Bio-Inspired Self-Healing, Shear-Thinning, and Adhesive Gallic Acid-Conjugated Chitosan/Carbon Black Composite Hydrogels as Suture Support Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
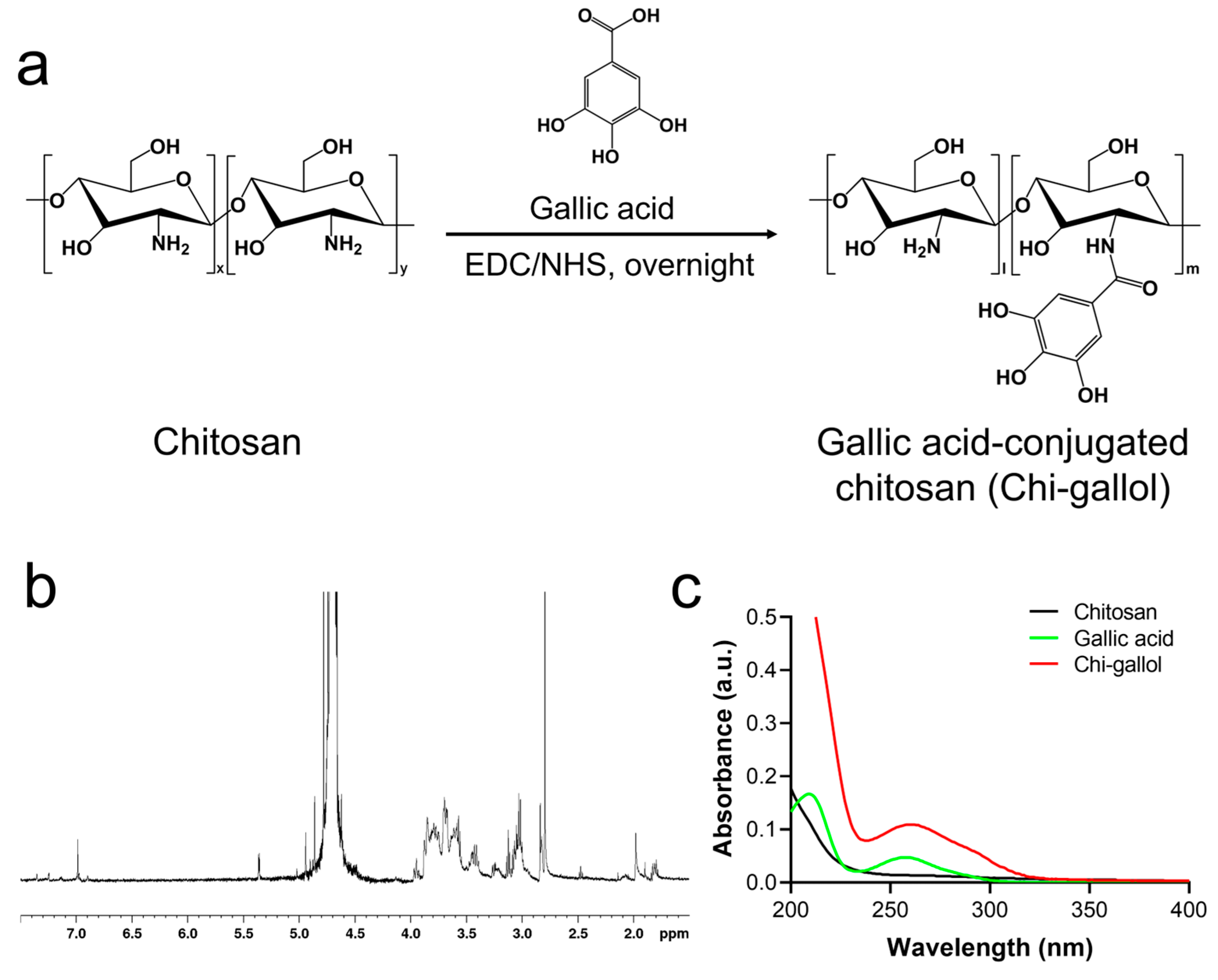
2.2. Synthesis of Chi-Gallol
2.3. 1H NMR and UV-Vis Spectroscopic Studies of Chi-Gallol
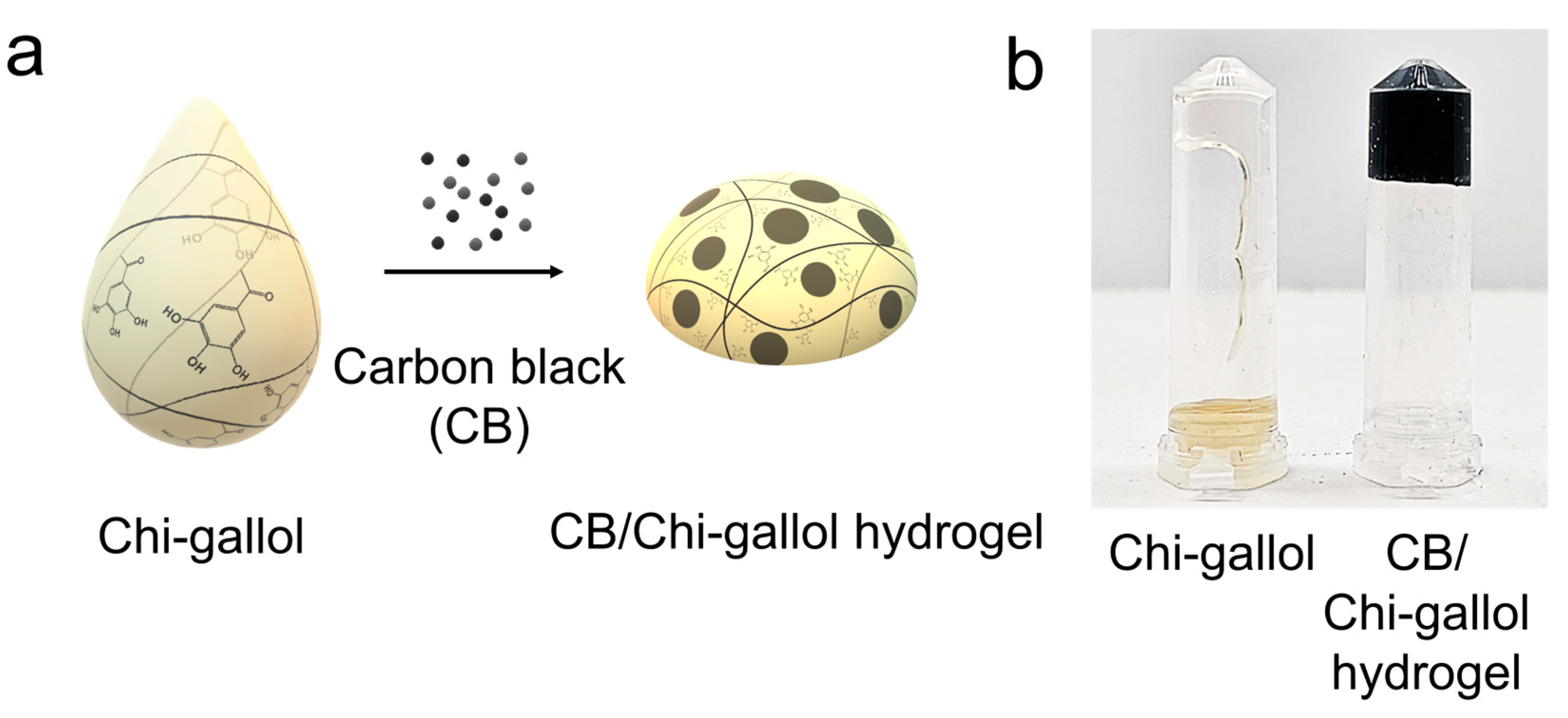
2.4. Preparation of CB/Chi-Gallol Hydrogels
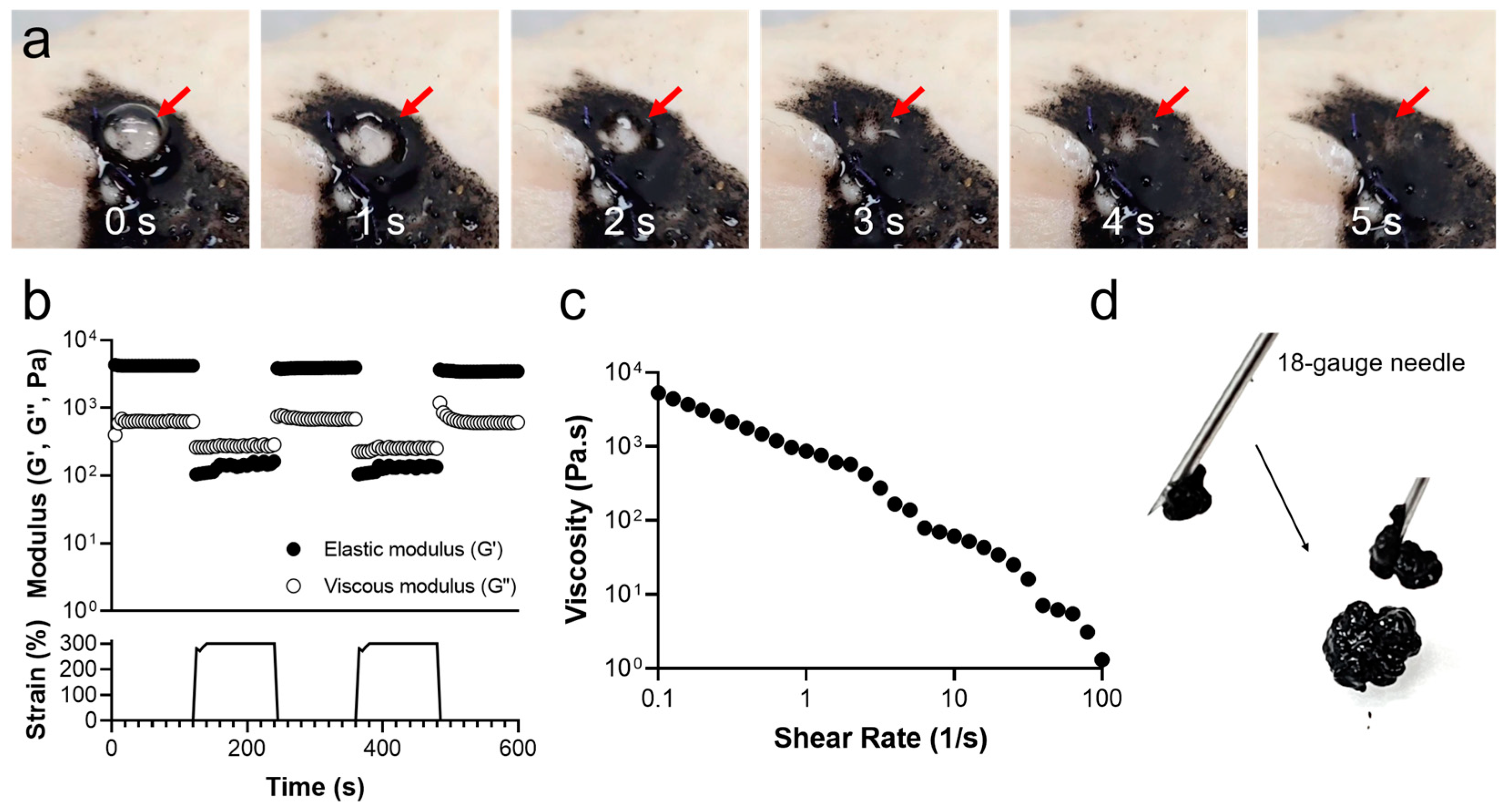
2.5. Rheological Analysis of CB/Chi-Gallol Hydrogels
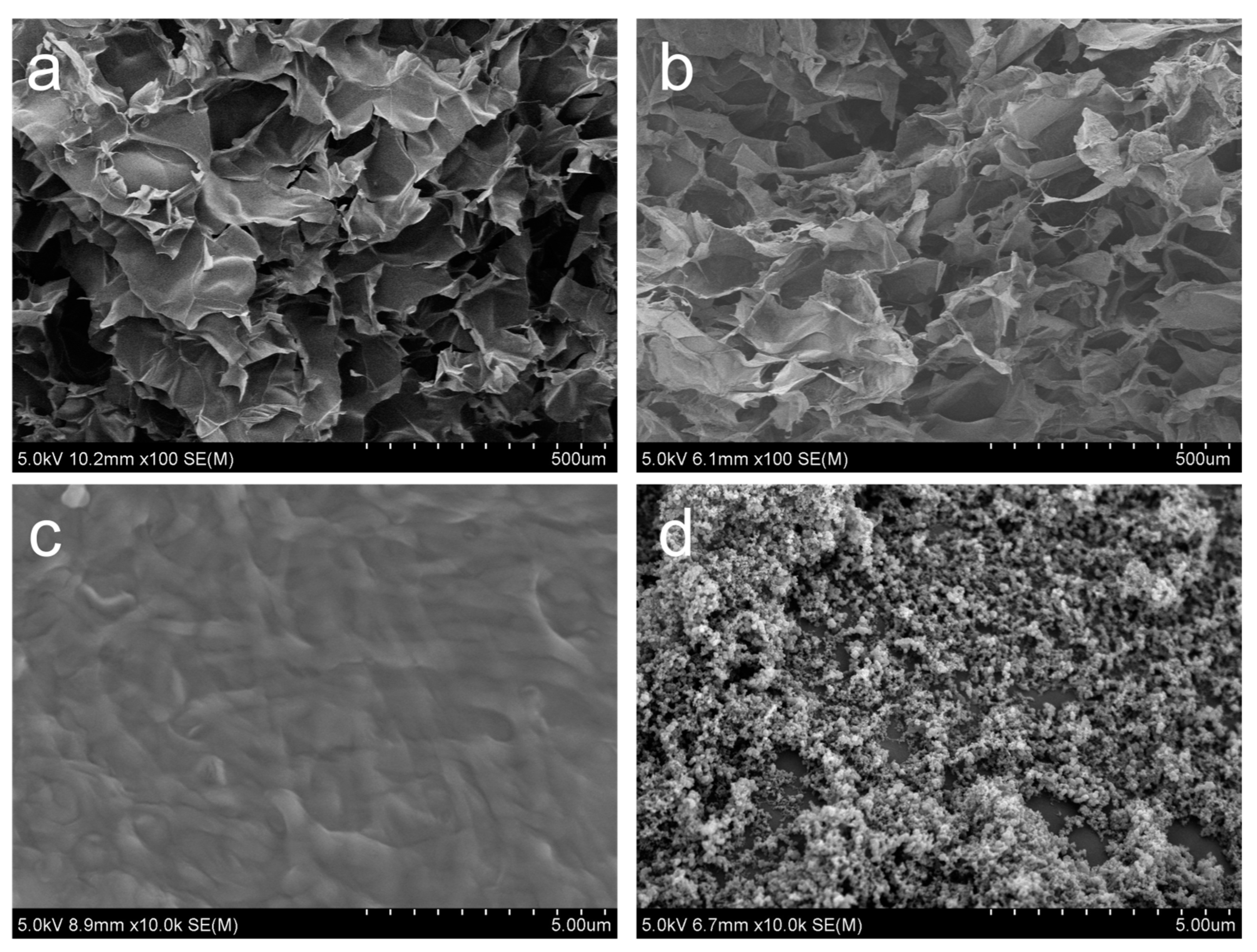
2.6. Morphological Analysis of CB/Chi-Gallol Hydrogels
2.7. Cell Viability of Extracts of CB/Chi-Gallol Hydrogels
2.8. Tissue-Adhesive Properties of CB/Chi-Gallol Hydrogels
2.9. In Vitro Bursting Pressure Measurements
2.10. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterizations of CB/Chi-Gallol Hydrogels
3.2. Self-Healing, Injectable, and Adhesive Properties of CB/Chi-Gallol Hydrogels
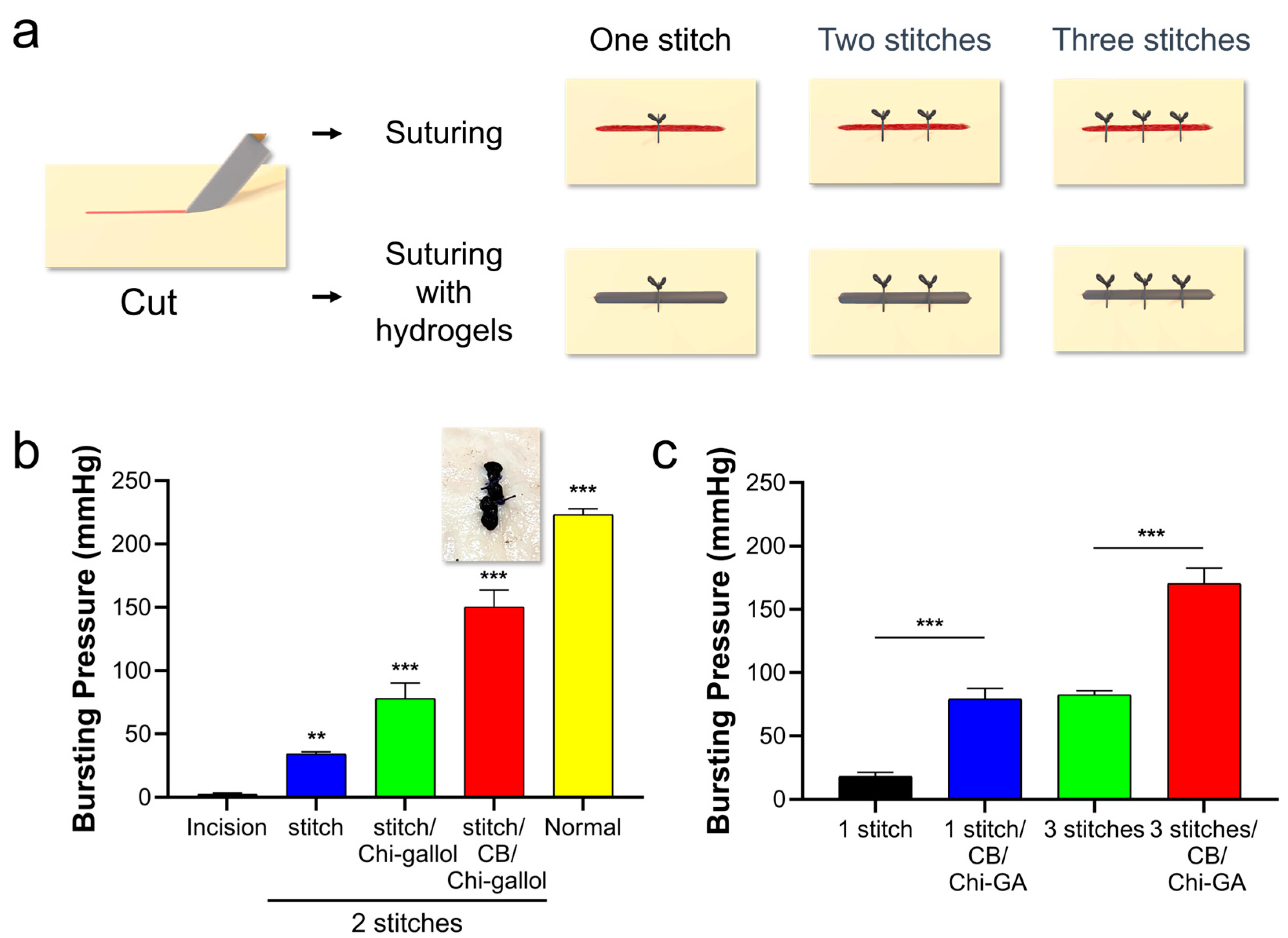
3.3. Assessments of CB/Chi-Gallol Hydrogels as Suture Support Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Moy, R.L.; Waldman, B.; Hein, D.W. A review of sutures and suturing techniques. J. Dermatol. Surg. Oncol. 1992, 18, 785–795. [Google Scholar] [CrossRef]
- Fry, D.E. The prevention of surgical site infection in elective colon surgery. Scientifica 2013, 2013, 896297. [Google Scholar] [CrossRef]
- Burkhardt, R.; Lang, N.P. Influence of suturing on wound healing. Periodontology 2000 2015, 68, 270–281. [Google Scholar] [CrossRef]
- Eto, K.; Urashima, M.; Kosuge, M.; Ohkuma, M.; Noaki, R.; Neki, K.; Ito, D.; Takeda, Y.; Sugano, H.; Yanaga, K. Standardization of surgical procedures to reduce risk of anastomotic leakage, reoperation, and surgical site infection in colorectal cancer surgery: A retrospective cohort study of 1189 patients. Int. J. Color. Dis. 2018, 33, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Blumetti, J.; Luu, M.; Sarosi, G.; Hartless, K.; McFarlin, J.; Parker, B.; Dineen, S.; Huerta, S.; Asolati, M.; Varela, E. Surgical site infections after colorectal surgery: