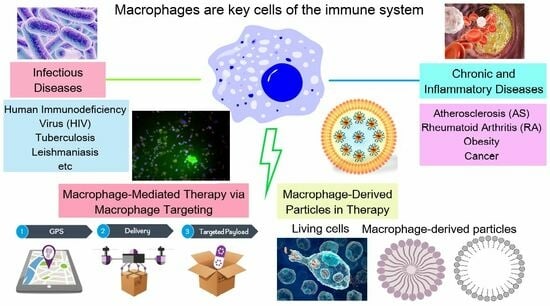
Biomimetic Systems Involving Macrophages and Their Potential for Targeted Drug Delivery
Abstract
:1. Introduction
2. Macrophages as Immune System Cells
2.1. Two Macrophage Phenotypes
| M1 | M2a | M2b | M2c | |
|---|---|---|---|---|
| Activation factors | IFN-γ, LPS, TNF-α | IL-4, IL-13 | Immune complexes, LPS, IL-1β | IL-10, TGF-β1 |
| Surface markers | CD80, CD86, TLR2, TLR4, MHC-II | CD163, CD206, MHC-II | CD86, MHC-II | CCR2, CD163, TLR1, TLR8 |
| Secreted cytokines | IL-1, IL-6, IL-12, IL-23, TNF-α | IL-10, IL-1Ra, TGF-β | IL-1, IL-6, IL-10, TNF-α | IL-10, TGF-β |
| Functions | Inflammatory response, phagocytosis of debris/cells, antigen presentation, stimulation of vascular sprouting | Inflammatory response, matrix deposition, fibrosis, angiogenesis | Immune regulation, T helper 2 activation | Matrix remodeling, fibrolysis, angiogenesis, phagocytosis |
| References | [49] | [50,51] | [52] | [53,54] |
2.2. Role of Macrophages in Chronic and Inflammatory Diseases
2.2.1. Atherosclerosis (AS)
2.2.2. Rheumatoid Arthritis (RA)
2.2.3. Obesity
2.2.4. Cancer
2.3. Role of Macrophages in Infectious Diseases
2.3.1. Human Immunodeficiency Virus (HIV)
2.3.2. Tuberculosis
2.3.3. Leishmaniasis
3. Application of Macrophage-Derived Particles in Therapy
3.1. Ex Vivo Preparation of Macrophage-Derived Carriers of Therapeutic Agents
3.1.1. Sources of Macrophages
3.1.2. Obtaining of Macrophage-Derived Carriers
Using of Living Cells (Figure 3)
- Encapsulation of drugs in macrophages via incubation
- b.
- Encapsulation of drugs in macrophages using hypotonic/resealing method
- c.
- Encapsulation of drugs in macrophage cell membranes using the electroporation/resealing method
- d.
- Adhesion of therapeutic particles to the macrophage membrane (cellular backpacks)
Encapsulation of Drugs in Macrophage-Derived Membrane Structures
- Encapsulation inside macrophage cellular membranes
- b.
- Encapsulation inside macrophage-derived vesicles
3.2. Macrophage-Derived Membranes (or Particles) as Anti-Inflammatory Agents
3.3. Macrophage-Derived Membranes (or Particles) as Anti-Tumor Agents
- Therapeutic effect obtained from macrophages
- b.
- Therapeutic effect due to drug-loaded nanoparticles inside macrophages
- c.
- Therapeutic effect due to surface engineering of macrophages
- d.
- Therapeutic effect due to bioengineered species
- e.
- Photothermal therapy
3.4. Macrophage-Derived Membranes (or Particles) for the Treatment of Infectious Diseases
- Treatment of viral infections
- b.
- Wound healing and treatment of bacterial infections
4. Macrophage-Mediated Therapy via Macrophage Targeting
4.1. Design of Therapeutic Agents Targeting Macrophages
4.1.1. Passive Macrophage-Targeting Therapeutic Agents
- Size
- b.
- Shape
- c.
- Surface charge and hydrophilicity
4.1.2. Active Macrophage-Targeting Therapeutic Agents
- Toll-like receptor targeting
- b.
- Scavenger receptor targeting
- c.
- Fc-receptor targeting
- d.
- Targeting of other receptors
4.2. Macrophage Targeting in Anti-Inflammation Therapy
4.3. Macrophage Targeting in Anti-Tumor Therapy
- Inhibition of macrophage recruitment
- b.
- Targeting Anti-Phagocytic Checkpoints
- c.
- TAM depletion
- d.
- Reprogramming of TAMs
4.4. Macrophage-Targeting in the Therapy of Infectious Diseases
- Viral infectious diseases
- b.
- Tuberculosis
- c.
- Protozoan infectious diseases
4.5. Potency of Macrophage Targeting via CD206 Receptor
5. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| Abbreviation | Definition |
| TAM | Tumor-Associated Macrophage |
| TME | Tumor Microenvironment |
| AS | Atherosclerosis |
| RA | Rheumatoid Arthritis |
| AT | Adipose Tissue |
| EGF | Epidermal Growth Factor |
| FGF | Fibroblast Growth Factor |
| HGF | Hepatocyte Growth Factor |
| MITF | Microphthalmia-associated Transcription Factor |
| HIV | Human Immunodeficiency Virus |
| AIDS | Acquired Immunodeficiency Syndrome |
| Mtb | Mycobacterium Tuberculosis |
| TB | Tuberculosis |
| ALL | Acute Lymphoblastic Leukemia |
| PRR | Pattern Recognition Receptor |
| TLR | Toll-Like Receptor |
| SR | Scavenger Receptor |
| FcR | Fc-Receptor |
| PAMP | Pathogen-Associated Molecular Pattern |
| DAMP | Damage-associated Molecular Pattern |
| LPS | Lipopolysaccharide |
| ROS | Reactive Oxygen Species |
| SA | Sialic Acid |
| NP | Nanoparticle |
| NR | Nanorod |
| SLN | Solid Lipid Nanoparticle |
| NDLs | Nano-Deformable Liposomes |
| PEG | Polyethylene Glycol |
| PLGA | Poly(D,L-Lactide-co-Glycolide Acid) |
| DPPC | Dipalmitoylphosphatidylcholine |
| DPPE | 1,2-Bis(diphenylphosphino) ethane |
| DPPG | Dipalmitoyl Phosphatidylglycerol |
| JAC | Lectin Jacalin |
| PAH | Poly(allylamine hydrochloride) |
| CAT | Catalase bovine liver |
| PAA | Poly(acrylic aid) |
| PNIPAAM | Poly(N-isopropylacrylamide) |
| PPiP | 2- aminoethyldiisopropyl |
| BMM | Bone Marrow-Derived Macrophages |
| siRNA | Small Interfering RNA |
| MPS | Mononuclear Phagocyte System |
| DOX | Doxorubicin |
| PTX | Paclitaxel |
| TPZ | Tirapazamine |
| EPI | Epirubicin |
| ZA | Zoledronic acid |
| INH | Isoniazid |
| SSG | Sodium Stibogluconate |
| AmB | Amphotericin B |
| PM | Paromomycin |
| EPR | Enhanced Permeability and Retention |
References
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Cagno, V.; Andreozzi, P.; D’Alicarnasso, M.; Silva, P.J.; Mueller, M.; Galloux, M.; Le Goffic, R.; Jones, S.T.; Vallino, M.; Hodek, J.; et al. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nature Mater. 2018, 17, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, K.M.; Macparland, S.A.; Ma, X.Z.; Spetzler, V.N.; Echeverri, J.; Ouyang, B.; Fadel, S.M.; Sykes, E.A.; Goldaracena, N.; Kaths, J.M.; et al. Mechanism of Hard-Nanomaterial Clearance by the Liver. Nat. Mater. 2016, 15, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage Biology in Development, Homeostasis and Disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage Plasticity, Polarization, and Function in Health and Disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Epelman, S.; Lavine, K.J.; Randolph, G.J. Origin and Functions of Tissue Macrophages. Immunity 2014, 41, 21–35. [Google Scholar] [CrossRef]
- Gautiar, E.L.; Shay, T.; Miller, J.; Greter, M.; Jakubzick, C.; Ivanov, S.; Helft, J.; Chow, A.; Elpek, K.G.; Gordonov, S.; et al. Gene-Expression Profiles and Transcriptional Regulatory Pathways That Underlie the Identity and Diversity of Mouse Tissue Macrophages. Nat. Immunol. 2012, 13, 1118–1128. [Google Scholar] [CrossRef]
- Hoeffel, G.; Ginhoux, F. Fetal Monocytes and the Origins of Tissue-Resident Macrophages. Cell. Immunol. 2018, 330, 5–15. [Google Scholar] [CrossRef]
- Wu, Y.; Hirschi, K.K. Tissue-Resident Macrophage Development and Function. Front. Cell Dev. Biol. 2021, 8, 617879. [Google Scholar] [CrossRef]
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef]
- Tacke, F. Monozyten-Subpopulationen in Entzündungsprozessen: Prinzip Und Perspektive. Dtsch. Med. Wochenschr. 2009, 134, 1645–1648. [Google Scholar] [CrossRef] [PubMed]
- Auffray, C.; Sieweke, M.H.; Geissmann, F. Blood Monocytes: Development, Heterogeneity, and Relationship with Dendritic Cells. Annu. Rev. Immunol. 2009, 27, 669–692. [Google Scholar] [CrossRef] [PubMed]
- Randolph, G.J. Immunology: No Need to Coax Monocytes. Science 2011, 332, 1268–1269. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Plüddemann, A. Tissue Macrophages: Heterogeneity and Functions. BMC Biol. 2017, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, L.; Cassol, E.; Poli, G. Macrophage Polarization in Health and Disease. Sci. World J. 2011, 11, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Stout, R.D.; Jiang, C.; Matta, B.; Tietzel, I.; Watkins, S.K.; Suttles, J. Macrophages Sequentially Change Their Functional Phenotype in Response to Changes in Microenvironmental Influences. J. Immunol. 2005, 175, 342–349. [Google Scholar] [CrossRef]
- Mosser, D.; Edwards, J. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative Activation of Macrophages: An Immunologic Functional Perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage Plasticity and Interaction with Lymphocyte Subsets: Cancer as a Paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Gordon, S. The Macrophage: Past, Present and Future. Eur. J. Immunol. 2007, 37, S9–S17. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 Polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Klose, C.S.N.; Artis, D. Innate Lymphoid Cells Control Signaling Circuits to Regulate Tissue-Specific Immunity. Cell Res. 2020, 30, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mosser, D.M. Macrophage Activation by Endogenous Danger Signals. J. Pathol. 2008, 214, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O. Regulators of Macrophage Activation. Eur. J. Immunol. 2011, 41, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Nakane, A.; Minagawa, T.; Kasai, N.; Yamamoto, K.-I.; Sato, N.; Tsuruoka, N. Human Tumor Necrosis Factor Increases the Resistance against Listeria Infection in Mice. Med. Microbiol. Immunol. 1989, 178, 337–346. [Google Scholar] [CrossRef]
- Hutchings, M.I.; Palmer, T.; Harrington, D.J.; Sutcliffe, I.C. Lipoprotein Biogenesis in Gram-Positive Bacteria: Knowing When to Hold ’em, Knowing When to Fold ’Em. Trends Microbiol. 2009, 17, 13–21. [Google Scholar] [CrossRef]
- Rumbo, M.; Nempont, C.; Kraehenbuhl, J.P.; Sirard, J.C. Mucosal Interplay among Commensal and Pathogenic Bacteria: Lessons from Flagellin and Toll-like Receptor 5. FEBS Lett. 2006, 580, 2976–2984. [Google Scholar] [CrossRef]
- Xue, Q.; Lu, Y.; Eisele, M.R.; Sulistijo, E.S.; Khan, N.; Fan, R.; Miller-Jensen, K. Analysis of Single-Cell Cytokine Secretion Reveals a Role for Paracrine Signaling in Coordinating Macrophage Responses to TLR4 Stimulation. Sci. Signal. 2015, 8, ra59. [Google Scholar] [CrossRef]
- Fieren, M.W.J.A. The Local Inflammatory Responses to Infection of the Peritoneal Cavity in Humans: Their Regulation by Cytokines, Macrophages, and Other Leukocytes. Mediat. Inflamm. 2012, 2012, 976241. [Google Scholar] [CrossRef]
- Wang, J.; Nikrad, M.P.; Travanty, E.A.; Zhou, B.; Phang, T.; Gao, B.; Alford, T.; Ito, Y.; Nahreini, P.; Hartshorn, K.; et al. Innate Immune Response of Human Alveolar Macrophages during Influenza a Infection. PLoS ONE 2012, 7, e29879. [Google Scholar] [CrossRef]
- Fingleton, B. Matrix Metalloproteinases as Regulators of Inflammatory Processes. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Lagente, V.; Le Quement, C.; Boichot, E. Macrophage Metalloelastase (MMP-12) as a Target for Inflammatory Respiratory Diseases. Expert Opin. Ther. Targets 2009, 13, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Van Lint, P.; Libert, C. Chemokine and Cytokine Processing by Matrix Metalloproteinases and Its Effect on Leukocyte Migration and Inflammation. J. Leukoc. Biol. 2007, 82, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Nénan, S.; Boichot, E.; Lagente, V.; Bertrand, C.P. Macrophage Elastase (MMP-12): A pro-Inflammatory Mediator? Mem. Inst. Oswaldo Cruz 2005, 100, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Alternative Activation of Macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Munitz, A.; Brandt, E.B.; Mingler, M.; Finkelman, F.D.; Rothenberg, M.E.; Austen, K.F. Distinct Roles for IL-13 and IL-4 via IL-13 Receptor 1 and the Type II IL-4 Receptor in Asthma Pathogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 7240–7245. [Google Scholar] [CrossRef]
- Villalta, S.A.; Nguyen, H.X.; Deng, B.; Gotoh, T.; Tidbal, J.G. Shifts in Macrophage Phenotypes and Macrophage Competition for Arginine Metabolism Affect the Severity of Muscle Pathology in Muscular Dystrophy. Hum. Mol. Genet. 2009, 18, 482–496. [Google Scholar] [CrossRef]
- Laskin, D.L.; Sunil, V.R.; Gardner, C.R.; Laskin, J.D. Macrophages and Tissue Injury: Agents of Defense or Destruction? Annu. Rev. Pharmacol. Toxicol. 2011, 51, 267–288. [Google Scholar] [CrossRef]
