Bone Regeneration Induced by Patient-Adapted Mg Alloy-Based Scaffolds for Bone Defects: Present and Future Perspectives
Abstract
:1. Introduction
2. Mg Alloys Effects on Bone Regeneration
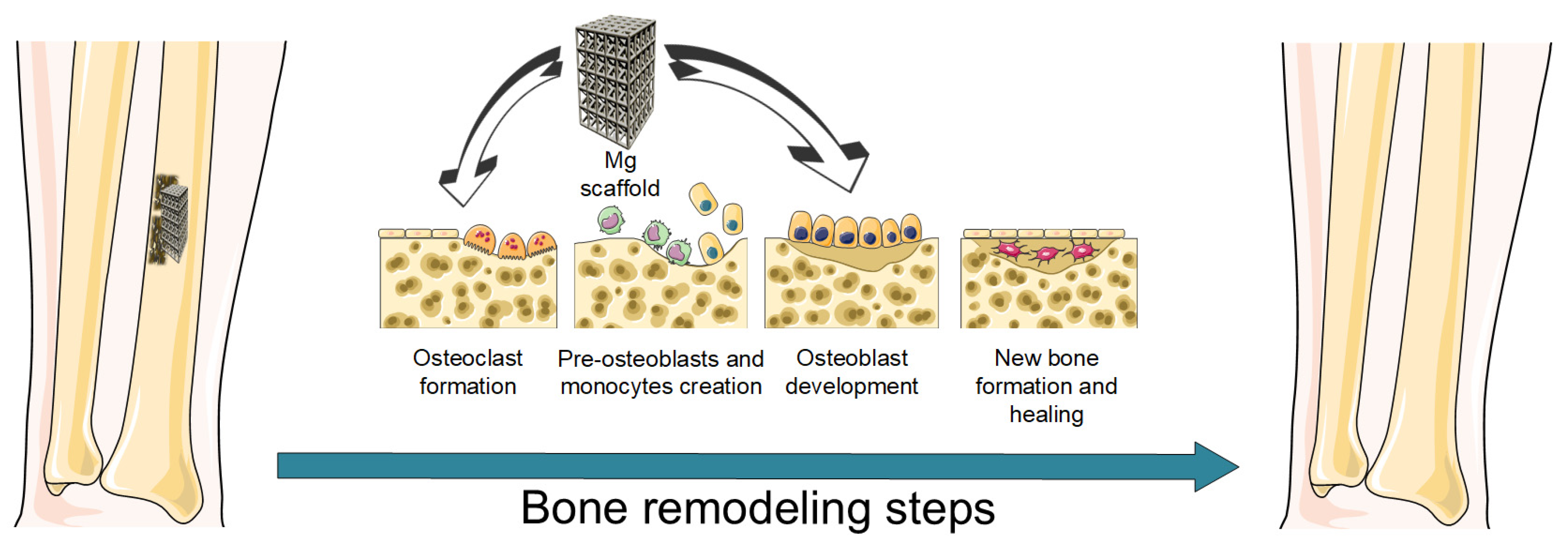
2.1. Bone Healing Process
2.2. Mg Alloys’ Role in Osteogenesis and Angiogenesis
2.2.1. Host Immune Response Modulation
2.2.2. Main Mechanisms of Mg-Based Alloys during the Angiogenesis
2.2.3. Osteogenic Mechanisms of Mg-Based Alloys
| Mg Alloy Effect | Main Role | Event | Alloy/Material | Remarks | Reference |
|---|---|---|---|---|---|
| Immune response modulation | Inflammatory activation at the injury site | Protein absorption. Fibrinogen (Fg)—key mediator for platelet activation and clot apparition | Mg-Zn | The Fg absorption was facilitated into the alloy with the lowest Zn content (Mg–1Zn) in comparison with Mg–2Zn and Mg–3Zn alloys | [45] |
| Recruitment of neutrophils and macrophage phagocytosis | Mg–Nd–Zn–Zr (JDBM) | A high concentration of magnesium ions determined an M1 macrophage polarization state due to the early degradation of Mg-based alloys that determined the apparition of inflammatory reactions. It was concluded that high concentrations of Mg-based ions determined the early debridement at the injury site | [48] | ||
| Activation of the adaptive immune system of the host | Nanoparticles containing Mg | The nanoparticles, which were enhanced with Mg, generated cell-mediated Th1 and antibody-mediated Th2 immunities after in vitro research. It was confirmed the positive influence of Mg-based materials on the T lymphocyte effect | [51] | ||
| Anti-inflammatory immune microenvironment apparition | Mg action on M2 macrophage polarization | Mg–Si–Ca | The alloy extract showed a significant effect on inhibiting the expression of pro-inflammatory cytokines, proving a reduction of the M1 macrophages | [52] | |
| Mg–10Gd, Mg–2Ag | The Mg-based alloys determined an increase of the M2 phenotype of macrophages concomitantly with a reduction of the M1 phenotype | [53] | |||
| M2 macrophages and MSCs interaction | Mg–Nd–Zn–Zr (JDBM) | An Mg-alloy scaffold induced a damping effect on the macrophage inflammatory profile simultaneously with chondrocyte differentiation of MSCs | [63] | ||
| M2 macrophages’ role in angiogenesis | Mg–Zn–Ca | The alloy was implanted in a rat femur and showed evidence of early angiogenesis | [54] | ||
| Immunomodulatory signaling pathways | Reduced ROS level | Mg–Nd–Zn–Zr (JDBM) | The Mg2+ ions resulted from the Mg–Nd–Zn–Zr degradation determined the apparition of an environment with a reduced number of ROS | [61] | |
| Activation of P13K/AKT signaling pathway | Mg-based alloy | The Mg2+ ions had a beneficial effect on P13K/AKT-signaling pathway activation due to lipopolysaccharide (LPS) inflammatory response decrease | [64] | ||
| Mechanisms of Mg alloys during angiogenesis | Apparition of factors that stimulate angiogenesis | Upregulation of hypoxia-inducible factor (HIF) and vascular endothelial growth factor (VEGF) | Mg2+ introduced on a Ti scaffold surface | An important upregulation of the MagT1 expression on the HUVECs surface was put in evidence | [71] |
| Pure Mg | Mg degradation products with a concentration between 2-to-8 mM promoted the expression of VEGFA and VEGFB under hypoxia conditions | [72] | |||
| Degradation of vascular basement membrane | MMPs that digest the basement membrane and liberate ECs | 6.25% Mg–Zn–Mn extract | The expression of MMP-2 was improved, showing evidence for the start of angiogenesis | [74] | |
| Endothelial cell proliferation, migration, and tube formation | Mg concentration | Mg-based alloy | At a concentration of Mg2+ ions equal to 1 mM, the proliferation of HUVECs is improved. This fact remains valid until an Mg2+ concentration of 5 mM. At higher than 10 mM concentrations, the angiogenic factor secretions and tube formation are drastically affected | [75] | |
| Neuropeptides involved in Mg-induced angiogenesis | Mg–Zn–Gd scaffold coated with Ca–P | The scaffold promoted and influenced the CGRP serum in a large bone defect in a canine animal model. The inhibition of CGRP generated a down-regulation of new blood vessel numbers at the defect place | [67] | ||
| Platelet-derived growth factor (PDGF-BB) positive influence on angiogenesis | Mg alloy | A concentration of Mg2+ ions between 1 and 5 mM had a good influence on PDGF-BB expression in HUVECs as a function of metallic ion concentration | [75] | ||
| Stability and maturation of the newly formed blood vessels | Mg–Cu | The Mg-based alloy extract determined an upregulation of the endothelial receptor tyrosine kinase TIE-1 and activin receptor-like kinase ACVRL1 in the HUVECs plasma membrane | [68] | ||
| Osteogenic mechanisms of Mg-based alloys | State and function of the osteoblast-related cells | Mg2+ concentration influence | Mg alloy | An Mg2+ concentration between 2.5-to-5 mM was found to increase the osteogenic differentiation of rBMSC cells | [87] |
| Mg alloy | A concentration of magnesium ions comprised between 5 and 10 mM had the best effect on ECM mineralization and osteogenic differentiation of hBMSCs. The viability of the cells was drastically decreased when the Mg2+ concentration exceeded 20 mM | [67] | |||
| pH influence | Mg–Ga layered double oxide nanosheets deposited on alkali-heat-treated titanium | The osteogenic differentiation of MSCs and autophagic activity were promoted by the alkaline microenvironment with a pH of about 8.5 | [91] | ||
| Mg alloy | At a pH between 9 and 10, an important decrease of hFOB 1.19 cells was reached | [93] | |||
| Integrin-dependent cell adhesion | Mg–1Zn, Mg–1Zn–0.5Sn | A promotion effect on the expression of integrin β1 and α1 was achieved | [98] | ||
| Mg–1.0Ca–0.2Si | The alloy extract stimulated the expression of the following integrins: α5, α4, α3, and β1, β5 on the surface of hMSCs | [20] | |||
| MAPK-signaling pathway activation | Mg–Zn | The osteogenic differentiation of BMSCs was sustained by activating the MAPK-signaling pathway, concomitantly with ERK1/2 signaling, JNK signaling, and p38 signaling | [99] | ||
| Mg–1Ca–2.0Sr | In this case, only ERK1/2 was activated, while p38 and JNK pathway proteins were not upregulated | [100] | |||
| Activation of Wnt pathway | Mg-3.5Li-0.5Ca | The activation of Wnt/β-Catenin was achieved, and the osteoblastic differentiation of hBMSCs was attained | [83] | ||
| Activation of Smad-dependent signaling pathway | Mg-1Y/Mg | The Smad-dependent signaling pathway upregulated the expression of BMP2 family members (TGF-β and TGF-β1) | [103] | ||
| Formation and function of the osteoclasts | Mg2+concentration influence | MgCl2 | At a concentration of Mg2+ ions of about 5 mM, the formation and activation of the osteoclast were encouraged, while at a concentration higher than 25 mM, the cell viability decreased | [84] | |
| pH influence | Mg/Mg alloy | The inhibitory effect of material extract regarding the osteoclast activity was reversed after the pH neutralization | [105] | ||
| H2 release | - | Different H2 concentrations were chosen to study osteoclastogenesis. At 50% and 75% H2, the process related to osteoclast-induced BMMCs was inhibited and led to cellular apoptosis | [96] |
3. Patient-Adapted Strategies for BTE
4. Patient-Adapted Mg-Based Scaffolds Manufacturing Technologies
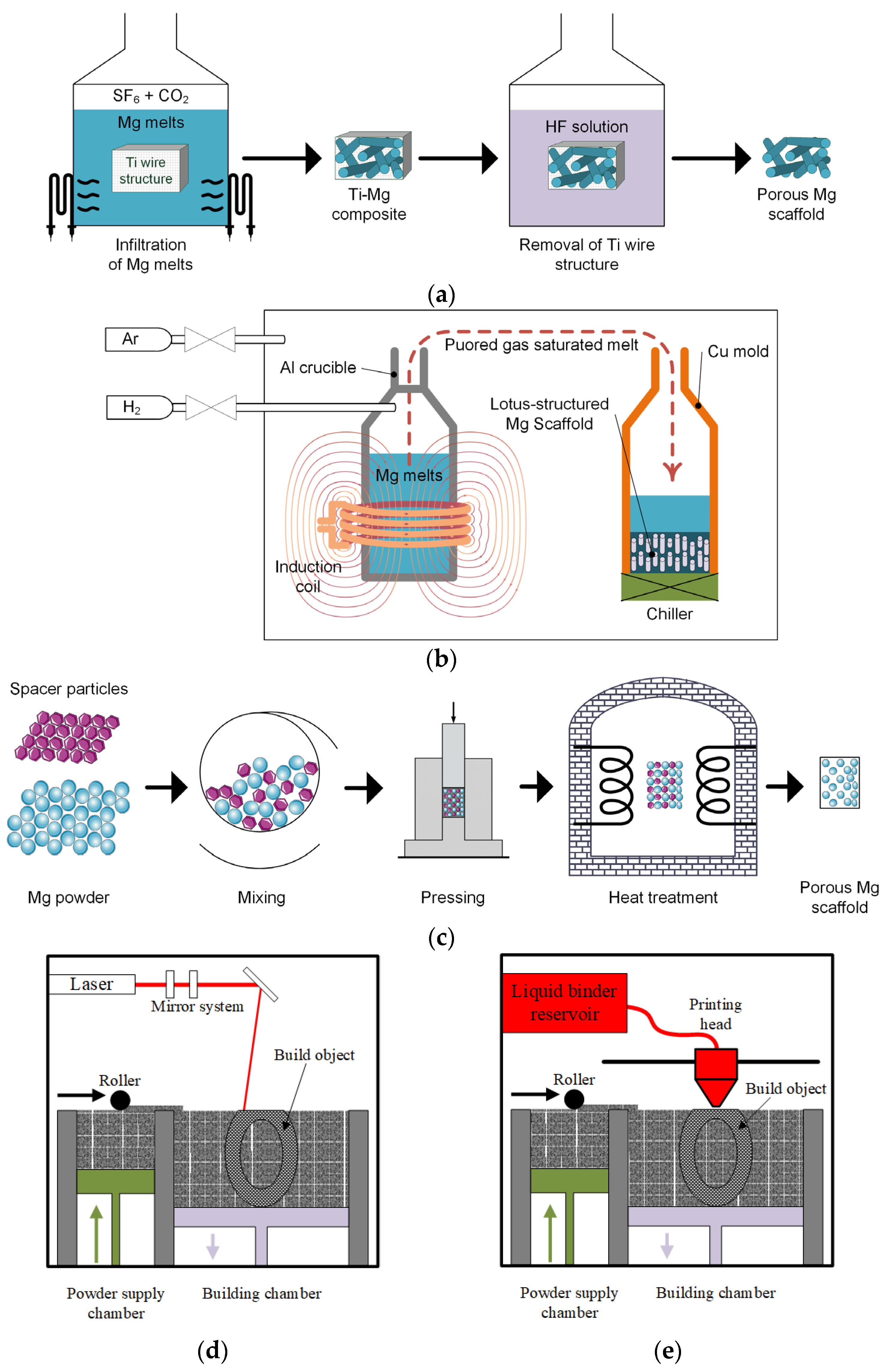
4.1. Titanium Wire Space Holder Technology
4.2. Hydrogen-Injection Technology
4.3. Powder Metallurgy
4.4. Laser-Perforation Technology
4.5. Laser-Based Additive Manufacturing Technologies
4.6. Powder-Bed Inkjet Additive Manufacturing Technology
4.7. Indirect Additive Manufacturing Technology
| Method | Advantages | Disadvantages | Mg-Based Alloy Scaffolds | Mechanical Properties | Corrosion Behavior | In Vitro and In Vivo Biocompatibility Tests | Ref. |
|---|---|---|---|---|---|---|---|