Do risk factors vary depending on the type of infection considered? Surgery 2007, 142, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Shogan, B.D.; Carlisle, E.M.; Alverdy, J.C.; Umanskiy, K. Do we really know why colorectal anastomoses leak? J. Gastrointest. Surg. 2013, 17, 1698–1707. [Google Scholar] [CrossRef]
- Fang, A.H.; Chao, W.; Ecker, M. Review of colonic anastomotic leakage and prevention methods. J. Clin. Med. 2020, 9, 4061. [Google Scholar] [CrossRef]
- Bruce, J.; Krukowski, Z.H.; Al-Khairy, G.; Russell, E.M.; Park, K. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br. J. Surg. 2001, 88, 1157–1168. [Google Scholar] [CrossRef]
- Paun, B.C.; Cassie, S.; MacLean, A.R.; Dixon, E.; Buie, W.D. Postoperative complications following surgery for rectal cancer. Ann. Surg. 2010, 251, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Akyol, A.; McGregor, J.; Galloway, D.; Murray, G.; George, W. Recurrence of colorectal cancer after sutured and stapled large bowel anastomoses. Br. J. Surg. 1991, 78, 1297–1300. [Google Scholar] [CrossRef]
- Alberts, J.; Parvaiz, A.; Moran, B. Predicting risk and diminishing the consequences of anastomotic dehiscence following rectal resection. Colorectal. Dis. 2003, 5, 478–482. [Google Scholar] [CrossRef]
- Tsalikidis, C.; Mitsala, A.; Mentonis, V.I.; Romanidis, K.; Pappas-Gogos, G.; Tsaroucha, A.K.; Pitiakoudis, M. Predictive factors for anastomotic leakage following colorectal cancer surgery: Where are we and where are we going? Curr. Oncol. 2023, 30, 3111–3137. [Google Scholar] [CrossRef] [PubMed]
- Zarnescu, E.C.; Zarnescu, N.O.; Costea, R. Updates of risk factors for anastomotic leakage after colorectal surgery. Diagnostics 2021, 11, 2382. [Google Scholar] [CrossRef] [PubMed]
- Akasu, T.; Takawa, M.; Yamamoto, S.; Yamaguchi, T.; Fujita, S.; Moriya, Y. Risk factors for anastomotic leakage following intersphincteric resection for very low rectal adenocarcinoma. J. Gastrointest. Surg. 2010, 14, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.B.; Shogan, B.D. The science of anastomotic healing. Semin. Colon Rectal Surg. 2022, 33, 100879. [Google Scholar] [CrossRef]