- Clouthier, D.E.; Comerford, S.A.; Hammer, R.E. Hepatic Fibrosis, Glomerulosclerosis, and a Lipodystrophy-like Syndrome in PEPCK-TGF-Β1 Transgenic Mice. J. Clin. Investig. 1997, 100, 2697–2713. [Google Scholar] [CrossRef]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage Polarization: Tumor-Associated Macrophages as a Paradigm for Polarized M2 Mononuclear Phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Gerber, J.S.; Mosser, D.M. Reversing Lipopolysaccharide Toxicity by Ligating the Macrophage Fcγ Receptors. J. Immunol. 2001, 166, 6861–6868. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The Chemokine System in Diverse Forms of Macrophage Activation and Polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Rantakari, P.; Patten, D.A.; Valtonen, J.; Karikoski, M.; Gerke, H.; Dawes, H.; Laurila, J.; Ohlmeier, S.; Elima, K.; Hübscher, S.G.; et al. Stabilin-1 Expression Defines a Subset of Macrophages That Mediate Tissue Homeostasis and Prevent Fibrosis in Chronic Liver Injury. Proc. Natl. Acad. Sci. USA 2016, 113, 9298–9303. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.Z.; Pollard, J.W. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef]
- Anderson, C.F.; Mosser, D.M. Cutting Edge: Biasing Immune Responses by Directing Antigen to Macrophage Fcγ Receptors. J. Immunol. 2002, 168, 3697–3701. [Google Scholar] [CrossRef]
- Pierce, G.F.; Mustoe, T.A.; Lingelbach, J.; Masakowski, V.R.; Griffin, G.L.; Senior, R.M.; Deuel, T.F. Plateletoderived Growth Factor and Transforming Growth Factor-Beta Enhance Tissue Repair Activities by Unique Mechanisms. J. Cell Biol. 1989, 109, 429–440. [Google Scholar] [CrossRef]
- Song, E.; Ouyang, N.; Hörbelt, M.; Antus, B.; Wang, M.; Exton, M.S. Influence of Alternatively and Classically Activated Macrophages on Fibrogenic Activities of Human Fibroblasts. Cell. Immunol. 2000, 204, 19–28. [Google Scholar] [CrossRef]
- Koch, A.E.; Kunkel, S.L.; Chensue, S.W.; Haines, G.K.; Strieter, R.M. Expression of Interleukin-I and Interleukin-I Receptor Antagonist by Human Rheumatoid Synovial Tissue Macrophages. Clin. Immunol. Immunopathol. 1992, 65, 23–29. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 Paradigm of Macrophage Activation: Time for Reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Pechkovsky, D.V.; Prasse, A.; Kollert, F.; Engel, K.M.Y.; Dentler, J.; Luttmann, W.; Friedrich, K.; Müller-Quernheim, J.; Zissel, G. Alternatively Activated Alveolar Macrophages in Pulmonary Fibrosis—Mediator Production and Intracellular Signal Transduction. Clin. Immunol. 2010, 137, 89–101. [Google Scholar] [CrossRef]
- Klopfleisch, R. Macrophage Reaction against Biomaterials in the Mouse Model—Phenotypes, Functions and Markers. Acta Biomater. 2016, 43, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, S.; Wu, H.; Rong, X.; Guo, J. M2b Macrophage Polarization and Its Roles in Diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Gharib, S.A.; McMahan, R.S.; Eddy, W.E.; Long, M.E.; Parks, W.C.; Aitken, M.L.; Manicone, A.M. Transcriptional and Functional Diversity of Human Macrophage Repolarization. J. Allergy Clin. Immunol. 2019, 143, 1536–1548. [Google Scholar] [CrossRef] [PubMed]
- Lurier, E.B.; Dalton, D.; Dampier, W.; Raman, P.; Nassiri, S.; Ferraro, N.M.; Rajagopalan, R.; Sarmady, M.; Spiller, K.L. Transcriptome Analysis of IL-10-Stimulated (M2c) Macrophages by next-Generation Sequencing. Immunobiology 2017, 222, 847–856. [Google Scholar] [CrossRef]
- Song, Y.; Huang, Y.; Zhou, F.; Ding, J.; Zhou, W. Macrophage-Targeted Nanomedicine for Chronic Diseases Immunotherapy. Chin. Chem. Lett. 2022, 33, 597–612. [Google Scholar] [CrossRef]
- Shashkin, P.; Dragulev, B.; Ley, K. Macrophage Differentiation to Foam Cells. Curr. Pharm. Des. 2005, 11, 3061–3072. [Google Scholar] [CrossRef]
- Gleissner, C.A.; Shaked, I.; Little, K.M.; Ley, K. CXC Chemokine Ligand 4 Induces a Unique Transcriptome in Monocyte-Derived Macrophages. J. Immunol. 2010, 184, 4810–4818. [Google Scholar] [CrossRef]
- Udalova, I.A.; Mantovani, A.; Feldmann, M. Macrophage Heterogeneity in the Context of Rheumatoid Arthritis. Nat. Rev. Rheumatol. 2016, 12, 472–485. [Google Scholar] [CrossRef]
- Russo, L.; Lumeng, C.N. Properties and Functions of Adipose Tissue Macrophages in Obesity. Immunology 2018, 155, 407–417. [Google Scholar] [CrossRef]
- Peterson, K.R.; Cottam, M.A.; Kennedy, A.J.; Hasty, A.H. Macrophage-Targeted Therapeutics for Metabolic Disease. Trends Pharmacol. Sci. 2018, 39, 536–546. [Google Scholar] [CrossRef]
- Rigo, A.; Gottardi, M.; Zamò, A.; Mauri, P.; Bonifacio, M.; Krampera, M.; Damiani, E.; Pizzolo, G.; Vinante, F. Macrophages May Promote Cancer Growth via a GM-CSF/HB-EGF Paracrine Loop That Is Enhanced by CXCL12. Mol. Cancer 2010, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- Belli, C.; Trapani, D.; Viale, G.; D’Amico, P.; Duso, B.A.; Della Vigna, P.; Orsi, F.; Curigliano, G. Targeting the Microenvironment in Solid Tumors. Cancer Treat. Rev. 2018, 65, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.-Q.; Du, W.-L.; Cai, M.-H.; Yao, J.-Y.; Zhao, Y.-Y.; Mou, X.-Z. The Roles of Tumor-Associated Macrophages in Tumor Angiogenesis and Metastasis. Cell. Immunol. 2020, 353, 104119. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-Associated Macrophages: An Accomplice in Solid Tumor Progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef] [PubMed]
- Kruize, Z.; Kootstra, N.A. The Role of Macrophages in HIV-1 Persistence and Pathogenesis. Front. Microbiol. 2019, 10, 2828. [Google Scholar] [CrossRef]
- Guirado, E.; Schlesinger, L.S.; Kaplan, G. Macrophages in Tuberculosis: Friend or Foe. Semin. Immunopathol. 2013, 35, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Nuermberger, E.; Bishai, W.R.; Grosset, J.H. Latent Tuberculosis Infection. Semin. Respir. Crit. Care Med. 2004, 25, 317–336. [Google Scholar] [CrossRef] [PubMed]
- Podinovskaia, M.; Descoteaux, A. Leishmania and the Macrophage: A Multifaceted Interaction. Future Microbiol. 2015, 10, 111–129. [Google Scholar] [CrossRef]
- Liang, T.; Zhang, R.; Liu, X.; Ding, Q.; Wu, S.; Li, C.; Lin, Y.; Ye, Y.; Zhong, Z.; Zhou, M. Recent Advances in Macrophage-Mediated Drug Delivery Systems. Int. J. Nanomed. 2021, 16, 2703–2714. [Google Scholar] [CrossRef]
- Gorantla, S.; Dou, H.; Boska, M.; Destache, C.J.; Nelson, J.; Poluektova, L.; Rabinow, B.E.; Gendelman, H.E.; Mosley, R.L. Quantitative Magnetic Resonance and SPECT Imaging for Macrophage Tissue Migration and Nanoformulated Drug Delivery. J. Leukoc. Biol. 2006, 80, 1165–1174. [Google Scholar] [CrossRef]
- Dou, H.; Destache, C.J.; Morehead, J.R.; Mosley, R.L.; Boska, M.D.; Kingsley, J.; Gorantla, S.; Poluektova, L.; Nelson, J.A.; Chaubal, M.; et al. Development of a Macrophage-Based Nanoparticle Platform for Antiretroviral Drug Delivery. Blood 2006, 108, 2827–2835. [Google Scholar] [CrossRef]
- Lee, S. Monocytes: A Novel Drug Delivery System Targeting Atherosclerosis. J. Drug Target. 2014, 22, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Evangelopoulos, M.; Yazdi, I.K.; Acciardo, S.; Palomba, R.; Giordano, F.; Pasto, A.; Sushnitha, M.; Martinez, J.O.; Basu, N.; Torres, A.; et al. Biomimetic Cellular Vectors for Enhancing Drug Delivery to the Lungs. Sci. Rep. 2020, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Thamphiwatana, S.; Angsantikul, P.; Escajadillo, T.; Zhang, Q.; Olson, J.; Luk, B.T.; Zhang, S.; Fang, R.H.; Gao, W.; Nizet, V.; et al. Macrophage-like Nanoparticles Concurrently Absorbing Endotoxins and Proinflammatory Cytokines for Sepsis Management. Proc. Natl. Acad. Sci. USA 2017, 114, 11488–11493. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as Drug Delivery Vehicles for Parkinson’s Disease Therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef]
- Li, R.; He, Y.; Zhu, Y.; Jiang, L.; Zhang, S.; Qin, J.; Wu, Q.; Dai, W.; Shen, S.; Pang, Z.; et al. Route to Rheumatoid Arthritis by Macrophage-Derived Microvesicle-Coated Nanoparticles. Nano Lett. 2019, 19, 124–134. [Google Scholar] [CrossRef]
- Weischenfeldt, J.; Porse, B. Bone Marrow-Derived Macrophages (BMM): Isolation and Applications. Cold Spring Harb. Protoc. 2008, 2008, pdb.prot5080. [Google Scholar] [CrossRef]
- Busch, C.; Favret, J.; Geirsdóttir, L.; Molawi, K.; Sieweke, M. Isolation and Long-Term Cultivation of Mouse Alveolar Macrophages. Bio Protoc. 2019, 9, e3302. [Google Scholar] [CrossRef]
- Pineda-Torra, I.; Gage, M.; De Juan, A.; Pello, O.M. Isolation, Culture, and Polarization of Murine Bone Marrow-Derived and Peritoneal Macrophages. Methods Mol. Biol. 2015, 1339, 101–109. [Google Scholar]
- Kunjachan, S.; Gupta, S.; Dwivedi, A.K.; Dube, A.; Chourasia, M.K. Chitosan-Based Macrophage-Mediated Drug Targeting for the Treatment of Experimental Visceral Leishmaniasis. J. Microencapsul. 2011, 28, 301–310. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Min, H.K.; Kim, D.H.; Kim, C.S.; Han, J.; Park, J.O.; Choi, E. Macrophage-Mediated Delivery of Multifunctional Nanotherapeutics for Synergistic Chemo-Photothermal Therapy of Solid Tumors. ACS Appl. Mater. Interfaces 2020, 12, 10130–10141. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Tan, T.; Zhu, D.; Yu, H.; Liu, Y.; Zhou, H.; Jin, Y.; Xia, Q. Paclitaxel-Loaded Macrophage Membrane Camouflaged Albumin Nanoparticles for Targeted Cancer Therapy. Int. J. Nanomed. 2020, 15, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Monmai, C.; Kim, J.-S.; Baek, S.-H. Use of Germination to Enhance Resveratrol Content and Its Anti-Inflammatory Activity in Lipopolysaccharide-Stimulated RAW264.7 Cells. Molecules 2023, 28, 4898. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Gupta, S. PLGA-Based Macrophage-Mediated Drug Targeting for the Treatment of Visceral Leishmaniasis. Pharm. Biomed. Res. 2017, 3, 41–47. [Google Scholar]
- Zhou, X.; Luo, B.; Kang, K.; Zhang, Y.; Jiang, P.; Lan, F.; Yi, Q.; Wu, Y. Leukocyte-Repelling Biomimetic Immunomagnetic Nanoplatform for High-Performance Circulating Tumor Cells Isolation. Small 2019, 15, e1900558. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, C.; O’Sullivan, M.P.; Sivadas, N.; O’Leary, S.; Gallagher, P.J.; Keane, J.; Cryan, S.A. The Application of High-Content Analysis in the Study of Targeted Particulate Delivery Systems for Intracellular Drug Delivery to Alveolar Macrophages. Mol. Pharm. 2011, 8, 1100–1112. [Google Scholar] [CrossRef]
- He, W.; Frueh, J.; Wu, Z.; He, Q. Leucocyte Membrane-Coated Janus Microcapsules for Enhanced Photothermal Cancer Treatment. Langmuir 2016, 32, 3637–3644. [Google Scholar] [CrossRef]
- Tushinski, R.J.; Oliver, I.T.; Guilbert, L.J.; Tynan, P.W.; Warner, J.R.; Stanley, E.R. Survival of Mononuclear Phagocytes Depends on a Lineage-Specific Growth Factor That the Differentiated Cells Selectively Destroy. Cell 1982, 28, 71–81. [Google Scholar] [CrossRef]
- Zhao, Y.; Haney, M.J. Active Targeted Macrophage-Mediated Delivery of Catalase to Affected Brain Regions in Models of Parkinson’s Disease. J. Nanomed. Nanotechnol. 2011, S4, 3. [Google Scholar] [CrossRef]
- Evans, M.A.; Huang, P.J.; Iwamoto, Y.; Ibsen, K.N.; Chan, E.M.; Hitomi, Y.; Ford, P.C.; Mitragotri, S. Macrophage-Mediated Delivery of Light Activated Nitric Oxide Prodrugs with Spatial, Temporal and Concentration Control. Chem. Sci. 2018, 9, 3729–3741. [Google Scholar] [CrossRef]
- Bain, C.C.; Jenkins, S.J. The biology of serous cavity macrophages. Cellular Immunology 2018, 330, 126–135. [Google Scholar] [CrossRef]
- Lv, Y.; Jun, Y.; Tang, Z.; Li, X.; Tao, M.; Zhang, Z.; Liu, L.; Sun, S.; Wang, Q.; Luo, C.; et al. Enhanced Antitumor Efficacy of Macrophage-Mediated Egg Yolk Lipid-Derived Delivery System against Breast Cancer. Int. J. Nanomed. 2020, 15, 10075–10084. [Google Scholar] [CrossRef] [PubMed]
- Kandekar, S.G.; Del Río-Sancho, S.; Lapteva, M.; Kalia, Y.N. Selective Delivery of Adapalene to the Human Hair Follicle under Finite Dose Conditions Using Polymeric Micelle Nanocarriers. Nanoscale 2018, 10, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; Tripathi, R.P.; Chen, L.; Caminade, A.M.; Shi, X.; Majoral, J.P. New Ways to Treat Tuberculosis Using Dendrimers as Nanocarriers. Pharmaceutics 2018, 10, 105. [Google Scholar] [CrossRef]
- Wang, K.; Lin, S.; Nune, K.C.; Misra, R.D.K. Chitosan-Gelatin-Based Microgel for Sustained Drug Delivery. J. Biomater. Sci. Polym. Ed. 2016, 27, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Salmaso, S.; Caliceti, P. Stealth Properties to Improve Therapeutic Efficacy of Drug Nanocarriers. J. Drug Deliv. 2013, 2013, 374252. [Google Scholar] [CrossRef]
- Rabanel, J.M.; Hildgen, P.; Banquy, X. Assessment of PEG on Polymeric Particles Surface, a Key Step in Drug Carrier Translation. J. Control. Release 2014, 185, 71–87. [Google Scholar] [CrossRef]
- García, K.P.; Zarschler, K.; Barbaro, L.; Barreto, J.A.; O’Malley, W.; Spiccia, L.; Stephan, H.; Graham, B. Zwitterionic-Coated “Stealth” Nanoparticles for Biomedical Applications: Recent Advances in Countering Biomolecular Corona Formation and Uptake by the Mononuclear Phagocyte System. Small 2014, 10, 2516–2529. [Google Scholar] [CrossRef]