| Titanium wire space holder (TWSH) | Controllable pore-size and scaffold structure; pipe-like structure | The necessity of HF acid use | High-purity Mg | Young’s moduli: 2.18 ± 0.06 GPa (250-PMg) and 2.37 ± 0.09 GPa (400-PMg); compression strength: 41.2 ± 2.14 MPa (250-PMg) and 46.3 ± 3.65 MPa (400-PMg) | Corrosion rates (CR) of 1.31 ± 0.11 mm/yr (250-PMg) and 1.53 ± 0.15 mm/yr (400-PMg) | High cell viability and decreased cytotoxicity for MG63 cells. In vivo tests made on rabbits showed moderate levels of severe inflammatory reactions | [123] |
| Pure Mg | Compressive yielding strength of 4.3–6.2 MPa; Young’s modulus in the range of 0.5–1.0 GPa | - | - | [124] | |||
| Hydrogen injection | Straight and upward unidirectional oriented pores | The increased cost of casting equipment | Pure Mg | Compressive yield strength of 23.9 ± 4.9 MPa for porous Mg sample before immersion | A weight loss of about 10% occurs after 250 h of immersion in SBF | Indirect cell experiments made on L-929 cells indicated that the developed scaffolds are safe for cellular applications with RGR Grade of 1 | [131] |
| Powder metallurgy | Easy to perform technique; interconnected pore network | Lack of corrosion resistance in the absence of coatings; low mechanical integrity | Complex polymeric coating from PCL and Gel reinforced with BaG particles for pure Mg | - | Difference in the pH values: 9.6 (pure Mg) and 7.7 (coated Mg) after 3 days of immersion. The uncoated samples were fully degraded | - | [132] |
| Mg-4 wt.% Zn, Mg-6 wt.% Zn | For the 550 °C heat treatment, Young’s modulus values decreased from 6000 MPa (Mg-4 wt.% Zn) and 7500 MPa (Mg-6 wt.% Zn) at a 20% porosity to about 2800 MPa (Mg-4 wt.% Zn) and 3500 MPa (Mg-6 wt.% Zn) at a porosity of 45%. The values of compressive strength: between 60 MPa (both alloys) and 15 MPa (both alloys) | - | - | [133] | |||
| Laser perforation | The structure, shape, and diameter size of the pores are easy to control and modify | Expensive programmable laser processing machine | β-TCP coating on pure Mg | Young’s modulus had values 0.4–0.6 GPa; mechanical strength had values between 8–12 MPa when scaffold porosity variated between 42% and 50% | pH values lower than 8 after 1 month of immersion; then it increased to 9 after 2 months of immersion | After 3 h of UMR106 cell incubation, the cell viability obtained in the case of coated Mg scaffold extract was comparable to control samples | [134] |
| Selective laser melting data | The powder can be recycled; a rapid solidification of the part can be achieved due to fast cooling and heating cycles; high-density parts with good mechanical properties and adequate biodegradation behavior are obtained | The evaporation process can be detrimental to the Mg powder bed due to its decreased weight, dust explosion danger, high energy-consuming process | Mg–Y–Nd–Gd–Zr (WE43) | The Young’s modulus was found to be between 0.7–0.8 GPa for scaffold samples | pH during the first 3 days increased from 7.4 to 8.1 and then gradually decreased; after 4 weeks of immersion, about 20% volume loss was reported | Reduced cytotoxicity of the scaffold on MG63 cells of less than 25% was determined | [139] |
| Mg–Y–Nd–Gd–Zr surface modification based on Plasma Electrolytic Oxidation (PEO) and/or heat treatment | The maximum compressive stress was determined as a function of pore size. It was noticed that PEO-modified structures with big pores had a reduced stress resistance of about 5 MPa in comparison with the ones with small pores (20 MPa) | The big pore PEO surface-modified samples had a lower value of hydrogen emission of 20 mL at 20 days compared to the small pore sample (60 mL at 20 days) | - | [143] | |||
| Mg–Zn–Zr (ZK60) | The hardness of SLM ZK60 porous material was about 0.78 GPa higher than that of cast ZK60 (0.55 GPa) | SLM-produced ZK60 exhibited a higher corrosion resistance in Hanks’ solution, being characterized by a decrease of 30% regarding hydrogen evolution rate and of about 50% in the corrosion current density compared with cast ZK60 | - | [144] | |||
| Binderless jetting | Faster and easy process | Post-processing steps are necessary due to the limited mechanical performance of the parts | Mg–Zn–Zr | At the optimal sintering temperature of 573 °C, the scaffold Young’s modulus was 18 GPa, and the compressive strength was 174 MPa | - | - | [145] |
| Indirect additive manufacturing procedure | Elimination explosion danger due to the volatile nature of the Mg | The geometry of pore size and strut can be designed at the macro-scale level; topological mismatch between the as-designed and as-produced Mg scaffolds | Co–Cr/Mg–Al–Zn (AZ31) | The hybrid scaffold mechanical stiffness shifted from 18.3 GPa to 5.5 GPa, directly proportional to the mass loss produced by implant immersion in SBF. The yield strength value dropped from 155 MPa (100% of initial AZ31 content) to 54 MPa (0% AZ31 content) | An increased degradation rate occurred due to galvanic corrosion apparition | - | [148] |
| Mg–Al–Zn (Die-cast) (AZ91D) | - | - | In vivo tests made on NZW rabbits. The result was sustained by the macrophage-specific antibody MAC387 test. Low inflammatory reactions for Mg-based implants were reported | [149] |
5. Artificial Intelligence Techniques for Patient-Adapted Mg-Based Scaffold Manufacture
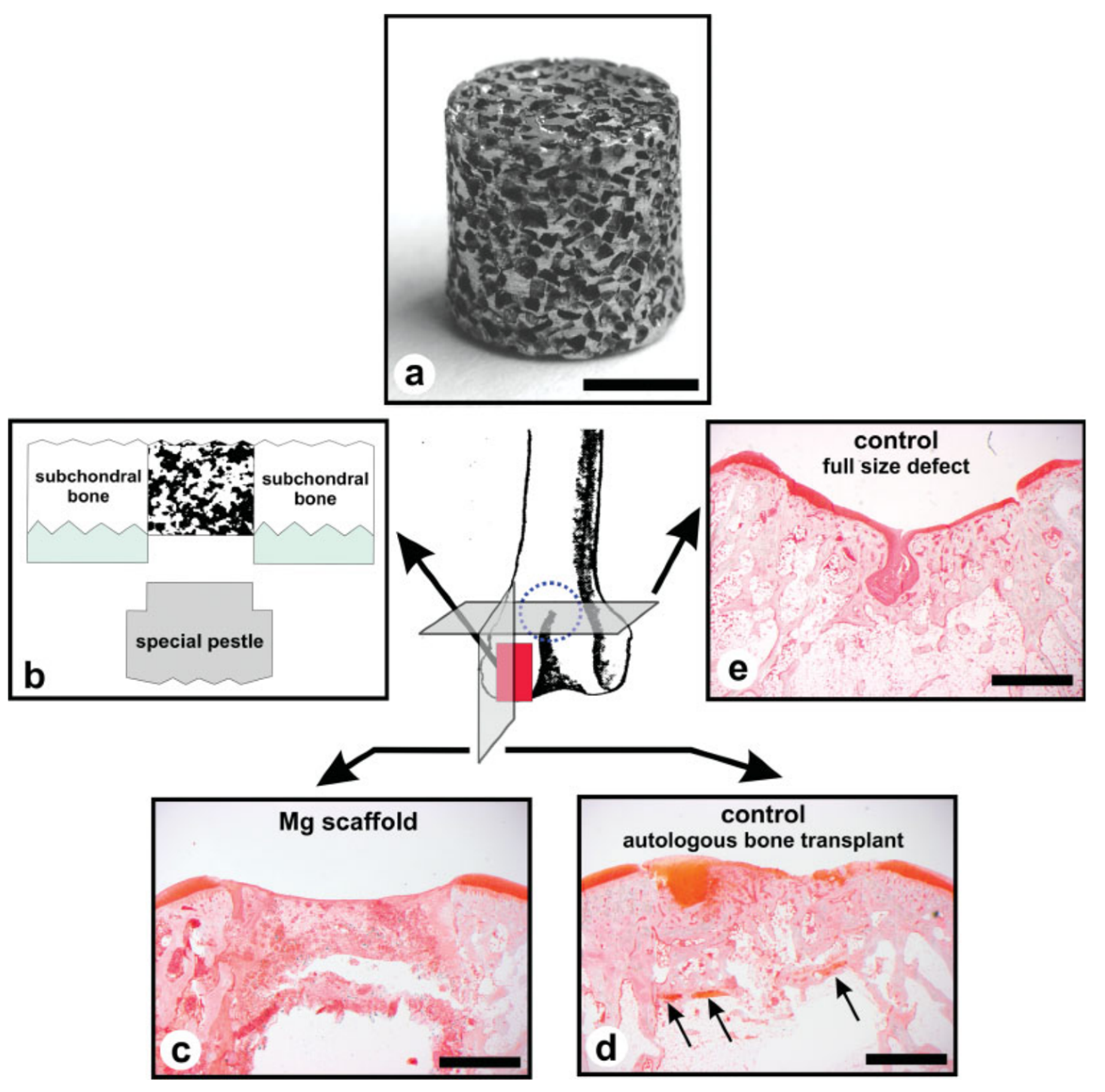
6. Challenges, Potential Clinical Applications, and Future Trends of Patient-Adapted Mg-Based Scaffolds
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hewett, T.E.; Olsen, G.; Atkinson, M. The Use of Big Data to Improve Human Health: How Experience from Other Industries Will Shape the Future. Int. J. Sport. Phys. Ther. 2021, 16, 1590–1594. [Google Scholar] [CrossRef]
- Schulze, M.; Gosheger, G.; Bockholt, S.; De Vaal, M.; Budny, T.; Tönnemann, M.; Pützler, J.; Bövingloh, A.S.; Rischen, R.; Hofbauer, V.; et al. Complex Bone Tumors of the Trunk—The Role of 3D Printing and Navigation in Tumor Orthopedics: A Case Series and Review of the Literature. J. Pers. Med. 2021, 11, 517. [Google Scholar] [CrossRef] [PubMed]
- Amin, T.; Parr, W.C.H.; Mobbs, R.J. Opinion Piece: Patient-Specific Implants May Be the Next Big Thing in Spinal Surgery. J. Pers. Med. 2021, 11, 498. [Google Scholar] [CrossRef] [PubMed]
- Savov, P.; Tuecking, L.-R.; Windhagen, H.; Ettinger, M. Individual Revision Knee Arthroplasty Is a Safe Limb Salvage Procedure. J. Pers. Med. 2021, 11, 572. [Google Scholar] [CrossRef] [PubMed]
- Rudert, M.; Holzapfel, B.M.; Pilge, H.; Rechl, H.; Gradinger, R. Beckenteilresektion (innere Hemipelvektomie) und endoprothetischer Ersatz bei hüftgelenksnahen Tumoren. Oper. Orthop. Traumatol. 2012, 24, 196–214. [Google Scholar] [CrossRef] [PubMed]
- Angelini, A.; Piazza, M.; Pagliarini, E.; Trovarelli, G.; Spertino, A.; Ruggieri, P. The Orthopedic-Vascular Multidisciplinary Approach Improves Patient Safety in Surgery for Musculoskeletal Tumors: A Large-Volume Center Experience. J. Pers. Med. 2021, 11, 462. [Google Scholar] [CrossRef] [PubMed]
- Pumilia, C.A.; Schroeder, L.; Sarpong, N.O.; Martin, G. Patient Satisfaction, Functional Outcomes, and Implant Survivorship in Patients Undergoing Customized Unicompartmental Knee Arthroplasty. J. Pers. Med. 2021, 11, 753. [Google Scholar] [CrossRef]
- Moret, C.S.; Schelker, B.L.; Hirschmann, M.T. Clinical and Radiological Outcomes after Knee Arthroplasty with Patient-Specific versus Off-the-Shelf Knee Implants: A Systematic Review. J. Pers. Med. 2021, 11, 590. [Google Scholar] [CrossRef]
- Georgeanu, V.A.; Gingu, O.; Antoniac, I.V.; Manolea, H.O. Current Options and Future Perspectives on Bone Graft and Biomaterials Substitutes for Bone Repair, from Clinical Needs to Advanced Biomaterials Research. Appl. Sci. 2023, 13, 8471. [Google Scholar] [CrossRef]
