Physical-Chemical and Microhardness Properties of Model Dental Composites Containing 1,2-Bismethacrylate-3-eugenyl Propane Monomer
Abstract
:1. Introduction
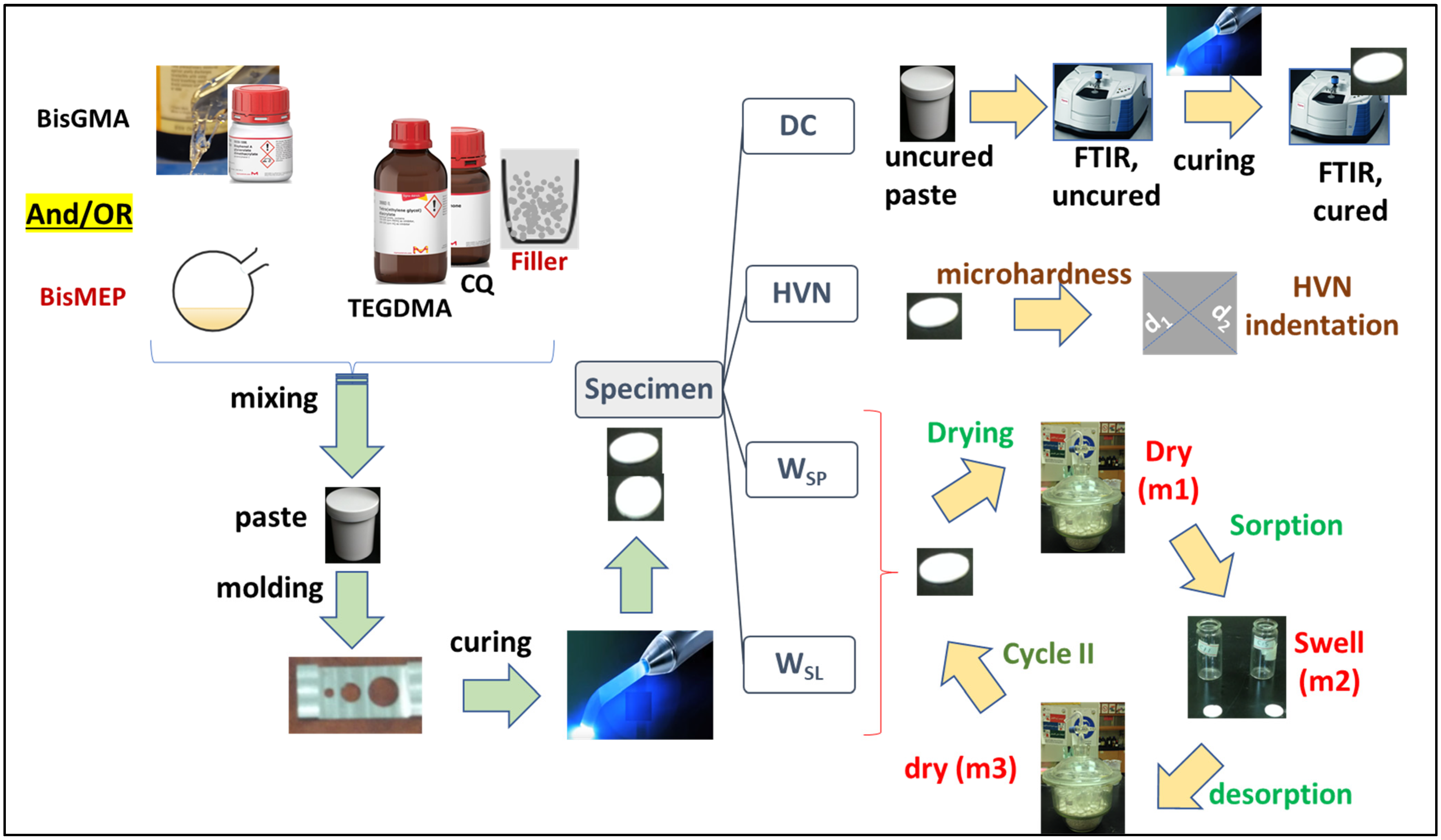
2. Materials and Methods
2.1. Chemistry-General
2.2. Preparation of Model Composites
2.3. Degree of Conversion and Dept of Cure
2.4. Vickers Hardness Test
2.5. Water Uptake and Solubility
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization
3.2. Analysis of Curing Degree
3.3. Vickers Hardness
3.4. Water Sorption and Solubility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Schricker, S. Composite resin polymerization and relevant parameters. In Orthodontic Applications of Biomaterials; Woodhead Publishing: Sawston, UK, 2017; pp. 153–170. [Google Scholar]
- Habib, E.; Wang, R.; Wang, Y.; Zhu, M.; Zhu, X. Inorganic fillers for dental resin composites: Present and future. ACS Biomater. Sci. Eng. 2016, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Barszczewska-Rybarek, I.M.; Chrószcz, M.W.; Chladek, G. Novel urethane-dimethacrylate monomers and compositions for use as matrices in dental restorative materials. Int. J. Mol. Sci. 2020, 21, 2644. [Google Scholar] [CrossRef] [PubMed]
- Geurtsen, W.; Leyhausen, G. Concise review biomaterials & bioengineering: Chemical-biological interactions of the resin monomer triethyleneglycol-dimethacrylate (TEGDMA). J. Dent. Res. 2001, 80, 2046–2050. [Google Scholar]
- Vouvoudi, E.C. Overviews on the Progress of Flowable Dental Polymeric Composites: Their Composition, Polymerization Process, Flowability and Radiopacity Aspects. Polymers 2022, 14, 4182. [Google Scholar] [CrossRef]
- Elliott, J.E.; Lovell, L.; Bowman, C. Primary cyclization in the polymerization of bis-GMA and TEGDMA: A modeling approach to understanding the cure of dental resins. Dent. Mater. 2001, 17, 221–229. [Google Scholar] [CrossRef]
- Al-Kahtani, H.M.; Al-Odayni, A.-B.; Saeed, W.S.; Robaian, A.; Al-Kahtani, A.; Aouak, T.; Alrahlah, A. Novel 1, 2-Bismethacrylate-3-Eugenyl Propane for Resin Composites: Synthesis, Characterization, Rheological, and Degree of Conversion. Polymers 2023, 15, 1481. [Google Scholar] [CrossRef]
- Al-Odayni, A.-B.; Alfotawi, R.; Khan, R.; Saeed, W.S.; Al-Kahtani, A.; Aouak, T.; Alrahlah, A. Synthesis of chemically modified BisGMA analog with low viscosity and potential physical and biological properties for dental resin composite. Dent. Mater. 2019, 35, 1532–1544. [Google Scholar] [CrossRef]
