Mechanical Resistance of the Largest Denticle on the Movable Claw of the Mud Crab
Abstract
:1. Introduction
2. Materials and Methods
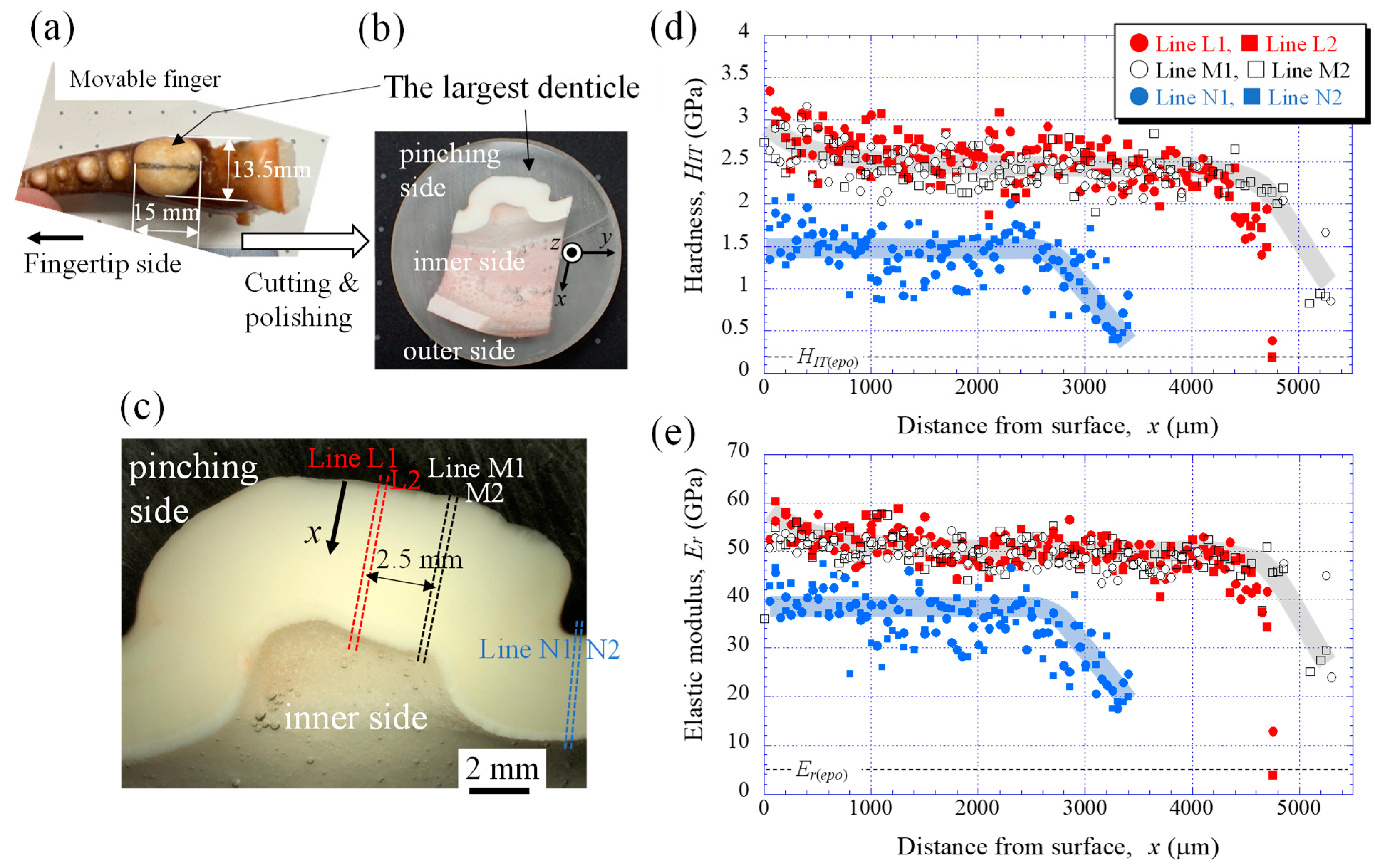
2.1. Sample Preparation
2.2. Nanoindentation Tests
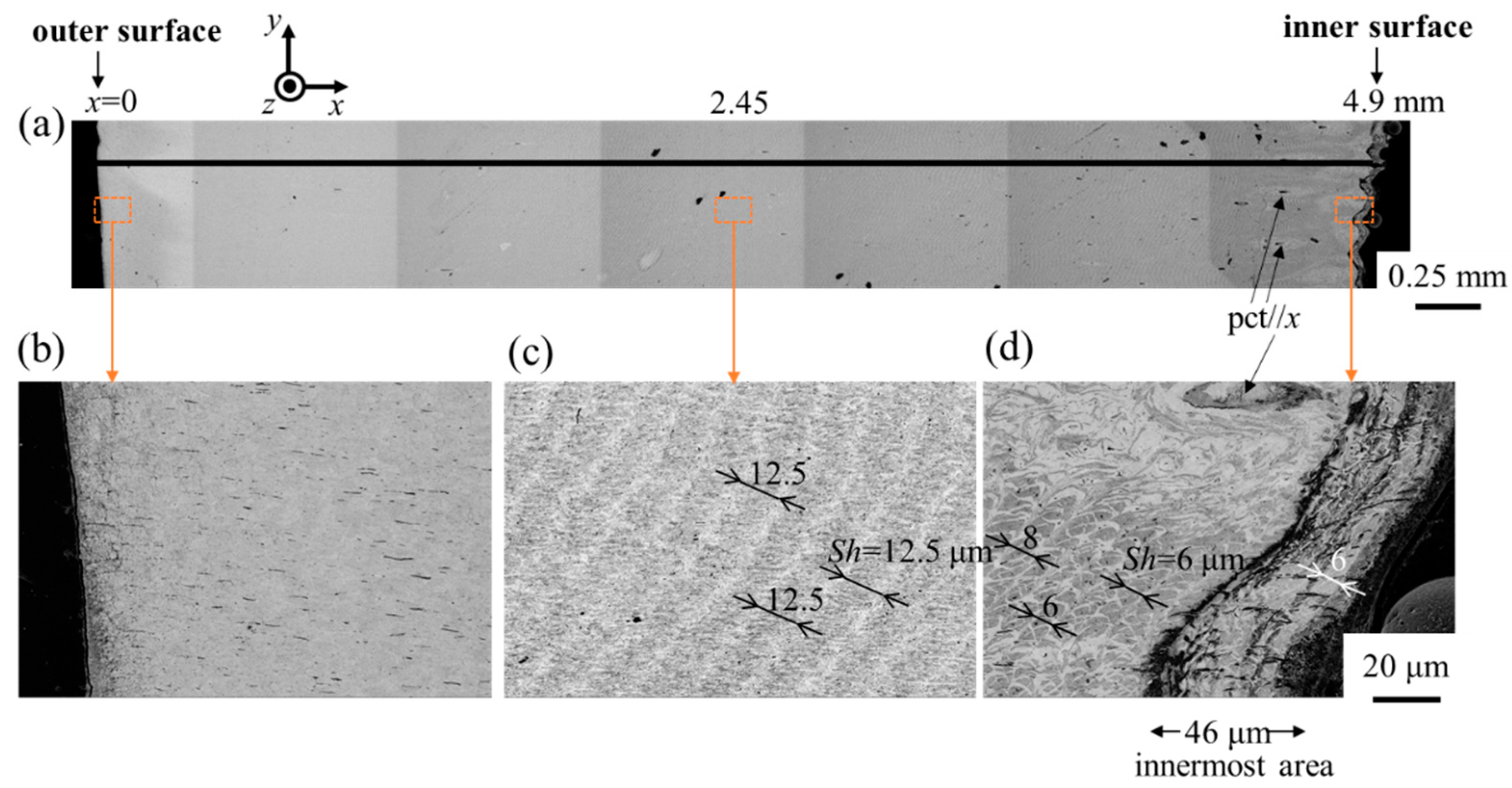
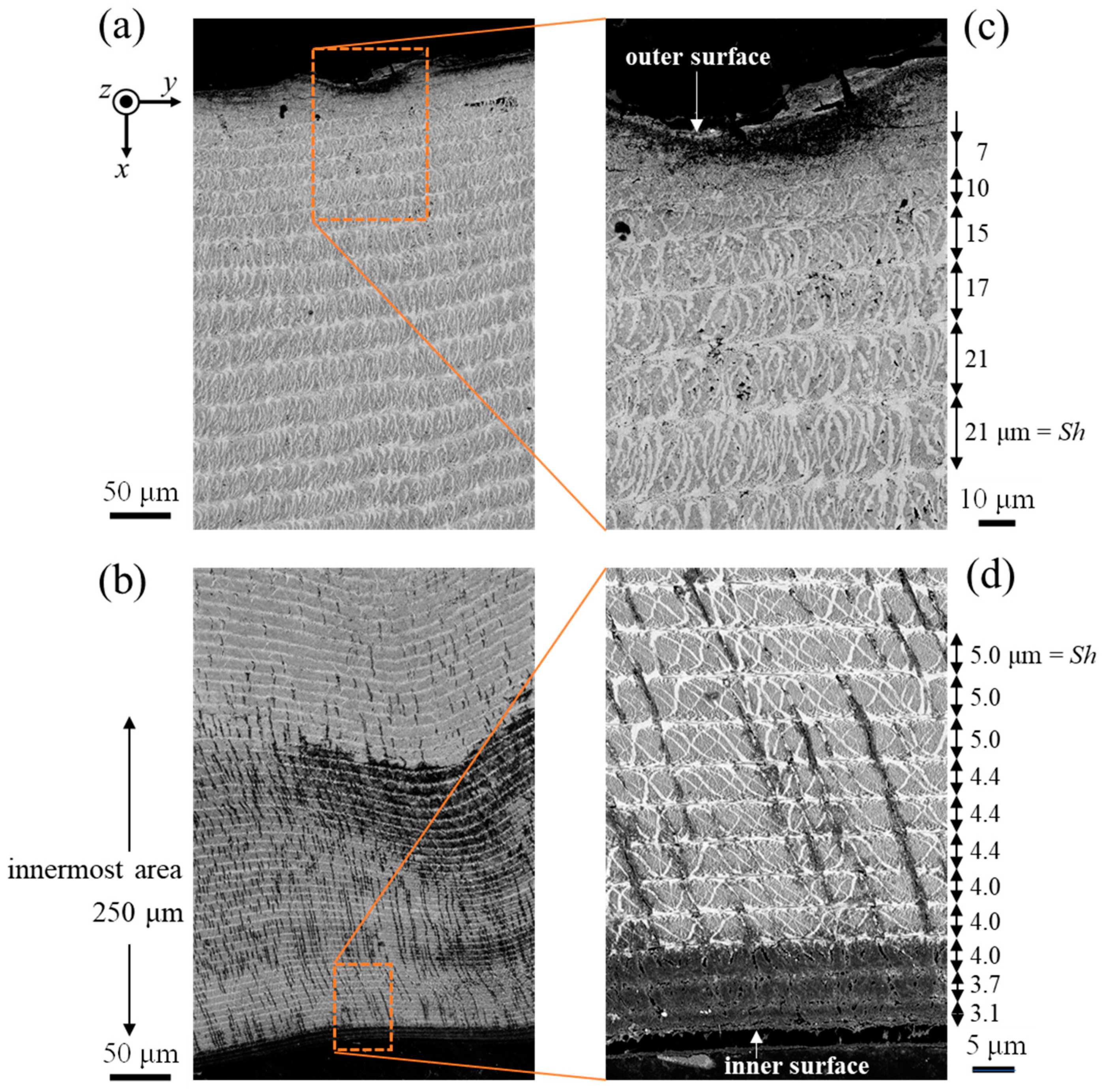
2.3. Microstructure Observation
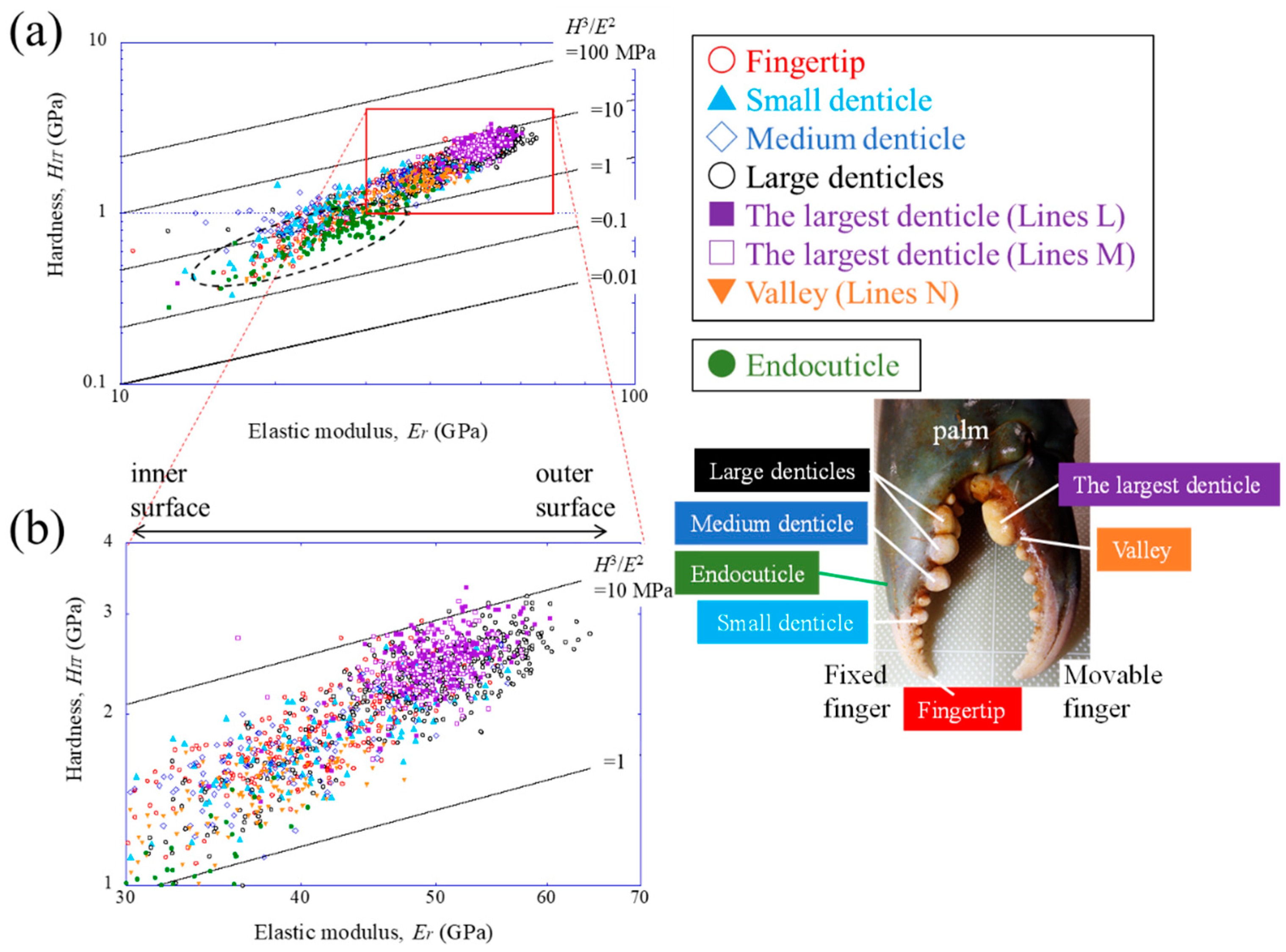
3. Results
3.1. Mechanical Properties
3.2. Microstructure and Elemental Composition
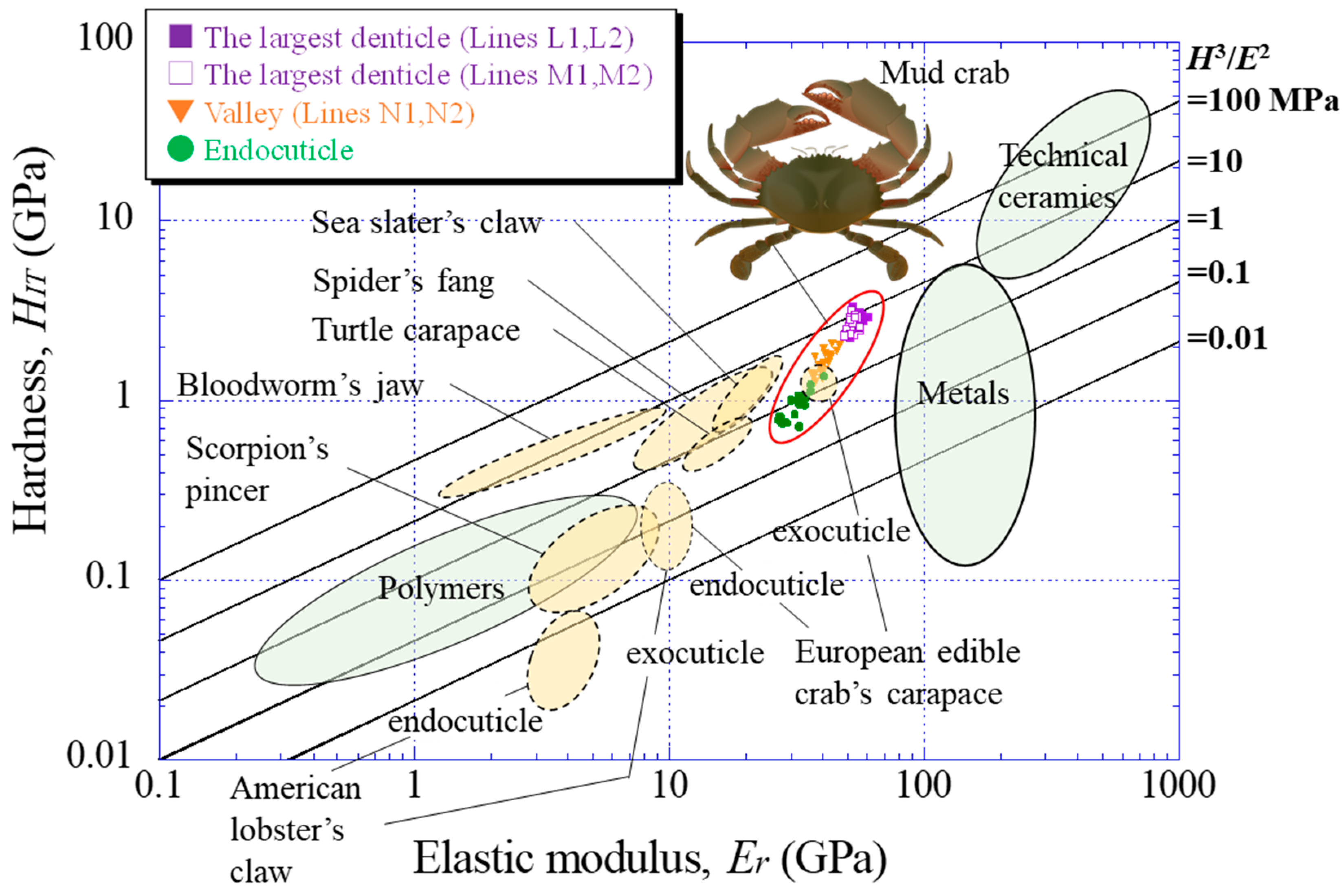
4. Discussion
4.1. Cuticle Change on the Pinching Side
4.2. Hardness (HIT)–Elastic Modulus (Er) Map
4.3. Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouligand, Y. Twisted fibrous arrangements in biological materials and cholesteric mesophases. Tissue Cell 1972, 4, 189–217. [Google Scholar] [CrossRef] [PubMed]
- Collado, A.G.; Blanco, J.M.; Gupta, M.K.; Vicentea, R.D. Advances in polymers based multi-material additive-manufacturing techniques: State-of-art review on properties and applications. Addit. Manuf. 2022, 50, 102577. [Google Scholar] [CrossRef]
- Li, X.; He, J.; Zhang, W.; Jiang, N.; Li, D. Additive manufacturing of biomedical constructs with biomimetic structural organizations. Materials 2016, 9, 909. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Song, Z.; Zhang, S.; Ni, Y.; Cai, S.; Gong, X.; He, L.; Yu, S. Discontinuous fibrous Bouligand architecture enabling formidable fracture resistance with crack orientation insensitivity. Proc. Natl. Acad. Sci. USA 2020, 117, 15465–15472. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.K.; Hazell, P.J.; Escobedo, J.P.; Wang, H. Biomimetic armour design strategies for additive manufacturing: A review. Mater. Des. 2021, 205, 109730. [Google Scholar] [CrossRef]
- Amorim, L.; Santos, A.; Nunes, J.P.; Viana, J.C. Bioinspired approaches for toughening of fibre reinforced polymer composites. Mater. Des. 2021, 199, 109336. [Google Scholar] [CrossRef]
- Tiismus, H.; Kallaste, A.; Vaimann, T.; Rassõlkin, A. State of the art of additively manufactured electromagnetic materials for topology optimized electrical machines. Addit. Manuf. 2022, 55, 102778. [Google Scholar] [CrossRef]
- Yao, H.; Zheng, G.; Li, W.; McDowell, M.T.; Seh, Z.; Liu, N.; Lu, Z.; Cui, Y. Crab shells as sustainable templates from nature for nanostructured battery electrodes. Nano Lett. 2013, 13, 3385–3390. [Google Scholar] [CrossRef]
- Fabritius, H.-O.; Karsten, E.S.; Balasundaram, K.; Hild, S.; Huemer, K.; Raabe, D. Correlation of structure, composition and local mechanical properties in the dorsal carapace of the edible crab Cancer pagurus. Z. Für Krist.-Cryst. Mater. 2012, 227, 766–776. