D Printing Technologies in Soft Grippers. Adv. Mater. 2024, 36, e2307963. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.L.; Lyu, Z.; Ariffin, M.Z.; Yeong, W.Y.; Lum, G.Z.; Campolo, D.; Han, B.S.; Wong, H.Y.A. 3D Printing of Robotic Soft Grippers: Toward Smart Actuation and Sensing. Adv. Mater. Technol. 2022, 7, 2101672. [Google Scholar] [CrossRef]
- Wang, M.; Li, W.; Tang, G.; Garciamendez-Mijares, C.E.; Zhang, Y.S. Engineering (Bio)Materials through Shrinkage and Expansion. Adv. Healthc. Mater. 2021, 10, 2100380. [Google Scholar] [CrossRef]
- Feng, W.; Wang, Z. Tailoring the Swelling-Shrinkable Behavior of Hydrogels for Biomedical Applications. Adv. Sci. 2023, 10, e2303326. [Google Scholar] [CrossRef]
- Lavrador, P.; Esteves, M.R.; Gaspar, V.M.; Mano, J.F. Stimuli-Responsive Nanocomposite Hydrogels for Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2005941. [Google Scholar] [CrossRef]
- Han, I.K.; Chung, T.; Han, J.; Kim, Y.S. Nanocomposite Hydrogel Actuators Hybridized with Various Dimensional Nanomaterials for Stimuli Responsiveness Enhancement. Nano Converg. 2019, 6, 18. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Y.; Xiao, J.; Chen, S.-W.; Tu, Q.; Yuan, M.-S.; Wang, J. Liquid Metal-Doped Conductive Hydrogel for Construction of Multifunctional Sensors. Anal. Chem. 2023, 95, 3811–3820. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ding, Q.; Wang, H.; Wu, Z.; Gui, X.; Li, C.; Hu, N.; Tao, K.; Wu, J. Engineering Smart Composite Hydrogels for Wearable Disease Monitoring. Nano-Micro Lett. 2023, 15, 105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ren, L.; Chen, Y.; Niu, S.; Han, Z.; Ren, L. Bio-Inspired Soft Grippers Based on Impactive Gripping. Adv. Sci. 2021, 8, 2002017. [Google Scholar] [CrossRef]
- Qu, J.; Mao, B.; Li, Z.; Xu, Y.; Zhou, K.; Cao, X.; Fan, Q.; Xu, M.; Liang, B.; Liu, H.; et al. Recent Progress in Advanced Tactile Sensing Technologies for Soft Grippers. Adv. Funct. Mater. 2023, 33, 2306249. [Google Scholar] [CrossRef]
- Ji, Z.; Yan, C.; Yu, B.; Zhang, X.; Cai, M.; Jia, X.; Wang, X.; Zhou, F. 3D Printing of Hydrogel Architectures with Complex and Controllable Shape Deformation. Adv. Mater. Technol. 2019, 4, 1800713. [Google Scholar] [CrossRef]
- Goudu, S.R.; Yasa, I.C.; Hu, X.; Ceylan, H.; Hu, W.; Sitti, M. Biodegradable Untethered Magnetic Hydrogel Milli-Grippers. Adv. Funct. Mater. 2020, 30, 2004975. [Google Scholar] [CrossRef]
- Ansari, M.J.; Rajendran, R.R.; Mohanto, S.; Agarwal, U.; Panda, K.; Dhotre, K.; Manne, R.; Deepak, A.; Zafar, A.; Yasir, M.; et al. Poly(N-Isopropylacrylamide)-Based Hydrogels for Biomedical Applications: A Review of the State-of-the-Art. Gels 2022, 8, 454. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Fu, W.; Yao, M.; Ding, Z.; Xuan, J.; Li, D.; Wang, S.; Xia, Y.; Cao, M. Poly(N-Isopropylacrylamide)-Based Thermoresponsive Composite Hydrogels for Biomedical Applications. Polymers 2020, 12, 580. [Google Scholar] [CrossRef]
- Shaibie, N.A.; Ramli, N.A.; Faizal, N.D.F.M.; Srichana, T.; Amin, M.C.I.M. Poly(N-Isopropylacrylamide)-Based Polymers: Recent Overview for the Development of Temperature-Responsive Drug Delivery and Biomedical Applications. Macromol. Chem. Phys. 2023, 224, 2300157. [Google Scholar] [CrossRef]
- Xiong, J.; Chen, J.; Lee, P.S. Functional Fibers and Fabrics for Soft Robotics, Wearables, and Human–Robot Interface. Adv. Mater. 2021, 33, e2002640. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pal, A.; Aghakhani, A.; Pena-Francesch, A.; Sitti, M. Soft Actuators for Real-World Applications. Nat. Rev. Mater. 2022, 7, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A. Poly(Acrylic Acid) Nanocomposites: Design of Advanced Materials. J. Plast. Film Sheeting 2021, 37, 409–428. [Google Scholar] [CrossRef]