- Roberts, T.T.; Rosenbaum, A.J. Bone Grafts, Bone Substitutes and Orthobiologics. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials Design for Bone-Tissue Engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Ferreira, N.; Tanwar, Y.S. Systematic Approach to the Management of Post-Traumatic Segmental Diaphyseal Long Bone Defects: Treatment Algorithm and Comprehensive Classification System. Strateg. Trauma Limb Reconstr. 2021, 15, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Pacha, T.O.; Mommsen, P.; Brauckmann, V.; Aktas, G.; Krempec, M.; Wilhelmi, B.; Clausen, J.-D.; März, V.; Krezdorn, N.; Vogt, P.M.; et al. Interdisziplinäres Extremitäten-Board in der Behandlung von Komplexverletzungen. Unfallchirurgie 2023, 126, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Thouas, G.A. Metallic Implant Biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Bradberry, S.M.; Wilkinson, J.M.; Ferner, R.E. Systemic Toxicity Related to Metal Hip Prostheses. Clin. Toxicol. 2014, 52, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Ma, J.-X.; Xu, L.; Gu, X.-S.; Ma, X.-L. Biodegradable Materials for Bone Defect Repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yang, S.; Shi, H.; Song, Y.; Sun, H.; Wang, Q.; Tan, L.; Guo, S. Magnesium Alloys for Orthopedic Applications:A Review on the Mechanisms Driving Bone Healing. J. Magnes. Alloys 2022, 10, 3327–3353. [Google Scholar] [CrossRef]
- Doppalapudi, S.; Jain, A.; Khan, W.; Domb, A.J. Biodegradable Polymers—An Overview. Polym. Adv. Technol. 2014, 25, 427–435. [Google Scholar] [CrossRef]
- Bairagi, D.; Mandal, S. A Comprehensive Review on Biocompatible Mg-Based Alloys as Temporary Orthopaedic Implants: Current Status, Challenges, and Future Prospects. J. Magnes. Alloys 2022, 10, 627–669. [Google Scholar] [CrossRef]
- Li, W.; Qiao, W.; Liu, X.; Bian, D.; Shen, D.; Zheng, Y.; Wu, J.; Kwan, K.Y.H.; Wong, T.M.; Cheung, K.M.C.; et al. Biomimicking Bone–Implant Interface Facilitates the Bioadaption of a New Degradable Magnesium Alloy to the Bone Tissue Microenvironment. Adv. Sci. 2021, 8, 2102035. [Google Scholar] [CrossRef]
- Xing, F.; Li, S.; Yin, D.; Xie, J.; Rommens, P.M.; Xiang, Z.; Liu, M.; Ritz, U. Recent Progress in Mg-Based Alloys as a Novel Bioabsorbable Biomaterials for Orthopedic Applications. J. Magnes. Alloys 2022, 10, 1428–1456. [Google Scholar] [CrossRef]
- Lee, J.-W.; Han, H.-S.; Han, K.-J.; Park, J.; Jeon, H.; Ok, M.-R.; Seok, H.-K.; Ahn, J.-P.; Lee, K.E.; Lee, D.-H.; et al. Long-Term Clinical Study and Multiscale Analysis of in Vivo Biodegradation Mechanism of Mg Alloy. Proc. Natl. Acad. Sci. USA 2016, 113, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg Alloys for Orthopedic Implants—A Review. J. Magnes. Alloys 2021, 9, 1884–1905. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Razavi, M.; Vashaee, D.; Moharamzadeh, K.; Boccaccini, A.R.; Tayebi, L. Porous Magnesium-Based Scaffolds for Tissue Engineering. Mater. Sci. Eng. C 2017, 71, 1253–1266. [Google Scholar] [CrossRef] [PubMed]
- Istrate, B.; Munteanu, C.; Antoniac, I.-V.; Lupescu, Ș.-C. Current Research Studies of Mg–Ca–Zn Biodegradable Alloys Used as Orthopedic Implants—Review. Crystals 2022, 12, 1468. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, S.; Lu, F.; Wang, B.; Yang, L.; Qin, L.; Yang, K.; Li, Y.; Li, W.; Wang, W.; et al. Vascularized Bone Grafting Fixed by Biodegradable Magnesium Screw for Treating Osteonecrosis of the Femoral Head. Biomaterials 2016, 81, 84–92. [Google Scholar] [CrossRef]
- Rau, J.V.; Antoniac, I.; Cama, G.; Komlev, V.S.; Ravaglioli, A. Bioactive Materials for Bone Tissue Engineering. BioMed Res. Int. 2016, 2016, 3741428. [Google Scholar] [CrossRef]
- Quan, P.H.; Paltanea, V.M.; Paltanea, G.; Antoniac, I.; Nemoianu, I.V. Potential of Biodegradable Magnesium Alloys for Medical Applications. Key Eng. Mater. 2022, 931, 55–61. [Google Scholar] [CrossRef]
- Paltanea, G.; Manescu, V.; Antoniac, I.; Antoniac, A.; Nemoianu, I.V.; Robu, A.; Dura, H. A Review of Biomimetic and Biodegradable Magnetic Scaffolds for Bone Tissue Engineering and Oncology. Int. J. Mol. Sci. 2023, 24, 4312. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Antoniac, I.; Miculescu, M.; Mănescu, V.; Stere, A.; Quan, P.H.; Păltânea, G.; Robu, A.; Earar, K. Magnesium-Based Alloys Used in Orthopedic Surgery. Materials 2022, 15, 1148. [Google Scholar] [CrossRef] [PubMed]
- Quan, P.H.; Antoniac, I.; Miculescu, F.; Antoniac, A.; Manescu, V.; Robu, A.; Bița, A.-I.; Miculescu, M.; Saceleanu, A.; Bodog, A.D.; et al. Fluoride Treatment and In Vitro Corrosion Behavior of Mg-Nd-Y-Zn-Zr Alloys Type. Materials 2022, 15, 566. [Google Scholar] [CrossRef] [PubMed]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold Design for Bone Regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, P.; Di Silvio, L. Osteoblasts in Bone Tissue Engineering. Proc. Inst. Mech. Eng. H 2010, 224, 1415–1440. [Google Scholar] [CrossRef] [PubMed]
- Franz-Odendaal, T.A.; Hall, B.K.; Witten, P.E. Buried Alive: How Osteoblasts Become Osteocytes. Dev. Dyn. 2006, 235, 176–190. [Google Scholar] [CrossRef]
- Wildemann, B.; Ignatius, A.; Leung, F.; Taitsman, L.A.; Smith, R.M.; Pesántez, R.; Stoddart, M.J.; Richards, R.G.; Jupiter, J.B. Non-Union Bone Fractures. Nat. Rev. Dis. Prim. 2021, 7, 57. [Google Scholar] [CrossRef]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.; Yao, Z.; Goodman, S.B. Inflammation, Fracture and Bone Repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef]
- Gibon, E.; Lu, L.; Goodman, S.B. Aging, Inflammation, Stem Cells, and Bone Healing. Stem Cell Res. Ther. 2016, 7, 44. [Google Scholar] [CrossRef]
- Schmidt-Bleek, K.; Kwee, B.J.; Mooney, D.J.; Duda, G.N. Boon and Bane of Inflammation in Bone Tissue Regeneration and Its Link with Angiogenesis. Tissue Eng. Part B Rev. 2015, 21, 354–364. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, Z.; Shi, Y.; Dong, L.; Wang, C. Modulating Macrophage Activities to Promote Endogenous Bone Regeneration: Biological Mechanisms and Engineering Approaches. Bioact. Mater. 2021, 6, 244–261. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone Physiological Microenvironment and Healing Mechanism: Basis for Future Bone-Tissue Engineering Scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [CrossRef] [PubMed]
- Marsell, R.; Einhorn, T.A. The Biology of Fracture Healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Chu, Z.; Ma, J.; Ouyang, L. Immunomodulation Effect of Biomaterials on Bone Formation. J. Funct. Biomater. 2022, 13, 103. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Niu, W.; Lei, B.; Boccaccini, A.R. Immunomodulatory Bioactive Glasses for Tissue Regeneration. Acta Biomater. 2021, 133, 168–186. [Google Scholar] [CrossRef]
- Wang, H.; Bai, M.; Yuan, H.; Hou, Y.; Liu, Y.; Fang, Z.; Sun, Y.; Wang, J.; Zhu, S.; Guan, S. Zn Content Mediated Fibrinogen Adsorption on Biodegradable Mg-Zn Alloys Surfaces. J. Magnes. Alloys 2021, 9, 2145–2154. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, H.; Wang, J.; Zhang, E.; Bai, M.; Sun, Y.; Wang, J.; Zhu, S.; Zheng, Y.; Guan, S. Influence of the Second Phase on Protein Adsorption on Biodegradable Mg Alloys’ Surfaces: Comparative Experimental and Molecular Dynamics Simulation Studies. Acta Biomater. 2021, 129, 323–332. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, N.; Betts, L.; Zhu, D. Bio-Adaption between Magnesium Alloy Stent and the Blood Vessel: A Review. J. Mater. Sci. Technol. 2016, 32, 815–826. [Google Scholar] [CrossRef]
- Xie, K.; Wang, N.; Guo, Y.; Zhao, S.; Tan, J.; Wang, L.; Li, G.; Wu, J.; Yang, Y.; Xu, W.; et al. Additively Manufactured Biodegradable Porous Magnesium Implants for Elimination of Implant-Related Infections: An in Vitro and in Vivo Study. Bioact. Mater. 2021, 8, 140–152. [Google Scholar] [CrossRef]
- Liu, C.; He, P.; Wan, P.; Li, M.; Wang, K.; Tan, L.; Zhang, Y.; Yang, K. The in Vitro Biocompatibility and Macrophage Phagocytosis of Mg17Al12 Phase in Mg–Al–Zn Alloys. J. Biomed. Mater. Res. Part A 2015, 103, 2405–2415. [Google Scholar] [CrossRef]
- Li, F.-Y.; Chaigne-Delalande, B.; Kanellopoulou, C.; Davis, J.C.; Matthews, H.F.; Douek, D.C.; Cohen, J.I.; Uzel, G.; Su, H.C.; Lenardo, M.J. Second Messenger Role for Mg2+ Revealed by Human T-Cell Immunodeficiency. Nature 2011, 475, 471–476. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Ito, A.; Watanabe, Y.; Sogo, Y.; Hirose, M.; Ohno, T.; Tsuji, N.M. Rod-Shaped and Substituted Hydroxyapatite Nanoparticles Stimulating Type 1 and 2 Cytokine Secretion. Colloids Surf. B Biointerfaces 2016, 139, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-K.; Han, H.-S.; Lee, K.-S.; Lee, D.-H.; Lee, J.W.; Jeon, H.; Cho, S.-Y.; Roh, H.-J.; Kim, Y.-C.; Seok, H.-K. Comprehensive Study on the Roles of Released Ions from Biodegradable Mg–5 Wt% Ca–1 Wt% Zn Alloy in Bone Regeneration. J. Tissue Eng. Regen. Med. 2017, 11, 2710–2724. [Google Scholar] [CrossRef] [PubMed]