- Thompson, S.K.; Chang, E.Y.; Jobe, B.A. Clinical review: Healing in gastrointestinal anastomoses, Part I. Microsurgery 2006, 26, 131–136. [Google Scholar] [CrossRef]
- Huh, J.W.; Kim, H.R.; Kim, Y.J. Anastomotic leakage after laparoscopic resection of rectal cancer: The impact of fibrin glue. Am. J. Surg. 2010, 199, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Kim, H.J.; Kim, K.; Yoon, G.; Wang, Y.; Choi, G.S.; Lee, H.; Park, J.S. Multipurpose intraperitoneal adhesive patches. Adv. Funct. Mater. 2019, 29, 1900495. [Google Scholar] [CrossRef]
- Nordentoft, T.; Rømer, J.; Sørensen, M. Sealing of gastrointestinal anastomoses with a fibrin glue-coated collagen patch: A safety study. J. Investig. Surg. 2007, 20, 363–369. [Google Scholar] [CrossRef]
- Aysan, E.; Bektas, H.; Ersoz, F.; Sari, S.; Kaygusuz, A. A novel colonic anastomosis technique involving fixed polyglycolic acid mesh. Int. J. Clin. Exp. 2010, 3, 341. [Google Scholar]
- Nordentoft, T. Sealing of gastrointestinal anastomoses with fibrin glue coated collagen patch. Dan. Med. J. 2015, 62, B5081. [Google Scholar] [PubMed]
- Dilek, O.; Bakir, B.; Dilek, F.; Demirel, H.; Yiğit, M. Protection of intestinal anastomoses in septic environment with peritoneal graft and polyglycolic acid mesh: An experimental study. Acta Chir. Belg. 1996, 96, 261–265. [Google Scholar]
- Girgin, S.; Ozturk, H.; Gedik, E.; Akpolat, V.; Kale, E.; Ozturk, H. Effect of a 50-Hz Sinusoidal electromagnetic field on the integrity of experimental colonic anastomoses covered with fibrin glue. Adv. Clin. Exp. Med. 2009, 18, 13–18. [Google Scholar]
- Wu, Z.; Vakalopoulos, K.A.; Boersema, G.S.; Kroese, L.F.; Lam, K.H.; van der Horst, P.H.; Mulder, I.M.; Bastiaansen-Jenniskens, Y.M.; Kleinrensink, G.J.; Jeekel, J.; et al. The prevention of colorectal anastomotic leakage with tissue adhesives in a contaminated environment is associated with the presence of anti-inflammatory macrophages. Int. J. Color. Dis. 2014, 29, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E.; King, M.W. Chitosan based bioadhesives for biomedical applications: A review. Carbohydr. Polym. 2022, 282, 119100. [Google Scholar] [CrossRef]