- Park, H.; Robinson, J.R. Mechanisms of Mucoadhesion of Poly(Acrylic Acid) Hydrogels. Pharm. Res. 1987, 4, 457–464. [Google Scholar] [CrossRef]
- Lamch, Ł.; Ronka, S.; Moszyńska, I.; Warszyński, P.; Wilk, K.A. Hydrophobically Functionalized Poly(Acrylic Acid) Comprising the Ester-Type Labile Spacer: Synthesis and Self-Organization in Water. Polymers 2020, 12, 1185. [Google Scholar] [CrossRef] [PubMed]
- Shahid, A.; Roveda, L.; Piga, D.; Braghin, F. Learning Continuous Control Actions for Robotic Grasping with Reinforcement Learning. In Proceedings of the 2020 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Toronto, ON, Canada, 11–14 October 2020; pp. 4066–4072. [Google Scholar]
- Deng, Z.; Jonetzko, Y.; Zhang, L.; Zhang, J. Grasping Force Control of Multi-Fingered Robotic Hands through Tactile Sensing for Object Stabilization. Sensors 2020, 20, 1050. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of Poly(Vinyl Alcohol) and Natural Polymers. Polym. Rev. 2018, 58, 247–287. [Google Scholar] [CrossRef]
- Yang, H.; Xu, S.; Jiang, L.; Dan, Y. Thermal Decomposition Behavior of Poly (Vinyl Alcohol) with Different Hydroxyl Content. J. Macromol. Sci. Part B 2012, 51, 464–480. [Google Scholar] [CrossRef]
- Hdidar, M.; Chouikhi, S.; Fattoum, A.; Arous, M. Effect of Hydrolysis Degree and Mass Molecular Weight on the Structure and Properties of PVA Films. Ionics 2017, 23, 3125–3135. [Google Scholar] [CrossRef]
- Wang, J.; Ye, L. Structure and Properties of Polyvinyl Alcohol/Polyurethane Blends. Compos. Part B Eng. 2015, 69, 389–396. [Google Scholar] [CrossRef]
- Karimineghlani, P.; Emmons, E.; Green, M.J.; Shamberger, P.; Sukhishvili, S.A. A Temperature-Responsive Poly(Vinyl Alcohol) Gel for Controlling Fluidity of an Inorganic Phase Change Material. J. Mater. Chem. A 2017, 5, 12474–12482. [Google Scholar] [CrossRef]
- Shcherbina, A.A.; Chalykh, A.E.; Artyukhov, A.A.; Bryukhanov, L.A.; Shtilman, M.I. Study of Diagrams of the Phase State of the PVA-Water System in a Wide Range of Temperatures and Compositions. Public Health Toxicol. 2021, 1 (Suppl. S1), A27. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, Y.; Yan, D. Hyperbranched Polymer Vesicles: From Self-Assembly, Characterization, Mechanisms, and Properties to Applications. Chem. Soc. Rev. 2015, 44, 3874–3889. [Google Scholar] [CrossRef] [PubMed]
- Mercado-Montijo, J.; Anstine, D.M.; Rukmani, S.J.; Colina, C.M.; Andrew, J.S. PEGDA Hydrogel Structure from Semi-Dilute Concentrations: Insights from Experiments and Molecular Simulations. Soft Matter 2022, 18, 3565–3574. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Wang, J.; Mi, S. Photo Processing for Biomedical Hydrogels Design and Functionality: A Review. Polymers 2018, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Khalili, M.H.; Afsar, A.; Zhang, R.; Wilson, S.; Dossi, E.; Goel, S.; Impey, S.A.; Aria, A.I. Thermal Response of Multi-Layer UV Crosslinked PEGDA Hydrogels. Polym. Degrad. Stab. 2022, 195, 109805. [Google Scholar] [CrossRef]
- McAvoy, K.; Jones, D.; Thakur, R.R.S. Synthesis and Characterisation of Photocrosslinked Poly(Ethylene Glycol) Diacrylate Implants for Sustained Ocular Drug Delivery. Pharm. Res. 2018, 35, 36. [Google Scholar] [CrossRef]
- Panzer, J.M. Holding It Together: Noncovalent Cross-Linking Strategies for Ionogels and Eutectogels. Mater. Adv. 2022, 3, 7709–7725. [Google Scholar] [CrossRef]
- Lee, Y.; Song, W.J.; Sun, J.-Y. Hydrogel Soft Robotics. Mater. Today Phys. 2020, 15, 100258. [Google Scholar] [CrossRef]
- Li, W.; Guan, Q.; Li, M.; Saiz, E.; Hou, X. Nature-Inspired Strategies for the Synthesis of Hydrogel Actuators and Their Applications. Prog. Polym. Sci. 2023, 140, 101665. [Google Scholar] [CrossRef]
