Nanomechanical Mapping of Three Dimensionally Printed Poly-ε-Caprolactone Single Microfibers at the Cell Scale for Bone Tissue Engineering Applications
Abstract
:1. Introduction
2. Materials and Methods
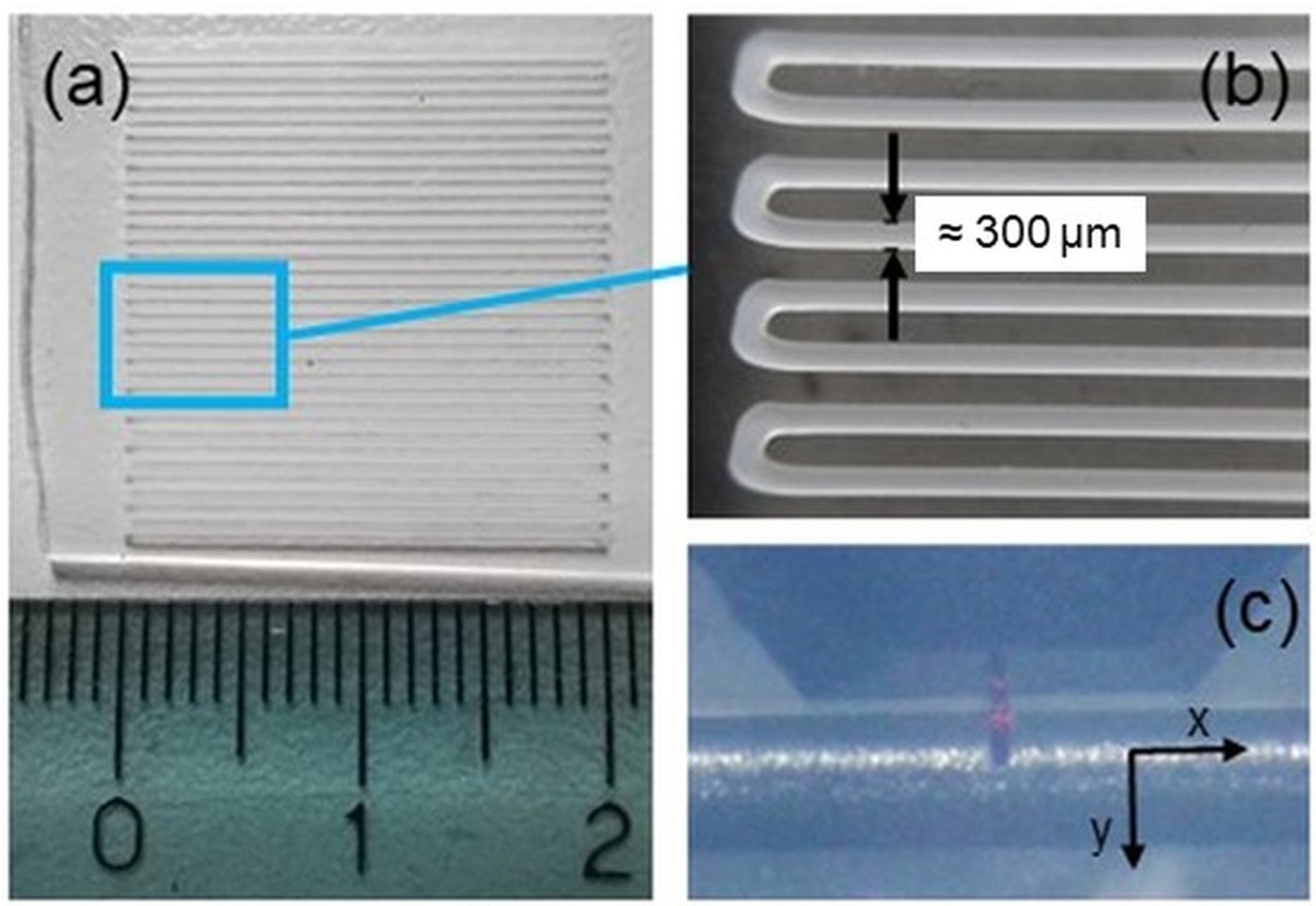
2.1. PCL Microfibers Fabrication
2.2. AFM Operation
2.3. Extraction of Young’s Modulus from Experimental Indentation Curves
3. Results
3.1. Determination of the Elastic-to-Plastic Deformation Threshold
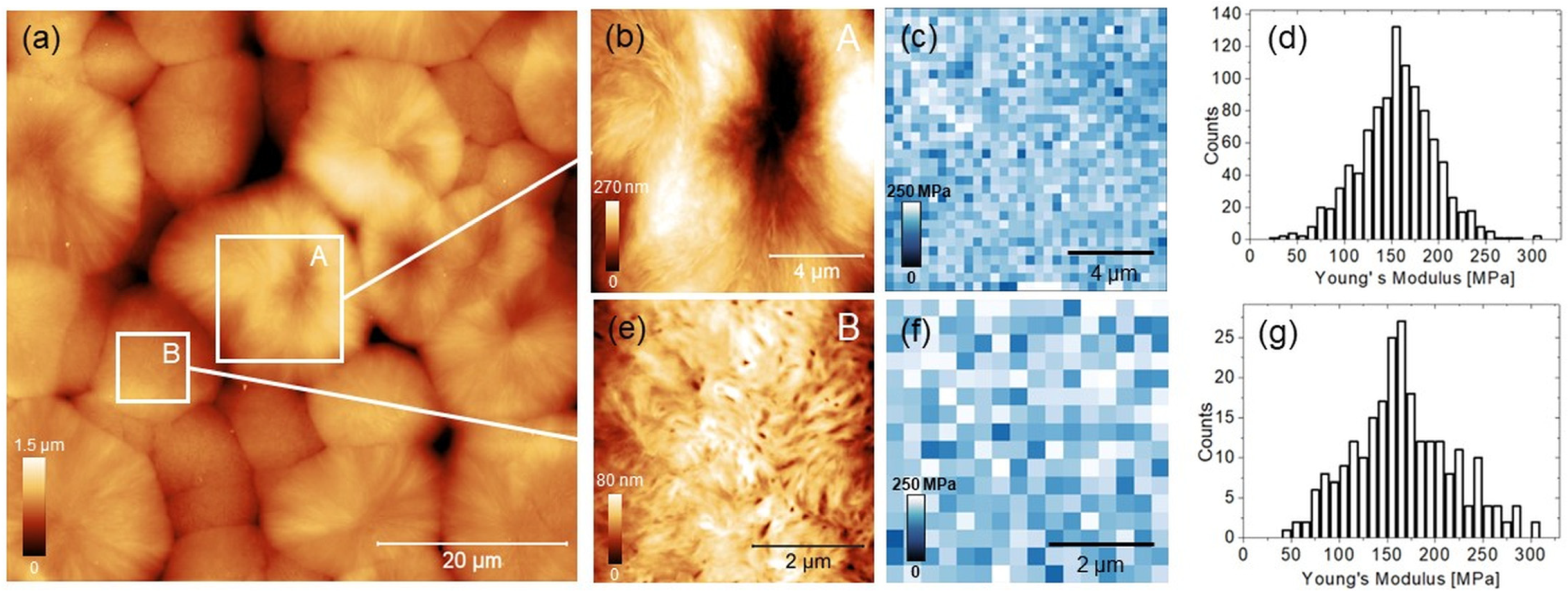
3.2. Mapping the Young’s Modulus
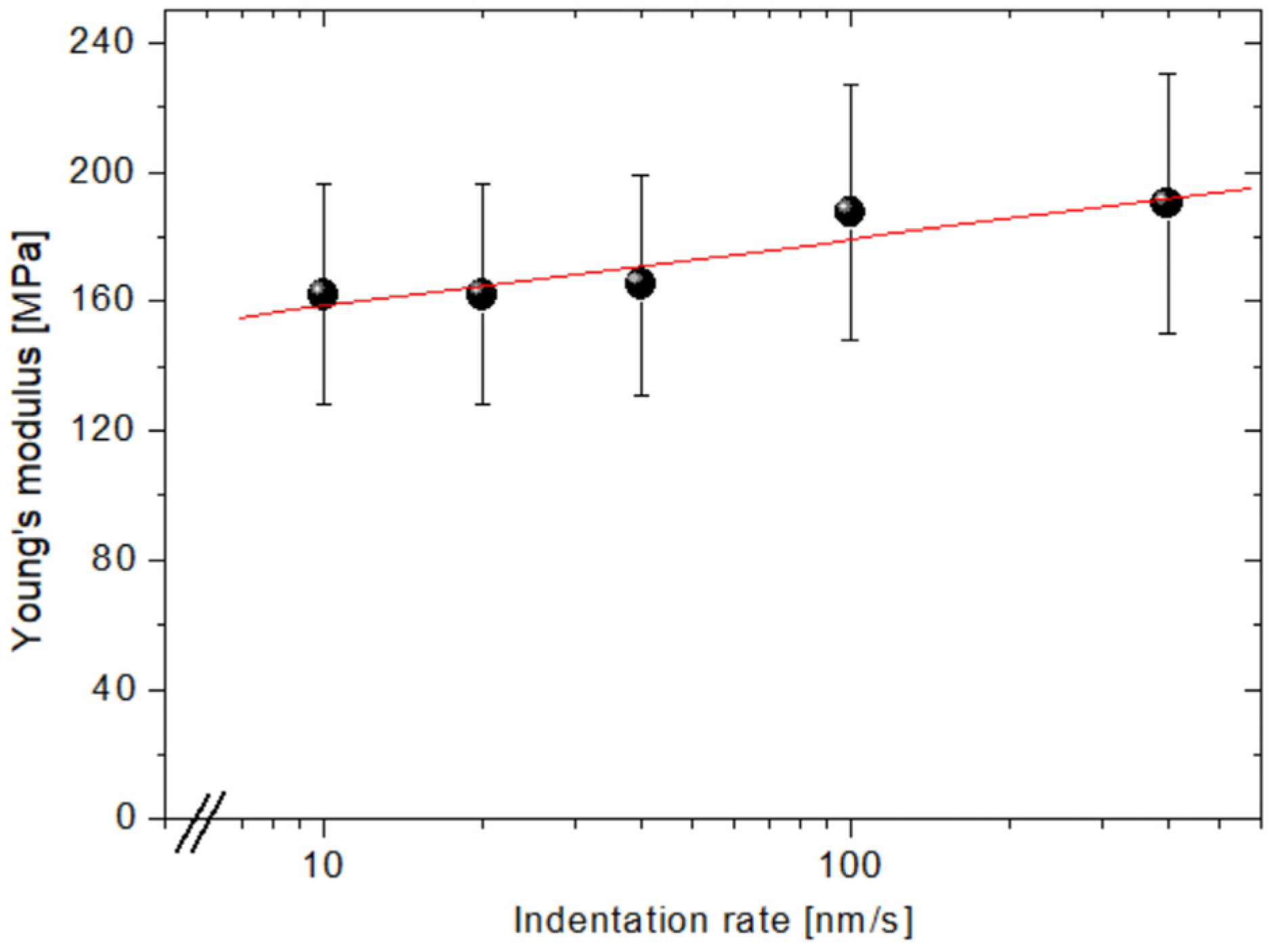
3.3. Viscoelasticity
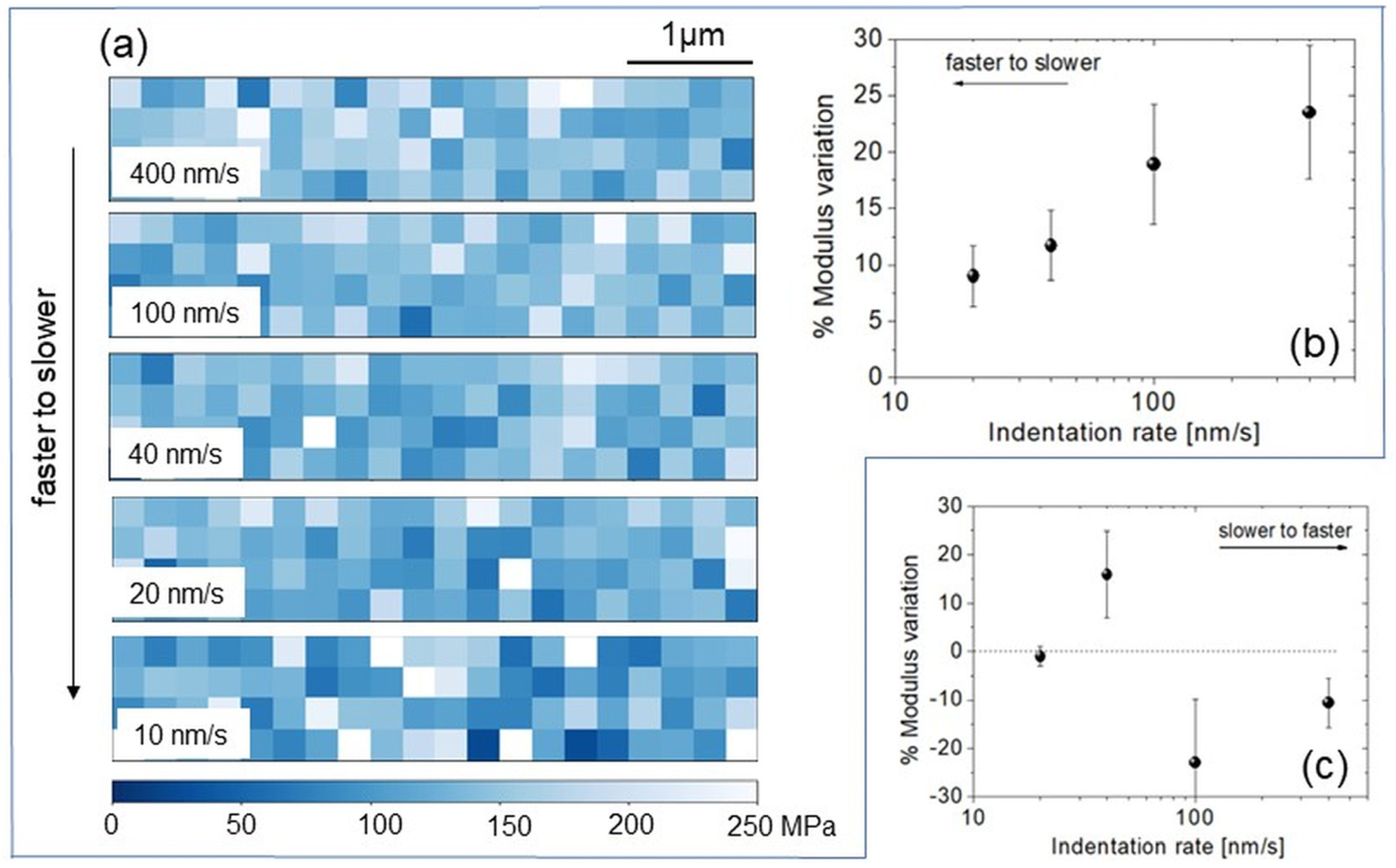
3.4. Dependence on the History of Nanoindentation
4. Discussion
4.1. Significance of the Study
4.2. Significance of the Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kozaniti, F.K.; Deligianni, D.D.; Georgiou, M.D.; Portan, D.V. The Role of Substrate Topography and Stiffness on MSC Cells Functions: Key Material Properties for Biomimetic Bone Tissue Engineering. Biomimetics 2022, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Breuls, R.G.; Jiya, T.U.; Smit, T.H. Scaffold stiffness influences cell behavior: Opportunities for skeletal tissue engineering. Open Orthop. J. 2008, 2, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Xu, Q.; Liu, W. An overview of substrate stiffness guided cellular response and its applications in tissue regeneration. Bioact. Mater. 2022, 15, 82–102. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Kharche, S.; More, N.; Ranglani, D.; Singh, G.; Kapusetti, G. Natural Biopolymers for Bone Tissue Engineering: A Brief Review. Eng. Regener. 2023, 4, 193–204. [Google Scholar] [CrossRef]
- Das, A.; Ringu, T.; Ghosh, S.; Pramanik, N. A comprehensive review on recent advances in preparation, physicochemical characterization, and bioengineering applications of biopolymers. Polym. Bull. 2023, 80, 7247–7312. [Google Scholar] [CrossRef] [PubMed]