- Rayamajhi, S.; Nguyen, T.D.T.; Marasini, R.; Aryal, S. Macrophage-Derived Exosome-Mimetic Hybrid Vesicles for Tumor Targeted Drug Delivery. Acta Biomater. 2019, 94, 482–494. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Ghosh, S.S. Transmembrane TNFα-Expressed Macrophage Membrane-Coated Chitosan Nanoparticles as Cancer Therapeutics. ACS Omega 2020, 5, 1572–1580. [Google Scholar] [CrossRef]
- Wu, L.; Li, L.; Li, S.; Liu, L.; Xin, W.; Li, C.; Yin, X.; Xu, X.; Bao, F.; Hua, Z. Macrophage-Mediated Tumor-Targeted Delivery of Engineered Salmonella Typhimurium VNP20009 in Anti-PD1 Therapy against Melanoma. Acta Pharm. Sin. B 2022, 12, 3952–3971. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, H.Y.; Ju, E.J.; Jung, J.; Park, J.; Chung, H.K.; Lee, J.S.; Lee, J.S.; Park, H.J.; Song, S.Y.; et al. Use of Macrophages to Deliver Therapeutic and Imaging Contrast Agents to Tumors. Biomaterials 2012, 33, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Nowacek, A.S.; Balkundi, S.; McMillan, J.; Roy, U.; Martinez-Skinner, A.; Mosley, R.L.; Kanmogne, G.; Kabanov, A.V.; Bronich, T.; Gendelman, H.E. Analyses of Nanoformulated Antiretroviral Drug Charge, Size, Shape and Content for Uptake, Drug Release and Antiviral Activities in Human Monocyte-Derived Macrophages. J. Control. Release 2011, 150, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Klyachko, N.L.; Polak, R.; Haney, M.J.; Zhao, Y.; Gomes Neto, R.J.; Hill, M.C.; Kabanov, A.V.; Cohen, R.E.; Rubner, M.F.; Batrakova, E.V. Macrophages with Cellular Backpacks for Targeted Drug Delivery to the Brain. Biomaterials 2017, 140, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Doshi, N.; Swiston, A.J.; Gilbert, J.B.; Alcaraz, M.L.; Cohen, R.E.; Rubner, M.F.; Mitragotri, S. Cell-Based Drug Delivery Devices Using Phagocytosis-Resistant Backpacks. Adv. Mater. 2011, 23, H105–H109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, K.; Li, C.; Guo, Q.; Chen, Q.; He, X.; Liu, L.; Zhang, Y.; Lu, Y.; Chen, X.; et al. Macrophage-Membrane-Coated Nanoparticles for Tumor-Targeted Chemotherapy. Nano Lett. 2018, 18, 1908–1915. [Google Scholar] [CrossRef]
- Huang, X.; Wang, L.; Guo, H.; Zhang, W. Macrophage Membrane-Coated Nanovesicles for Dual-Targeted Drug Delivery to Inhibit Tumor and Induce Macrophage Polarization. Bioact. Mater. 2023, 23, 69–79. [Google Scholar] [CrossRef]
- Zhao, H.; Li, L.; Zhang, J.; Zheng, C.; Ding, K.; Xiao, H.; Wang, L.; Zhang, Z. C-C Chemokine Ligand 2 (CCL2) Recruits Macrophage-Membrane-Camouflaged Hollow Bismuth Selenide Nanoparticles to Facilitate Photothermal Sensitivity and Inhibit Lung Metastasis of Breast Cancer. ACS Appl. Mater. Interfaces 2018, 10, 31124–31135. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Huang, Q.; Peng, C.; Yao, L.; Chen, H.; Qiu, Z.; Wu, Y.; Wang, L.; Chen, W. Exosomes from M1-Polarized Macrophages Enhance Paclitaxel Antitumor Activity by Activating Macrophages-Mediated Inflammation. Theranostics 2019, 9, 1714–1727. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, Y.; Banks, W.A.; Bullock, K.M.; Haney, M.; Batrakova, E.; Kabanov, A.V. Macrophage Exosomes as Natural Nanocarriers for Protein Delivery to Inflamed Brain. Biomaterials 2017, 142, 1–12. [Google Scholar] [CrossRef]
- Pei, Y.; Yeo, Y. Drug Delivery to Macrophages: Challenges and Opportunities. J. Control. Release 2016, 240, 202–211. [Google Scholar] [CrossRef]
- Choi, M.R.; Stanton-Maxey, K.J.; Stanley, J.K.; Levin, C.S.; Bardhan, R.; Akin, D.; Badve, S.; Sturgis, J.; Robinson, J.P.; Bashir, R.; et al. A Cellular Trojan Horse for Delivery of Therapeutic Nanoparticles into Tumors. Nano Lett. 2007, 7, 3759–3765. [Google Scholar] [CrossRef] [PubMed]
- Batrakova, E.V.; Li, S.; Reynolds, A.D.; Mosley, R.L.; Bronich, T.K.; Kabanov, A.V.; Gendelman, H.E. A Macrophage-Nanozyme Delivery System for Parkinson’s Disease. Bioconjug. Chem. 2007, 18, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Tabata, Y.; Ikada, Y. Effect of the Size and Surface Charge of Polymer Microspheres on Their Phagocytosis by Macrophage. Biomaterials 1988, 9, 356–362. [Google Scholar] [CrossRef]
- Otsuka, H.; Nagasaki, Y.; Kataoka, K. PEGylated Nanoparticles for Biological and Pharmaceutical Applications. Adv. Drug Deliv. Rev. 2003, 55, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, A.; Andersson, B.; Thurnherr, T.; Krug, H.; Scheynius, A.; Fadeel, B. Toxicology of Engineered Nanomaterials: Focus on Biocompatibility, Biodistribution and Biodegradation. Biochim. Biophys. Acta Gen. Subj. 2011, 1810, 361–373. [Google Scholar] [CrossRef]
- Madsen, S.J.; Christie, C.; Hong, S.J.; Trinidad, A.; Peng, Q.; Uzal, F.A.; Hirschberg, H. Nanoparticle-Loaded Macrophage-Mediated Photothermal Therapy: Potential for Glioma Treatment. Lasers Med. Sci. 2015, 30, 1357–1365. [Google Scholar] [CrossRef]
- Favretto, M.E.; Cluitmans, J.C.A.; Bosman, G.J.C.G.M.; Brock, R. Human Erythrocytes as Drug Carriers: Loading Efficiency and Side Effects of Hypotonic Dialysis, Chlorpromazine Treatment and Fusion with Liposomes. J. Control. Release 2013, 170, 343–351. [Google Scholar] [CrossRef]
- Hamidi, M.; Rafiei, P.; Azadi, A.; Mohammadi-Samani, S. Encapsulation of Valproate-Loaded Hydrogel Nanoparticles in Intact Human Erythrocytes: A Novel Nano-Cell Composite for Drug Delivery. J. Pharm. Sci. 2011, 100, 1702–1711. [Google Scholar] [CrossRef]
- Tsong, T.Y. Electroporation of Cell Membranes. Biophys. J. 1991, 60, 297–306. [Google Scholar] [CrossRef]
- IV, C.W.S.; Evans, M.A.; Wang, L.L.W.; Baugh, N.; Iyer, S.; Wu, D.; Zhao, Z.; Pusuluri, A.; Ukidve, A.; Pan, D.C.; et al. Cellular backpacks for macrophage immunotherapy. Sci. Adv. 2020, 6. [Google Scholar] [CrossRef]
- Yoo, J.W.; Irvine, D.; Discher, D.; Mitragotri, S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat. Rev. Drug Discov. 2011, 10, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Vogel, S.N. Toll Receptors, CD14, and Macrophage Activation and Deactivation by LPS. Microbes Infect. 2002, 4, 903–914. [Google Scholar] [CrossRef]
- Schmid-Schönbein, G.W. Analysis of Inflammation. Annu. Rev. Biomed. Eng. 2006, 8, 93–151. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Pamer, E.G. Monocyte Recruitment during Infection and Inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef]
- Dinarello, C.A. Proinflammatory Cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef]
- Tan, Q.; He, L.; Meng, X.; Wang, W.; Pan, H.; Yin, W.; Zhu, T.; Huang, X.; Shan, H. Macrophage Biomimetic Nanocarriers for Anti-Inflammation and Targeted Antiviral Treatment in COVID-19. J. Nanobiotechnol. 2021, 19, 173. [Google Scholar] [CrossRef]
- Gao, C.; Huang, Q.; Liu, C.; Kwong, C.H.T.; Yue, L.; Wan, J.B.; Lee, S.M.Y.; Wang, R. Treatment of Atherosclerosis by Macrophage-Biomimetic Nanoparticles via Targeted Pharmacotherapy and Sequestration of Proinflammatory Cytokines. Nat. Commun. 2020, 11, 2622. [Google Scholar] [CrossRef]
- Tao, Y.; Ning, M.; Dou, H. A Novel Therapeutic System for Malignant Glioma: Nanoformulation, Pharmacokinetic, and Anticancer Properties of Cell-Nano-Drug Delivery. Nanomedicine 2013, 9, 222–232. [Google Scholar] [CrossRef]
- Evans, M.A.; Shields, C.W.; Krishnan, V.; Wang, L.L.; Zhao, Z.; Ukidve, A.; Lewandowski, M.; Gao, Y.; Mitragotri, S. Macrophage-Mediated Delivery of Hypoxia-Activated Prodrug Nanoparticles. Adv. Ther. 2020, 3, 1900162. [Google Scholar] [CrossRef]
- Wayne, E.C.; Long, C.; Haney, M.J.; Batrakova, E.V.; Leisner, T.M.; Parise, L.V.; Kabanov, A.V. Targeted Delivery of SiRNA Lipoplexes to Cancer Cells Using Macrophage Transient Horizontal Gene Transfer. Adv. Sci. 2019, 6, 1900582. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, S.; Iwasaki, Y. Surface Modification of Macrophages with Nucleic Acid Aptamers for Enhancing the Immune Response against Tumor Cells. Bioconjug. Chem. 2018, 29, 4160–4167. [Google Scholar] [CrossRef] [PubMed]
- Muthana, M.; Giannoudis, A.; Scott, S.D.; Fang, H.Y.; Coffelt, S.B.; Morrow, F.J.; Murdoch, C.; Burton, J.; Cross, N.; Burke, B.; et al. Use of Macrophages to Target Therapeutic Adenovirus to Human Prostate Tumors. Cancer Res. 2011, 71, 1805–1815. [Google Scholar] [CrossRef] [PubMed]
- Xuan, M.; Shao, J.; Dai, L.; Li, J.; He, Q. Macrophage Cell Membrane Camouflaged Au Nanoshells for in Vivo Prolonged Circulation Life and Enhanced Cancer Photothermal Therapy. ACS Appl. Mater. Interfaces 2016, 8, 9610–9618. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Qiu, Y.; Yu, Q.; He, J.; Mei, L.; Liu, Y.; Li, J.; Wang, X.; Li, M.; Zhang, Z.; et al. Macrophage-Mediated Multi-Mode Drug Release System for Photothermal Combined with Anti-Inflammatory Therapy against Postoperative Recurrence of Triple Negative Breast Cancer. Int. J. Pharm. 2021, 607, 120975. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Qin, J.; Han, L.; Zhao, W.; Liang, J.; Xie, Z.; Yang, P.; Wang, J. Exploiting Macrophages as Targeted Carrier to Guide Nanoparticles into Glioma. Oncotarget 2016, 7, 37081–37091. [Google Scholar] [CrossRef]
- Swiston, A.J.; Gilbert, J.B.; Irvine, D.J.; Cohen, R.E.; Rubner, M.F. Freely Suspended Cellular “Backpacks” Lead to Cell Aggregate Self-Assembly. Biomacromolecules 2010, 11, 1826–1832. [Google Scholar] [CrossRef]
- Swiston, A.J.; Cheng, C.; Um, S.H.; Irvine, D.J.; Cohen, R.E.; Rubner, M.F. Surface Functionalization of Living Cells with Multilayer Patches. Nano Lett. 2008, 8, 4446–4453. [Google Scholar] [CrossRef]
- Movva, R.H.; Yarraguntla, S.R.; Paravastu, V.K.K. Cellular Backpacks for Macrophage Immunotherapy—A Review. GSC Biol. Pharm. Sci. 2022, 20, 126–133. [Google Scholar] [CrossRef]
- Holden, C.A.; Yuan, Q.; Yeudall, W.A.; Lebman, D.A.; Yang, H. Surface Engineering of Macrophages with Nanoparticles to Generate a Cell-Nanoparticle Hybrid Vehicle for Hypoxia-Targeted Drug Delivery. J. Nanomed. 2009, 5, 25–36. [Google Scholar] [CrossRef]
- de Melo-Diogo, D.; Pais-Silva, C.; Dias, D.R.; Moreira, A.F.; Correia, I.J. Strategies to Improve Cancer Photothermal Therapy Mediated by Nanomaterials. Adv. Healthc. Mater. 2017, 6, 1700073. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, F.; Ju, Y.; Hong, J.; Ding, Y. Gold Nanomaterial Engineering for Macrophage-Mediated Inflammation and Tumor Treatment. Adv. Healthc. Mater. 2021, 10, 2000818. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Grotepas, C.B.; McMillan, J.M.; Destache, C.J.; Chaubal, M.; Werling, J.; Kipp, J.; Rabinow, B.; Gendelman, H.E. Macrophage Delivery of Nanoformulated Antiretroviral Drug to the Brain in a Murine Model of NeuroAIDS. J. Immunol. 2009, 183, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Yang, J.; Liu, W. Bacteria Activated-Macrophage Membrane-Coated Tough Nanocomposite Hydrogel with Targeted Photothermal Antibacterial Ability for Infected Wound Healing. Chem. Eng. J. 2021, 420, 127638. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Zhang, L.; Miron, R.J.; Liang, J.; Shi, M.; Mo, W.; Zheng, S.; Zhao, Y.; Zhang, Y. Pretreated Macrophage-Membrane-Coated Gold Nanocages for Precise Drug Delivery for Treatment of Bacterial Infections. Adv. Mater. 2018, 30, 1804023. [Google Scholar] [CrossRef]
- Guo, S.; Gu, J.; Jiang, Y.; Cui, W.; Chen, J.; Li, L.; Zheng, K.; Xu, Y. Pretreatment of Macrophage-Membrane-Coated Nanoparticles for Therapeutical Targeting of P. Gingivalis-Accelerated Atherosclerosis. Mater. Des. 2022, 223, 111155. [Google Scholar] [CrossRef]
- Meng, Z.; Pan, L.; Qian, S.; Yang, X.; Pan, L.; Chi, R.; Chen, J.; Pan, J.; Shi, C. Antimicrobial Peptide Nanoparticles Coated with Macrophage Cell Membrane for Targeted Antimicrobial Therapy of Sepsis. Mater. Des. 2023, 229, 111883. [Google Scholar] [CrossRef]
- Chono, S.; Tanino, T.; Seki, T.; Morimoto, K. Uptake Characteristics of Liposomes by Rat Alveolar Macrophages: Influence of Particle Size and Surface Mannose Modification. J. Pharm. Pharmacol. 2010, 59, 75–80. [Google Scholar] [CrossRef]
- Yue, H.; Wei, W.; Yue, Z.; Lv, P.; Wang, L.; Ma, G.; Su, Z. Particle Size Affects the Cellular Response in Macrophages. Eur. J. Pharm. Sci. 2010, 41, 650–657. [Google Scholar] [CrossRef]
- Champion, J.A.; Mitragotri, S. Role of Target Geometry in Phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging Macrophages with Nanoparticles. Nat. Mater. 2014, 13, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Gao, X.; Du, Y.; Zhang, H.; Gao, J.; Zheng, A. Size, Shape, Charge and “Stealthy” Surface: Carrier Properties Affect the Drug Circulation Time in Vivo. Asian J. Pharm. Sci. 2021, 16, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Stylianopoulos, T. Delivering Nanomedicine to Solid Tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef]