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, C.; Dai, N.; Shen, J.; Guan, J. Structural characterization and regulation of the mechanical properties of the carapace cuticle in tri-spine horseshoe crab (Tachypleus tridentatus). J. Mecha. Behav. Biomed. Mater. 2022, 125, 104954. [Google Scholar] [CrossRef]
- Politi, Y.; Priewasser, M.; Pippel, E.; Zaslansky, P.; Hartmann, J.; Siegel, S.; Li, C.; Barth, F.G.; Fratzl, P. A Spider’s Fang: How to design an injection needle using chitin-based composite material. Adv. Funct. Mater. 2012, 22, 2519–2528. [Google Scholar] [CrossRef]
- Inoue, T.; Oka, S.; Hara, T. Three-dimensional microstructure of robust claw of coconut crab, one of the largest terrestrial crustaceans. Mater. Des. 2021, 206, 109765. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Shu, T.; Feng, X.Q. Study of biomechanical, anatomical, and physiological properties of scorpion stingers for developing biomimetic materials. Mater. Sci. Eng. C 2016, 58, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Han, Q.; Zhang, J.; Han, Z.; Niu, S.; Ren, L. Advanced bio-inspired structural materials: Local properties determine overall performance. Mater. Today 2020, 41, 177–199. [Google Scholar] [CrossRef]
- Natarajan, B.; Gilman, J.W. Bioinspired Bouligand cellulose nanocrystal composites: A review of mechanical properties. Phil. Trans. R. Soc. A 2017, 376, 20170050. [Google Scholar] [CrossRef] [PubMed]