- Li, M.; Zhou, S.; Guan, Q.; Li, W.; Li, C.; Bouville, F.; Bai, H.; Saiz, E. Robust Underwater Oil-Repellent Biomimetic Ceramic Surfaces: Combining the Stability and Reproducibility of Functional Structures. ACS Appl. Mater. Interfaces 2022, 14, 46077–46085. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Dai, C.F.; Wagner, D.; Khoruzhenko, O.; Hong, W.; Breu, J.; Zheng, Q.; Wu, Z.L. Patterned Electrode Assisted One-Step Fabrication of Biomimetic Morphing Hydrogels with Sophisticated Anisotropic Structures. Adv. Sci. 2021, 8, 2102353. [Google Scholar] [CrossRef]
- Zhan, Z.; Chen, L.; Duan, H.; Chen, Y.; He, M.; Wang, Z. 3D Printed Ultra-Fast Photothermal Responsive Shape Memory Hydrogel for Microrobots. Int. J. Extrem. Manuf. 2021, 4, 015302. [Google Scholar] [CrossRef]
- Hua, M.; Wu, D.; Wu, S.; Ma, Y.; Alsaid, Y.; He, X. 4D Printable Tough and Thermoresponsive Hydrogels. ACS Appl. Mater. Interfaces 2021, 13, 12689–12697. [Google Scholar] [CrossRef] [PubMed]
- Arslan, H.; Nojoomi, A.; Jeon, J.; Yum, K. 3D Printing of Anisotropic Hydrogels with Bioinspired Motion. Adv. Sci. 2019, 6, 1800703. [Google Scholar] [CrossRef]
- Li, W.; Zhou, R.; Ouyang, Y.; Guan, Q.; Shen, Y.; Saiz, E.; Li, M.; Hou, X. Harnessing Biomimicry for Controlled Adhesion on Material Surfaces. Small 2024, e2401859. [Google Scholar] [CrossRef] [PubMed]
- Shim, T.S.; Kim, S.-H.; Heo, C.-J.; Jeon, H.C.; Yang, S.-M. Inside Back Cover: Controlled Origami Folding of Hydrogel Bilayers with Sustained Reversibility for Robust Microcarriers. Angew. Chem. Int. Ed. 2012, 51, 1489. [Google Scholar] [CrossRef]
- Zheng, W.J.; An, N.; Yang, J.H.; Zhou, J.; Chen, Y.M. Tough Al-Alginate/Poly(N-Isopropylacrylamide) Hydrogel with Tunable LCST for Soft Robotics. ACS Appl. Mater. Interfaces 2015, 7, 1758–1764. [Google Scholar] [CrossRef]
- Yao, C.; Liu, Z.; Yang, C.; Wang, W.; Ju, X.-J.; Xie, R.; Chu, L.-Y. Poly(N-Isopropylacrylamide)-Clay Nanocomposite Hydrogels with Responsive Bending Property as Temperature-Controlled Manipulators. Adv. Funct. Mater. 2015, 25, 2980–2991. [Google Scholar] [CrossRef]
- Kim, D.; Kim, H.; Lee, E.; Jin, K.S.; Yoon, J. Programmable Volume Phase Transition of Hydrogels Achieved by Large Thermal Hysteresis for Static-Motion Bilayer Actuators. Chem. Mater. 2016, 28, 8807–8814. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, T.; Hong, W.; Sun, W.; Liu, X.; Tong, Z. Infrared-Driving Actuation Based on Bilayer Graphene Oxide-Poly(N-Isopropylacrylamide) Nanocomposite Hydrogels. J. Mater. Chem. A 2014, 2, 15633–15639. [Google Scholar] [CrossRef]
- Wu, J.; Wu, B.; Xiong, J.; Sun, S.; Wu, P. Entropy-Mediated Polymer–Cluster Interactions Enable Dramatic Thermal Stiffening Hydrogels for Mechanoadaptive Smart Fabrics. Angew. Chem. Int. Ed. 2022, 61, e202204960. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Zhao, C.; Guan, X.; Lu, J.; Zhang, J. Cold-Induced Shape Memory Hydrogels for Strong and Programmable Artificial Muscles. Sci. China Mater. 2022, 65, 2274–2280. [Google Scholar] [CrossRef]
- Ni, C.; Chen, D.; Yin, Y.; Wen, X.; Chen, X.; Yang, C.; Chen, G.; Sun, Z.; Wen, J.; Jiao, Y.; et al. Shape Memory Polymer with Programmable Recovery Onset. Nature 2023, 622, 748–753. [Google Scholar] [CrossRef]
- Li, M.; Mao, A.; Guan, Q.; Saiz, E. Nature-Inspired Adhesive Systems. Chem. Soc. Rev. 2024, 53, 8240–8305. [Google Scholar] [CrossRef]