- Takai, E.; Costa, K.D.; Shaheen, A.; Hung, C.T.; Guo, X.E. Osteoblast Elastic Modulus Measured by Atomic Force Microscopy Is Substrate Dependent. Ann. Biomed. Eng. 2005, 33, 963–971. [Google Scholar] [CrossRef]
- Basso, N.; Heersche, J.N.M. Characteristics of in vitro osteoblastic cell loading models. Bone 2002, 30, 347–351. [Google Scholar] [CrossRef]
- Marrese, M.; Guarino, V.; Ambrosio, L. Atomic Force Microscopy: A Powerful Tool to Address Scaffold Design in Tissue Engineering. J. Funct Biomater. 2017, 8, 7. [Google Scholar] [CrossRef]
- Suriano, R.; Credi, C.; Levi, M.; Turri, S. AFM nanoscale indentation in air of polymeric and hybrid materials with highly different stiffness. Appl. Surf. Sci. 2014, 311, 558–566. [Google Scholar] [CrossRef]
- Joshi, J.; Homburg, S.V.; Ehrmann, A. Atomic Force Microscopy (AFM) on Biopolymers and Hydrogels for Biotechnological Applications—Possibilities and Limits. Polymers 2022, 14, 1267. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Mehrotra, D. 3D bioprinting and craniofacial regeneration. J. Oral Biol. Craniofac. Res. 2020, 10, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Harrysson, O.L.A.; Rao, P.K.; Tamayol, A.; Cormier, D.R.; Zhang, Y.; Rivero, I.V. Extrusion bioprinting: Recent progress, challenges, and future opportunities. Bioprinting 2021, 21, e00116. [Google Scholar] [CrossRef]
- Ng, W.L.; Yeong, W.Y.; Naing, M.W. Polyvinylpyrrolidone-Based Bio-Ink Improves Cell Viability and Homogeneity during Drop-On-Demand Printing. Materials 2017, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.L.; Lee, J.M.; Zhou, M.; Chen, Y.W.; Lee, K.A.; Yeong, W.Y.; Shen, Y.F. Vat polymerization-based bioprinting-process, materials, applications and regulatory challenges. Biofabrication 