- Gao, W.J.; Liu, J.X.; Liu, M.N.; Yao, Y.D.; Liu, Z.Q.; Liu, L.; He, H.H.; Zhou, H. Macrophage 3D Migration: A Potential Therapeutic Target for Inflammation and Deleterious Progression in Diseases. Pharmacol. Res. 2021, 167, 105563. [Google Scholar] [CrossRef]
- Zhang, G.; Ma, L.; Bai, L.; Li, M.; Guo, T.; Tian, B.; He, Z.; Fu, Q. Inflammatory Microenvironment-Targeted Nanotherapies. J. Control. Release 2021, 334, 114–126. [Google Scholar] [CrossRef]
- Sharma, G.; Valenta, D.T.; Altman, Y.; Harvey, S.; Xie, H.; Mitragotri, S.; Smith, J.W. Polymer Particle Shape Independently Influences Binding and Internalization by Macrophages. J. Control. Release 2010, 147, 408–412. [Google Scholar] [CrossRef]
- Herd, H.; Daum, N.; Jones, A.T.; Huwer, H.; Ghandehari, H.; Lehr, C.M. Nanoparticle Geometry and Surface Orientation Influence Mode of Cellular Uptake. ACS Nano 2013, 7, 1961–1973. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of Particle Size and Surface Charge on Cellular Uptake and Biodistribution of Polymeric Nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef]
- Zahr, A.S.; Davis, C.A.; Pishko, M.V. Macrophage Uptake of Core-Shell Nanoparticles Surface Modified with Poly(Ethylene Glycol). Langmuir 2006, 22, 8178–8185. [Google Scholar] [CrossRef]
- Epstein-Barash, H.; Gutman, D.; Markovsky, E.; Mishan-Eisenberg, G.; Koroukhov, N.; Szebeni, J.; Golomb, G. Physicochemical Parameters Affecting Liposomal Bisphosphonates Bioactivity for Restenosis Therapy: Internalization, Cell Inhibition, Activation of Cytokines and Complement, and Mechanism of Cell Death. J. Control. Release 2010, 146, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xie, X.; Jiang, J.; Lin, M.; Zheng, E.; Qiu, W.; Yeung, I.; Zhu, M.; Li, Q.; Xia, T.; et al. Use of Nanoformulation to Target Macrophages for Disease Treatment. Adv. Funct. Mater. 2021, 31, 2104487. [Google Scholar] [CrossRef]
- Mosqueira, V.C.F.; Legrand, P.; Gulik, A.; Bourdon, O.; Gref, R.; Labarre, D.; Barratt, G. Relationship between Complement Activation, Cellular Uptake and Surface Physicochemical Aspects of Novel PEG-Modified Nanocapsules. Biomaterials 2001, 22, 2967–2979. [Google Scholar] [CrossRef] [PubMed]
- Motskin, M.; Müller, K.H.; Genoud, C.; Monteith, A.G.; Skepper, J.N. The Sequestration of Hydroxyapatite Nanoparticles by Human Monocyte-Macrophages in a Compartment That Allows Free Diffusion with the Extracellular Environment. Biomaterials 2011, 32, 9470–9482. [Google Scholar] [CrossRef] [PubMed]
- Sarparanta, M.; Bimbo, L.M.; Rytkoänen, J.; Mäkilä, E.; Laaksonen, T.J.; Laaksonen, P.; Nyman, M.; Salonen, J.; Linder, M.B.; Hirvonen, J.; et al. Intravenous Delivery of Hydrophobin-Functionalized Porous Silicon Nanoparticles: Stability, Plasma Protein Adsorption and Biodistribution. Mol. Pharm. 2012, 9, 654–663. [Google Scholar] [CrossRef]
- Shi, D.; Beasock, D.; Fessler, A.; Szebeni, J.; Ljubimova, J.Y.; Afonin, K.A.; Dobrovolskaia, M.A. To PEGylate or Not to PEGylate: Immunological Properties of Nanomedicine’s Most Popular Component, Polyethylene Glycol and Its Alternatives. Adv. Drug Deliv. Rev. 2022, 180, 114079. [Google Scholar] [CrossRef]
- Hattori, Y.; Kawakami, S.; Suzuki, S.; Yamashita, F.; Hashida, M. Enhancement of Immune Responses by DNA Vaccination through Targeted Gene Delivery Using Mannosylated Cationic Liposome Formulations Following Intravenous Administration in Mice. Biochem. Biophys. Res. Commun. 2004, 317, 992–999. [Google Scholar] [CrossRef]
- Ortega, R.A.; Barham, W.; Sharman, K.; Tikhomirov, O.; Giorgio, T.D.; Yull, F.E. Manipulating the NF-ΚB Pathway in Macrophages Using Mannosylated, SiRNA-Delivering Nanoparticles Can Induce Immunostimulatory and Tumor Cytotoxic Functions. Int. J. Nanomed. 2016, 11, 2163–2177. [Google Scholar] [CrossRef]
- Locke, L.W.; Mayo, M.W.; Yoo, A.D.; Williams, M.B.; Berr, S.S. PET Imaging of Tumor Associated Macrophages Using Mannose Coated 64Cu Liposomes. Biomaterials 2012, 33, 7785–7793. [Google Scholar] [CrossRef]
- Pi, J.; Shen, L.; Yang, E.; Shen, H.; Huang, D.; Wang, R.; Hu, C.; Jin, H.; Cai, H.; Cai, J.; et al. Macrophage-Targeted Isoniazid–Selenium Nanoparticles Promote Antimicrobial Immunity and Synergize Bactericidal Destruction of Tuberculosis Bacilli. Angew. Chem. Int. Ed. 2020, 59, 3226–3234. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Z.; Jiang, Y.; Zhang, D.; Chen, J.; Dong, L.; Zhang, J. Targeted Delivery of Oligonucleotides into Tumor-Associated Macrophages for Cancer Immunotherapy. J. Control. Release 2012, 158, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, C.; Yin, C. Galactosylated Trimethyl Chitosan-Cysteine Nanoparticles Loaded with Map4k4 SiRNA for Targeting Activated Macrophages. Biomaterials 2013, 34, 3667–3677. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, M.; Ali, H.; Ain, Q.U.; Mukhtar, M.; Gul, R.; Sarwar, A.; Khan, S. A Holistic QBD Approach to Design Galactose Conjugated PLGA Polymer and Nanoparticles to Catch Macrophages during Intestinal Inflammation. Mater. Sci. Eng. C 2021, 126, 112183. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Gou, J.; Sun, W.; Tao, X.; Tan, X.; Wang, P.; Zhang, Y.; He, H.; Yin, T.; Tang, X. Entrapping of Nanoparticles in Yeast Cell Wall Microparticles for Macrophage-Targeted Oral Delivery of Cabazitaxel. Mol. Pharm. 2018, 15, 2870–2882. [Google Scholar] [CrossRef]
- Soto, E.R.; Caras, A.C.; Kut, L.C.; Castle, M.K.; Ostroff, G.R. Glucan Particles for Macrophage Targeted Delivery of Nanoparticles. J. Drug Deliv. 2012, 2012, 143524. [Google Scholar] [CrossRef]
- Jain, S.; Amiji, M. Tuftsin-Modified Alginate Nanoparticles as a Noncondensing Macrophage-Targeted DNA Delivery System. Biomacromolecules 2012, 13, 1074–1085. [Google Scholar] [CrossRef]
- Nagai, T.; Tanaka, M.; Tsuneyoshi, Y.; Xu, B.; Michie, S.A.; Hasui, K.; Hirano, H.; Arita, K.; Matsuyama, T. Targeting Tumor-Associated Macrophages in an Experimental Glioma Model with a Recombinant Immunotoxin to Folate Receptor β. Cancer Immunol. Immunother. 2009, 58, 1577–1586. [Google Scholar] [CrossRef]
- Thomas, T.P.; Goonewardena, S.N.; Majoros, I.J.; Kotlyar, A.; Cao, Z.; Leroueil, P.R.; Baker, J.R. Folate-Targeted Nanoparticles Show Efficacy in the Treatment of Inflammatory Arthritis. Arthritis Rheum. 2011, 63, 2671–2680. [Google Scholar] [CrossRef]
- Rollett, A.; Reiter, T.; Nogueira, P.; Cardinale, M.; Loureiro, A.; Gomes, A.; Cavaco-Paulo, A.; Moreira, A.; Carmo, A.M.; Guebitz, G.M. Folic Acid-Functionalized Human Serum Albumin Nanocapsules for Targeted Drug Delivery to Chronically Activated Macrophages. Int. J. Pharm. 2012, 427, 460–466. [Google Scholar] [CrossRef]
- Gao, Y.; Sarfraz, M.K.; Clas, S.D.; Roa, W.; Löbenberg, R. Hyaluronic Acid-Tocopherol Succinate-Based Self-Assembling Micelles for Targeted Delivery of Rifampicin to Alveolar Macrophages. J. Biomed. Nanotechnol. 2014, 11, 1312–1329. [Google Scholar] [CrossRef]
- Gouveia, V.M.; Lopes-De-Araújo, J.; Costa Lima, S.A.; Nunes, C.; Reis, S. Hyaluronic Acid-Conjugated PH-Sensitive Liposomes for Targeted Delivery of Prednisolone on Rheumatoid Arthritis Therapy. Nanomedicine 2018, 13, 1037–1049. [Google Scholar] [CrossRef]
- Hlaing, S.P.; Cao, J.; Lee, J.; Kim, J.; Saparbayeva, A.; Kwak, D.; Kim, H.; Hwang, S.; Yun, H.; Moon, H.R.; et al. Hyaluronic Acid-Conjugated PLGA Nanoparticles Alleviate Ulcerative Colitis via CD44-Mediated Dual Targeting to Inflamed Colitis Tissue and Macrophages. Pharmaceutics 2022, 14, 2118. [Google Scholar] [CrossRef]
- Ding, J.; Sui, D.; Liu, M.; Su, Y.; Wang, Y.; Liu, M.; Luo, X.; Liu, X.; Deng, Y.; Song, Y. Sialic Acid Conjugate-Modified Liposomes Enable Tumor Homing of Epirubicin via Neutrophil/Monocyte Infiltration for Tumor Therapy. Acta Biomater. 2021, 134, 702–715. [Google Scholar] [CrossRef]
- Tang, X.; Sui, D.; Liu, M.; Zhang, H.; Liu, M.; Wang, S.; Zhao, D.; Sun, W.; Liu, M.; Luo, X.; et al. Targeted Delivery of Zoledronic Acid through the Sialic Acid—Siglec Axis for Killing and Reversal of M2 Phenotypic Tumor-Associated Macrophages—A Promising Cancer Immunotherapy. Int. J. Pharm. 2020, 590, 119929. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Kaisho, T.; Akira, S. Toll-like Receptor Function and Signaling. J. Allergy Clin. Immunol. 2006, 117, 979–987. [Google Scholar] [CrossRef]
- Federico, S.; Pozzetti, L.; Papa, A.; Carullo, G.; Gemma, S.; Butini, S.; Campiani, G.; Relitti, N. Modulation of the Innate Immune Response by Targeting Toll-like Receptors: A Perspective on Their Agonists and Antagonists. J. Med. Chem. 2020, 63, 13466–13513. [Google Scholar] [CrossRef]
- Zeng, Q.; Jewell, C.M. Directing Toll-like Receptor Signaling in Macrophages to Enhance Tumor Immunotherapy. Curr. Opin. Biotechnol. 2019, 60, 138–145. [Google Scholar] [CrossRef]
- Canton, J.; Neculai, D.; Grinstein, S. Scavenger Receptors in Homeostasis and Immunity. Nat. Rev. Immunol. 2013, 13, 621–634. [Google Scholar] [CrossRef]
- Areschoug, T.; Gordon, S. Scavenger Receptors: Role in Innate Immunity and Microbial Pathogenesis. Cell. Microbiol. 2009, 11, 1160–1169. [Google Scholar] [CrossRef]
- Lepenies, B.; Lee, J.; Sonkaria, S. Targeting C-Type Lectin Receptors with Multivalent Carbohydrate Ligands. Adv. Drug Deliv. Rev. 2013, 65, 1271–1281. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Ezhov, A.A.; Petrov, R.A.; Vigovskiy, M.A.; Grigorieva, O.A.; Belogurova, N.G.; Kudryashova, E.V. Mannosylated Polymeric Ligands for Targeted Delivery of Antibacterials and Their Adjuvants to Macrophages for the Enhancement of the Drug Efficiency. Pharmaceuticals 2022, 15, 1172. [Google Scholar] [CrossRef]
- Serrasqueiro, F.; Barbosa, A.I.; Lima, S.A.C.; Reis, S. Targeting the Mannose Receptor with Functionalized Fucoidan/Chitosan Nanoparticles Triggers the Classical Activation of Macrophages. Int. J. Mol. Sci. 2023, 24, 9908. [Google Scholar] [CrossRef]
- Ma, C.; Wu, M.; Ye, W.; Huang, Z.; Ma, X.; Wang, W.; Wang, W.; Huang, Y.; Pan, X.; Wu, C. Inhalable Solid Lipid Nanoparticles for Intracellular Tuberculosis Infection Therapy: Macrophage-Targeting and PH-Sensitive Properties. Drug Deliv. Transl. Res. 2021, 11, 1218–1235. [Google Scholar] [CrossRef]
- Peng, Y.; Yao, W.; Wang, B.; Zong, L. Mannosylated Chitosan Nanoparticles Based Macrophage-Targeting Gene Delivery System Enhanced Cellular Uptake and Improved Transfection Efficiency. J. Nanosci. Nanotechnol. 2015, 15, 2619–2627. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, X.; Jia, L.; Prud’Homme, R.K.; Szekely, Z.; Sinko, P.J. Optimal Structural Design of Mannosylated Nanocarriers for Macrophage Targeting. J. Control. Release 2014, 194, 341–349. [Google Scholar] [CrossRef]
- Napoletano, C.; Zizzari, I.G.; Rughetti, A.; Rahimi, H.; Irimura, T.; Clausen, H.; Nuti, M. Targeting of macrophage galactose-type C-type lectin (MGL) induces DC signaling and activation. Eur. J. Immunol. 2012, 42, 936–945. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.; Li, R.; Li, C.; Xu, L.; Qiao, W.; Dong, N. Galactose-Modified Nanoparticles for Delivery of MicroRNA to Mitigate the Progress of Abdominal Aortic Aneurysms via Regulating Macrophage Polarization. Nanomedicine 2022, 44, 102564. [Google Scholar] [CrossRef]
- Sharma, R.; Liaw, K.; Sharma, A.; Jimenez, A.; Chang, M.; Salazar, S.; Amlani, I.; Kannan, S.; Kannan, R.M. Glycosylation of PAMAM Dendrimers Significantly Improves Tumor Macrophage Targeting and Specificity in Glioblastoma. J. Control. Release 2021, 337, 179–192. [Google Scholar] [CrossRef]
- Foerster, F.; Bamberger, D.; Schupp, J.; Weilbächer, M.; Kaps, L.; Strobl, S.; Radi, L.; Diken, M.; Strand, D.; Tuettenberg, A.; et al. Dextran-Based Therapeutic Nanoparticles for Hepatic Drug Delivery. Nanomedicine 2016, 11, 2663–2677. [Google Scholar] [CrossRef]
- Shah, N.K.; Gupta, S.K.; Wang, Z.; Meenach, S.A. Enhancement of Macrophage Uptake via Phosphatidylserine-Coated Acetalated Dextran Nanoparticles. J. Drug Deliv. Sci. Technol. 2019, 50, 57–65. [Google Scholar] [CrossRef]