- Ryu, J.H.; Hong, S.; Lee, H. Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: A mini review. Acta Biomater. 2015, 27, 101–115. [Google Scholar] [CrossRef]
- Khan, A.; Alamry, K.A. Recent advances of emerging green chitosan-based biomaterials with potential biomedical applications: A review. Carbohydr. Res. 2021, 506, 108368. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, Y.; Jiang, Y.; Quan, W.; Luo, H.; Wu, K.; Li, S.; Ouyang, Q. Progress in research of chitosan chemical modification technologies and their applications. Mar. Drugs. 2022, 20, 536. [Google Scholar] [CrossRef]
- Adnan, S.; Ranjha, N.M.; Hanif, M.; Asghar, S. O-Carboxymethylated chitosan; A promising tool with in-vivo anti-inflammatory and analgesic properties in albino rats. Int. J Biol. Macromol. 2020, 156, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Yang, Z.; Chen, J.; Wang, D.; Zhang, Y. Recent advances in antiviral activities and potential mechanisms of sulfated polysaccharides. Carbohydr. Polym. 2021, 272, 118526. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Luo, Y. Polyphenol-chitosan conjugates: Synthesis, characterization, and applications. Carbohydr. Polym. 2016, 151, 624–639. [Google Scholar] [CrossRef] [PubMed]
- Hauptstein, S.; Bonengel, S.; Griessinger, J.; Bernkop-Schnürch, A. Synthesis and characterization of pH tolerant and mucoadhesive (thiol–polyethylene glycol) chitosan graft polymer for drug delivery. J. Pharm. Sci. 2014, 103, 594–601. [Google Scholar] [CrossRef]
- Jayakumar, R.; Reis, R.L.; Mano, J.F. Synthesis and characterization of pH-sensitive thiol-containing chitosan beads for controlled drug delivery applications. Drug Deliv. 2007, 14, 9–17. [Google Scholar] [CrossRef]
- Abdelgawad, A.M.; El-Naggar, M.E.; Hudson, S.M.; Rojas, O.J. Fabrication and characterization of bactericidal thiol-chitosan and chitosan iodoacetamide nanofibres. Int. J. Biol. Macromol. 2017, 94, 96–105. [Google Scholar] [CrossRef]