2020, 12, 022001. [Google Scholar] [CrossRef] [PubMed]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Hossain, M.; Shi, H.H.; Tariq, A.; Ramakrishna, S.; Umer, R. Additive manufacturing of sustainable biomaterials for biomedical applications. Asian J. Pharm Sci. 2023, 18, 100812. [Google Scholar] [CrossRef] [PubMed]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Sadeghianmaryan, A.; Jalalvand, M.; Hossain, M. Recent advances in 3D-printed polylactide and polycaprolactone-based biomaterials for tissue engineering applications. Int. J. Biol. Macromol. 2022, 218, 930–968. [Google Scholar] [CrossRef]
- Petretta, M.; Gambardella, A.; Boi, M.; Berni, M.; Cavallo, C.; Marchiori, G.; Maltarello, M.C.; Bellucci, D.; Fini, M.; Baldini, N.; et al. Composite Scaffolds for Bone Tissue Regeneration Based on PCL and Mg-Containing Bioactive Glasses. Biology 2021, 10, 398. [Google Scholar] [CrossRef]
- Watts, S.; Hill, R.; O’Donnell, M.; Law, R. Influence of magnesia on the structure and properties of bioactive glasses. J. Non-Cryst. Solids 2010, 356, 517–524. [Google Scholar] [CrossRef]
- Zreiqat, H.; Howlett, C.R.; Zannettino, A.; Evans, P.; Schulze-Tanzil, G.; Knabe, C.; Shakibaei, M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J. Biomed. Mater. Res. 2020, 62, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Bontempi, M.; Capozza, R.; Visani, A.; Fini, M.; Giavaresi, G.; Gambardella, A. Near-Surface Nanomechanics of Medical-Grade PEEK Measured by Atomic Force Microscopy. Polymers 2023, 15, 718. [Google Scholar] [CrossRef] [PubMed]
- Olubowale, O.H.; Biswas, S.; Azom, G.; Prather, B.L.; Owoso, S.D.; Rinee, K.C.; Marroquin, K.; Gates, K.A.; Chambers, M.B.; Xu, A.; et al. “May the Force Be with You!” Force–Volume Mapping with Atomic Force Microscopy. ACS Omega 2021, 6, 25860–25875. [Google Scholar] [CrossRef] [PubMed]