- Costantino, M.D.; Schuster, A.; Helmholz, H.; Meyer-Rachner, A.; Willumeit-Römer, R.; Luthringer-Feyerabend, B.J.C. Inflammatory Response to Magnesium-Based Biodegradable Implant Materials. Acta Biomater. 2020, 101, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Stötzel, S.; Khassawna, T.E.; Iskhahova, K.; Florian Wieland, D.; Zeller Plumhoff, B.; Haugen, H.J. Early Osteoimmunomodulatory Effects of Magnesium–Calcium–Zinc Alloys. J. Tissue Eng. 2021, 12, 20417314211047100. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Witte, F.; Li, J.; Guan, S. The Increased Ratio of Mg2+/Ca2+ from Degrading Magnesium Alloys Directs Macrophage Fate for Functionalized Growth of Endothelial Cells. Smart Mater. Med. 2022, 3, 188–198. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, L.; Willumeit-Römer, R.; Luthringer-Feyerabend, B.J.C. Macrophage-Derived Oncostatin M/Bone Morphogenetic Protein 6 in Response to Mg-Based Materials Influences pro-Osteogenic Activity of Human Umbilical Cord Perivascular Cells. Acta Biomater. 2021, 133, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Cheng, S.; Zhang, R.; Li, M.; Zhou, J.; Wang, D.; Zhang, Y. Zn-Contained Mussel-Inspired Film on Mg Alloy for Inhibiting Bacterial Infection and Promoting Bone Regeneration. Regen. Biomater. 2021, 8, rbaa044. [Google Scholar] [CrossRef]
- Wang, M.; Yu, Y.; Dai, K.; Ma, Z.; Liu, Y.; Wang, J.; Liu, C. Improved Osteogenesis and Angiogenesis of Magnesium-Doped Calcium Phosphate Cement via Macrophage Immunomodulation. Biomater. Sci. 2016, 4, 1574–1583. [Google Scholar] [CrossRef]
- Ye, J.; Xie, C.; Wang, C.; Huang, J.; Yin, Z.; Heng, B.C.; Chen, X.; Shen, W. Promoting Musculoskeletal System Soft Tissue Regeneration by Biomaterial-Mediated Modulation of Macrophage Polarization. Bioact. Mater. 2021, 6, 4096–4109. [Google Scholar] [CrossRef]
- Sun, L.; Li, X.; Xu, M.; Yang, F.; Wang, W.; Niu, X. In Vitro Immunomodulation of Magnesium on Monocytic Cell toward Anti-Inflammatory Macrophages. Regen. Biomater. 2020, 7, 391–401. [Google Scholar] [CrossRef]
- Jin, L.; Chen, C.; Li, Y.; Yuan, F.; Gong, R.; Wu, J.; Zhang, H.; Kang, B.; Yuan, G.; Zeng, H.; et al. A Biodegradable Mg-Based Alloy Inhibited the Inflammatory Response of THP-1 Cell-Derived Macrophages Through the TRPM7–PI3K–AKT1 Signaling Axis. Front. Immunol. 2019, 10, 2798. [Google Scholar] [CrossRef] [PubMed]
- Mazur, A.; Maier, J.A.M.; Rock, E.; Gueux, E.; Nowacki, W.; Rayssiguier, Y. Magnesium and the Inflammatory Response: Potential Physiopathological Implications. Arch. Biochem. Biophys. 2007, 458, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wu, H.; Wang, L.; Jiang, D.; Wang, W.; Yuan, G.; Pei, J.; Jia, W. The Beneficial Potential of Magnesium-Based Scaffolds to Promote Chondrogenesis through Controlled Mg2+ Release in Eliminating the Destructive Effect of Activated Macrophages on Chondrocytes. Biomater. Adv. 2022, 134, 112719. [Google Scholar] [CrossRef] [PubMed]
- Su, N.-Y.; Peng, T.-C.; Tsai, P.-S.; Huang, C.-J. Phosphoinositide 3-Kinase/Akt Pathway Is Involved in Mediating the Anti-Inflammation Effects of Magnesium Sulfate. J. Surg. Res. 2013, 185, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Istrate, B.; Rau, J.V.; Munteanu, C.; Antoniac, I.V.; Saceleanu, V. Properties and in Vitro Assessment of ZrO2-Based Coatings Obtained by Atmospheric Plasma Jet Spraying on Biodegradable Mg-Ca and Mg-Ca-Zr Alloys. Ceram. Int. 2020, 46, 15897–15906. [Google Scholar] [CrossRef]
- Han, H.; Jun, I.; Seok, H.; Lee, K.; Lee, K.; Witte, F.; Mantovani, D.; Kim, Y.; Glyn-Jones, S.; Edwards, J.R. Biodegradable Magnesium Alloys Promote Angio-Osteogenesis to Enhance Bone Repair. Adv. Sci. 2020, 7, 2000800. [Google Scholar] [CrossRef]
- Zhang, D.; Ni, N.; Su, Y.; Miao, H.; Tang, Z.; Ji, Y.; Wang, Y.; Gao, H.; Ju, Y.; Sun, N.; et al. Targeting Local Osteogenic and Ancillary Cells by Mechanobiologically Optimized Magnesium Scaffolds for Orbital Bone Reconstruction in Canines. ACS Appl. Mater. Interfaces 2020, 12, 27889–27904. [Google Scholar] [CrossRef]
- Liu, C.; Fu, X.; Pan, H.; Wan, P.; Wang, L.; Tan, L.; Wang, K.; Zhao, Y.; Yang, K.; Chu, P.K. Biodegradable Mg-Cu Alloys with Enhanced Osteogenesis, Angiogenesis, and Long-Lasting Antibacterial Effects. Sci. Rep. 2016, 6, 27374. [Google Scholar] [CrossRef]
- Yoshizawa, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium Ion Stimulation of Bone Marrow Stromal Cells Enhances Osteogenic Activity, Simulating the Effect of Magnesium Alloy Degradation. Acta Biomater. 2014, 10, 2834–2842. [Google Scholar] [CrossRef]
- Bai, Y.; Yin, G.; Huang, Z.; Liao, X.; Chen, X.; Yao, Y.; Pu, X. Localized Delivery of Growth Factors for Angiogenesis and Bone Formation in Tissue Engineering. Int. Immunopharmacol. 2013, 16, 214–223. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, G.; Xue, Y.; Wang, D.; Liu, X.; Sun, J. Multifunctions of Dual Zn/Mg Ion Co-Implanted Titanium on Osteogenesis, Angiogenesis and Bacteria Inhibition for Dental Implants. Acta Biomater. 2017, 49, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Willumeit-Römer, R.; Luthringer-Feyerabend, B.J.C. Effect of Magnesium-Degradation Products and Hypoxia on the Angiogenesis of Human Umbilical Vein Endothelial Cells. Acta Biomater. 2019, 98, 269–283. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Chapter One—Biochemical and Biological Attributes of Matrix Metalloproteinases. In Progress in Molecular Biology and Translational Science; Khalil, R.A., Ed.; Matrix Metalloproteinases and Tissue Remodeling in Health and Disease: Cardiovascular Remodeling; Academic Press: Cambridge, MA, USA, 2017; Volume 147, pp. 1–73. [Google Scholar]
- Li, D.; Yuan, Q.; Yu, K.; Xiao, T.; Liu, L.; Dai, Y.; Xiong, L.; Zhang, B.; Li, A. Mg–Zn–Mn Alloy Extract Induces the Angiogenesis of Human Umbilical Vein Endothelial Cells via FGF/FGFR Signaling Pathway. Biochem. Biophys. Res. Commun. 2019, 514, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Guo, S.; Tang, Z.; Wei, X.; Gao, P.; Wang, N.; Li, X.; Guo, Z. Magnesium Promotes Bone Formation and Angiogenesis by Enhancing MC3T3-E1 Secretion of PDGF-BB. Biochem. Biophys. Res. Commun. 2020, 528, 664–670. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Mi, J.; He, X.; Pan, Q.; Zheng, L.; Zu, H.; Chen, Z.; Dai, B.; Li, X.; et al. Biodegradable Magnesium Combined with Distraction Osteogenesis Synergistically Stimulates Bone Tissue Regeneration via CGRP-FAK-VEGF Signaling Axis. Biomaterials 2021, 275, 120984. [Google Scholar] [CrossRef]
- Xie, H.; Cui, Z.; Wang, L.; Xia, Z.; Hu, Y.; Xian, L.; Li, C.; Xie, L.; Crane, J.; Wan, M.; et al. PDGF-BB Secreted by Preosteoclasts Induces Angiogenesis during Coupling with Osteogenesis. Nat. Med. 2014, 20, 1270–1278. [Google Scholar] [CrossRef]
- Ge, W.; Chen, K.; Tang, H.; Arken, X.; Zhang, X.; Gu, X.; Zhu, C. Degradability and in Vivo Biocompatibility of Micro-Alloyed Mg-Ca-La Alloys as Orthopedic Implants. Mater. Lett. 2022, 310, 131510. [Google Scholar] [CrossRef]
- Gu, X.; Wang, F.; Xie, X.; Zheng, M.; Li, P.; Zheng, Y.; Qin, L.; Fan, Y. In Vitro and in Vivo Studies on As-Extruded Mg- 5.25wt.%Zn-0.6wt.%Ca Alloy as Biodegradable Metal. Sci. China Mater. 2018, 61, 619–628. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, Q.; Wang, J.; Yang, Q.; Zhao, W.; Qiao, B.; Li, Y.; Jiang, D. Improving in Vitro and in Vivo Corrosion Resistance and Biocompatibility of Mg–1Zn–1Sn Alloys by Microalloying with Sr. Bioact. Mater. 2021, 6, 4654–4669. [Google Scholar] [CrossRef]
- Wang, W.-H.; Wang, F.; Zhao, H.-F.; Yan, K.; Huang, C.-L.; Yin, Y.; Huang, Q.; Chen, Z.-Z.; Zhu, W.-Y. Injectable Magnesium-Zinc Alloy Containing Hydrogel Complex for Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 617585. [Google Scholar] [CrossRef]