- Liu, S.; Gao, G.; Xiao, Y.; Fu, J. Tough and Responsive Oppositely Charged Nanocomposite Hydrogels for Use as Bilayer Actuators Assembled through Interfacial Electrostatic Attraction. J. Mater. Chem. B 2016, 4, 3239–3246. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, Y.; Yue, Y.; He, S.; Jiang, S.; Mei, C.; Xu, X.; Wu, Q.; Xiao, H.; Han, J. Nanocellulose-Mediated Bilayer Hydrogel Actuators with Thermo-Responsive, Shape Memory and Self-Sensing Performances. Carbohydr. Polym. 2024, 335, 122067. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Gao, J.; Yu, X.; Shi, J.; Chen, J.; Yu, L.; Chen, S.; Ding, J. 3D-Printed Porous Scaffolds of Hydrogels Modified with TGF-Β1 Binding Peptides to Promote In Vivo Cartilage Regeneration and Animal Gait Restoration. ACS Appl. Mater. Interfaces 2022, 14, 15982–15995. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, G.; Hao, D.; Chen, L.; Liu, K.; Liu, M. Macroscopic Layered Organogel–Hydrogel Hybrids with Controllable Wetting and Swelling Performance. Adv. Funct. Mater. 2018, 28, 1800793. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, X.; Luo, M.; Cao, K.; Lu, Y.; Xu, B.B.; Pan, H.; Tao, K.; Jiang, Y. Amino Acid-Induced Interface Charge Engineering Enables Highly Reversible Zn Anode. Adv. Funct. Mater. 2021, 31, 2103514. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, L.; Liu, A.; He, S.; Shao, W. Ultrafast Thermo-Responsive Bilayer Hydrogel Actuator Assisted by Hydrogel Microspheres. Sens. Actuators B Chem. 2022, 357, 131434. [Google Scholar] [CrossRef]
- Koo, H.B.; Heo, E.; Cho, I.; Kim, S.H.; Kang, J.; Chang, J.-B. Human Hand-Inspired All-Hydrogel Gripper with a High Load Capacity Formed by the Split-Brushing Adhesion of Diverse Hydrogels. Mater. Horiz. 2023, 10, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Xue, Y.; Sun, Y.; Chen, L.; Zhang, C.; Wu, Q.; Peng, S.; Ma, C.; Liu, Z.; Jiang, S.; et al. A Robust Anisotropic Light-Responsive Hydrogel for Ultrafast and Complex Biomimetic Actuation via Poly(Pyrrole)-Coated Electrospun Nanofiber. Chem. Eng. J. 2023, 452, 139373. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Liu, H.; Guo, Q.; Yu, H.; Yuan, Z.; Yang, W. Bionic Sea Anemone Actuator with a Double-Layered Gripper Driven by Multiple Physical Fields. ACS Appl. Polym. Mater. 2023, 5, 5582–5591. [Google Scholar] [CrossRef]
- Armon, S.; Efrati, E.; Kupferman, R.; Sharon, E. Geometry and Mechanics in the Opening of Chiral Seed Pods. Science 2011, 333, 1726–1730. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Dong, X.; Zhao, H.; Hu, T.S.; Lan, X.; Ding, L.; Li, J.; Ni, H.; Contreras, J.A.; Zeng, H.; et al. Near-Infrared Responsive Gecko-Inspired Flexible Arm Gripper. Mater. Today Phys. 2022, 29, 100919. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Cai, W.; Zhang, X.; Wang, Z.; Street, J.; Ong, W.-J.; Xia, Z.; Xu, Q. A Self-Healing Hydrogel with Pressure Sensitive Photoluminescence for Remote Force Measurement and Healing Assessment. Mater. Horiz. 2019, 6, 703–710. [Google Scholar] [CrossRef]
- An, N.; Li, M.; Zhou, J. Predicting Origami-Inspired Programmable Self-Folding of Hydrogel Trilayers. Smart Mater. Struct. 2016, 25, 11LT02. [Google Scholar] [CrossRef]
- Basak, S.; Bandyopadhyay, A. Next-Gen Biomimetic Actuators: Bilayer Hydrogel Evolution in the 21st Century and Its Advancements from a Post-2020 Perspective. RSC Appl. Polym. 2024, 2, 583–605. [Google Scholar] [CrossRef]
- Chen, L.; Liu, F.; Abdiryim, T.; Liu, X. Stimuli-Responsive Hydrogels as Promising Platforms for Soft Actuators. Mater. Today Phys. 2024, 40, 101281. [Google Scholar] [CrossRef]
- Li, H.; Hai, N.; Wu, X.; Yuan, Z.; Chen, X.; Zhang, J. Thermo-Hardening Hydrogel Actuators as Self-Locking Grippers. Sci. China Mater. 2024, 67, 2115–2122. [Google Scholar] [CrossRef]
- Peng, X.; Wang, H. Shape Changing Hydrogels and Their Applications as Soft Actuators. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 1314–1324. [Google Scholar] [CrossRef]
- Sano, K.; Ishida, Y.; Aida, T. Synthesis of Anisotropic Hydrogels and Their Applications. Angew. Chem. Int. Ed. 2018, 57, 2532–2543. [Google Scholar] [CrossRef] [PubMed]