- Garcìa, R. Nanomechanical mapping of soft materials with the atomic force microscope: Methods, theory and applications. Chem. Soc. Rev. 2020, 49, 5850–5884. [Google Scholar] [CrossRef] [PubMed]
- Kontomaris, S.V.; Malamou, A. Hertz model or Oliver & Pharr analysis? Tutorial regarding AFM nanoindentation experiments on biological samples. Mater. Res. Express 2020, 7, 033001. [Google Scholar]
- Kontomaris, S.V.; Malamou, A. A novel approximate method to calculate the force applied on an elastic half space by a rigid sphere. Eur. J. Phys. 2021, 42, 025010. [Google Scholar] [CrossRef]
- Bontempi, M.; Salamanna, F.; Capozza, R.; Visani, A.; Fini, M.; Gambardella, A. Nanomechanical mapping of hard tissues by atomic force microscopy: An application to cortical bone. Materials 2022, 15, 7512. [Google Scholar] [CrossRef]
- Tranchida, D.; Kiflie, Z.; Acierno, S.; Piccarolo, S. Nanoscale mechanical characterization of polymers by atomic force microscopy (AFM) nanoindentations: Viscoelastic characterization of a model material. Meas. Sci. Technol. 2009, 20, 095702. [Google Scholar] [CrossRef]
- Collinson, D.W.; Sheridan, R.J.; Palmeri, M.J.; Brinson, L.C. Best practices and recommendations for accurate nanomechanical characterization of heterogeneous polymer systems with atomic force microscopy. Prog. Polym. Sci. 2021, 119, 101420. [Google Scholar] [CrossRef]
- Kotani, R.; Yokoyama, S.; Nobusue, S.; Yamaguchi, S.; Osuka, A.; Yabu, H.; Saito, S. Bridging pico-to-nanonewtons with a ratiometric force probe for monitoring nanoscale polymer physics before damage. Nat. Commun. 2022, 13, 303. [Google Scholar] [CrossRef]