- Li, D.; Zhang, D.; Yuan, Q.; Liu, L.; Li, H.; Xiong, L.; Guo, X.; Yan, Y.; Yu, K.; Dai, Y.; et al. In Vitro and in Vivo Assessment of the Effect of Biodegradable Magnesium Alloys on Osteogenesis. Acta Biomater. 2022, 141, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Liu, Y.; Wang, S.; Zeng, R.-C.; Liu, Y.; Zheng, Y.; Zhou, Y. In Vitro and in Vivo Investigation on Biodegradable Mg-Li-Ca Alloys for Bone Implant Application. Sci. China Mater. 2019, 62, 256–272. [Google Scholar] [CrossRef]
- Wu, L.; Feyerabend, F.; Schilling, A.F.; Willumeit-Römer, R.; Luthringer, B.J.C. Effects of Extracellular Magnesium Extract on the Proliferation and Differentiation of Human Osteoblasts and Osteoclasts in Coculture. Acta Biomater. 2015, 27, 294–304. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhang, H.; Qiu, J. Osteogenic Effects of Bioabsorbable Magnesium Implant in Rat Mandibles and in Vitro. J. Periodontol. 2021, 92, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Geng, Z.; Huang, Y.; Jia, Z.; Cui, Z.; Li, Z.; Wu, S.; Liang, Y.; Zhu, S.; Yang, X.; et al. Unraveling the Osteogenesis of Magnesium by the Activity of Osteoblasts in Vitro. J. Mater. Chem. B 2018, 6, 6615–6621. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yang, G.; Jiang, F.; Zhou, M.; Yin, S.; Tang, Y.; Tang, T.; Zhang, Z.; Zhang, W.; Jiang, X. A Magnesium-Enriched 3D Culture System That Mimics the Bone Development Microenvironment for Vascularized Bone Regeneration. Adv. Sci. 2019, 6, 1900209. [Google Scholar] [CrossRef]
- Wang, C.-X.; Ma, T.; Wang, M.-Y.; Guo, H.-Z.; Ge, X.-Y.; Zhang, Y.; Lin, Y. Facile Distribution of an Alkaline Microenvironment Improves Human Bone Marrow Mesenchymal Stem Cell Osteogenesis on a Titanium Surface through the ITG/FAK/ALP Pathway. Int. J. Implant. Dent. 2021, 7, 56. [Google Scholar] [CrossRef]
- Pan, H.; Shen, Y.; Wen, C.; Peng, S.; Lu, W.W. Role of pH—The Essential Step for Osteoporotic Bone Regeneration. Bone 2010, 47, S444. [Google Scholar] [CrossRef]
- Galow, A.-M.; Rebl, A.; Koczan, D.; Bonk, S.M.; Baumann, W.; Gimsa, J. Increased Osteoblast Viability at Alkaline pH in Vitro Provides a New Perspective on Bone Regeneration. Biochem. Biophys. Rep. 2017, 10, 17–25. [Google Scholar] [CrossRef]
- Chen, M.; Hu, Y.; Hou, Y.; Li, M.; Tan, L.; Chen, M.; Geng, W.; Tao, B.; Jiang, H.; Luo, Z.; et al. Magnesium/Gallium-Layered Nanosheets on Titanium Implants Mediate Osteogenic Differentiation of MSCs and Osseointegration under Osteoporotic Condition. Chem. Eng. J. 2022, 427, 130982. [Google Scholar] [CrossRef]
- Tan, J.; Wang, D.; Cao, H.; Qiao, Y.; Zhu, H.; Liu, X. Effect of Local Alkaline Microenvironment on the Behaviors of Bacteria and Osteogenic Cells. ACS Appl. Mater. Interfaces 2018, 10, 42018–42029. [Google Scholar] [CrossRef] [PubMed]
- Wagener, V.; Schilling, A.; Mainka, A.; Hennig, D.; Gerum, R.; Kelch, M.-L.; Keim, S.; Fabry, B.; Virtanen, S. Cell Adhesion on Surface-Functionalized Magnesium. ACS Appl. Mater. Interfaces 2016, 8, 11998–12006. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.; Fischerauer, S.; Treichler, S.; Martinelli, E.; Eichler, J.; Myrissa, A.; Zötsch, S.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A.M. The Influence of Biodegradable Magnesium Implants on the Growth Plate. Acta Biomater. 2018, 66, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, A.F.; Lin, J.; Lin, A.; Sallee, A.; Le, B.; Cortez Alcaraz, M.C.; Guan, R.-G.; Botimer, G.; Inceoğlu, S.; Liu, H. Degradation of Bioresorbable Mg–4Zn–1Sr Intramedullary Pins and Associated Biological Responses in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2017, 9, 44332–44355. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, D.-L.; Huang, Y.-C.; Wang, T.-B.; Zeng, H. Hydrogen Inhibits the Osteoclastogenesis of Mouse Bone Marrow Mononuclear Cells. Mater. Sci. Eng. C 2020, 110, 110640. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, Y.; Liu, R.; Wu, S.; Fang, J.; Huang, B.; Li, Z.; Chen, Z.; Chen, Z. Tuning Surface Properties of Bone Biomaterials to Manipulate Osteoblastic Cell Adhesion and the Signaling Pathways for the Enhancement of Early Osseointegration. Colloids Surf. B Biointerfaces 2018, 164, 58–69. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, J.; Weiyang, J.; Qiao, B.; Wang, Y.; Li, Y.; Jiang, D. A Novel Biodegradable Mg-1Zn-0.5Sn Alloy: Mechanical Properties, Corrosion Behavior, Biocompatibility, and Antibacterial Activity. J. Magnes. Alloys 2020, 8, 374–386. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Liu, C.; Tan, W.; Tang, M.; Zhou, X.; Sun, T.; Deng, Y. Mg2+ in β-TCP/Mg–Zn Composite Enhances the Differentiation of Human Bone Marrow Stromal Cells into Osteoblasts through MAPK-Regulated Runx2/Osx. J. Cell. Physiol. 2020, 235, 5182–5191. [Google Scholar] [CrossRef]
- Li, M.; He, P.; Wu, Y.; Zhang, Y.; Xia, H.; Zheng, Y.; Han, Y. Stimulatory Effects of the Degradation Products from Mg-Ca-Sr Alloy on the Osteogenesis through Regulating ERK Signaling Pathway. Sci. Rep. 2016, 6, 32323. [Google Scholar] [CrossRef]
- Schupbach, D.; Comeau-Gauthier, M.; Harvey, E.; Merle, G. Wnt Modulation in Bone Healing. Bone 2020, 138, 115491. [Google Scholar] [CrossRef]
- Haffner-Luntzer, M. Experimental Agents to Improve Fracture Healing: Utilizing the WNT Signaling Pathway. Injury 2021, 52, S44–S48. [Google Scholar] [CrossRef] [PubMed]
- Li, R.W.; Kirkland, N.T.; Truong, J.; Wang, J.; Smith, P.N.; Birbilis, N.; Nisbet, D.R. The Influence of Biodegradable Magnesium Alloys on the Osteogenic Differentiation of Human Mesenchymal Stem Cells. J. Biomed. Mater. Res. Part A 2014, 102, 4346–4357. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.-L.; Xu, M.-H.; Li, X.; Xinlong, H.; Fang, W.; Dong, J. The Roles of Acidosis in Osteoclast Biology. Front. Physiol. 2016, 7, 222. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.M.; Risser, G.E.; Spiller, K.L. Sequential Drug Delivery to Modulate Macrophage Behavior and Enhance Implant Integration. Adv. Drug Deliv. Rev. 2019, 149–150, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Jessop, Z.M.; Al-Sabah, A.; Francis, W.R.; Whitaker, I.S. Transforming Healthcare through Regenerative Medicine. BMC Med. 2016, 14, 115. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Avilés, F.; Sanz-Ruiz, R.; Climent, A.M.; Badimon, L.; Bolli, R.; Charron, D.; Fuster, V.; Janssens, S.; Kastrup, J.; Kim, H.-S.; et al. Global Position Paper on Cardiovascular Regenerative Medicine. Eur. Heart J. 2017, 38, 2532–2546. [Google Scholar] [CrossRef] [PubMed]
- Stoor, P.; Suomalainen, A.; Lindqvist, C.; Mesimäki, K.; Danielsson, D.; Westermark, A.; Kontio, R.K. Rapid Prototyped Patient Specific Implants for Reconstruction of Orbital Wall Defects. J. Cranio-Maxillofac. Surg. 2014, 42, 1644–1649. [Google Scholar] [CrossRef]
- Kotecha, M.; Magin, R.; Mao, J. Magnetic Resonance Imaging in Tissue Engineering; Wiley: Hoboken, NJ, USA, 2017; ISBN 978-1-119-19335-7. [Google Scholar]
- Ryu, J.H.; Kim, H.S.; Lee, K.H. Contour-Based Algorithms for Generating 3D CAD Models from Medical Images. Int. J. Adv. Manuf. Technol. 2004, 24, 112–119. [Google Scholar] [CrossRef]
- He, J.; Chen, G.; Liu, M.; Xu, Z.; Chen, H.; Yang, L.; Lv, Y. Scaffold Strategies for Modulating Immune Microenvironment during Bone Regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110411. [Google Scholar] [CrossRef]
- Chen, H.; Han, Q.; Wang, C.; Liu, Y.; Chen, B.; Wang, J. Porous Scaffold Design for Additive Manufacturing in Orthopedics: A Review. Front. Bioeng. Biotechnol. 2020, 8, 609. [Google Scholar] [CrossRef]
- Al-Barqawi, M.O.; Church, B.; Thevamaran, M.; Thoma, D.J.; Rahman, A. Design and Validation of Additively Manufactured Metallic Cellular Scaffold Structures for Bone Tissue Engineering. Materials 2022, 15, 3310. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zou, S.; Mu, Y.; Wang, J.; Jin, Y. Additively Manufactured Scaffolds with Optimized Thickness Based on Triply Periodic Minimal Surface. Materials 2022, 15, 7084. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Chen, Y.; Yan, X.-T.; Zhang, G. Design of the Artificial Bone Scaffolds Based on the Multi-Field Coupling Model. Procedia CIRP 2016, 56, 95–99. [Google Scholar] [CrossRef]