- Sydney Gladman, A.; Matsumoto, E.A.; Nuzzo, R.G.; Mahadevan, L.; Lewis, J.A. Biomimetic 4D Printing. Nat. Mater. 2016, 15, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tan, Y.; Wang, X.; An, W.; Xu, S.; Liao, W.; Wang, Y. Photothermal Nanocomposite Hydrogel Actuator with Electric-Field-Induced Gradient and Oriented Structure. ACS Appl. Mater. Interfaces 2018, 10, 7688–7692. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tian, F.; Wang, X.; Xu, P.; An, W.; Hu, Y.; Xu, S. Biomimetic Color-Changing Hierarchical and Gradient Hydrogel Actuators Based on Salt-Induced Microphase Separation. ACS Appl. Mater. Interfaces 2019, 11, 48428–48436. [Google Scholar] [CrossRef]
- Gao, F.; Xu, Z.; Liang, Q.; Liu, B.; Li, H.; Wu, Y.; Zhang, Y.; Lin, Z.; Wu, M.; Ruan, C.; et al. Direct 3D Printing of High Strength Biohybrid Gradient Hydrogel Scaffolds for Efficient Repair of Osteochondral Defect. Adv. Funct. Mater. 2018, 28, 1706644. [Google Scholar] [CrossRef]
- Luo, R.; Wu, J.; Dinh, N.-D.; Chen, C.-H. Gradient Porous Elastic Hydrogels with Shape-Memory Property and Anisotropic Responses for Programmable Locomotion. Adv. Funct. Mater. 2015, 25, 7272–7279. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Chen, Z.; Fang, S.; Zhu, Y.; Baughman, R.H.; Jiang, L. Tunable, Fast, Robust Hydrogel Actuators Based on Evaporation-Programmed Heterogeneous Structures. Chem. Mater. 2017, 29, 9793–9801. [Google Scholar] [CrossRef]
- Wang, E.; Desai, M.S.; Lee, S.-W. Light-Controlled Graphene-Elastin Composite Hydrogel Actuators. Nano Lett. 2013, 13, 2826–2830. [Google Scholar] [CrossRef]
- Li, C.; Li, M.; Ni, Z.; Guan, Q.; Blackman, B.R.K.; Saiz, E. Stimuli-Responsive Surfaces for Switchable Wettability and Adhesion. J. R. Soc. Interface 2021, 18, 20210162. [Google Scholar] [CrossRef]
- Li, M.; Li, C.; Blackman, B.R.K.; Eduardo, S. Mimicking Nature to Control Bio-Material Surface Wetting and Adhesion. Int. Mater. Rev. 2022, 67, 658–681. [Google Scholar] [CrossRef]
- Spratte, T.; Arndt, C.; Wacker, I.; Hauck, M.; Adelung, R.; Schröder, R.R.; Schütt, F.; Selhuber-Unkel, C. Thermoresponsive Hydrogels with Improved Actuation Function by Interconnected Microchannels. Adv. Intell. Syst. 2022, 4, 2100081. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Xu, H.; Cheng, Z.; Yan, J.; Xie, X.-M. Biomimetic Gradient Hydrogel Actuators with Ultrafast Thermo-Responsiveness and High Strength. ACS Appl. Mater. Interfaces 2022, 14, 32541–32550. [Google Scholar] [CrossRef]
- Han, G.; Cheng, H.; Cheng, Y.; Zhou, B.; Liu, C.; Feng, Y. Scalable Sol–Gel Permeation Assembly of Phase Change Layered Film toward Thermal Management and Light-Thermal Driving Applications. Adv. Funct. Mater. 2024, 34, 2401295. [Google Scholar] [CrossRef]
- Liu, H.; Chu, H.; Yuan, H.; Li, D.; Deng, W.; Fu, Z.; Liu, R.; Liu, Y.; Han, Y.; Wang, Y.; et al. Bioinspired Multifunctional Self-Sensing Actuated Gradient Hydrogel for Soft-Hard Robot Remote Interaction. Nano Micro Lett. 2024, 16, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, S.; Li, B.; Yu, B.; Lee, H.; Cai, M.; Gorb, S.N.; Zhou, F.; Liu, W. Gecko’s Feet-Inspired Self-Peeling Switchable Dry/Wet Adhesive. Chem. Mater. 2021, 33, 2785–2795. [Google Scholar] [CrossRef]
- Kobayashi, K.; Yoon, C.; Oh, S.H.; Pagaduan, J.V.; Gracias, D.H. Biodegradable Thermomagnetically Responsive Soft Untethered Grippers. ACS Appl. Mater. Interfaces 2019, 11, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, Q.; Wu, X.; Li, W.; Lan, W.; Heng, L.; Street, J.; Xia, Z. Tough Reversible Adhesion Properties of a Dry Self-Cleaning Biomimetic Surface. ACS Appl. Mater. Interfaces 2018, 10, 26787–26794. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Guan, Q.; Dai, X.; Lv, J.; Xia, Z.; Ong, W.-J.; Saiz, E.; Hou, X. A Tough Reversible Biomimetic Transparent Adhesive Tape with Pressure-Sensitive and Wet-Cleaning Properties. ACS Nano 2021, 15, 19194–19201. [Google Scholar] [CrossRef]