- Han, J.; Na, R.; Zhao, N.; Yuan, X.; Fu, L.; Jing, J.; Qian, A.; Ye, W. Macrophage-Targeted Dextran Sulfate-Dexamethasone Conjugate Micelles for Effective Treatment of Rheumatoid Arthritis. Molecules 2023, 28, 591. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tatematsu, K.; Somiya, M.; Iijima, M.; Kuroda, S. Development of a Macrophage-Targeting and Phagocytosis-Inducing Bio-Nanocapsule-Based Nanocarrier for Drug Delivery. Acta Biomater. 2018, 73, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, Y.; Tomizawa, K.; Nagita, M.; Michiue, H.; Nishiki, T.; Ohmori, I.; Seno, M.; Matsui, H. Development of Bionanocapsules Targeting Brain Tumors. J. Control. Release 2007, 122, 159–164. [Google Scholar] [CrossRef]
- Xie, S.; Li, S.; Zhang, Z.; Chen, M.; Ran, P.; Li, X. Bacterial Ghosts for Targeting Delivery and Subsequent Responsive Release of Ciprofloxacin to Destruct Intracellular Bacteria. Chem. Eng. J. 2020, 399, 125700. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Ravetch, J.V. Fc-Receptors as Regulators of Immunity. Adv. Immunol. 2007, 96, 179–204. [Google Scholar]
- Mkaddem, S.B.; Benhamou, M.; Monteiro, R.C. Understanding Fc Receptor Involvement in Inflammatory Diseases: From Mechanisms to New Therapeutic Tools. Front. Immunol. 2019, 10, 811. [Google Scholar] [CrossRef]
- Bar-Shavit, Z.; Stabinsky, Y.; Fridkin, M.; Goldman, R. Tuftsin-Macrophage Interaction: Specific Binding and Augmentation of Phagocytosis. J. Cell. Physiol. 1979, 100, 55–62. [Google Scholar] [CrossRef]
- Khan, M.A. Targeted Drug Delivery Using Tuftsin-Bearing Liposomes: Implications in the Treatment of Infectious Diseases and Tumors. Curr. Drug Targets 2020, 22, 770–778. [Google Scholar] [CrossRef]
- Liang, D.S.; Wen, Z.J.; Wang, J.H.; Zhu, F.F.; Guo, F.; Zhou, J.L.; Xu, J.J.; Zhong, H.J. Legumain Protease-Sheddable PEGylated, Tuftsin-Modified Nanoparticles for Selective Targeting to Tumour-Associated Macrophages. J. Drug Target. 2022, 30, 82–93. [Google Scholar] [CrossRef]
- Horváti, K.; Bacsa, B.; Szabó, N.; Dávid, S.; Mezo, G.; Grolmusz, V.; Vértessy, B.; Hudecz, F.; Bo’sze, S. Enhanced Cellular Uptake of a New, in Silico Identified Antitubercular Candidate by Peptide Conjugation. Bioconjug Chem. 2012, 23, 900–907. [Google Scholar] [CrossRef]
- Scaranti, M.; Cojocaru, E.; Banerjee, S.; Banerji, U. Exploiting the Folate Receptor α in Oncology. Nat. Rev. Clin. Oncol. 2020, 17, 349–359. [Google Scholar] [CrossRef]
- Rios de la Rosa, J.M.; Tirella, A.; Gennari, A.; Stratford, I.J.; Tirelli, N. The CD44-Mediated Uptake of Hyaluronic Acid-Based Carriers in Macrophages. Adv. Healthc. Mater. 2017, 6, 1601012. [Google Scholar] [CrossRef] [PubMed]
- Paulos, C.M.; Turk, M.J.; Breur, G.J.; Low, P.S. Folate Receptor-Mediated Targeting of Therapeutic and Imaging Agents to Activated Macrophages in Rheumatoid Arthritis. Adv. Drug Deliv. Rev. 2004, 56, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gao, S.; Kjems, J. Folic Acid Conjugated Chitosan for Targeted Delivery of SiRNA to Activated Macrophages in Vitro and in Vivo. J. Mater. Chem. B 2014, 2, 8608–8615. [Google Scholar] [CrossRef] [PubMed]
- Poh, S.; Chelvam, V.; Ayala-López, W.; Putt, K.S.; Low, P.S. Selective Liposome Targeting of Folate Receptor Positive Immune Cells in Inflammatory Diseases. Nanomedicine 2018, 14, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Sager, H.B.; Dutta, P.; Dahlman, J.E.; Hulsmans, M.; Courties, G.; Sun, Y.; Heidt, T.; Vinegoni, C.; Borodovsky, A.; Fitzgerald, K.; et al. RNAi Targeting Multiple Cell Adhesion Molecules Reduces Immune Cell Recruitment and Vascular Inflammation after Myocardial Infarction. Sci. Transl. Med. 2016, 8, 342ra80. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Sahu, A.; Hwang, Y.; Kim, G.B.; Nam, G.H.; Kim, I.-S.; Chan Kwon, I.; Tae, G. Targeted Delivery of Anti-Inflammatory Cytokine by Nanocarrier Reduces Atherosclerosis in Apo E−/− Mice. Biomaterials 2020, 226, 119550. [Google Scholar] [CrossRef]
- Kim, H.; Kim, B.H.; Huh, B.K.; Yoo, Y.C.; Heo, C.Y.; Bin Choy, Y.; Park, J.H. Surgical Suture Releasing Macrophage-Targeted Drug-Loaded Nanoparticles for an Enhanced Anti-Inflammatory Effect. Biomater. Sci. 2017, 5, 1670–1677. [Google Scholar] [CrossRef]
- Ospelt, C.; Gay, S. TLRs and Chronic Inflammation. Int. J. Biochem. Cell Biol. 2010, 42, 495–505. [Google Scholar] [CrossRef]
- Murgueitio, M.S.; Henneke, P.; Glossmann, H.; Santos-Sierra, S.; Wolber, G. Prospective Virtual Screening in a Sparse Data Scenario: Design of Small-Molecule TLR2 Antagonists. ChemMedChem 2014, 9, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, Y.; Xia, R.; Wei, Y.; Wei, X. Role of the CCL2-CCR2 Signalling Axis in Cancer: Mechanisms and Therapeutic Targeting. Cell Prolif. 2021, 54, e13115. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xiao, Q.; Dong, M.; Guo, D.; Wu, X.; Wang, B. Glioblastoma Immunotherapy Targeting the Innate Immune Checkpoint CD47-SIRPα Axis. Front. Immunol. 2020, 11, 593219. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, P.F.; Sivapurapu, N.; Jovanovic, B.; Kajdacsy-Balla, A. Monocyte-Induced Prostate Cancer Cell Invasion Is Mediated by Chemokine Ligand 2 and Nuclear Factor-ΚB Activity. J. Clin. Cell. Immunol. 2015, 6, 308. [Google Scholar] [CrossRef] [PubMed]
- Grossman, J.G.; Nywening, T.M.; Belt, B.A.; Panni, R.Z.; Krasnick, B.A.; DeNardo, D.G.; Hawkins, W.G.; Goedegebuure, S.P.; Linehan, D.C.; Fields, R.C. Recruitment of CCR2+ Tumor Associated Macrophage to Sites of Liver Metastasis Confers a Poor Prognosis in Human Colorectal Cancer. Oncoimmunology 2018, 7, e1470729. [Google Scholar] [CrossRef] [PubMed]
- Flores-Toro, J.A.; Luo, D.; Gopinath, A.; Sarkisian, M.R.; Campbell, J.J.; Charo, I.F.; Singh, R.; Schall, T.J.; Datta, M.; Jain, R.K.; et al. CCR2 Inhibition Reduces Tumor Myeloid Cells and Unmasks a Checkpoint Inhibitor Effect to Slow Progression of Resistant Murine Gliomas. Proc. Natl. Acad. Sci. USA 2020, 117, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Logtenberg, M.E.W.; Scheeren, F.A.; Schumacher, T.N. The CD47-SIRPα Immune Checkpoint. Immunity 2020, 52, 742–752. [Google Scholar] [CrossRef]
- Russ, A.; Hua, A.B.; Montfort, W.R.; Rahman, B.; Bin Riaz, I.; Khalid, M.U.; Carew, J.S.; Nawrocki, S.T.; Persky, D.; Anwer, F. Blocking “Don’t Eat Me” Signal of CD47-SIRPα in Hematological Malignancies, an in-Depth Review. Blood Rev. 2018, 32, 480–489. [Google Scholar] [CrossRef]
- Ho, C.C.M.; Guo, N.; Sockolosky, J.T.; Ring, A.M.; Weiskopf, K.; Özkan, E.; Mori, Y.; Weissman, I.L.; Garcia, K.C. “Velcro” Engineering of High Affinity CD47 Ectodomain as Signal Regulatory Protein α (SIRPα) Antagonists That Enhance Antibody-Dependent Cellular Phagocytosis. J. Biol. Chem. 2015, 290, 12650–12663. [Google Scholar] [CrossRef]
- Murata, Y.; Tanaka, D.; Hazama, D.; Yanagita, T.; Saito, Y.; Kotani, T.; Oldenborg, P.A.; Matozaki, T. Anti-Human SIRPα Antibody Is a New Tool for Cancer Immunotherapy. Cancer Sci. 2018, 109, 1300–1308. [Google Scholar] [CrossRef]
- Ring, N.G.; Herndler-Brandstetter, D.; Weiskopf, K.; Shan, L.; Volkmer, J.P.; George, B.M.; Lietzenmayer, M.; McKenna, K.M.; Naik, T.J.; McCarty, A.; et al. Anti-SIRPα Antibody Immunotherapy Enhances Neutrophil and Macrophage Antitumor Activity. Proc. Natl. Acad. Sci. USA 2017, 114, E10578–E10585. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhang, T.; Peng, B.; Luo, X.; Liu, X.; Hu, L.; Liu, Y.; Di, D.; Song, Y.; Deng, Y. Targeted Delivery of Epirubicin to Tumor-Associated Macrophages by Sialic Acid-Cholesterol Conjugate Modified Liposomes with Improved Antitumor Activity. Int. J. Pharm. 2017, 523, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Song, X.Y.; Li, Y.; Ye, L.L.; Zhou, Q.; Yang, W.B. Tumor-Associated Macrophages: A Promising Target for a Cancer Immunotherapeutic Strategy. Pharmacol. Res. 2020, 161, 105111. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Ren, X.; Yu, H.; Zhan, Y. Targeting the CCL2/CCR2 Axis in Cancer Immunotherapy: One Stone, Three Birds? Front. Immunol. 2021, 12, 771210. [Google Scholar] [CrossRef] [PubMed]
- Loberg, R.D.; Ying, C.; Craig, M.; Yan, L.; Snyder, L.A.; Pienta, K.J. CCL2 as an Important Mediator of Prostate Cancer Growth in Vivo through the Regulation of Macrophage Infiltration. Neoplasia 2007, 9, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Loberg, R.D.; Ying, C.; Craig, M.; Day, L.L.; Sargent, E.; Neeley, C.; Wojno, K.; Snyder, L.A.; Yan, L.; Pienta, K.J. Targeting CCL2 with Systemic Delivery of Neutralizing Antibodies Induces Prostate Cancer Tumor Regression in Vivo. Cancer Res. 2007, 67, 9417–9424. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, Y.; Chen, K.G.; Luo, Y.L.; Wang, J. Cationic Polymeric Nanoparticle Delivering CCR2 SiRNA to Inflammatory Monocytes for Tumor Microenvironment Modification and Cancer Therapy. Mol. Pharm. 2018, 15, 3642–3653. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.; Huang, L. Macrophage-Mediated Tumor Cell Phagocytosis: Opportunity for Nanomedicine Intervention. Adv. Funct. Mater. 2021, 31, 2006220. [Google Scholar] [CrossRef]
- Veillette, A.; Tang, Z. Signaling Regulatory Protein (SIRP)a-CD47 Blockade Joins the Ranks of Immune Checkpoint Inhibition. J. Clin. Oncol. 2019, 37, 1012–1014. [Google Scholar] [CrossRef]
- Yanagita, T.; Murata, Y.; Tanaka, D.; Motegi, S.; Arai, E.; Daniwijaya, E.W.; Hazama, D.; Washio, K.; Saito, Y.; Kotani, T.; et al. Anti-SIRPα Antibodies as a Potential New Tool for Cancer Immunotherapy. JCI Insight 2017, 2, 89140. [Google Scholar] [CrossRef]
- Chao, M.P.; Alizadeh, A.A.; Tang, C.; Jan, M.; Weissman-Tsukamoto, R.; Zhao, F.; Park, C.Y.; Weissman, I.L.; Majeti, R. Therapeutic Antibody Targeting of CD47 Eliminates Human Acute Lymphoblastic Leukemia. Cancer Res. 2011, 71, 1374–1384. [Google Scholar] [CrossRef]
- Koh, E.; Lee, E.J.; Nam, G.H.; Hong, Y.; Cho, E.; Yang, Y.; Kim, I.S. Exosome-SIRPα, a CD47 Blockade Increases Cancer Cell Phagocytosis. Biomaterials 2017, 121, 121–129. [Google Scholar] [CrossRef]
- Paul, B.; Liedtke, M.; Khouri, J.; Rifkin, R.; Gandhi, M.D.; Kin, A.; Levy, M.Y.; Silbermann, R.; Cottini, F.; Sborov, D.W.; et al. A Phase II Multi-Arm Study of Magrolimab Combinations in Patients with Relapsed/Refractory Multiple Myeloma. Future Oncol. 2023, 19, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Voets, E.; Paradé, M.; Lutje Hulsik, D.; Spijkers, S.; Janssen, W.; Rens, J.; Reinieren-Beeren, I.; Van Den Tillaart, G.; Van Duijnhoven, S.; Driessen, L.; et al. Functional Characterization of the Selective Pan-Allele Anti-SIRPα Antibody ADU-1805 That Blocks the SIRPα-CD47 Innate Immune Checkpoint. J. Immunother. Cancer 2019, 7, 340. [Google Scholar] [CrossRef]
- Cui, X.; Ma, C.; Vasudevaraja, V.; Serrano, J.; Tong, J.; Peng, Y.; Delorenzo, M.; Shen, G.; Frenster, J.; Morales, R.-T.T.; et al. Dissecting the Immunosuppressive Tumor Microenvironments in Glioblastoma-on-a-Chip for Optimized PD-1 Immunotherapy. eLife 2020, 9, e52253. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Eissler, N.; Le Blanc, K.; Johnsen, J.I.; Kogner, P.; Kiessling, R. Targeting Suppressive Myeloid Cells Potentiates Checkpoint Inhibitors to Control Spontaneous Neuroblastoma. Clin. Cancer Res. 2016, 22, 3849–3859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Velez-Delgado, A.; Mathew, E.; Li, D.; Mendez, F.M.; Flannagan, K.; Rhim, A.D.; Simeone, D.M.; Beatty, G.L.; Di Magliano, M.P. Myeloid Cells Are Required for PD-1/PD-L1 Checkpoint Activation and the Establishment of an Immunosuppressive Environment in Pancreatic Cancer. Gut 2017, 66, 124–136. [Google Scholar] [CrossRef]
- Li, C.; Lai, C.; Qiu, Q.; Luo, X.; Hu, L.; Zheng, H.; Lu, Y.; Liu, M.; Zhang, H.; Liu, X.; et al. Dual-Ligand Modification of PEGylated Liposomes Used for Targeted Doxorubicin Delivery to Enhance Anticancer Efficacy. AAPS PharmSciTech 2019, 20, 188. [Google Scholar] [CrossRef]
- Datta, M.; Coussens, L.M.; Nishikawa, H.; Hodi, F.S.; Jain, R.K. Reprogramming the Tumor Microenvironment to Improve Immunotherapy: Emerging Strategies and Combination Therapies. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 165–174. [Google Scholar] [CrossRef]
- Sousa, S.; Auriola, S.; Mönkkönen, J.; Määttä, J. Liposome Encapsulated Zoledronate Favours M1-like Behaviour in Murine Macrophages Cultured with Soluble Factors from Breast Cancer Cells. BMC Cancer 2015, 15, 4. [Google Scholar] [CrossRef]
- Giraudo, E.; Inoue, M.; Hanahan, D. An Amino-Bisphosphonate Targets MMP-9–Expressing Macrophages and Angiogenesis to Impair Cervical Carcinogenesis. J. Clin. Investig. 2004, 114, 623–633. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.X.; Qiao, S.L.; An, H.W.; Ma, Y.; Qiao, Z.Y.; Rajapaksha, R.P.Y.J.; Wang, H. Polymeric Nanoparticles Enable Reversing Macrophage in Tumor Microenvironment for Immunotherapy. Biomaterials 2017, 112, 153–163. [Google Scholar] [CrossRef]