- Sachs, C.; Fabritius, H.; Raabe, D. Experimental investigation of the elastic–plastic deformation of mineralized lobster cuticle by digital image correlation. J. Struct. Biol. 2006, 155, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Zeng, H.; Luo, H.; Wu, X. Construction of Wearable Touch Sensors by Mimicking the Properties of Materials and Structures in Nature. Biomimetics 2023, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Thomas, A.; Glancey, J.L.; Karlsson, A.M. Mechanical behavior of bio-inspired laminated composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 211–220. [Google Scholar] [CrossRef]
- Pan, L.; Wang, F.; Cheng, Y.; Leow, W.R.; Zhang, Y.; Wang, M.; Cai, P.; Ji, B.; Li, D.; Chen, X. A supertough electro-tendon based on spider silk composites. Nat. Commun. 2020, 11, 1332. [Google Scholar] [CrossRef]
- Alexander, R.M. The maximum forces exerted by animals. J. Exp. Biol. 1985, 115, 231–238. Available online: https://jeb.biologists.org/content/jexbio/115/1/231.full.pdf (accessed on 4 September 2020). [CrossRef]
- Huber, D.R.; Eason, T.G.; Hueter, R.E.; Motta, P.J. Analysis of the bite force and mechanical design of the feeding mechanism of the durophagous horn shark Heterodontus francisci. J. Exp. Biol. 2005, 208, 3553–3571. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.M. Maximum force production: Why are crabs so strong? Proc. Biol. Sci. 2000, 267, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Hara, T.; Nakazato, K.; Oka, S. Superior mechanical resistance in the exoskeleton of the coconut crab, Birgus latro. Mater. Today Bio 2021, 12, 100132. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Li, J.; Qiu, F. Microstructure and mechanical properties of the dactylopodites of the chinese mitten crab (Eriocheir sinensis). Appl. Sci. 2018, 8, 674. [Google Scholar] [CrossRef]
- Sayekti, P.R.; Fahrunnida, F.; Cerniauskas, G.; Robert, C.; Retnoaji, B.; Alam, P. The impact behaviour of crab carapaces in relation to morphology. Materials 2020, 13, 3994. [Google Scholar] [CrossRef] [PubMed]
- Vittori, M.; Srot, V.; Korat, L.; Rejec, M.; Sedmak, P.; Bussmann, B.; Predel, F.; Aken, P.A.; Štrus, J. The mechanical consequences of the interplay of mineral distribution and organic matrix orientation in the claws of the sea slater Ligia pallasii. Minerals 2021, 11, 1373. [Google Scholar] [CrossRef]
- Inoue, T.; Oka, S.; Nakazato, K.; Hara, T. Structural changes and mechanical resistance of claws and denticles in coconut crabs of different sizes. Biology 2021, 10, 1304. [Google Scholar] [CrossRef] [PubMed]
- Waugh, D.A.; Feldmann, R.M.; Schroeder, A.M.; Mutel, M.H. Differential cuticle architecture and its preservation in fossil and extant Callinectes and Scylla claws. J. Crustac. Biol. 2006, 26, 271–282. [Google Scholar] [CrossRef]