- Na, H.; Kang, Y.-W.; Park, C.S.; Jung, S.; Kim, H.-Y.; Sun, J.-Y. Hydrogel-Based Strong and Fast Actuators by Electroosmotic Turgor Pressure. Science 2022, 376, 301–307. [Google Scholar] [CrossRef]
- Shin, Y.; Choi, M.-Y.; Choi, J.; Na, J.-H.; Kim, S.Y. Design of an Electro-Stimulated Hydrogel Actuator System with Fast Flexible Folding Deformation under a Low Electric Field. ACS Appl. Mater. Interfaces 2021, 13, 15633–15646. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Kim, D.; Song, Y.; Lee, S.; Kwon, M.; Han, S.; Kang, D.; Kim, Y.; Huh, J.; Koh, J.-S.; et al. Electroosmosis-Driven Hydrogel Actuators Using Hydrophobic/Hydrophilic Layer-By-Layer Assembly-Induced Crack Electrodes. ACS Nano 2020, 14, 11906–11918. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.; Palleau, E.; Dickey, M.D.; Velev, O.D. Electro-Actuated Hydrogel Walkers with Dual Responsive Legs. Soft Matter 2014, 10, 1337–1348. [Google Scholar] [CrossRef]
- Podstawczyk, D.; Nizioł, M.; Szymczyk, P.; Wiśniewski, P.; Guiseppi-Elie, A. 3D Printed Stimuli-Responsive Magnetic Nanoparticle Embedded Alginate-Methylcellulose Hydrogel Actuators. Addit. Manuf. 2020, 34, 101275. [Google Scholar] [CrossRef]
- Tang, J.; Sun, B.; Yin, Q.; Yang, M.; Hu, J.; Wang, T. 3D Printable, Tough, Magnetic Hydrogels with Programmed Magnetization for Fast Actuation. J. Mater. Chem. B 2021, 9, 9183–9190. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, Z.; Qi, H.; Lu, X.; Jiang, T.; Wang, L.; Zhang, G.; Xiao, R.; Wu, H. Magnetic-Field Induced Shape Memory Hydrogels for Deformable Actuators. Soft Matter 2024, 20, 5314–5323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wei, H.; Tang, J. Self-Sensing Magnetic Actuators of Bilayer Hydrogels. Int. J. Smart Nano Mater. 2023, 14, 496–509. [Google Scholar] [CrossRef]
- Shao, G.; Ware, H.O.T.; Huang, J.; Hai, R.; Li, L.; Sun, C. 3D Printed Magnetically-Actuating Micro-Gripper Operates in Air and Water. Addit. Manuf. 2021, 38, 101834. [Google Scholar] [CrossRef]
- Kim, D.; Song, S.; Jang, S.; Kim, G.; Lee, J.; Lee, Y.; Park, S. Untethered Gripper-Type Hydrogel Millirobot Actuated by Electric Field and Magnetic Field. Smart Mater. Struct. 2020, 29, 085024. [Google Scholar] [CrossRef]
- Tachibana, D.; Murakami, K.; Kozaki, T.; Matsuda, R.; Isoda, Y.; Nakamura, F.; Isano, Y.; Ueno, K.; Fuchiwaki, O.; Ota, H. Ultrafast and Highly Deformable Electromagnetic Hydrogel Actuators Assembled from Liquid Metal Gel Fiber. Adv. Intell. Syst. 2022, 4, 2100212. [Google Scholar] [CrossRef]
- Jang, S.; Park, S. 4D Printed Untethered Milli-Gripper Fabricated Using a Biodegradable and Biocompatible Electro- and Magneto-Active Hydrogel. Sens. Actuators B Chem. 2023, 384, 133654. [Google Scholar] [CrossRef]
- Huang, H.; Han, L.; Fu, X.; Wang, Y.; Yang, Z.; Pan, L.; Xu, M. Multiple Stimuli Responsive and Identifiable Zwitterionic Ionic Conductive Hydrogel for Bionic Electronic Skin. Adv. Electron. Mater. 2020, 6, 2000239. [Google Scholar] [CrossRef]
- Ribeiro, M.; Boudoukhani, M.; Belmonte-Reche, E.; Genicio, N.; Sillankorva, S.; Gallo, J.; Rodríguez-Abreu, C.; Moulai-Mostefa, N.; Bañobre-López, M. Xanthan-Fe3O4 Nanoparticle Composite Hydrogels for Non-Invasive Magnetic Resonance Imaging and Magnetically Assisted Drug Delivery. ACS Appl. Nano Mater. 2021, 4, 7712–7729. [Google Scholar] [CrossRef]