- Park, S.G.; Li, M.-X.; Cho, W.K.; Joung, Y.K.; Huh, K.M. Thermosensitive gallic acid-conjugated hexanoyl glycol chitosan as a novel wound healing biomaterial. Carbohydr. Polym. 2021, 260, 117808. [Google Scholar] [CrossRef]
- Ramirez-Barron, S.N.; Sanchez-Valdes, S.; Betancourt, R.; Gallardo, C.A.; Puente-Urbina, B.; Rodriguez-Fernández, O.S.; Carneiro-da Cunha, M.G.; dos Santos-Correia, M.T.; Sanchez-Martinez, Z.V. Preparation and characterization of gelatin-gallic acid/ZnO nanocomposite with antibacterial properties as a promising multi-functional bioadhesive for wound dressing applications. Int. J. Adhes. 2021, 104, 102749. [Google Scholar] [CrossRef]
- Puertas-Bartolomé, M.; Vázquez-Lasa, B.; San Román, J. Bioactive and bioadhesive catechol conjugated polymers for tissue regeneration. Polymers 2018, 10, 768. [Google Scholar] [CrossRef]
- Kim, K.; Ryu, J.H.; Lee, D.Y.; Lee, H. Bio-inspired catechol conjugation converts water-insoluble chitosan into a highly water-soluble, adhesive chitosan derivative for hydrogels and LbL assembly. Biomater. Sci. 2013, 1, 783–790. [Google Scholar] [CrossRef]
- Ryu, J.H.; Lee, Y.; Kong, W.H.; Kim, T.G.; Park, T.G.; Lee, H. Catechol-functionalized chitosan/Pluronic hydrogels for tissue adhesives and hemostatic materials. Biomacromolecules 2011, 12, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Lee, Y.; Do, M.J.; Jo, S.D.; Kim, J.S.; Kim, B.-S.; Im, G.-I.; Park, T.G.; Lee, H. Chitosan-g-hematin: Enzyme-mimicking polymeric catalyst for adhesive hydrogels. Acta Biomater. 2014, 10, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Choi, J.S.; Park, E.; Eom, M.R.; Jo, S.; Lee, M.S.; Kwon, S.K.; Lee, H. Chitosan oral patches inspired by mussel adhesion. J. Control. Release 2020, 317, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Jo, S.; Koh, M.Y.; Lee, H. Bio-inspired, water-soluble to insoluble self-conversion for flexible, biocompatible, transparent, catecholamine polysaccharide thin films. Adv. Funct. Mater. 2014, 24, 7709–7716. [Google Scholar] [CrossRef]