- Al-Odayni, A.-B.; Saeed, W.S.; Ahmed, A.Y.B.H.; Alrahlah, A.; Al-Kahtani, A.; Aouak, T. New monomer based on eugenol methacrylate, synthesis, polymerization and copolymerization with methyl methacrylate–characterization and thermal properties. Polymers 2020, 12, 160. [Google Scholar] [CrossRef]
- Fugolin, A.P.; de Paula, A.B.; Dobson, A.; Huynh, V.; Consani, R.; Ferracane, J.L.; Pfeifer, C.S. Alternative monomer for BisGMA-free resin composites formulations. Dent. Mater. 2020, 36, 884–892. [Google Scholar] [CrossRef]
- Riva, Y.R.; Rahman, S.F. Dental composite resin: A review. In Proceedings of the 4th Biomedical Engineering’s Recent Progress in Biomaterials, Drugs Development, Health, and Medical Devices, Padang, Indonesia, 22–24 July 2019. [Google Scholar]
- Yiu, C.; King, N.; Pashley, D.H.; Suh, B.; Carvalho, R.M.d.; Carrilho, M.; Tay, F. Effect of resin hydrophilicity and water storage on resin strength. Biomaterials 2004, 25, 5789–5796. [Google Scholar] [CrossRef]
- Contreras, R.G.; Vilchis, R.J.S.; Torres, L.S.A.; Arrocena, M.C.A.; García-Garduño, R.; de la Fuente Hernández, J. Vickers microhardness comparison of 4 composite resins with different types of filler. J. Oral Res. 2015, 4, 313–320. [Google Scholar] [CrossRef]
- da Silva Segalin, A.; Fernandez, D.M.; Roberto De Oliveira Bauer, J.; Loguercio, A.D.; Reis, A. Marginal adaptation and hardness of resin composite restorations activated with four energies. J. Esthet. Restor. Dent. 2005, 17, 303–310. [Google Scholar] [CrossRef]
- Muradbegovic, A.; Par, M.; Panduric, V.; Zugec, P.; Tauböck, T.T.; Attin, T.; Tarle, Z.; Marovic, D. Water-Induced Changes in Experimental Resin Composites Functionalized with Conventional (45S5) and Customized Bioactive Glass. J. Funct. Biomater. 2023, 14, 298. [Google Scholar] [CrossRef]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Baroudi, K.; Rodrigues, J.C. Flowable resin composites: A systematic review and clinical considerations. J. Clin. Diagn. Res. JCDR 2015, 9, ZE18. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, S.A.; Zidan, A.Z. Effect of water sorption and solubility on color stability of bulk-fill resin composite. J. Contemp. Dent. Pract. 2018, 19, 1129–1134. [Google Scholar]
- Davies, P.; Rajapakse, Y.D.; Verdu, J. Durability of Composites in A Marine Environment; Springer: Berlin/Heidelberg, Germany, 2014; Volume 10. [Google Scholar]
- David, F.; Moretti, P.; Tagliaferri, V.; Trovalusci, F. FIMEC test to evaluate the water uptake of coated and uncoated CFRP composites. Materials 2020, 13, 1154. [Google Scholar] [CrossRef]
- Merdas, I.; Thominette, F.; Tcharkhtchi, A.; Verdu, J. Factors governing water absorption by composite matrices. Compos. Sci. Technol. 2002, 62, 487–492. [Google Scholar] [CrossRef]