- Rosen, M.N.; Baran, K.A.; Sison, J.N.; Steffel, B.V.; Long, W.C.; Foy, R.J.; Smith, K.E.; Aronson, R.B.; Dickinson, G.H. Mechanical resistance in decapod claw denticles: Contribution of structure and composition. Acta Biomater. 2010, 110, 196–207. [Google Scholar] [CrossRef]
- Alberts, H.H.; Lee, S.Y.; Meynecke, J.-O.; Diele, K.; Nordhaus, I.; Wolff, M. Life-history, movement, and habitat use of Scylla serrata (decapoda, portunidae): Current knowledge and future challenges. Hydrobiologia 2015, 763, 5–21. [Google Scholar] [CrossRef]
- Fisheries Fact Sheet (Mud Crab). 2013. Available online: https://www.fish.wa.gov.au/Documents/recreational_fishing/fact_sheets/fact_sheet_mud_crab.pdf (accessed on 25 September 2023).
- Khaksari, H.; Safaie, M.; Salarzadeh, A. Population dynamics and reproductive biology of Scylla serrata (Forskal, 1775) on the shores overlooking the mangrove forest of the Persian Gulf. Reg. Stud. Mar. Sci. 2023, 57, 102758. [Google Scholar] [CrossRef]
- Inoue, T.; Hiroto, T.; Hara, Y.; Nakazato, K.; Oka, S. Tissue structure and mechanical properties of the exoskeleton of the huge claws of the mud crab. Scylla serrata. J. Mater. Sci. 2023, 58, 1099–1115. [Google Scholar] [CrossRef]
- Yoshihama, T. (Irabu Island, Okinawa, Japan). Personal communication. 2022.