- Sanandiya, N.D.; Lee, S.; Rho, S.; Lee, H.; Kim, I.S.; Hwang, D.S.; Sanandiya, N.D. Tunichrome-inspired pyrogallol functionalized chitosan for tissue adhesion and hemostasis. Carbohydr. Polym. 2019, 208, 77–85. [Google Scholar] [CrossRef]
- Park, G.R.; Gwak, M.A.; Choi, Y.H.; Park, W.H. pH-sensitive gallol-rich chitosan hydrogel beads for on-off controlled drug delivery. Int. J. Biol. Macromol. 2023, 240, 124346. [Google Scholar] [CrossRef]
- Gwak, M.A.; Lee, S.J.; Lee, D.; Park, S.A.; Park, W.H. Highly gallol-substituted, rapidly self-crosslinkable, and robust chitosan hydrogel for 3D bioprinting. Int. J. Biol. Macromol. 2023, 227, 493–504. [Google Scholar] [CrossRef]
- Xie, M.; Hu, B.; Wang, Y.; Zeng, X. Grafting of gallic acid onto chitosan enhances antioxidant activities and alters rheological properties of the copolymer. J. Agric. Food Chem. 2014, 62, 9128–9136. [Google Scholar] [CrossRef]
- Wu, J.; Lee, J.; Jung, J.Y.; Hwang, J.H.; Kim, K.S.; Shin, M.; Lee, H.; Park, S.H. Less-suture vascular anastomosis: Development of alternative protocols with multifunctional self-wrapping, transparent, adhesive, elastic biomaterials. Adv. Mater. 2023, 35, 2301098. [Google Scholar] [CrossRef]
- Kang, S.I.; Shin, H.H.; Yoon, G.; Park, J.S.; Ryu, J.H. Double-layer adhesives for preventing anastomotic leakage and reducing post-surgical adhesion. Mater. Today Bio 2023, 23, 100806. [Google Scholar] [CrossRef]
- Yan, A.; Lau, B.W.; Weissman, B.S.; Külaots, I.; Yang, N.Y.C.; Kane, A.B.; Hurt, R.H. Biocompatible, hydrophilic, supramolecular carbon nanoparticles for cell delivery. Adv. Mater. 2006, 18, 2373–2378. [Google Scholar] [CrossRef]
- Haider, M.K.; Sun, L.; Ullah, A.; Ullah, S.; Suzuki, Y.; Park, S.; Kato, Y.; Tamada, Y.; Kim, I.S. Polyacrylonitrile/carbon black nanoparticle/nano-hydroxyapatite (PAN/nCB/HA) composite nanofibrous matrix as a potential biomaterial scaffold for bone regenerative applications. Mater. Today Commun. 2021, 27, 102259. [Google Scholar] [CrossRef]
- Al Habis, N.; El Moumen, A.; Tarfaoui, M.; Lafdi, K. Mechanical properties of carbon black/poly(ε-caprolactone)-based tissue scaffolds. Arabian J. Chem. 2020, 13, 3210–3217. [Google Scholar] [CrossRef]