- He, X.Y.; Liu, B.Y.; Ai, S.L.; Xu, L.; Zhuo, R.X.; Cheng, S.X. Functional Polymer/Inorganic Hybrid Nanoparticles for Macrophage Targeting Delivery of Oligodeoxynucleotides in Cancer Immunotherapy. Mater. Today Chem. 2017, 4, 106–116. [Google Scholar] [CrossRef]
- Sun, Y.; Cronin, M.F.; Mendonça, M.C.P.; Guo, J.; O’Driscoll, C.M. Sialic Acid-Targeted Cyclodextrin-Based Nanoparticles Deliver CSF-1R SiRNA and Reprogram Tumour-Associated Macrophages for Immunotherapy of Prostate Cancer. Eur. J. Pharm. Sci. 2023, 185, 106427. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.; Castro, F.; Domingues, M.; Lage, A.; Alves, É.; de Oliveira, R.; de Melo, C.; Eduardo Calzavara-Silva, C.; Sarmento, B. Reprogramming of Tumor-Associated Macrophages by Polyaniline-Coated Iron Oxide Nanoparticles Applied to Treatment of Breast Cancer. Int. J. Pharm. 2023, 636, 122866. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, B.; Wu, L.; Xiao, H.; Ding, K.; Zheng, C.; Song, Q.; Sun, L.; Wang, L.; Zhang, Z. Amplified Cancer Immunotherapy of a Surface-Engineered Antigenic Microparticle Vaccine by Synergistically Modulating Tumor Microenvironment. ACS Nano 2019, 13, 12553–12566. [Google Scholar] [CrossRef]
- Yoon, J.; Le, X.T.; Kim, J.; Lee, H.; Nguyen, N.T.; Lee, W.T.; Lee, E.S.; Oh, K.T.; Choi, H.-G.; Youn, Y.S. Macrophage-Reprogramming Upconverting Nanoparticles for Enhanced TAM-Mediated Antitumor Therapy of Hypoxic Breast Cancer. J. Control. Release 2023, 360, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Djaldetti, M.; Salman, H.; Bergman, M.; Djaldetti, R.; Bessler, H. Phagocytosis—The Mighty Weapon of the Silent Warriors. Microsc. Res. Tech. 2002, 57, 421–431. [Google Scholar] [CrossRef]
- Kirsh, R.; Bugelski, P.J.; Poste, G. Drug Delivery to Macrophages for the Therapy of Cancer and Infectious Diseases. Ann. N. Y. Acad. Sci. 1987, 507, 141–154. [Google Scholar] [CrossRef]
- Mosaiab, T.; Farr, D.C.; Kiefel, M.J.; Houston, T.A. Carbohydrate-Based Nanocarriers and Their Application to Target Macrophages and Deliver Antimicrobial Agents. Adv. Drug Deliv. Rev. 2019, 151–152, 94–129. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Herbein, G. The Macrophage: A Therapeutic Target in HIV-1 Infection. Mol. Cell. Ther. 2014, 2, 10. [Google Scholar] [CrossRef]
- Dutta, T.; Garg, M.; Jain, N.K. Targeting of Efavirenz Loaded Tuftsin Conjugated Poly(Propyleneimine) Dendrimers to HIV Infected Macrophages in Vitro. Eur. J. Pharm. Sci. 2008, 34, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Asthana, A.; Agashe, H.B.; Agrawal, G.P.; Jain, N.K. Stavudine-Loaded Mannosylated Liposomes: In-Vitro Anti-HIV-I Activity, Tissue Distribution and Pharmacokinetics. J. Pharm. Pharmacol. 2010, 58, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Adlin Jino Nesalin, J.; Anton Smith, A. Preparation and Evaluation of Stavudine Loaded Chitosan Nanoparticles. J. Pharm. Res. 2013, 6, 268–274. [Google Scholar] [CrossRef]
- Dev, A.; Binulal, N.S.; Anitha, A.; Nair, S.V.; Furuike, T.; Tamura, H.; Jayakumar, R. Preparation of Poly(Lactic Acid)/Chitosan Nanoparticles for Anti-HIV Drug Delivery Applications. Carbohydr. Polym. 2010, 80, 833–838. [Google Scholar] [CrossRef]
- Varshosaz, J.; Taymouri, S.; Jafari, E.; Jahanian-Najafabadi, A.; Taheri, A. Formulation and Characterization of Cellulose Acetate Butyrate Nanoparticles Loaded with Nevirapine for HIV Treatment. J. Drug Deliv. Sci. Technol. 2018, 48, 9–20. [Google Scholar] [CrossRef]
- Ramana, L.N.; Sharma, S.; Sethuraman, S.; Ranga, U.; Krishnan, U.M. Evaluation of Chitosan Nanoformulations as Potent Anti-HIV Therapeutic Systems. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 476–484. [Google Scholar] [CrossRef]
- Pieters, J. Mycobacterium Tuberculosis and the Macrophage: Maintaining a Balance. Cell Host Microbe 2008, 3, 399–407. [Google Scholar] [CrossRef]
- Gairola, A.; Benjamin, A.; Weatherston, J.D.; Cirillo, J.D.; Wu, H. Recent Developments in Drug Delivery for Treatment of Tuberculosis by Targeting Macrophages. Adv. Ther. 2022, 5, 2100193. [Google Scholar] [CrossRef]
- Mukhtar, M.; Csaba, N.; Robla, S.; Varela-Calviño, R.; Nagy, A.; Burian, K.; Kókai, D.; Ambrus, R. Dry Powder Comprised of Isoniazid-Loaded Nanoparticles of Hyaluronic Acid in Conjugation with Mannose-Anchored Chitosan for Macrophage-Targeted Pulmonary Administration in Tuberculosis. Pharmaceutics 2022, 14, 1543. [Google Scholar] [CrossRef]
- Kaye, P.; Scott, P. Leishmaniasis: Complexity at the host–pathogen interface. Nat. Rev. Microbiol. 2011, 9, 604–615. [Google Scholar] [CrossRef]
- Kunjachan, S.; Jose, S.; Thomas, C.A.; Joseph, E.; Kiessling, F.; Lammers, T. Physicochemical and Biological Aspects of Macrophage-Mediated Drug Targeting in Anti-Microbial Therapy. Fundam. Clin. Pharmacol. 2012, 26, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.J.; Din, F.U.; Khan, G.M. Sodium Stibogluconate Loaded Nano-Deformable Liposomes for Topical Treatment of Leishmaniasis: Macrophage as a Target Cell. Drug Deliv. 2018, 25, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, F.; Motazedian, M.H.; Asgari, Q.; Morowvat, M.H.; Molaei, M.; Heli, H. Erratum: Paromomycin-Loaded Mannosylated Chitosan Nanoparticles: Synthesis, Characterization and Targeted Drug Delivery against Leishmaniasis (Acta Tropica (2019) 197 (105045) PII: S0001-706X(19)30864-2). Acta Trop. 2019, 197, 105072. [Google Scholar] [CrossRef] [PubMed]
- Dowari, P.; Roy, S.; Das, S.; Chowdhuri, S.; Kushwaha, R.; Das, B.K.; Ukil, A.; Das, D. Mannose-Decorated Composite Peptide Hydrogel with Thixotropic and Syneresis Properties and Its Application in Treatment of Leishmaniasis. Chem. Asian J. 2022, 17, e202200550. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Kudryashova, E.V. Computer Simulation of the Receptor–Ligand Interactions of Mannose Receptor CD206 in Comparison with the Lectin Concanavalin A Model. Biochemistry 2022, 87, 54–69. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Ezhov, A.A.; Vigovskiy, M.A.; Grigorieva, O.A.; Dyachkova, U.D.; Belogurova, N.G.; Kudryashova, E.V. Application Prospects of FTIR Spectroscopy and CLSM to Monitor the Drugs Interaction with Bacteria Cells Localized in Macrophages for Diagnosis and Treatment Control of Respiratory Diseases. Diagnostics 2023, 13, 698. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Kudryashova, E.V. Spectroscopy Approach for Highly-Efficient Screening of Lectin-Ligand Interactions in Application for Mannose Receptor and Molecular Containers for Antibacterial Drugs. Pharmaceuticals 2022, 15, 625. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Vanichkin, D.A.; Kudryashova, E.V. Methods for Determining the Parameters of Receptor-Ligand Interactions on the Model of Concanavalin A and Mannosylated Chitosans Promising Carriers for Drug Delivery to Alveolar Macrophages. Biotekhnologiya 2021, 37, 28–40. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Davydova, M.P.; Danilov, M.R.; Krylov, S.S.; Belogurova, N.G.; Kudryashova, E.V. Covalent Conjugates of Allylbenzenes and Terpenoids as Antibiotics Enhancers with the Function of Prolonged Action. Pharmaceuticals 2023, 16, 1102. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Ezhov, A.A.; Ferberg, A.S.; Krylov, S.S.; Semenova, M.N.; Semenov, V.V.; Kudryashova, E.V. Polymeric Micelles Formulation of Combretastatin Derivatives with Enhanced Solubility, Cytostatic Activity and Selectivity against Cancer Cells. Pharmaceutics 2023, 15, 1613. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Malashkeevich, S.M.; Belogurova, N.G.; Kudryashova, E.V. Thermoreversible Gels Based on Chitosan Copolymers as “Intelligent” Drug Delivery System with Prolonged Action for Intramuscular Injection. Pharmaceutics 2023, 15, 1478. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Vigovskiy, M.A.; Davydova, M.P.; Danilov, M.R.; Dyachkova, U.D.; Grigorieva, O.A.; Kudryashova, E.V. Mannosylated Systems for Targeted Delivery of Antibacterial Drugs to Activated Macrophages. Int. J. Mol. Sci. 2022, 23, 16144. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Belogurova, N.G.; Krylov, S.S.; Semenova, M.N.; Semenov, V.V.; Kudryashova, E.V. Plant Alkylbenzenes and Terpenoids in the Form of Cyclodextrin Inclusion Complexes as Antibacterial Agents and Levofloxacin Synergists. Pharmaceuticals 2022, 15, 861. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Streltsov, D.A.; Belogurova, N.G.; Kudryashova, E.V. Chitosan or Cyclodextrin Grafted with Oleic Acid Self-Assemble into Stabilized Polymeric Micelles with Potential of Drug Carriers. Life 2023, 13, 446. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Streltsov, D.A.; Ezhov, A.A.; Kudryashova, E.V. Smart pH- and Temperature-Sensitive Micelles Based on Chitosan Grafted with Fatty Acids to Increase the Efficiency and Selectivity of Doxorubicin and Its Adjuvant Regarding the Tumor Cells. Pharmaceutics 2023, 15, 1135. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Dobryakova, N.V.; Ezhov, A.A.; Kudryashova, E.V. Achievement of the Selectivity of Cytotoxic Agents against Cancer Cells by Creation of Combined Formulation with Terpenoid Adjuvants as Prospects to Overcome Multidrug Resistance. Int. J. Mol. Sci. 2023, 24, 8023. [Google Scholar] [CrossRef]
- Le-Deygen, I.M.; Skuredina, A.A.; Uporov, I.V.; Kudryashova, E.V. Thermodynamics and Molecular Insight in Guest–Host Complexes of Fluoroquinolones with β-Cyclodextrin Derivatives, as Revealed by ATR-FTIR Spectroscopy and Molecular Modeling Experiments. Anal. Bioanal. Chem. 2017, 409, 6451–6462. [Google Scholar] [CrossRef]
- Hill, L.E.; Gomes, C.; Taylor, T.M. Characterization of Beta-Cyclodextrin Inclusion Complexes Containing Essential Oils (Trans-Cinnamaldehyde, Eugenol, Cinnamon Bark, and Clove Bud Extracts) for Antimicrobial Delivery Applications. LWT 2013, 51, 86–93. [Google Scholar] [CrossRef]
- Cardoso, N.N.R.; Alviano, C.S.; Blank, A.F.; Romanos, M.T.V.; Fonseca, B.B.; Rozental, S.; Rodrigues, I.A.; Alviano, D.S. Synergism Effect of the Essential Oil from Ocimum Basilicum Var. Maria Bonita and Its Major Components with Fluconazole and Its Influence on Ergosterol Biosynthesis. Evid. Based Complement. Altern. Med. 2016, 2016, 5647182. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef] [PubMed]
- Boire, N.A.; Riedel, S.; Parrish, N.M. Essential Oils and Future Antibiotics: New Weapons against Emerging’Superbugs’? J. Anc. Dis. Prev. Rem. 2013, 1, 105. [Google Scholar] [CrossRef]
- Samet, A.V.; Shevchenko, O.G.; Rusak, V.V.; Chartov, E.M.; Myshlyavtsev, A.B.; Rusanov, D.A.; Semenova, M.N.; Semenov, V.V. Antioxidant Activity of Natural Allylpolyalkoxybenzene Plant Essential Oil Constituents. J. Nat. Prod. 2019, 82, 1451–1458. [Google Scholar] [CrossRef]
- Bin Yoo, C.; Han, K.T.; Cho, K.S.; Ha, J.; Park, H.J.; Nam, J.H.; Kil, U.H.; Lee, K.T. Eugenol Isolated from the Essential Oil of Eugenia Caryophyllata Induces a Reactive Oxygen Species-Mediated Apoptosis in HL-60 Human Promyelocytic Leukemia Cells. Cancer Lett. 2005, 225, 41–52. [Google Scholar] [CrossRef]
- Tadtong, S.; Watthanachaiyingcharoen, R.; Kamkaen, N. Antimicrobial Constituents and Synergism Effect of the Essential Oils from Cymbopogon Citratus and Alpinia Galanga. Nat. Prod. Commun. 2014, 9, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Teles, A.M.; Silva-Silva, J.V.; Fernandes, J.M.P.; Abreu-Silva, A.L.; Calabrese, K.D.S.; Mendes Filho, N.E.; Mouchrek, A.N.; Almeida-Souza, F. GC-MS Characterization of Antibacterial, Antioxidant, and Antitrypanosomal Activity of Syzygium Aromaticum Essential Oil and Eugenol. Evid. Based Complement. Altern. Med. 2021, 2021, 6663255. [Google Scholar] [CrossRef]
- Arana-Sánchez, A.; Estarrón-Espinosa, M.; Obledo-Vázquez, E.N.; Padilla-Camberos, E.; Silva-Vázquez, R.; Lugo-Cervantes, E. Antimicrobial and Antioxidant Activities of Mexican Oregano Essential Oils (Lippia Graveolens H. B. K.) with Different Composition When Microencapsulated Inβ-Cyclodextrin. Lett. Appl. Microbiol. 2010, 50, 585–590. [Google Scholar] [CrossRef]
- Herman, A.; Tambor, K.; Herman, A. Linalool Affects the Antimicrobial Efficacy of Essential Oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef]
- Razzaghi-Abyaneh, M.; Yoshinari, T.; Shams-Ghahfarokhi, M.; Rezaee, M.B.; Nagasawa, H.; Sakuda, S. Dillapiol and Apiol as Specific Inhibitors of the Biosynthesis of Aflatoxin G1 in Aspergillus Parasiticus. Biosci. Biotechnol. Biochem. 2007, 71, 2329–2332. [Google Scholar] [CrossRef]