- Van Bael, S.; Chai, Y.C.; Truscello, S.; Moesen, M.; Kerckhofs, G.; Van Oosterwyck, H.; Kruth, J.-P.; Schrooten, J. The Effect of Pore Geometry on the in Vitro Biological Behavior of Human Periosteum-Derived Cells Seeded on Selective Laser-Melted Ti6Al4V Bone Scaffolds. Acta Biomater. 2012, 8, 2824–2834. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Chen, Q.; Pugno, N.; Li, Z.-Y. Effect of Rehabilitation Exercise Durations on the Dynamic Bone Repair Process by Coupling Polymer Scaffold Degradation and Bone Formation. Biomech. Model. Mechanobiol. 2018, 17, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Cheong, V.S.; Fromme, P.; Mumith, A.; Coathup, M.J.; Blunn, G.W. Novel Adaptive Finite Element Algorithms to Predict Bone Ingrowth in Additive Manufactured Porous Implants. J. Mech. Behav. Biomed. Mater. 2018, 87, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, S.; Li, Q. Microstructure Design of Biodegradable Scaffold and Its Effect on Tissue Regeneration. Biomaterials 2011, 32, 5003–5014. [Google Scholar] [CrossRef]
- Yang, Y.; He, C.; Dianyu, E.; Yang, W.; Qi, F.; Xie, D.; Shen, L.; Peng, S.; Shuai, C. Mg Bone Implant: Features, Developments and Perspectives. Mater. Des. 2020, 185, 108259. [Google Scholar] [CrossRef]
- Salmi, M. Additive Manufacturing Processes in Medical Applications. Materials 2021, 14, 191. [Google Scholar] [CrossRef]
- Antoniac, I.; Manescu, V.; Paltanea, G.; Antoniac, A.; Nemoianu, I.V.; Petrescu, M.I.; Dura, H.; Bodog, A.D. Additive Manufactured Magnesium-Based Scaffolds for Tissue Engineering. Materials 2022, 15, 8693. [Google Scholar] [CrossRef]
- Cheng, M.; Wahafu, T.; Jiang, G.; Liu, W.; Qiao, Y.; Peng, X.; Cheng, T.; Zhang, X.; He, G.; Liu, X. A Novel Open-Porous Magnesium Scaffold with Controllable Microstructures and Properties for Bone Regeneration. Sci. Rep. 2016, 6, 24134. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; He, G. A New Approach to the Fabrication of Porous Magnesium with Well-Controlled 3D Pore Structure for Orthopedic Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 43, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Dezfuli, S.N.; Sadrnezhaad, S.K.; Shokrgozar, M.A.; Bonakdar, S. Fabrication of Biocompatible Titanium Scaffolds Using Space Holder Technique. J. Mater. Sci. Mater. Med. 2012, 23, 2483–2488. [Google Scholar] [CrossRef] [PubMed]
- Li, D.S.; Zhang, Y.P.; Ma, X.; Zhang, X.P. Space-Holder Engineered Porous NiTi Shape Memory Alloys with Improved Pore Characteristics and Mechanical Properties. J. Alloys Compd. 2009, 474, L1–L5. [Google Scholar] [CrossRef]
- Torres, Y.; Pavón, J.J.; Rodríguez, J.A. Processing and Characterization of Porous Titanium for Implants by Using NaCl as Space Holder. J. Mater. Process. Technol. 2012, 212, 1061–1069. [Google Scholar] [CrossRef]
- Manonukul, A.; Muenya, N.; Léaux, F.; Amaranan, S. Effects of Replacing Metal Powder with Powder Space Holder on Metal Foam Produced by Metal Injection Moulding. J. Mater. Process. Technol. 2010, 210, 529–535. [Google Scholar] [CrossRef]
- Toghyani, S.; Khodaei, M.; Razavi, M. Magnesium Scaffolds with Two Novel Biomimetic Designs and MgF2 Coating for Bone Tissue Engineering. Surf. Coat. Technol. 2020, 395, 125929. [Google Scholar] [CrossRef]
- Nakajima, H. Fabrication, Properties, and Applications of Porous Metals with Directional Pores. Proc. Jpn. Acad. Ser. B 2010, 86, 884–899. [Google Scholar] [CrossRef]
- Gu, X.N.; Zhou, W.R.; Zheng, Y.F.; Liu, Y.; Li, Y.X. Degradation and Cytotoxicity of Lotus-Type Porous Pure Magnesium as Potential Tissue Engineering Scaffold Material. Mater. Lett. 2010, 64, 1871–1874. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Razavi, M.; Vashaee, D.; Pothineni, V.R.; Rajadas, J.; Tayebi, L. Significant Degradability Enhancement in Multilayer Coating of Polycaprolactone-Bioactive Glass/Gelatin-Bioactive Glass on Magnesium Scaffold for Tissue Engineering Applications. Appl. Surf. Sci. 2015, 338, 137–145. [Google Scholar] [CrossRef]
- Seyedraoufi, Z.S.; Mirdamadi, S. Synthesis, Microstructure and Mechanical Properties of Porous Mg Zn Scaffolds. J. Mech. Behav. Biomed. Mater. 2013, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Tan, L.; Zhang, B.; Wu, C.; He, Y.; Yang, J.; Yang, K. Study on β-TCP Coated Porous Mg as a Bone Tissue Engineering Scaffold Material. J. Mater. Sci. Technol. 2009, 25, 123. [Google Scholar]
- Fu, Q.; Liang, W.; Huang, J.; Jin, W.; Guo, B.; Li, P.; Xu, S.; Chu, P.K.; Yu, Z. Research Perspective and Prospective of Additive Manufacturing of Biodegradable Magnesium-Based Materials. J. Magnes. Alloys 2023, 11, 1485–1504. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological Design and Additive Manufacturing of Porous Metals for Bone Scaffolds and Orthopaedic Implants: A Review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Staiger, M.P.; Dias, G.J.; Woodfield, T.B.F. A Novel Manufacturing Route for Fabrication of Topologically-Ordered Porous Magnesium Scaffolds. Adv. Eng. Mater. 2011, 13, 872–881. [Google Scholar] [CrossRef]
- Zhu, H.X.; Windle, A.H. Effects of Cell Irregularity on the High Strain Compression of Open-Cell Foams. Acta Mater. 2002, 50, 1041–1052. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Pavanram, P.; Leeflang, M.A.; Fockaert, L.I.; Pouran, B.; Tümer, N.; Schröder, K.-U.; Mol, J.M.C.; Weinans, H.; et al. Additively Manufactured Biodegradable Porous Magnesium. Acta Biomater. 2018, 67, 378–392. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, M.; Chen, C. Effect of Laser Processing Parameters on Porosity, Microstructure and Mechanical Properties of Porous Mg-Ca Alloys Produced by Laser Additive Manufacturing. Mater. Sci. Eng. A 2017, 703, 359–371. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, P.; Lin, X.; Liu, Y.; Bian, H.; Zhou, Y.; Gao, C.; Shuai, C. System Development, Formability Quality and Microstructure Evolution of Selective Laser-Melted Magnesium. Virtual Phys. Prototyp. 2016, 11, 173–181. [Google Scholar] [CrossRef]
- Matena, J.; Petersen, S.; Gieseke, M.; Teske, M.; Beyerbach, M.; Kampmann, A.; Escobar, H.M.; Gellrich, N.-C.; Haferkamp, H.; Nolte, I. Comparison of Selective Laser Melted Titanium and Magnesium Implants Coated with PCL. Int. J. Mol. Sci. 2015, 16, 13287–13301. [Google Scholar] [CrossRef]
- Kopp, A.; Derra, T.; Müther, M.; Jauer, L.; Schleifenbaum, J.H.; Voshage, M.; Jung, O.; Smeets, R.; Kröger, N. Influence of Design and Postprocessing Parameters on the Degradation Behavior and Mechanical Properties of Additively Manufactured Magnesium Scaffolds. Acta Biomater. 2019, 98, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Zai, W.; Man, H.C. Additive Manufacturing of ZK60 Magnesium Alloy by Selective Laser Melting: Parameter Optimization, Microstructure and Biodegradability. Mater. Today Commun. 2021, 26, 101922. [Google Scholar] [CrossRef]
- Salehi, M.; Maleksaeedi, S.; Sapari, M.A.B.; Nai, M.L.S.; Meenashisundaram, G.K.; Gupta, M. Additive Manufacturing of Magnesium–Zinc–Zirconium (ZK) Alloys via Capillary-Mediated Binderless Three-Dimensional Printing. Mater. Des. 2019, 169, 107683. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Koç, M. Additive Manufacturing of Biodegradable Magnesium Implants and Scaffolds: Review of the Recent Advances and Research Trends. J. Magnes. Alloys 2021, 9, 392–415. [Google Scholar] [CrossRef]
- Kleger, N.; Cihova, M.; Masania, K.; Studart, A.R.; Löffler, J.F. 3D Printing of Salt as a Template for Magnesium with Structured Porosity. Adv. Mater. 2019, 31, 1903783. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Franco, B.E.; Karaman, I.; Elwany, A.; Ma, J. Evolution of Mechanical Behavior of Magnesium Alloy Infiltrated 3D-Printed CoCr Scaffolds under Corrosion in Simulated Body Fluid. Mater. Sci. Eng. C 2019, 105, 109747. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Ulrich, H.; Rudert, M.; Willbold, E. Biodegradable Magnesium Scaffolds: Part 1: Appropriate Inflammatory Response. J. Biomed. Mater. Res. Part A 2007, 81A, 748–756. [Google Scholar] [CrossRef]
- Asadi-Eydivand, M.; Solati-Hashjin, M.; Fathi, A.; Padashi, M.; Abu Osman, N.A. Optimal Design of a 3D-Printed Scaffold Using Intelligent Evolutionary Algorithms. Appl. Soft Comput. 2016, 39, 36–47. [Google Scholar] [CrossRef]