- Kwon, H.J.; Shin, H.H.; Hyun, D.H.; Yoon, G.; Park, J.S.; Ryu, J.H. Carbon black-containing self-healing adhesive hydrogels for endoscopic tattooing. Sci. Rep. 2023, 13, 1880. [Google Scholar] [CrossRef]
- Lindner, K.; Ströbele, M.; Schlick, S.; Webering, S.; Jenckel, A.; Kopf, J.; Danov, O.; Sewald, K.; Buj, C.; Creutzenberg, O.; et al. Biological effects of carbon black nanoparticles are changed by surface coating with polycyclic aromatic hydrocarbons. Part. Fibre Toxicol. 2017, 14, 8. [Google Scholar] [CrossRef]
- Fiorito, S.; Serafino, A.; Andreola, F.; Togna, A.; Togna, G. Toxicity and biocompatibility of carbon nanoparticles. J. Nanosci. Nanotechnol. 2006, 6, 591–599. [Google Scholar] [CrossRef]
- Aslam, I.; Roeffaers, M.B.J. Carbonaceous nanoparticle air pollution: Toxicity and detection in biological samples. Nanomaterials 2022, 12, 3948. [Google Scholar] [CrossRef]
- Yuan, X.; Nie, W.; He, Z.; Yang, J.; Shao, B.; Ma, X.; Zhang, X.; Bi, Z.; Sun, L.; Liang, X.; et al. Carbon black nanoparticles induce cell necrosis through lysosomal membrane permeabilization and cause subsequent inflammatory response. Theranostics 2020, 10, 4589–4605. [Google Scholar] [CrossRef]
- Kim, H.; Park, K.; Lee, M.Y. Biocompatible dispersion methods for carbon black. Toxicol. Res. 2012, 28, 209–216. [Google Scholar] [CrossRef]
- Popov, V.K.; Antonov, E.N.; Bagratashvili, V.N.; Barry, J.J.A.; Ivanov, A.L.; Konovalov, A.N.; Howdle, S.M. Biodegradable scaffolds for tissue engineering fabricated by surface selective laser sintering. IFMBE Proc. 2007, 15, 676–679. [Google Scholar]
- Chen, H.; Zheng, T.; Wu, C.; Wang, J.; Ye, F.; Cui, M.; Sun, S.; Zhang, Y.; Li, Y.; Dong, Z. A shape-adaptive gallic acid driven multifunctional adhesive hydrogel loaded with scolopin2 for wound repair. Pharmaceuticals 2022, 15, 1422. [Google Scholar] [CrossRef] [PubMed]
- Lunkov, A.; Shagdarova, B.; Konovalova, M.; Zhuikova, Y.; Drozd, N.; Il’ina, A.; Varlamov, V. Synthesis of silver nanoparticles using gallic acid-conjugated chitosan derivatives. Carbohydr. Polym. 2020, 234, 115916. [Google Scholar] [CrossRef] [PubMed]