- Fryń, P.; Jewłoszewicz, B.; Bogdanowicz, K.A.; Przybył, W.; Gonciarz, A.; Pich, R.; Marzec, M.; Iwan, A. Research of Binary and Ternary Composites Based on Selected Aliphatic or Aliphatic–Aromatic Polymers, 5CB or SWCN toward Biodegradable Electrodes. Materials 2020, 13, 2480. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Duwez, A.-S.; Jérôme, C.; Léonard, A.F.; van der Werf, K.O.; Dijkstra, P.J.; Bennink, M.L. Mechanical testing of electrospun PCL fibers. Acta Biomater. 2012, 8, 218–224. [Google Scholar] [CrossRef]
- Kurniawan, D.; Nor, F.M.; Lee, H.Y.; Lim, J.Y. Elastic properties of polycaprolactone at small strains are significantly affected by strain rate and temperature. Proc. Inst. Mech. Eng. H 2011, 225, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Catledge, S.A.; Clem, W.C.; Shrikishen, N.; Chowdhury, S.; Stanishevsky, A.V.; Koopman, M.; Vohra, Y.K. An electrospun triphasic nanofibrous scaffold for bone tissue engineering. Biomed. Mater. 2007, 2, 142–150. [Google Scholar] [CrossRef]
- Costa, J.B.; Park, J.; Jorgensen, A.M.; Silva-Correia, J.; Reis, R.L.; Oliveira, J.M.; Atala, A.; Yoo, J.J.; Lee, S.J. 3D Bioprinted Highly Elastic Hybrid Constructs for Advanced Fibrocartilaginous Tissue Regeneration. Chem. Mater. 2020, 32, 8733–8746. [Google Scholar] [CrossRef] [PubMed]
- Dufrêne, Y.F. Recent progress in the application of atomic force microscopy imaging and force spectroscopy to microbiology. Curr. Opin. Microbiol. 2003, 6, 317–323. [Google Scholar] [CrossRef]
- Marion, G.; Saez, A.; Trichet, L.; Xayaphoummine, A.; Browaeys, J.; Silberzan, P.; Buguinb, A.; Ladoux, B. Traction forces and rigidity sensing regulate cell functions. Soft Matter 2008, 4, 1836–1843. [Google Scholar] [CrossRef]
- Wutticharoenmongkol, P.; Pavasant, P.; Supaphol, P. Osteoblastic Phenotype Expression of MC3T3-E1 Cultured on Electro-spun Polycaprolactone Fiber Mats Filled with Hydroxyapatite Nanoparticles. Biomacromolecules 2007, 8, 2602–2610. [Google Scholar] [CrossRef]