- Bermejillo Barrera, M.D.; Franco-Martínez, F.; Díaz Lantada, A. Artificial Intelligence Aided Design of Tissue Engineering Scaffolds Employing Virtual Tomography and 3D Convolutional Neural Networks. Materials 2021, 14, 5278. [Google Scholar] [CrossRef]
- Tourlomousis, F.; Jia, C.; Karydis, T.; Mershin, A.; Wang, H.; Kalyon, D.M.; Chang, R.C. Machine Learning Metrology of Cell Confinement in Melt Electrowritten Three-Dimensional Biomaterial Substrates. Microsyst. Nanoeng. 2019, 5, 15. [Google Scholar] [CrossRef]
- Lim, M.K.; Xiong, W.; Lei, Z. Theory, Supporting Technology and Application Analysis of Cloud Manufacturing: A Systematic and Comprehensive Literature Review. Ind. Manag. Data Syst. 2020, 120, 1585–1614. [Google Scholar] [CrossRef]
- Boccella, A.R.; Centobelli, P.; Cerchione, R.; Murino, T.; Riedel, R. Evaluating Centralized and Heterarchical Control of Smart Manufacturing Systems in the Era of Industry 4.0. Appl. Sci. 2020, 10, 755. [Google Scholar] [CrossRef]
- Cho, J.; Kang, S.; Kim, K. Real-Time Precise Object Segmentation Using a Pixel-Wise Coarse-Fine Method with Deep Learning for Automated Manufacturing. J. Manuf. Syst. 2022, 62, 114–123. [Google Scholar] [CrossRef]
- Sari, T.; Gules, H.K.; Yigitol, B. Awareness and Readiness of Industry 4.0: The Case of Turkish Manufacturing Industry. Adv. Prod. Eng. Manag. 2020, 15, 57–68. [Google Scholar] [CrossRef]
- Thames, L.; Schaefer, D. Industry 4.0: An Overview of Key Benefits, Technologies, and Challenges. In Cybersecurity for Industry 4.0: Analysis for Design and Manufacturing; Thames, L., Schaefer, D., Eds.; Springer Series in Advanced Manufacturing; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–33. ISBN 978-3-319-50660-9. [Google Scholar]
- Gajsek, B.; Marolt, J.; Rupnik, B.; Lerher, T.; Sternad, M. Using Maturity Model and Discrete-Event Simulation for Industry 4.0 Implementation. Int. J. Simul. Model. 2019, 18, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Caggiano, A. Cloud-Based Manufacturing Process Monitoring for Smart Diagnosis Services. Int. J. Comput. Integr. Manuf. 2018, 31, 612–623. [Google Scholar] [CrossRef]
- Cheng, K.; Niu, Z.-C.; Wang, R.C.; Rakowski, R.; Bateman, R. Smart Cutting Tools and Smart Machining: Development Approaches, and Their Implementation and Application Perspectives. Chin. J. Mech. Eng. 2017, 30, 1162–1176. [Google Scholar] [CrossRef]
- Araújo, N.; Pacheco, V.; Costa, L. Smart Additive Manufacturing: The Path to the Digital Value Chain. Technologies 2021, 9, 88. [Google Scholar] [CrossRef]
- Kunovjanek, M.; Knofius, N.; Reiner, G. Additive Manufacturing and Supply Chains—A Systematic Review. Prod. Plan. Control. 2022, 33, 1231–1251. [Google Scholar] [CrossRef]
- Wang, C.; Tan, X.P.; Tor, S.B.; Lim, C.S. Machine Learning in Additive Manufacturing: State-of-the-Art and Perspectives. Addit. Manuf. 2020, 36, 101538. [Google Scholar] [CrossRef]
- Oleff, A.; Küster, B.; Stonis, M.; Overmeyer, L. Process Monitoring for Material Extrusion Additive Manufacturing: A State-of-the-Art Review. Prog. Addit. Manuf. 2021, 6, 705–730. [Google Scholar] [CrossRef]
- Du, W.; Bai, Q.; Zhang, B. A Novel Method for Additive/Subtractive Hybrid Manufacturing of Metallic Parts. Procedia Manuf. 2016, 5, 1018–1030. [Google Scholar] [CrossRef]
- Kerbrat, O.; Mognol, P.; Hascoët, J.-Y. A New DFM Approach to Combine Machining and Additive Manufacturing. Comput. Ind. 2011, 62, 684–692. [Google Scholar] [CrossRef]
- Pragana, J.P.M.; Sampaio, R.F.V.; Bragança, I.M.F.; Silva, C.M.A.; Martins, P.A.F. Hybrid Metal Additive Manufacturing: A State–of–the-Art Review. Adv. Ind. Manuf. Eng. 2021, 2, 100032. [Google Scholar] [CrossRef]
- Grzesik, W. Hybrid Additive and Subtractive Manufacturing Processes and Systems: A Review. J. Mach. Eng. 2018, 18, 5–24. [Google Scholar] [CrossRef]
- Jena, M.C.; Mishra, S.K.; Moharana, H.S. Application of Industry 4.0 to Enhance Sustainable Manufacturing. Environ. Prog. Sustain. Energy 2020, 39, 13360. [Google Scholar] [CrossRef]
- Aggour, K.S.; Gupta, V.K.; Ruscitto, D.; Ajdelsztajn, L.; Bian, X.; Brosnan, K.H.; Chennimalai Kumar, N.; Dheeradhada, V.; Hanlon, T.; Iyer, N.; et al. Artificial Intelligence/Machine Learning in Manufacturing and Inspection: A GE Perspective. MRS Bull. 2019, 44, 545–558. [Google Scholar] [CrossRef]
- Rahman, M.A.; Saleh, T.; Jahan, M.P.; McGarry, C.; Chaudhari, A.; Huang, R.; Tauhiduzzaman, M.; Ahmed, A.; Mahmud, A.A.; Bhuiyan, M.S.; et al. Review of Intelligence for Additive and Subtractive Manufacturing: Current Status and Future Prospects. Micromachines 2023, 14, 508. [Google Scholar] [CrossRef]
- Ng, G.K.L.; Jarfors, A.E.W.; Bi, G.; Zheng, H.Y. Porosity Formation and Gas Bubble Retention in Laser Metal Deposition. Appl. Phys. A 2009, 97, 641–649. [Google Scholar] [CrossRef]
- Bidare, P.; Bitharas, I.; Ward, R.M.; Attallah, M.M.; Moore, A.J. Laser Powder Bed Fusion in High-Pressure Atmospheres. Int. J. Adv. Manuf. Technol. 2018, 99, 543–555. [Google Scholar] [CrossRef]
- Ferrar, B.; Mullen, L.; Jones, E.; Stamp, R.; Sutcliffe, C.J. Gas Flow Effects on Selective Laser Melting (SLM) Manufacturing Performance. J. Mater. Process. Technol. 2012, 212, 355–364. [Google Scholar] [CrossRef]
- Talib Mohammed, M. Mechanical Properties of SLM-Titanium Materials for Biomedical Applications: A Review. Mater. Today Proc. 2018, 5, 17906–17913. [Google Scholar] [CrossRef]
- Bax, B.; Rajput, R.; Kellet, R.; Reisacher, M. Systematic Evaluation of Process Parameter Maps for Laser Cladding and Directed Energy Deposition. Addit. Manuf. 2018, 21, 487–494. [Google Scholar] [CrossRef]
- McCann, R.; Obeidi, M.A.; Hughes, C.; McCarthy, É.; Egan, D.S.; Vijayaraghavan, R.K.; Joshi, A.M.; Acinas Garzon, V.; Dowling, D.P.; McNally, P.J.; et al. In-Situ Sensing, Process Monitoring and Machine Control in Laser Powder Bed Fusion: A Review. Addit. Manuf. 2021, 45, 102058. [Google Scholar] [CrossRef]
- Honarvar, F.; Varvani-Farahani, A. A Review of Ultrasonic Testing Applications in Additive Manufacturing: Defect Evaluation, Material Characterization, and Process Control. Ultrasonics 2020, 108, 106227. [Google Scholar] [CrossRef]
- Wasmer, K.; Le-Quang, T.; Meylan, B.; Shevchik, S.A. In Situ Quality Monitoring in AM Using Acoustic Emission: A Reinforcement Learning Approach. J. Mater. Eng. Perform. 2019, 28, 666–672. [Google Scholar] [CrossRef]
- Shevchik, S.A.; Kenel, C.; Leinenbach, C.; Wasmer, K. Acoustic Emission for in Situ Quality Monitoring in Additive Manufacturing Using Spectral Convolutional Neural Networks. Addit. Manuf. 2018, 21, 598–604. [Google Scholar] [CrossRef]
- du Plessis, A.; Yadroitsev, I.; Yadroitsava, I.; Le Roux, S.G. X-Ray Microcomputed Tomography in Additive Manufacturing: A Review of the Current Technology and Applications. 3D Print. Addit. Manuf. 2018, 5, 227–247. [Google Scholar] [CrossRef]
- du Plessis, A.; Yadroitsava, I.; Yadroitsev, I. Effects of Defects on Mechanical Properties in Metal Additive Manufacturing: A Review Focusing on X-ray Tomography Insights. Mater. Des. 2020, 187, 108385. [Google Scholar] [CrossRef]
- Kanko, J.A.; Sibley, A.P.; Fraser, J.M. In Situ Morphology-Based Defect Detection of Selective Laser Melting through Inline Coherent Imaging. J. Mater. Process. Technol. 2016, 231, 488–500. [Google Scholar] [CrossRef]
- Mahato, V.; Obeidi, M.A.; Brabazon, D.; Cunningham, P. An Evaluation of Classification Methods for 3D Printing Time-Series Data⁎⁎This Publication Has Resulted from Research Supported in Part by a Grant from Science Foundation Ireland (SFI) under Grant Number 16/RC/3872 and Is Co-Funded under the European Regional Development Fund. IFAC-PapersOnLine 2020, 53, 8211–8216. [Google Scholar] [CrossRef]
- Berumen, S.; Bechmann, F.; Lindner, S.; Kruth, J.-P.; Craeghs, T. Quality Control of Laser- and Powder Bed-Based Additive Manufacturing (AM) Technologies. Phys. Procedia 2010, 5, 617–622. [Google Scholar] [CrossRef]
- Mani, M.; Lane, B.; Donmez, A.; Feng, S.; Moylan, S.; Fesperman, R. Measurement Science Needs for Real-Time Control of Additive Manufacturing Powder Bed Fusion Processes; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2015; p. NIST IR 8036.