- Bielecki, K.; Gajda, A. The causes and prevention of anastomotic leak after colorectal surgery. Klin. Onkol. 1999, 12, 25–30. [Google Scholar]
- Kingham, P.T.; Pachter, L.H. Colonic anastomotic leak: Risk factors, diagnosis, and treatment. J. Am. Coll. Surg. 2009, 208, 269–278. [Google Scholar] [CrossRef]
- Platell, C.; Barwood, N.; Dorfmann, G.; Makin, G. The incidence of anastomotic leaks in patients undergoing colorectal surgery. Color. Dis. 2007, 9, 71–79. [Google Scholar] [CrossRef]
- Telem, D.A.; Chin, E.H.; Nguyen, S.Q.; Divino, C.M. Risk factors for anastomotic leak following colorectal surgery: A case-control study. Arch. Surg. 2010, 145, 371–376. [Google Scholar] [CrossRef]
- Park, J.S.; Huh, J.W.; Park, Y.A.; Cho, Y.B.; Yun, S.H.; Kim, H.C.; Lee, W.Y. Risk factors of anastomotic leakage and long-term survival after colorectal surgery. Medicine 2016, 95, e2890. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, H.H.; Ryu, J.H. Bio-Inspired Self-Healing, Shear-Thinning, and Adhesive Gallic Acid-Conjugated Chitosan/Carbon Black Composite Hydrogels as Suture Support Materials. Biomimetics 2023, 8, 542. https://doi.org/10.3390/biomimetics8070542
Shin HH, Ryu JH. Bio-Inspired Self-Healing, Shear-Thinning, and Adhesive Gallic Acid-Conjugated Chitosan/Carbon Black Composite Hydrogels as Suture Support Materials. Biomimetics. 2023; 8(7):542. https://doi.org/10.3390/biomimetics8070542
Chicago/Turabian StyleShin, Hyun Ho, and Ji Hyun Ryu. 2023. "Bio-Inspired Self-Healing, Shear-Thinning, and Adhesive Gallic Acid-Conjugated Chitosan/Carbon Black Composite Hydrogels as Suture Support Materials" Biomimetics 8, no. 7: 542. https://doi.org/10.3390/biomimetics8070542
APA StyleShin, H. H., & Ryu, J. H. (2023). Bio-Inspired Self-Healing, Shear-Thinning, and Adhesive Gallic Acid-Conjugated Chitosan/Carbon Black Composite Hydrogels as Suture Support Materials. Biomimetics, 8(7), 542. https://doi.org/10.3390/biomimetics8070542