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Moses, D.N.; Mattoni, M.A.; Slack, N.L.; Waite, J.H.; Zok, F.W. Role of melanin in mechanical properties of Glycera jaws. Acta Biomater. 2006, 2, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.C.; Milliron, G.W.; Miserez, A.; Lutterodt, K.E.; Herrera, S.; Gallana, I.; Mershon, W.J.; Swanson, B.; Zavattieri, P.; DiMasi, E.; et al. The stomatopod dactyl club: A formidable damage-tolerant biological hammer. Science 2012, 336, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Hara, Y.; Nakazato, K. Mechanical resistance and tissue structure of claw denticles of various sizes in the mud crab, Scylla serrata. Materials 2023, 16, 4114. [Google Scholar] [CrossRef] [PubMed]
- Zok, F.W.; Miserez, A. Property maps for abrasion resistance of materials. Acta Mater. 2007, 55, 6365–6371. [Google Scholar] [CrossRef]
- Raabe, D.; Sachs, C.; Romano, P. The crustacean exoskeleton as an example of a structurally and mechanically graded biological nanocomposite material. Acta Mater. 2005, 53, 4281–4292. [Google Scholar] [CrossRef]
- Kellersztein, I.; Cohen, S.R.; Bar-On, B.; Wagner, H.D. The exoskeleton of scorpions’ pincers: Structure and micro-mechanical properties. Acta Biomater. 2019, 94, 565–573. [Google Scholar] [CrossRef]
- Achrai, B.; Wagner, H.D. Micro-structure and mechanical properties of the turtle carapace as a biological composite shield. Acta Biomater. 2013, 9, 5890–5902. [Google Scholar] [CrossRef]
- Inoue, T.; Yin, F.; Kimura, Y.; Tsuzaki, K.; Ochiai, S. Delamination effect on impact properties of ultrafine-grained low-carbon steel processed by warm caliber rolling. Metall. Mater. Trans. A 2010, 41, 341–355. [Google Scholar] [CrossRef]
- Kum, D.W.; Oyama, T.; Wadsworth, J.; Sherby, O.D. The impact properties of laminated composites containing ultrahigh carbon (UHC) steels. J. Mech. Phys. 1983, 31, 173–186. [Google Scholar] [CrossRef]
- Pozuelo, M.; Carreno, F.; Ruano, O.A. Delamination effect on the impact toughness of an ultrahigh carbon–mild steel laminate composite. Compos. Sci. Technol. 2006, 66, 2671–2676. [Google Scholar] [CrossRef]