- Wei, Y.-J.; Silikas, N.; Zhang, Z.-T.; Watts, D.C. Diffusion and concurrent solubility of self-adhering and new resin–matrix composites during water sorption/desorption cycles. Dent. Mater. 2011, 27, 197–205. [Google Scholar] [CrossRef]
- Hampe, T.; Wiessner, A.; Frauendorf, H.; Alhussein, M.; Karlovsky, P.; Bürgers, R.; Krohn, S. Monomer Release from Dental Resins: The Current Status on Study Setup, Detection and Quantification for In Vitro Testing. Polymers 2022, 14, 1790. [Google Scholar] [CrossRef] [PubMed]
- Alrahlah, A.; Al-Odayni, A.-B.; Al-Mutairi, H.F.; Almousa, B.M.; Alsubaie, F.S.; Khan, R.; Saeed, W.S. A Low-Viscosity BisGMA Derivative for Resin Composites: Synthesis, Characterization, and Evaluation of Its Rheological Properties. Materials 2021, 14, 338. [Google Scholar] [CrossRef] [PubMed]
- Örtengren, U.; Wellendorf, H.; Karlsson, S.; Ruyter, I. Water sorption and solubility of dental composites and identification of monomers released in an aqueous environment. J. Oral Rehabil. 2001, 28, 1106–1115. [Google Scholar] [CrossRef]
- Suryawanshi, A.; Behera, N. Dental composite resin: A review of major mechanical properties, measurements and its influencing factors. Mater. Und Werkst. 2022, 53, 617–635. [Google Scholar] [CrossRef]
- Anfe, T.E.d.A.; Caneppele, T.M.F.; Agra, C.M.; Vieira, G.F. Microhardness assessment of different commercial brands of resin composites with different degrees of translucence. Braz. Oral Res. 2008, 22, 358–363. [Google Scholar] [CrossRef]
- Tapety, C.M.; Carneiro, Y.K.; Chagas, Y.M.; Souza, L.C.; de O Souza, N.; Valadas, L.A. Degree of Conversion and Mechanical Properties of a Commercial Composite with an Advanced Polymerization System. Acta Odontol. Latinoam. 2023, 36, 112. [Google Scholar] [CrossRef]
- Colombo, M.; Gallo, S.; Poggio, C.; Ricaldone, V.; Arciola, C.R.; Scribante, A. New resin-based bulk-fill composites: In vitro evaluation of micro-hardness and depth of cure as infection risk indexes. Materials 2020, 13, 1308. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Dave, F.; Mokhtari, M.; Ali, M.M.; Sherlock, R.; McIlhagger, A.; Tormey, D.; McFadden, S. On the application of Vickers micro hardness testing to isotactic polypropylene. Polymers 2022, 14, 1804. [Google Scholar] [CrossRef]
- Molina-Gutiérrez, S.; Manseri, A.; Ladmiral, V.; Bongiovanni, R.; Caillol, S.; Lacroix-Desmazes, P. Eugenol: A promising building block for synthesis of radically polymerizable monomers. Macromol. Chem. Phys. 2019, 220, 1900179. [Google Scholar] [CrossRef]