- Semenov, V.V.; Rusak, V.V.; Chartov, E.M.; Zaretskii, M.I.; Konyushkin, L.D.; Firgang, S.I.; Chizhov, A.O.; Elkin, V.V.; Latin, N.N.; Bonashek, V.M.; et al. Polyalkoxybenzenes from Plant Raw Materials 1. Isolation of Polyalkoxybenzenes from CO2 Extracts of Umbelliferae Plant Seeds. Russ. Chem. Bull. 2007, 56, 2448–2455. [Google Scholar] [CrossRef]
- Leite, A.M.; Lima, E.D.O.; De Souza, E.L.; Diniz, M.D.F.F.M.; Trajano, V.N.; De Medeiros, I.A. Inhibitory Effect of β-Pinene, α-Pinene and Eugenol on the Growth of Potential Infectious Endocarditis Causing Gram-Positive Bacteria. Braz. J. Pharm. Sci. 2007, 43, 121–126. [Google Scholar] [CrossRef]
- Puiu, R.A.; Bîrcă, A.C.; Grumezescu, V.; Duta, L.; Oprea, O.C.; Holban, A.M.; Hudiță, A.; Gălățeanu, B.; Balaure, P.C.; Grumezescu, A.M.; et al. Multifunctional Polymeric Biodegradable and Biocompatible Coatings Based on Silver Nanoparticles: A Comparative In Vitro Study on Their Cytotoxicity towards Cancer and Normal Cell Lines of Cytostatic Drugs versus Essential-Oil-Loaded Nanoparticles and on Their Antimicrobial and Antibiofilm Activities. Pharmaceutics 2023, 15, 1882. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Kudryashova, E.V. Mannose Receptors of Alveolar Macrophages as a Target for the Addressed Delivery of Medicines to the Lungs. Russ. J. Bioorg. Chem. 2022, 48, 46–75. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Savchenko, I.V.; Kudryashova, E.V. Fluorescent Probes with Förster Resonance Energy Transfer Function for Monitoring the Gelation and Formation of Nanoparticles Based on Chitosan Copolymers. J. Funct. Biomater. 2023, 14, 401. [Google Scholar] [CrossRef]
- Pflumm, M.N.; Wang, J.L.; Edelman, G.M. Conformational Changes in Concanavalin A. J. Biol. Chem. 1971, 246, 4369–4370. [Google Scholar] [CrossRef]
- Skuredina, A.A.; Tychinina, A.S.; Le-Deygen, I.M.; Golyshev, S.A.; Belogurova, N.G.; Kudryashova, E.V. The Formation of Quasi-Regular Polymeric Network of Cross-Linked Sulfobutyl Ether Derivative of β-Cyclodextrin Synthesized with Moxifloxacin as a Template. React. Funct. Polym. 2021, 159, 104811. [Google Scholar] [CrossRef]
- Rodriguez-perez, A.N.A.I.; Rodriguez-tenreiro, C.; Alvarez-lorenzo, C.; Taboada, P.; Concheiro, A.; Torres-labandeira, J.J. Sertaconazole/hydroxypropyl-beta-cyclodextrin complexation: Isothermal titration calorimetry and solubility approaches. J. Pharm. Sci. 2006, 95, 1751–1762. [Google Scholar] [CrossRef]
- Castro, S.; Duff, M.; Snyder, N.L.; Morton, M.; Kumar, C.V.; Peczuh, M.W. Recognition of Septanose Carbohydrates by Concanavalin A. Org. Biomol. Chem. 2005, 3, 3869–3872. [Google Scholar] [CrossRef]
- Thiele, C.; Auerbach, D.; Jung, G.; Wenz, G. Inclusion of Chemotherapeutic Agents in Substituted β-Cyclodextrin Derivatives. J. Incl. Phenom. Macrocycl. Chem. 2011, 69, 303–307. [Google Scholar] [CrossRef]
- Du, X.; Li, Y.; Xia, Y.L.; Ai, S.M.; Liang, J.; Sang, P.; Ji, X.L.; Liu, S.Q. Insights into Protein–Ligand Interactions: Mechanisms, Models, and Methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef]
- Białas, N.; Sokolova, V.; van der Meer, S.B.; Knuschke, T.; Ruks, T.; Klein, K.; Westendorf, A.M.; Epple, M. Bacteria (E. coli) Take up Ultrasmall Gold Nanoparticles (2 Nm) as Shown by Different Optical Microscopic Techniques (CLSM, SIM, STORM). Nano Sel. 2022, 3, 1407–1420. [Google Scholar] [CrossRef]
- Faradji, A.; Bohbot, A.; Frost, H.; Schmitt-Goguel, M.; Siffert, J.C.; Dufour, P.; Eber, M.; Lallot, C.; Wiesel, M.L.; Bergerat, J.P. Phase I Study of Liposomal MTP-PE-Activated Autologous Monocytes Administered Intraperitoneally to Patients with Peritoneal Carcinomatosis. J. Clin. Oncol. 1991, 9, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Hennemann, B.; Scheibenbogen, C.; SchuUmichen, C.; Andreesen, R. Intrahepatic Adoptive Immunotherapy with Autologous Tumorcytotoxic Macrophages in Patients with Cancer. J. Immunother. 1995, 18, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Andreesen, R.; Hennemann, B.; Krause, S.W. Adoptive Immunotherapy of Cancer Using Monocyte-Derived Macrophages: Rationale, Current Status, and Perspectives. J. Leukoc. Biol. 1998, 64, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Panjwani, A.; Sison, C.; Rosen, L.; Chugh, R.; Metz, C.; Bloom, O. Pilot Study: Elevated Circulating Levels of the Proinflammatory Cytokine Macrophage Migration Inhibitory Factor in Patients with Chronic Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2013, 94, 1498–1507. [Google Scholar] [CrossRef]
- Fu, J.; Wang, D.; Mei, D.; Zhang, H.; Wang, Z.; He, B.; Zhang, Q. Macrophage mediated biomimetic delivery system for the treatment of lung metastasis of breast cancer. J. Control. Release 2015, 204, 11–19. [Google Scholar] [CrossRef]
- Bressani, R.F.; Nowacek, A.S.; Singh, S.; Balkundi, S.; Rabinow, B.; McMillan, J.; Gendelman, H.E.; Kanmogne, G.D. Pharmacotoxicology of Monocyte-Macrophage Nanoformulated Antiretroviral Drug Uptake and Carriage. Nanotoxicology 2011, 5, 592–605. [Google Scholar] [CrossRef]
- Zhao, Y.; Haney, M.J.; Gupta, R.; Bohnsack, J.P.; He, Z.; Kabanov, A.V.; Batrakova, E.V. GDNF-Transfected Macrophages Produce Potent Neuroprotective Effects in Parkinson’s Disease Mouse Model. PLoS ONE 2014, 9, e106867. [Google Scholar] [CrossRef]
- Yang, T.D.; Choi, W.; Yoon, T.H.; Lee, K.J.; Lee, J.-S.; Joo, J.H.; Lee, M.-G.; Yim, H.S.; Choi, K.M.; Kim, B.; et al. In Vivo Photothermal Treatment by the Peritumoral Injection of Macrophages Loaded with Gold Nanoshells. Biomed. Opt. Express 2016, 7, 185. [Google Scholar] [CrossRef]
- Tack, C.J.; Kleijwegt, F.S.; Van Riel, P.L.C.M.; Roep, B.O. Development of Type 1 Diabetes in a Patient Treated with Anti-TNF-α Therapy for Active Rheumatoid Arthritis. Diabetologia 2009, 52, 1442–1444. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Krishnan, V.; Mitragotri, S. Effect of Physicochemical and Surface Properties on in Vivo Fate of Drug Nanocarriers. Adv. Drug Deliv. Rev. 2019, 143, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Kudryashova, E.V. Biomimetic System Based on Reconstituted Macrophage Membranes for Analyzing and Selection of Higher-Affinity Ligands Specific to Mannose Receptor to Develop the Macrophage-Focused Medicines. Biomedicines 2023, 11, 2769. [Google Scholar] [CrossRef] [PubMed]
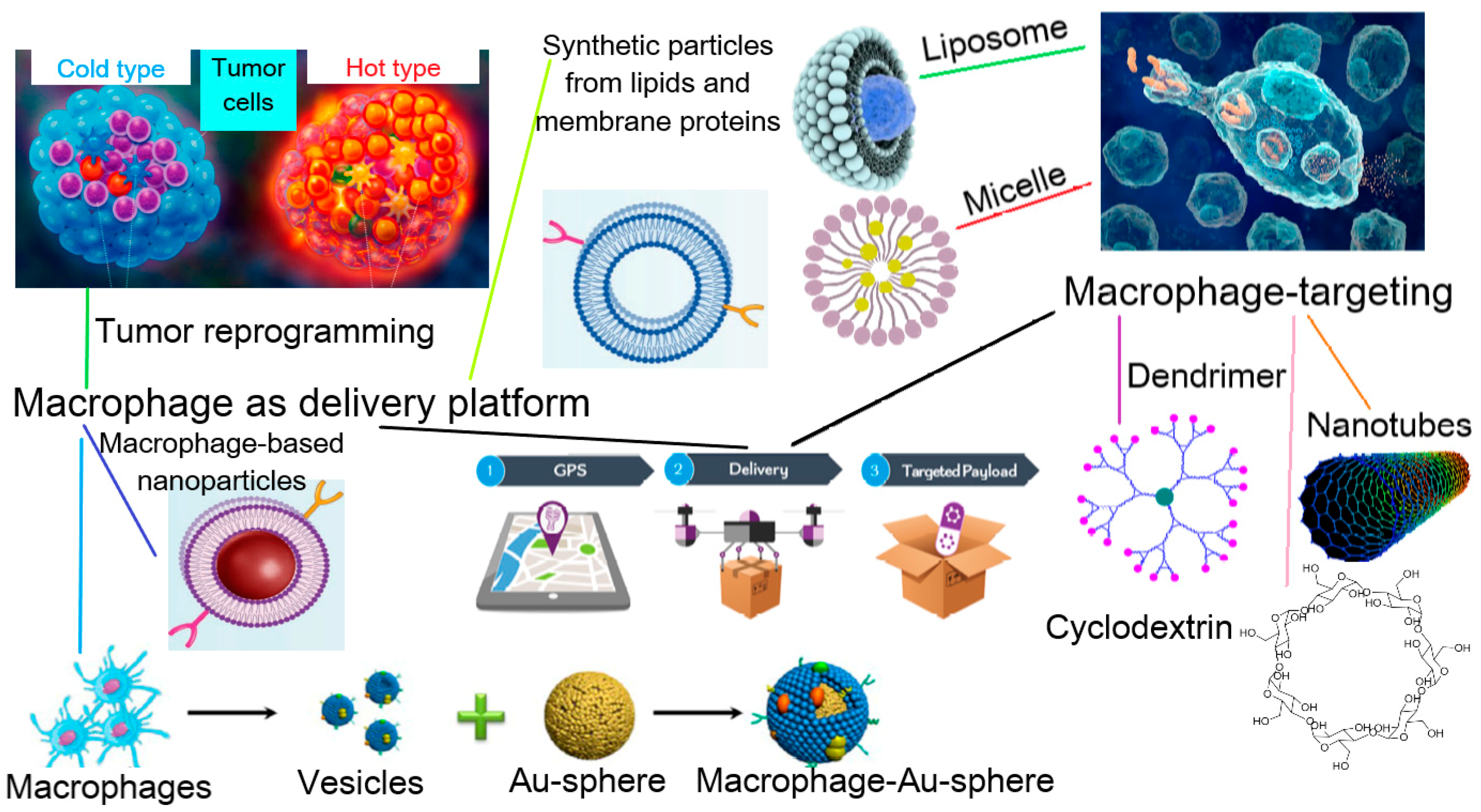
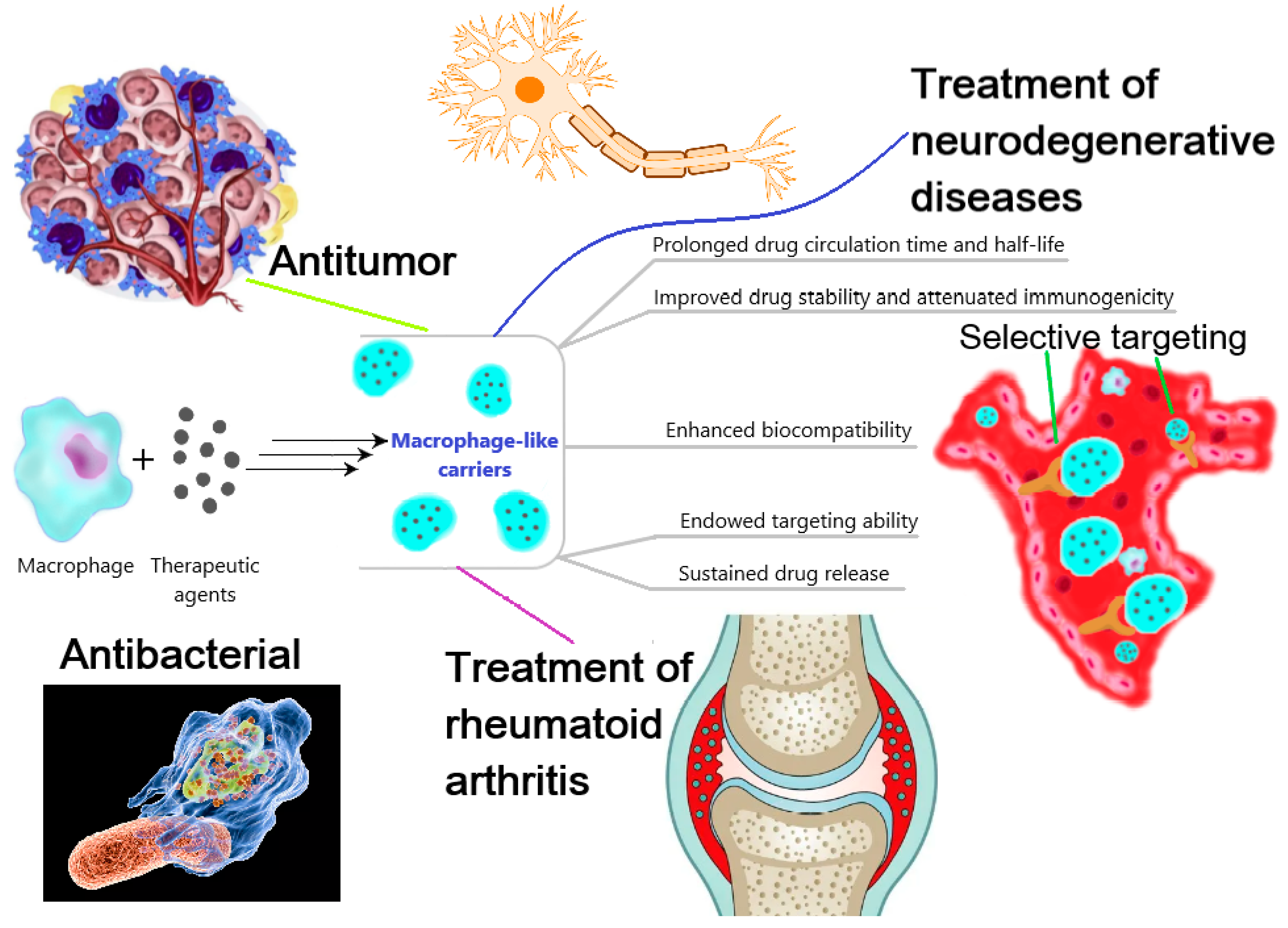

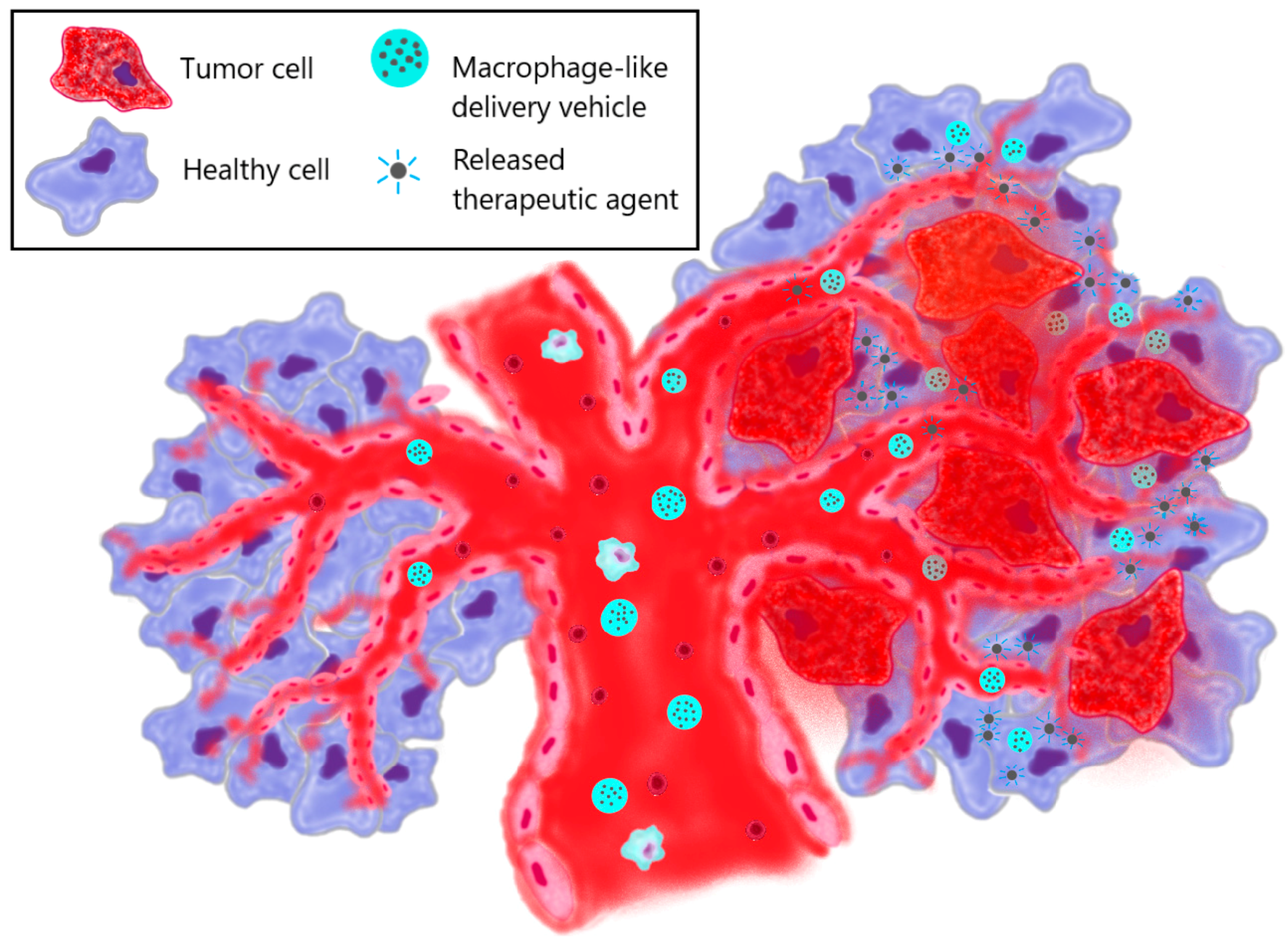
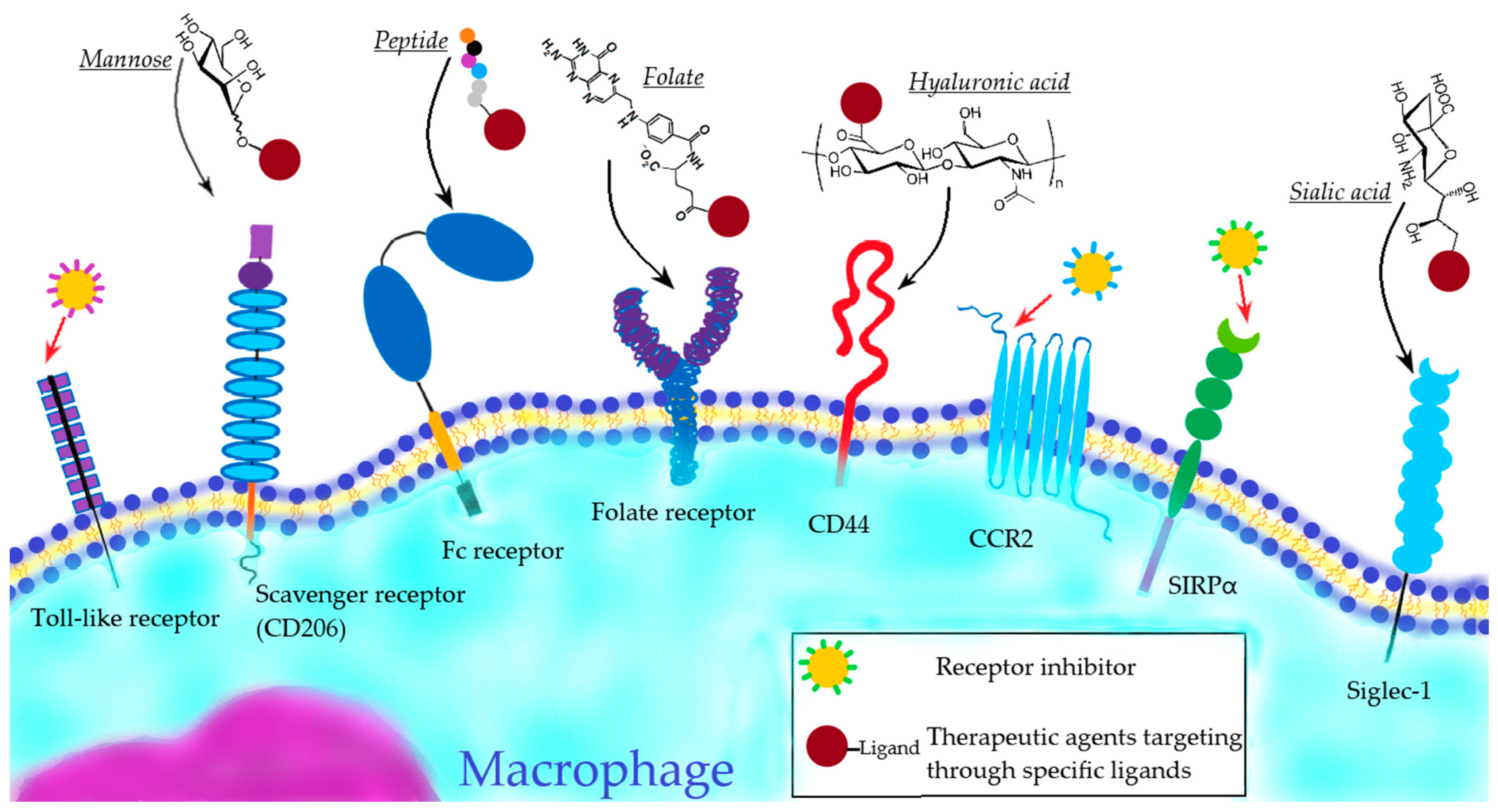

| Utilization of Living Cells | ||||||
|---|---|---|---|---|---|---|
| Method of Binding to Macrophages | Source | Carrier Particle | Cargo | Loading Efficiency (Related to the Drug) | Cell Viability | Refs. |
| Incubation (engulfment) | RAW264.7 | - | Doxorubicin (400 µg/mL) | ≈14% (after 10 s of incubation) | 79% at 72 h after incubation | [83] |
| Liposomes (diameter of 145 nm; composed of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine and 1-myristoyl-2-stearoylsn-glycero-3-phosphocholine) | AuNRs (150 µg/mL) + Doxorubicin (25 µg/mL) | 13.34% (after 6 h of incubation) 35.2% | 85% after 6 h of incubation | [81] | ||
| - | Bioengineered Salmonella typhimurium | 220 ± 13 CFU/100 cells (after 60 min of incubation) | >90% after 60 min of incubation | [101] | ||
| Mouse peritoneal macrophages | - | Doxorubicin (1–200 µg/mL) | No data | about 30–60% after 12 h of incubation | [102] | |
| Liposomes (diameter of 150 nm; composed of DPPC, DPPE, DPPG-Na and cholesterol) | Doxorubicin (1–200 µg/mL) | No data | about 80–90% at 12 h after incubation | |||
| BMM | Polymeric NPs (diameter of 0.35–2 µm; composed of PLGA) (100 µg/mL) | Nitric oxide | ≈77% (after 2 h of incubation) | ≈100% for incubation period of 24 h and 48 h | [90] | |
| Human monocyte-derived macrophages | Liposomes (size of 332 nm; composed of surfactants P188 and mPEG2000-DSPE) (100 μM) | Indinavir | 85% (after 4 h of incubation) | No effect of drug encapsulation on macrophage viability was observed | [103] | |
| Hypotonic dialysis | THP-1 | - | Catalase (osmolality of 75.67 mOsm/L during 15 min of dialysis) | 53% | 89% after encapsulation | [72] |
| Electroporation | J774 | - | Doxorubicin (20 mg/mL) | 5% (after <20 s of electroporation) | Drug-loading significantly decreased cell viability | [73] |
| Adhesion | Raw 264.7 | Multilayer microfilm (“backpack”) in disc-shaped polymer patches 7 µm in diameter (release region, magnetic region, payload region, and cell attachment region composed of BSM and JAC, PAH and MNP, PAH and CAT, and PAA and PAH-biotin, respectively) | Catalase (2.3 µU/cell backpack) | 80% (after a brief incubation with the “backpacks”) | Attachment of cell backpacks to macrophages did not alter their major functions | [104] |
| J774 | Multilayer microfilm (“backpack”) in disc-shaped polymer patches 7 µm in diameter (composed of PMA, PNIPAAM, PAH, Chitosan and Hyaluronic Acid) | Bovine serum albumin | ≈95% (after incubation with the “backpacks” for 4 h) | “Cellular backpacks” did not affect macrophage biological functions | [105] | |
| Application of Macrophage-Derived Membrane Structures | ||||||
| Source | Carrier Formulation | Cargo | Method of Encapsulation | Detected Proteins | Ref. | |
| Cellular membranes | J774 | Polymeric NPs (diameter of 84.5 nm; composed of PLGA) | - | Sonication | CD126, CD130, CD120, CD119, CD14 and TLR4 | [74] |
| Mouse peritoneal macrophages | Polymeric macrophage-membrane-coated NPs (diameter of 82.3 ± 7.5 nm; composed of IGF1R-targeting polymer cskc-PPiP) | Paclitaxel | Sonication | No data | [106] | |
| RAW264.7 | - | Methyltransferase like 14 + RS09 | Coextrusion | No data | [107] | |
| RAW 264.7 | Bi2Se3 hollow mesoporous NPs (diameter of 110 nm) | Quercetin | Coextrusion | α4 integrin, CCR2 | [108] | |
| Vesicles | RAW264.7 | - | Paclitaxel | Sonication | Alix, TSG101, CD9, iNOS, Arg-1 | [109] |
| J774A.1 | Liposomes (diameter of 100 nm; composed of L-a-phosphatidylcholine and Cholesterol) | Doxorubicin | Vortexing, sonication and coextrusion | CD81, CD63 and CD9 | [99] | |
| RAW 264.7 | Polymeric NPs (diameter of 96 ± 6.9 nm; composed of PLGA) | - | Sonication | CD45, CD14, CD44, CD18, Mac-1, etc. | [76] | |
| RAW264.7 | - | Brain-derived neurotrophic factor | Simple mixing | Alix, Tsg 101, LAMP 2 and cytosolic protein β-actin | [110] | |
| Vehicle | Carrier Formulation | Cargo | Target | Highlighted Features of Macrophage-Derived Particles | Therapeutic Effect | Refs. |
|---|---|---|---|---|---|---|
| Macrophage membrane | Chitosan NPs | - | Tumor cells: HeLa, MCF7 and MDA-MB-231 (in vitro) | Stability Biocompatibility and hemocompatibility Triggering apoptosis due to the presence of TNFα in macrophage membrane | Dose-dependent anti-tumor proliferative properties and triggering of apoptosis after 48 h of coculture | [100] |
| Macrophage | - | Doxorubicin | 4T1 mouse breast cancer cells (in vivo) | Meaningful content of the drug High targeting ability | Significant inhibition of tumor growth and increasing the survival rate among tumor-bearing mice compared to saline and DOX groups after systemic injection for 15 days on days 6, 8, 10, 13 and 15 | [83] |
| Macrophage | Poly(D,L-lactide-co-glycolide) micelles and pluronic block copolymer micelles | Paclitaxel | Human glioma cell line U87 (in vitro) | Main biological functions of macrophages were preserved Anti-tumor effect was enhanced compared to nano-paclitaxel | Significant tumor cell growth inhibition after 3 days of coculture | [130] |
| Macrophage | Poly(D,L-lactide-co-glycolide) NPs | Tirapazamine | 4T1 mouse breast cancer cells (in vivo) | Targeting ability Enhanced accumulation in hypoxic areas of tumor | Inhibition of tumor growth and extension in the median survival time compared to saline and tirapazamine groups after two injections with an interval of 3 days. Especially high efficacy was attained in the synergetic chemotherapy. | [131] |
| Macrophage | - | siRNA lipoplexes | MDA-MB-468 breast cancer model (in vivo) | Ability for horizontal gene transfer of siRNA in tumor site Anti-tumor effect was enhanced compared to pure siRNA Results indicated that exosomal secretion via M2 activation is involved with gene transfer | A significant reduction in the tumor spheres growth after single administration (no control group) | [132] |