- Chen, W.; Wen, Y.; Fan, X.; Sun, M.; Tian, C.; Yang, M.; Xie, H. Magnetically Actuated Intelligent Hydrogel-Based Child-Parent Microrobots for Targeted Drug Delivery. J. Mater. Chem. B 2021, 9, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Wang, X.; Chen, X.-Z.; Mushtaq, F.; Deng, S.; Zhu, C.; Torlakcik, H.; Terzopoulou, A.; Qin, X.-H.; Xiao, X.; et al. 3D-Printed Soft Magnetoelectric Microswimmers for Delivery and Differentiation of Neuron-Like Cells. Adv. Funct. Mater. 2020, 30, 1910323. [Google Scholar] [CrossRef]
- Liu, D.; Liu, X.; Li, P.; Tang, X.; Kojima, M.; Huang, Q.; Arai, T. Magnetic Driven Two-Finger Micro-Hand with Soft Magnetic End-Effector for Force-Controlled Stable Manipulation in Microscale. Micromachines 2021, 12, 410. [Google Scholar] [CrossRef] [PubMed]
- Merhebi, S.; Mayyas, M.; Abbasi, R.; Christoe, M.J.; Han, J.; Tang, J.; Rahim, M.A.; Yang, J.; Tan, T.T.; Chu, D.; et al. Magnetic and Conductive Liquid Metal Gels. ACS Appl. Mater. Interfaces 2020, 12, 20119–20128. [Google Scholar] [CrossRef]
- Li, M.; Li, C.; Blackman, B.R.K.; Saiz, E. Energy Conversion Based on Bio-Inspired Superwetting Interfaces. Matter 2021, 4, 3400–3414. [Google Scholar] [CrossRef]
- Ye, S.; Ma, W.; Fu, G. A Novel Nature-Inspired Anisotropic Hydrogel with Programmable Shape Deformations. Chem. Eng. J. 2022, 450, 137908. [Google Scholar] [CrossRef]
- Jiang, S.; Xia, L.; Ma, H.; Yang, T.; Qian, L. pH and Temperature Dual-Responsive Hydrogel Actuator with Bidirectional Bending Behavior and Ultra Large Bending Angle. Eur. Polym. J. 2023, 197, 112296. [Google Scholar] [CrossRef]
- Yang, C.; Shi, X.; Deng, H.; Du, Y. Antifatigue Hydration-Induced Polysaccharide Hydrogel Actuators Inspired by Crab Joint Wrinkles. ACS Appl. Mater. Interfaces 2022, 14, 6251–6260. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wu, B.; Zhou, Q.; Jian, Y.; Le, X.; Lu, H.; Zhang, D.; Zhang, J.; Zhang, Z.; Chen, T. Ionic Strength and Thermal Dual-Responsive Bilayer Hollow Spherical Hydrogel Actuator. Macromol. Rapid Commun. 2020, 41, e1900543. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yang, C.; Jian, Y.; Deng, H.; Du, Y.; Shi, X. Ion-Responsive Chitosan Hydrogel Actuator Inspired by Carrotwood Seed Pod. Carbohydr. Polym. 2022, 276, 118759. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, L.; Jiang, Y.; Zhang, X.; Yao, X.; Soh, S. Soft Stimuli-Responsive Grippers and Machines with High Load-to-Weight Ratios. Mater. Horiz. 2019, 6, 160–168. [Google Scholar] [CrossRef]
- Liu, H.; Jia, X.; Liu, R.; Chen, K.; Wang, Z.; Lyu, T.; Cui, X.; Zhao, Y.; Tian, Y. Multifunctional Gradient Hydrogel with Ultrafast Thermo-Responsive Actuation and Ultrahigh Conductivity. J. Mater. Chem. A 2022, 10, 21874–21883. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, B.; Li, B.; Fu, H. Advanced Design of Soft Robots with Artificial Intelligence. Nano-Micro Lett. 2024, 16, 214. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Z. Hydrogel and Machine Learning for Soft Robots’ Sensing and Signal Processing: A Review. J. Bionic Eng. 2023, 20, 845–857. [Google Scholar] [CrossRef]
- Cui, Y.; Li, D.; Gong, C.; Chang, C. Bioinspired Shape Memory Hydrogel Artificial Muscles Driven by Solvents. ACS Nano 2021, 15, 13712–13720. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, G.; Xu, Z.; Tang, D.; Chen, T. Recent Progress in Bionic Skin Based on Conductive Polymer Gels. Macromol. Rapid Commun. 2021, 42, 2100480. [Google Scholar] [CrossRef]
- Lin, X.; Wang, X.; Zeng, L.; Wu, Z.L.; Guo, H.; Hourdet, D. Stimuli-Responsive Toughening of Hydrogels. Chem. Mater. 2021, 33, 7633–7656. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Zhang, J.; Hou, Y.; Xu, X.; Liu, Q. A Programmable Bilayer Hydrogel Actuator Based on the Asymmetric Distribution of Crystalline Regions. J. Mater. Chem. B 2022, 10, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guan, Q.; Li, C.; Saiz, E. Self-Powered Hydrogel Sensors. Device 2023, 1, 100007. [Google Scholar] [CrossRef]