- Kaufman, T.S. The multiple faces of eugenol. A versatile starting material and building block for organic and bio-organic synthesis and a convenient precursor toward bio-based fine chemicals. J. Braz. Chem. Soc. 2015, 26, 1055–1085. [Google Scholar] [CrossRef]
- Kristufek, S.L.; Wacker, K.T.; Tsao, Y.-Y.T.; Su, L.; Wooley, K.L. Monomer design strategies to create natural product-based polymer materials. Nat. Prod. Rep. 2017, 34, 433–459. [Google Scholar] [CrossRef] [PubMed]
- Kouznetsov, V.V.; Vargas Méndez, L.Y. Synthesis of eugenol-based monomers for sustainable epoxy thermoplastic polymers. J. Appl. Polym. Sci. 2022, 139, 52237. [Google Scholar] [CrossRef]
- Rojo, L.; Vazquez, B.; Parra, J.; López Bravo, A.; Deb, S.; San Roman, J. From natural products to polymeric derivatives of “eugenol”: A new approach for preparation of dental composites and orthopedic bone cements. Biomacromolecules 2006, 7, 2751–2761. [Google Scholar] [CrossRef] [PubMed]
- Alrahlah, A.; Al-Odayni, A.-B.; Saeed, W.S.; Al-Kahtani, A.; Alkhtani, F.M.; Al-Maflehi, N.S. Water Sorption, Water Solubility, and Rheological Properties of Resin-Based Dental Composites Incorporating Immobilizable Eugenol-Derivative Monomer. Polymers 2022, 14, 366. [Google Scholar] [CrossRef]
- Shahavi, M.H.; Hosseini, M.; Jahanshahi, M.; Meyer, R.L.; Darzi, G.N. Evaluation of critical parameters for preparation of stable clove oil nanoemulsion. Arab. J. Chem. 2019, 12, 3225–3230. [Google Scholar] [CrossRef]
- Bazan, A.; Nowicki, P.; Półrolniczak, P.; Pietrzak, R. Thermal analysis of activated carbon obtained from residue after supercritical extraction of hops. J. Therm. Anal. Calorim. 2016, 125, 1199–1204. [Google Scholar] [CrossRef]
- Habib, E.; Wang, R.; Zhu, X. Monodisperse silica-filled composite restoratives mechanical and light transmission properties. Dent. Mater. 2017, 33, 280–287. [Google Scholar] [CrossRef]
- Par, M.; Spanovic, N.; Tauböck, T.T.; Attin, T.; Tarle, Z. Degree of conversion of experimental resin composites containing bioactive glass 45S5: The effect of post-cure heating. Sci. Rep. 2019, 9, 17245. [Google Scholar] [CrossRef]
- Al-Odayni, A.-B.; Saeed, W.S.; Khan, R.; Al-Kahtani, A.; Aouak, T.; Almutairi, K.; Alrahlah, A. Viscosity, Degree of Polymerization, Water Uptake, and Water Solubility Studies on Experimental Dichloro-BisGMA-Based Dental Composites. Appl. Sci. 2021, 11, 3577. [Google Scholar] [CrossRef]
- Rezaei, S.; Abbasi, M.; Sadeghi Mahounak, F.; Moradi, Z. Curing depth and degree of conversion of five bulk-fill composite resins compared to a conventional composite. Open Dent. J. 2019, 13, 422–429. [Google Scholar] [CrossRef]