| Macrophage | N-methacryloyl mannosamine (conjugated to macrophage surface) | Nucleic acid aptamers | CCRF-CEM tumor cells (in vitro) | Surface modification did not affect macrophage phenotype and viability The capture of tumor cells was improved | High anticancer immune response via macrophages was observed after 30 h of coculture | [133] |
| Macrophage | - | Oncolytic adenovirus | Human prostate tumor model (in vivo) | Targeting ability Accumulation in hypoxic/perinecrotic areas of the tumor | A lasting antitumor effect, enhanced in comparison with saline group, with negligible metastatic frequency was observed after 48 h of single injection | [134] |
| Macrophage membrane | Gold nanoshells (AuNSs) | Cy7 | 4T1 cancer cells (in vivo) | Active targeting ability High tumoritropic accumulation Good biocompatibility Prolonged circulation time Membrane coating did not affect NIR optical properties of AuNSs | Effective inhibition of tumor growth and its complete eradication after systemic daily injection with NIR irradiation for 25 days. Antitumor effect was enhanced in comparison with Cy7-AuNSs and saline groups | [135] |
| Macrophage | Liposomes | AuNRs + Doxorubicin | 4T1 mouse breast cancer cells (in vivo) | High targeting ability Effective infiltration into the tumor tissue High thermal sensitivity Controlled drug release via photothermal performance | Synergetic chemo- and phototherapy allowed enhanced tumor growth inhibition compared to pure liposomes and saline groups after 24 h of single injection | [81] |
| Macrophage | Liposomes | ICG (photothermal agent) + Resveratrol (anti-inflammatory drug) | 4T1 post-operative model (in vivo) | Tumor-targeting ability Good inflammatory tropism Release of the liposomes was enhanced due to membrane destruction via phototherapy Excellent photothermal performance | Ablation of residual tumor tissues, inhibiting tumor postoperative relapse and reduction in postoperative inflammation. The inhibition of tumor growth was enhanced with the delivery of macrophage-derived particles compared to liposome and saline groups after systemic injection, following NIR irradiation every 2 days for 29 days | [136] |
| Receptor Targeting | Carrier Formulation | Ligand Modification/Coating | Cargo | Purpose | Result | Refs. |
|---|---|---|---|---|---|---|
| Mannose receptor | Liposomes | Mannose | DNA | Stimulation of immune response | Mannosylated cationic liposomes exhibited significantly improved DNA delivery compared to unmodified liposomes | [167] |
| Polymeric micelles | siRNA | TAM repolarization | Modified micelles could selectively deliver efficacious amounts of functional siRNA into TAMs | [168] | ||
| Liposomes | 64Cu | PET imaging of TAMs | Highly selective accumulation of the liposomes in TAMs was observed | [169] | ||
| Selenium NPs | Isoniazid | Treatment of tuberculosis | The NPs preferentially entered macrophages and accumulated in lysosomes, releasing isoniazid | [170] | ||
| Galactose receptor | Dextran NPs | Galactose | CpG, anti-IL-10 and anti-IL-10 receptor oligonucleotides | TAM repolarization | NPs accumulated in the tumor and was taken up predominantly by TAMs | [171] |
| Chitosan-cysteine NPs | siRNA | Treatment of ulcerative colitis | Galactose modification significantly facilitated the uptake by macrophages and targeting ability of the NPs | [172] | ||
| Poly(lactic-co-glycolic acid) NPs | Dexamethasone | Development of the strategy to catch macrophages during intestinal inflammation | NPs were effectively captured by macrophages | [173] | ||
| Dectin-1 | Polymer–lipid hybrid NPs | Yeast cell wall microparticles, containing β-1,3-D-glucan | Cabazitaxel | Development of oral targeted drug delivery | The microparticles were rapidly and efficiently taken up by macrophages | [174] |
| Mesoporous silica NPs | Doxorubicin | Development of anti-tumor therapy | Drug delivery to macrophages was enhanced compared to uncoated silica NPs | [175] | ||
| Fc receptor | Alginate NPs | Tuftsin | DNA | Development of anti-inflammatory agents | Tuftsin-modified NPs were rapidly internalized in murine macrophages | [176] |
| Folate receptor-β (FRβ) | - | Anti-mouse FRβ monoclonal antibody | Pseudomonas exotoxin A | TAM depletion | Direct eliminating of TAMs was attained | [177] |
| Poly(amidoamine) dendrimers | Folic acid | Methotrexate | Alleviating of the inflammatory disease of arthritis | High degree of specific binding and internalization of the dendrimers into macrophages was observed | [178] | |
| Human serum albumin nanocapsules | - | Evaluating targeting ability of folic acid-modified agents | The internalization of nanocapsules was enhanced via FR specificity | [179] | ||
| CD44 | Hyaluronic acid–tocopherol succinate micelles | Hyaluronic acid | Rifampicin | Development of tuberculosis treatment | Micelles exhibited significant phagocytosis and a CD44-dependent uptake in comparison to free drug | [180] |
| Liposomes | Prednisolone | Development of rheumatoid arthritis therapy | Enhanced cellular uptake, mainly mediated by caveolae- and clathrin-dependent endocytosis, was achieved | [181] | ||
| Poly(lactic-co-glycolic acid) NPs | Curcumin | Alleviation of ulcerative colitis | Enhanced drug delivery to intestinal macrophages and selective accumulation in inflamed colitis tissue with minimal accumulation in healthy colon tissue was observed | [182] | ||
| Siglec-1 | Liposomes | Sialic acid | Epirubicin | Tumor therapy | The tumor-targeting efficiency and the accumulation of epirubicin in monocytes was improved compared to unmodified liposomes | [183] |
| Zoledronic acid | TAM depletion and repolarization | High targeting ability was observed | [184] |
| Method | Applications | Brief Description | Refs. |
|---|---|---|---|
| FTIR spectroscopy | Macrophage CD206 receptor—ligand interaction studies on the example of the ConA model and mannosylated polymers | The use of a model receptor protein allows for the rapid primary screening of ligands and selection of the most affine ones, and it is not necessary to isolate hard-to-reach CD206 | [278,283] |
| Drug—delivery system (to macrophages) interactions | Registration of FTIR spectra of drug complexes with different polymer ratios and calculation of dissociation constants and entrapment efficiency. Study of molecular details of binding (functional groups) | [283,284,286] | |
| Cell—drug formulation interactions. The effect of the drug on the cells. Selection of the optimal composition of the drug formulation | Provide information about the main components of the cell interacting with the drug. Using this technique, efflux and its inhibition on bacterial and cancer cells were demonstrated | [277,286] | |
| Quantification of living cells | Centrifugation of cell suspension and registration of the FTIR spectra of sediment. Low analysis time: does not require seeding of bacteria on a Petri dish | [280] | |
| Characterization of polymeric drug delivery systems | The presence of all components, and the success of crosslinking. Molecular architecture | [192,278,280,281,282,283,284,285,286,287] | |
| NMR spectroscopy | Drug interaction with the delivery system | The NMR spectrum provides valuable information about the functional groups involved | [284] |
| Characterization of polymer drug delivery systems | The presence of all components, and the success of crosslinking | [192,280,283] | |
| Fluorescence spectroscopy | Macrophage CD206 receptor—ligand interaction studies on the example of ConA model and mannosylated polymers | Quenching of tryptophan fluorescence in the receptor protein and an increase in fluorescence anisotropy during ligand binding. An alternative is using a FITC-labeled ligand | [279] |
| Inclusion of fluorophore drugs in polymer particles | Change in fluorescent properties, the position and intensity of the maximum, as well as FRET | [304] | |
| Interaction of ligands with cells, adsorption and permeability over time, and the effect of efflux inhibitors on drug permeability and retention | |||
| UV spectroscopy | Macrophage CD206 receptor—ligand interaction studies on the example of ConA model and mannosylated polymers | Change in protein uptake during ligand binding and change in secondary structure | [279] |
| Loading and release of drugs from polymer carriers | Absorption characterizes the amount of drug loaded or released from nanoparticles | [278,285] | |
| Antibacterial activity | A600 correlates with the number of colony-forming units | [280,284,285] | |
| Circular dichroism spectroscopy | Secondary structure of macrophage CD206 receptor (or its model protein on the example of ConA model) during ligand binding | Changing the circular dichroism sometimes with a cardinal reversal of the spectrum | [305] |
| Loading of chiral drugs into polymeric particles | [306] | ||
| Isothermal titration calorimetry | Study of macrophage CD206 receptor—ligand interaction studies on the example of ConA model and mannosylated polymers | Thermodynamic parameters (enthalpy, entropy and Gibbs energy) of ligand–receptor complex formation | [307,308,309,310] |
| Atomic force microscopy, SEM and TEM | Study of the morphology of nanoparticles, simulating epitopes of pathogenic microorganisms recognized by macrophages. Study of the morphology of bacterial and macrophage cells with adsorbed polymers | High-quality images providing information about the structure of nanoparticles and their effect on bacteria | [281,304,306] |
| Nanoparticle tracking analysis (NTA) | Characterization of macrophage target drug delivery system (nanoparticles) | The rate of particle movement is related to a sphere equivalent hydrodynamic radius as calculated through the Stokes–Einstein equation | [285] |
| Dynamic light scattering | Detection of polymeric nanoparticles interaction with cells surface by changing of zeta potential of bacteria and macrophages cells during polymer adsorption | The zeta potential characterizes the stability of nanoparticles. For cells, there is a recharge during the adsorption of polymers | [192,281,304] |
| Confocal laser scanning microscopy | Interaction of drug formulations with bacterial and eukaryotic (macrophage and cancerous) cells | Images from multiple cells at the micro and nanoscale. Inhibition of efflux (reverse release of drugs from cells) has been demonstrated | [192,277,311] |
| Microbiological studies | Study of the antibacterial effect of drugs, including effect on bacteria inside macrophage | The strengthening and prolonged (in vitro) of antibacterial drugs due to the addition of adjuvants to them has been demonstrated | [192,278,280,283,284,285] |
| Pharmacokinetics studies | Testing the macrophage-targeted drug delivery system in terms of the drug circulation time in the bloodstream and bio-distribution | A multiple increase in the half-life of the drug is shown, especially for covalent pro-drugs, and accumulation in the lungs | [280,283,285] |
| Flow cytometry | The existence of fluorescent nanoparticles with the drug (not debris) | [281,304,306] | |
| Nanoparticles adsorption on E. coli cells, and quantification of living cells by DAPI staining | |||
| Computer modeling | Molecular dynamics and neural network analysis of macrophage CD206 ligand and drug–polymer interaction | The study of ligands does not require synthesis in the laboratory and complex experiments—as does the primary stage of selecting candidates for drug delivery systems to macrophages. Molecular architecture of complexes, binding sites and prediction of binding energy. | [276,288] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savchenko, I.V.; Zlotnikov, I.D.; Kudryashova, E.V. Biomimetic Systems Involving Macrophages and Their Potential for Targeted Drug Delivery. Biomimetics 2023, 8, 543. https://doi.org/10.3390/biomimetics8070543
Savchenko IV, Zlotnikov ID, Kudryashova EV. Biomimetic Systems Involving Macrophages and Their Potential for Targeted Drug Delivery. Biomimetics. 2023; 8(7):543. https://doi.org/10.3390/biomimetics8070543
Chicago/Turabian StyleSavchenko, Ivan V., Igor D. Zlotnikov, and Elena V. Kudryashova. 2023. "Biomimetic Systems Involving Macrophages and Their Potential for Targeted Drug Delivery" Biomimetics 8, no. 7: 543. https://doi.org/10.3390/biomimetics8070543
APA StyleSavchenko, I. V., Zlotnikov, I. D., & Kudryashova, E. V. (2023). Biomimetic Systems Involving Macrophages and Their Potential for Targeted Drug Delivery. Biomimetics, 8(7), 543. https://doi.org/10.3390/biomimetics8070543