- Ochiai, S.; Osamura, K. Influences of interfacial bonding strength and scatter of fibre strength on tensile behaviour of unidirectional metal matrix composites. J. Mater. Sci. 1988, 23, 886–893. [Google Scholar] [CrossRef]
- Inoue, T.; Kimura, Y. Effect of delamination and grain refinement on fracture energy of ultrafine-grained steel determined using an instrumented Charpy impact test. Materials 2022, 15, 867. [Google Scholar] [CrossRef] [PubMed]

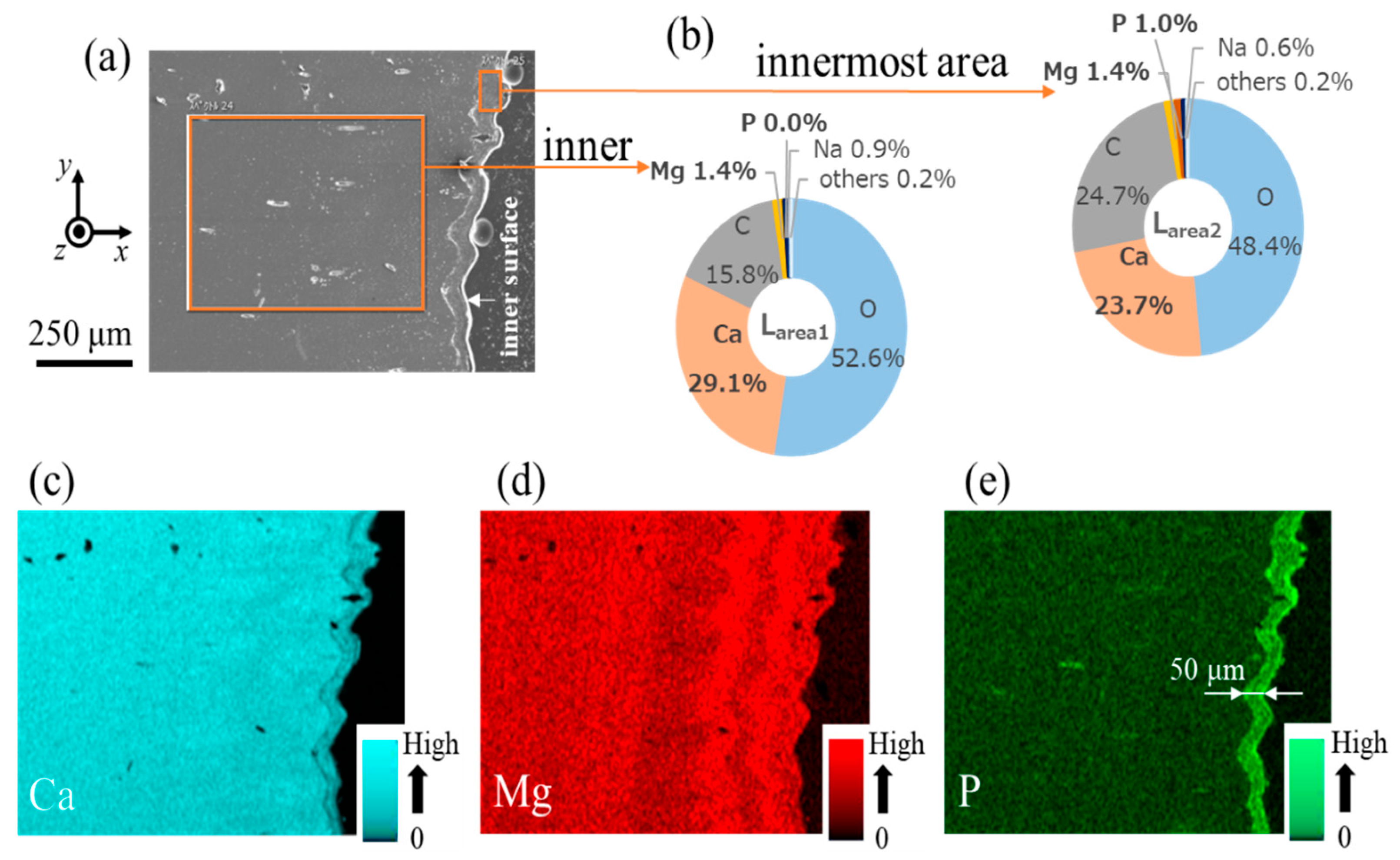


| Area | Site | Ca | Mg | P | C | O | Na | Cl | S |
|---|---|---|---|---|---|---|---|---|---|
| Line L1 (peak) | Inner (denticle) Innermost (endocuticle) | 29.1 23.7 | 1.4 1.4 | 0.0 1.0 | 15.8 24.7 | 52.6 48.4 | 0.9 0.6 | 0.0 0.0 | 0.1 0.2 |
| Line N1 (valley) | Inner (denticle) Innermost (endocuticle) | 26.7 24.5 | 1.9 2.2 | 0.0 0.8 | 18.4 20.7 | 52.1 50.9 | 0.8 0.8 | 0.0 0.0 | 0.1 0.1 |
| Fingertip | Surface Innermost (endocuticle) | 30.3 24.9 | 0.9 2.2 | 0.0 0.7 | 16.2 20.9 | 51.7 50.4 | 0.9 0.8 | 0.0 0.1 | 0.0 0.1 |
| Large denticle on fixed finger | Inner (denticle) Innermost (endocuticle) | 29.8 25.5 | 1.4 2.3 | 0.0 0.6 | 15.6 22.1 | 52.1 48.6 | 1.0 0.7 | 0.1 0.2 | 0.0 0.1 |
| Claw outer side | Endocuticle Endocuticle | 24.8 23.5 | 2.0 1.9 | 0.8 0.7 | 20.8 21.4 | 50.6 51.3 | 0.8 0.8 | 0.1 0.2 | 0.1 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, T.; Hara, Y.; Nakazato, K. Mechanical Resistance of the Largest Denticle on the Movable Claw of the Mud Crab. Biomimetics 2023, 8, 602. https://doi.org/10.3390/biomimetics8080602
Inoue T, Hara Y, Nakazato K. Mechanical Resistance of the Largest Denticle on the Movable Claw of the Mud Crab. Biomimetics. 2023; 8(8):602. https://doi.org/10.3390/biomimetics8080602
Chicago/Turabian StyleInoue, Tadanobu, Yuka Hara, and Koji Nakazato. 2023. "Mechanical Resistance of the Largest Denticle on the Movable Claw of the Mud Crab" Biomimetics 8, no. 8: 602. https://doi.org/10.3390/biomimetics8080602
APA StyleInoue, T., Hara, Y., & Nakazato, K. (2023). Mechanical Resistance of the Largest Denticle on the Movable Claw of the Mud Crab. Biomimetics, 8(8), 602. https://doi.org/10.3390/biomimetics8080602