- Gharibshahian, M.; Salehi, M.; Beheshtizadeh, N.; Kamalabadi-Farahani, M.; Atashi, A.; Nourbakhsh, M.S.; Alizadeh, M. Recent advances on 3D-printed PCL-based composite scaffolds for bone tissue engineering. Front. Bioeng. Biotechnol. 2023, 11, 1168504. [Google Scholar] [CrossRef]
- Wu, J.; Peng, Z. The investigation of nanotribology of UHMWPE in fluid using atomic force microscopy. J. Biomed. Mater. Res. 2015, 103B, 751–763. [Google Scholar] [CrossRef]
- Williamson, M.R.; Woollard, K.; Griffiths, H.R.; Coombes, A. Gravity Spun Polycaprolactone Fibers for Applications in Vascular Tissue Engineering: Proliferation and Function of Human Vascular Endothelial Cells. Tissue Eng. 2006, 12, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.J.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 23, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Huang, J.; Narayan, R.J. Gradient scaffolds for osteochondral tissue engineering and regeneration. J. Mater. Chem. B 2020, 8, 8149–8170. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Li, N.; Du, Z.; Li, X. Integrated gradient tissue-engineered osteochondral scaffolds: Challenges, current efforts and future perspectives. Bioact. Mater. 2023, 20, 574–597. [Google Scholar] [CrossRef]
- Chen, L.; Wei, L.; Su, X.; Qin, L.; Xu, Z.; Huang, X.; Chen, H.; Hu, N. Preparation and Characterization of Biomimetic Functional Scaffold with Gradient Structure for Osteochondral Defect Repair. Bioengineering 2023, 10, 213. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, S.; Shao, X.; Zhang, Q.; Xue, C.; Zhang, S.; Lin, Y.; Zhu, B.; Cai, X. Effect of matrix stiffness on osteoblast functionalization. Cell Prolif. 2017, 50, 12338. [Google Scholar] [CrossRef]