- Cheng, Y.; Ren, K.; Yang, D.; Wei, J. Bilayer-Type Fluorescence Hydrogels with Intelligent Response Serve as Temperature/pH Driven Soft Actuators. Sens. Actuators B Chem. 2018, 255, 3117–3126. [Google Scholar] [CrossRef]
- Zhao, T.; Tan, Y.; Li, Y.; Wang, X. Ionic Fuel-Powered Hydrogel Actuators for Soft Robotics. J. Colloid Interface Sci. 2025, 677, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Liu, S.; Wu, F.; Zhang, H.; Fu, J. Shape Memory Effect and Rapid Reversible Actuation of Nanocomposite Hydrogels with Electrochemically Controlled Local Metal Ion Coordination and Crosslinking. J. Mater. Chem. B 2020, 8, 9679–9685. [Google Scholar] [CrossRef] [PubMed]
- Rus, D.; Tolley, M.T. Design, Fabrication and Control of Soft Robots. Nature 2015, 521, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xiao, P.; Le, X.; Lu, W.; Théato, P.; Ma, C.; Du, B.; Zhang, J.; Huang, Y.; Chen, T. Mimosa Inspired Bilayer Hydrogel Actuator Functioning in Multi-Environments. J. Mater. Chem. C 2018, 6, 1320–1327. [Google Scholar] [CrossRef]
- Xue, Y.; Zhou, Z.; Lei, J.; Liu, Z. A Constitutive Model of Water-Triggered Shape Memory Hydrogels and Its Finite Element Implementation. J. Appl. Mech. 2023, 90, 071005. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Cui, Z.; Bao, L.; Xia, Z.; Liu, Z.; Zhou, X. High Performance and Multifunction Moisture-Driven Yin–Yang-Interface Actuators Derived from Polyacrylamide Hydrogel. Small 2023, 19, 2303228. [Google Scholar] [CrossRef]
- Cangialosi, A.; Yoon, C.; Liu, J.; Huang, Q.; Guo, J.; Nguyen, T.D.; Gracias, D.H.; Schulman, R. DNA Sequence–Directed Shape Change of Photopatterned Hydrogels via High-Degree Swelling. Science 2017, 357, 1126–1130. [Google Scholar] [CrossRef]
- Lu, D.; Zhu, M.; Wu, S.; Lian, Q.; Wang, W.; Adlam, D.; Hoyland, J.A.; Saunders, B.R. Programmed Multiresponsive Hydrogel Assemblies with Light-Tunable Mechanical Properties, Actuation, and Fluorescence. Adv. Funct. Mater. 2020, 30, 1909359. [Google Scholar] [CrossRef]
- Son, H.; Byun, E.; Yoon, Y.J.; Nam, J.; Song, S.H.; Yoon, C. Untethered Actuation of Hybrid Hydrogel Gripper via Ultrasound. ACS Macro Lett. 2020, 9, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, W.; Guan, Q.; Lv, J.; Wang, Z.; Ding, L.; Li, C.; Saiz, E.; Hou, X. Sweat-Resistant Bioelectronic Skin Sensor. Device 2023, 1, 100006. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Wang, C.; Song, H.; Shao, J.; Lan, G.; Zhang, J.; Li, X.; Li, M. Advancement in Soft Hydrogel Grippers: Comprehensive Insights into Materials, Fabrication Strategies, Grasping Mechanism, and Applications. Biomimetics 2024, 9, 585. https://doi.org/10.3390/biomimetics9100585
Dong X, Wang C, Song H, Shao J, Lan G, Zhang J, Li X, Li M. Advancement in Soft Hydrogel Grippers: Comprehensive Insights into Materials, Fabrication Strategies, Grasping Mechanism, and Applications. Biomimetics. 2024; 9(10):585. https://doi.org/10.3390/biomimetics9100585
Chicago/Turabian StyleDong, Xiaoxiao, Chen Wang, Haoxin Song, Jinqiang Shao, Guiyao Lan, Jiaming Zhang, Xiangkun Li, and Ming Li. 2024. "Advancement in Soft Hydrogel Grippers: Comprehensive Insights into Materials, Fabrication Strategies, Grasping Mechanism, and Applications" Biomimetics 9, no. 10: 585. https://doi.org/10.3390/biomimetics9100585
APA StyleDong, X., Wang, C., Song, H., Shao, J., Lan, G., Zhang, J., Li, X., & Li, M. (2024). Advancement in Soft Hydrogel Grippers: Comprehensive Insights into Materials, Fabrication Strategies, Grasping Mechanism, and Applications. Biomimetics, 9(10), 585. https://doi.org/10.3390/biomimetics9100585