- Yokesh, C.A.; Hemalatha, P.; Muthalagu, M.; Justin, M.R. Comparative evaluation of the depth of cure and degree of conversion of two bulk fill flowable composites. J. Clin. Diagn. Res. JCDR 2017, 11, ZC86. [Google Scholar] [CrossRef] [PubMed]
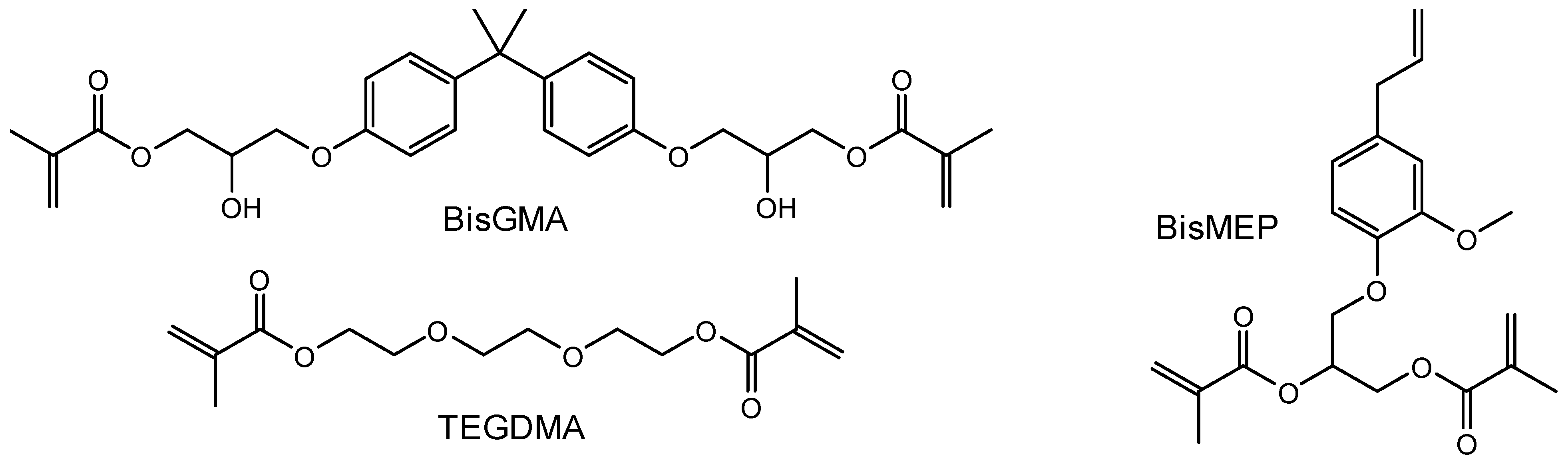
| Composite (Group) | Filler, 66 wt% | Matrix, 34 wt% | ||
|---|---|---|---|---|
| Silanized Silica | TEGDMA | BisGMA | BisMEP | |
| CEa0 | 66.00 | 17.00 | 17.00 | 0.00 |
| CEa25 | 66.00 | 17.00 | 12.75 | 4.25 |
| CEa50 | 66.00 | 17.00 | 8.50 | 8.50 |
| CEa100 | 66.00 | 17.00 | 0.00 | 17.00 |
| Composite | VHN: Mean (SD); n = 5 | WSP (%): Mean (SD); n = 3 | WSL (%): Mean (SD); n = 3 | DC1 (%): Mean (SD); n = 5 | DC2 (%): Mean (SD); n = 5 |
|---|---|---|---|---|---|
| CEa0 | 50.04 a (1.23) | 2.39 a (0.25) | 0.97 a (0.35) | 60.28 a,A (5.54) | 59.19 a,A (3.71) |
| CEa25 | 48.33 a (2.23) | 2.19 a (0.21) | 1.07 a (0.44) | 58.72 a,c,A (7.40) | 58.48 a,A (1.77) |
| CEa50 | 46.66 a (3.74) | 2.13 a (0.91) | 1.20 a (0.64) | 57.04 a,c,A (5.65) | 44.83 b,B (7.17) |
| CEa100 | 43.12 b (1.55) | 2.03 a (0.77) | 1.77 a (0.24) | 49.50 b,c,A (4.16) | 42.93 b,A (10.14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Odayni, A.-B.; Al-Kahtani, H.M.; Sharaf Saeed, W.; Al-Kahtani, A.; Aouak, T.; Khan, R.; De Vera, M.A.T.; Alrahlah, A. Physical-Chemical and Microhardness Properties of Model Dental Composites Containing 1,2-Bismethacrylate-3-eugenyl Propane Monomer. Biomimetics 2023, 8, 511. https://doi.org/10.3390/biomimetics8070511
Al-Odayni A-B, Al-Kahtani HM, Sharaf Saeed W, Al-Kahtani A, Aouak T, Khan R, De Vera MAT, Alrahlah A. Physical-Chemical and Microhardness Properties of Model Dental Composites Containing 1,2-Bismethacrylate-3-eugenyl Propane Monomer. Biomimetics. 2023; 8(7):511. https://doi.org/10.3390/biomimetics8070511
Chicago/Turabian StyleAl-Odayni, Abdel-Basit, Haifa Masfeer Al-Kahtani, Waseem Sharaf Saeed, Abdullah Al-Kahtani, Taieb Aouak, Rawaiz Khan, Merry Angelyn Tan De Vera, and Ali Alrahlah. 2023. "Physical-Chemical and Microhardness Properties of Model Dental Composites Containing 1,2-Bismethacrylate-3-eugenyl Propane Monomer" Biomimetics 8, no. 7: 511. https://doi.org/10.3390/biomimetics8070511
APA StyleAl-Odayni, A.-B., Al-Kahtani, H. M., Sharaf Saeed, W., Al-Kahtani, A., Aouak, T., Khan, R., De Vera, M. A. T., & Alrahlah, A. (2023). Physical-Chemical and Microhardness Properties of Model Dental Composites Containing 1,2-Bismethacrylate-3-eugenyl Propane Monomer. Biomimetics, 8(7), 511. https://doi.org/10.3390/biomimetics8070511