- Tweedie, C.A.; Constantinides, G.; Lehman, K.E.; Brill, D.J.; Blackman, G.S.; Van Vliet, K.J. Enhanced Stiffness of Amorphous Polymer Surfaces under Confinement of Localized Contact Loads. Adv. Mater. 2007, 19, 2540–2546. [Google Scholar] [CrossRef]

| Maximum Force Fmax (Curve) [nN] | Maximum Penetration Depth hmax (Curve) [nm] | Residual Depth After 5 min hr (Image) [nm] |
|---|---|---|
| 300 | 72 | 67 ± 11 |
| 220 | 66 | 15.5 ± 5.5 |
| 160 | 61 | 7.5 ± 2.5 |
| 120 | 45 | 12.5 ± 5.5 |
| 70 | 29 | 3.5 ± 2.5 |
| 50 | 21 | 0 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bontempi, M.; Marchiori, G.; Petretta, M.; Capozza, R.; Grigolo, B.; Giavaresi, G.; Gambardella, A. Nanomechanical Mapping of Three Dimensionally Printed Poly-ε-Caprolactone Single Microfibers at the Cell Scale for Bone Tissue Engineering Applications. Biomimetics 2023, 8, 617. https://doi.org/10.3390/biomimetics8080617
Bontempi M, Marchiori G, Petretta M, Capozza R, Grigolo B, Giavaresi G, Gambardella A. Nanomechanical Mapping of Three Dimensionally Printed Poly-ε-Caprolactone Single Microfibers at the Cell Scale for Bone Tissue Engineering Applications. Biomimetics. 2023; 8(8):617. https://doi.org/10.3390/biomimetics8080617
Chicago/Turabian StyleBontempi, Marco, Gregorio Marchiori, Mauro Petretta, Rosario Capozza, Brunella Grigolo, Gianluca Giavaresi, and Alessandro Gambardella. 2023. "Nanomechanical Mapping of Three Dimensionally Printed Poly-ε-Caprolactone Single Microfibers at the Cell Scale for Bone Tissue Engineering Applications" Biomimetics 8, no. 8: 617. https://doi.org/10.3390/biomimetics8080617
APA StyleBontempi, M., Marchiori, G., Petretta, M., Capozza, R., Grigolo, B., Giavaresi, G., & Gambardella, A. (2023). Nanomechanical Mapping of Three Dimensionally Printed Poly-ε-Caprolactone Single Microfibers at the Cell Scale for Bone Tissue Engineering Applications. Biomimetics, 8(8), 617. https://doi.org/10.3390/biomimetics8080617