- Vlasea, M.L.; Lane, B.; Lopez, F.; Mekhontsev, S.; Donmez, A. Development of Powder Bed Fusion Additive Manufacturing Test Bed for Enhanced Real-Time Process Control. In Proceedings of the 2015 International Solid Freeform Fabrication Symposium, Austin, TX, USA, 10–12 August 2015; University of Texas at Austin: Austin, TX, USA, 2015; pp. 527–539. [Google Scholar]
- O’Regan, P.; Prickett, P.; Setchi, R.; Hankins, G.; Jones, N. Metal Based Additive Layer Manufacturing: Variations, Correlations and Process Control. Procedia Comput. Sci. 2016, 96, 216–224. [Google Scholar] [CrossRef]
- Krauss, H.; Eschey, C.; Zaeh, M.F. Thermography for Monitoring the Selective Laser Melting Process. In Proceedings of the 2012 International Solid Freeform Fabrication Symposium, Austin, TX, USA, 6–8 August 2012; pp. 999–1014. [Google Scholar]
- Körner, C.; Attar, E.; Heinl, P. Mesoscopic Simulation of Selective Beam Melting Processes. J. Mater. Process. Technol. 2011, 211, 978–987. [Google Scholar] [CrossRef]
- Islam, M.; Purtonen, T.; Piili, H.; Salminen, A.; Nyrhilä, O. Temperature Profile and Imaging Analysis of Laser Additive Manufacturing of Stainless Steel. Phys. Procedia 2013, 41, 835–842. [Google Scholar] [CrossRef]
- Razvi, S.S.; Feng, S.; Narayanan, A.; Lee, Y.-T.T.; Witherell, P. A Review of Machine Learning Applications in Additive Manufacturing. In Proceedings of the ASME 2019 International Design Engineering Technical Conferences and Computers and Information in Engineering Conference. Volume 1: 39th Computers and Information in Engineering Conference, Anaheim, CA, USA, 18–21 August 2019. V001T02A040. American Society of Mechanical Engineers Digital Collection: 2019. [Google Scholar]
- Chen, D.; Wang, P.; Pan, R.; Zha, C.; Fan, J.; Kong, S.; Li, N.; Li, J.; Zeng, Z. Research on in Situ Monitoring of Selective Laser Melting: A State of the Art Review. Int. J. Adv. Manuf. Technol. 2021, 113, 3121–3138. [Google Scholar] [CrossRef]
- Wang, R.; Cheng, M.N.; Loh, Y.M.; Wang, C.; Fai Cheung, C. Ensemble Learning with a Genetic Algorithm for Surface Roughness Prediction in Multi-Jet Polishing. Expert Syst. Appl. 2022, 207, 118024. [Google Scholar] [CrossRef]
- Seetharaman, S.; Sankaranarayanan, D.; Gupta, M. Magnesium-Based Temporary Implants: Potential, Current Status, Applications, and Challenges. J. Funct. Biomater. 2023, 14, 324. [Google Scholar] [CrossRef]
- Streza, A.; Antoniac, A.; Manescu (Paltanea), V.; Paltanea, G.; Robu, A.; Dura, H.; Verestiuc, L.; Stanica, E.; Voicu, S.I.; Antoniac, I.; et al. Effect of Filler Types on Cellulose-Acetate-Based Composite Used as Coatings for Biodegradable Magnesium Implants for Trauma. Materials 2023, 16, 554. [Google Scholar] [CrossRef]
- Antoniac, I.; Miculescu, F.; Cotrut, C.; Ficai, A.; Rau, J.V.; Grosu, E.; Antoniac, A.; Tecu, C.; Cristescu, I. Controlling the Degradation Rate of Biodegradable Mg–Zn-Mn Alloys for Orthopedic Applications by Electrophoretic Deposition of Hydroxyapatite Coating. Materials 2020, 13, 263. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.V.; Antoniac, I.; Filipescu, M.; Cotrut, C.; Fosca, M.; Nistor, L.C.; Birjega, R.; Dinescu, M. Hydroxyapatite Coatings on Mg-Ca Alloy Prepared by Pulsed Laser Deposition: Properties and Corrosion Resistance in Simulated Body Fluid. Ceram. Int. 2018, 44, 16678–16687. [Google Scholar] [CrossRef]
- Antoniac, I.V.; Filipescu, M.; Barbaro, K.; Bonciu, A.; Birjega, R.; Cotrut, C.M.; Galvano, E.; Fosca, M.; Fadeeva, I.V.; Vadalà, G.; et al. Iron Ion-Doped Tricalcium Phosphate Coatings Improve the Properties of Biodegradable Magnesium Alloys for Biomedical Implant Application. Adv. Mater. Interfaces 2020, 7, 2000531. [Google Scholar] [CrossRef]
- Dragomir, L.; Antoniac, A.; Manescu, V.; Robu, A.; Dinu, M.; Pana, I.; Cotrut, C.M.; Kamel, E.; Antoniac, I.; Rau, J.V.; et al. Preparation and Characterization of Hydroxyapatite Coating by Magnetron Sputtering on Mg–Zn–Ag Alloys for Orthopaedic Trauma Implants. Ceram. Int. 2023, 49, 26274–26288. [Google Scholar] [CrossRef]
- Antoniac, I.; Manescu, V.; Antoniac, A.; Paltanea, G. Magnesium-Based Alloys with Adapted Interfaces for Bone Implants and Tissue Engineering. Regen. Biomater. 2023, 10, rbad095. [Google Scholar] [CrossRef] [PubMed]
- Dragomir, L.; Antoniac, I.; Manescu, V.; Antoniac, A.; Miculescu, M.; Trante, O.; Streza, A.; Cotruț, C.M.; Forna, D.A. Microstructure and Corrosion Behaviour of Mg-Ca and Mg-Zn-Ag Alloys for Biodegradable Hard Tissue Implants. Crystals 2023, 13, 1213. [Google Scholar] [CrossRef]
- Qiao, S.; Wang, Y.; Zan, R.; Wu, H.; Sun, Y.; Peng, H.; Zhang, R.; Song, Y.; Ni, J.; Zhang, S.; et al. Biodegradable Mg Implants Suppress the Growth of Ovarian Tumor. ACS Biomater. Sci. Eng. 2020, 6, 1755–1763. [Google Scholar] [CrossRef]
- Yang, N.; Gong, F.; Cheng, L.; Lei, H.; Li, W.; Sun, Z.; Ni, C.; Wang, Z.; Liu, Z. Biodegradable Magnesium Alloy with Eddy Thermal Effect for Effective and Accurate Magnetic Hyperthermia Ablation of Tumors. Natl. Sci. Rev. 2021, 8, nwaa122. [Google Scholar] [CrossRef]
- Zan, R.; Ji, W.; Qiao, S.; Wu, H.; Wang, W.; Ji, T.; Yang, B.; Zhang, S.; Luo, C.; Song, Y.; et al. Biodegradable Magnesium Implants: A Potential Scaffold for Bone Tumor Patients. Sci. China Mater. 2021, 64, 1007–1020. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen Acts as a Therapeutic Antioxidant by Selectively Reducing Cytotoxic Oxygen Radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Yuan, B.; Chen, H.; Zhao, R.; Deng, X.; Chen, G.; Yang, X.; Xiao, Z.; Aurora, A.; Iulia, B.A.; Zhang, K.; et al. Construction of a Magnesium Hydroxide/Graphene Oxide/Hydroxyapatite Composite Coating on Mg–Ca–Zn–Ag Alloy to Inhibit Bacterial Infection and Promote Bone Regeneration. Bioact. Mater. 2022, 18, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Saheban, M.; Bakhsheshi-Rad, H.R.; Kasiri-Asgarani, M.; Hamzah, E.; Ismail, A.F.; Aziz, M.; Dayaghi, E. Effect of Zeolite on the Corrosion Behavior, Biocompatibility and Antibacterial Activity of Porous Magnesium/Zeolite Composite Scaffolds. Mater. Technol. 2019, 34, 258–269. [Google Scholar] [CrossRef]
- Ferreira, L.; Fonseca, A.M.; Botelho, G.; Aguiar, C.A.-; Neves, I.C. Antimicrobial Activity of Faujasite Zeolites Doped with Silver. Microporous Mesoporous Mater. 2012, 160, 126–132. [Google Scholar] [CrossRef]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver Nanoparticles: A New View on Mechanistic Aspects on Antimicrobial Activity. Nanomedicine 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Trivedi, P.; Nune, K.C.; Misra, R.D.K.; Patel, A.K.; Balani, K.; Jayganthan, R. Cellular Response of Escherichia Coli to Mg-2Zn-2Gd Alloy with Different Grain Structure: Mechanism of Disruption of Colonisation. Mater. Technol. 2016, 31, 836–844. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manescu, V.; Antoniac, I.; Antoniac, A.; Laptoiu, D.; Paltanea, G.; Ciocoiu, R.; Nemoianu, I.V.; Gruionu, L.G.; Dura, H. Bone Regeneration Induced by Patient-Adapted Mg Alloy-Based Scaffolds for Bone Defects: Present and Future Perspectives. Biomimetics 2023, 8, 618. https://doi.org/10.3390/biomimetics8080618
Manescu V, Antoniac I, Antoniac A, Laptoiu D, Paltanea G, Ciocoiu R, Nemoianu IV, Gruionu LG, Dura H. Bone Regeneration Induced by Patient-Adapted Mg Alloy-Based Scaffolds for Bone Defects: Present and Future Perspectives. Biomimetics. 2023; 8(8):618. https://doi.org/10.3390/biomimetics8080618
Chicago/Turabian StyleManescu (Paltanea), Veronica, Iulian Antoniac, Aurora Antoniac, Dan Laptoiu, Gheorghe Paltanea, Robert Ciocoiu, Iosif Vasile Nemoianu, Lucian Gheorghe Gruionu, and Horatiu Dura. 2023. "Bone Regeneration Induced by Patient-Adapted Mg Alloy-Based Scaffolds for Bone Defects: Present and Future Perspectives" Biomimetics 8, no. 8: 618. https://doi.org/10.3390/biomimetics8080618
APA StyleManescu, V., Antoniac, I., Antoniac, A., Laptoiu, D., Paltanea, G., Ciocoiu, R., Nemoianu, I. V., Gruionu, L. G., & Dura, H. (2023). Bone Regeneration Induced by Patient-Adapted Mg Alloy-Based Scaffolds for Bone Defects: Present and Future Perspectives. Biomimetics, 8(8), 618. https://doi.org/10.3390/biomimetics8080618




.png)






