A Bionic Venus Flytrap Soft Microrobot Driven by Multiphysics for Intelligent Transportation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
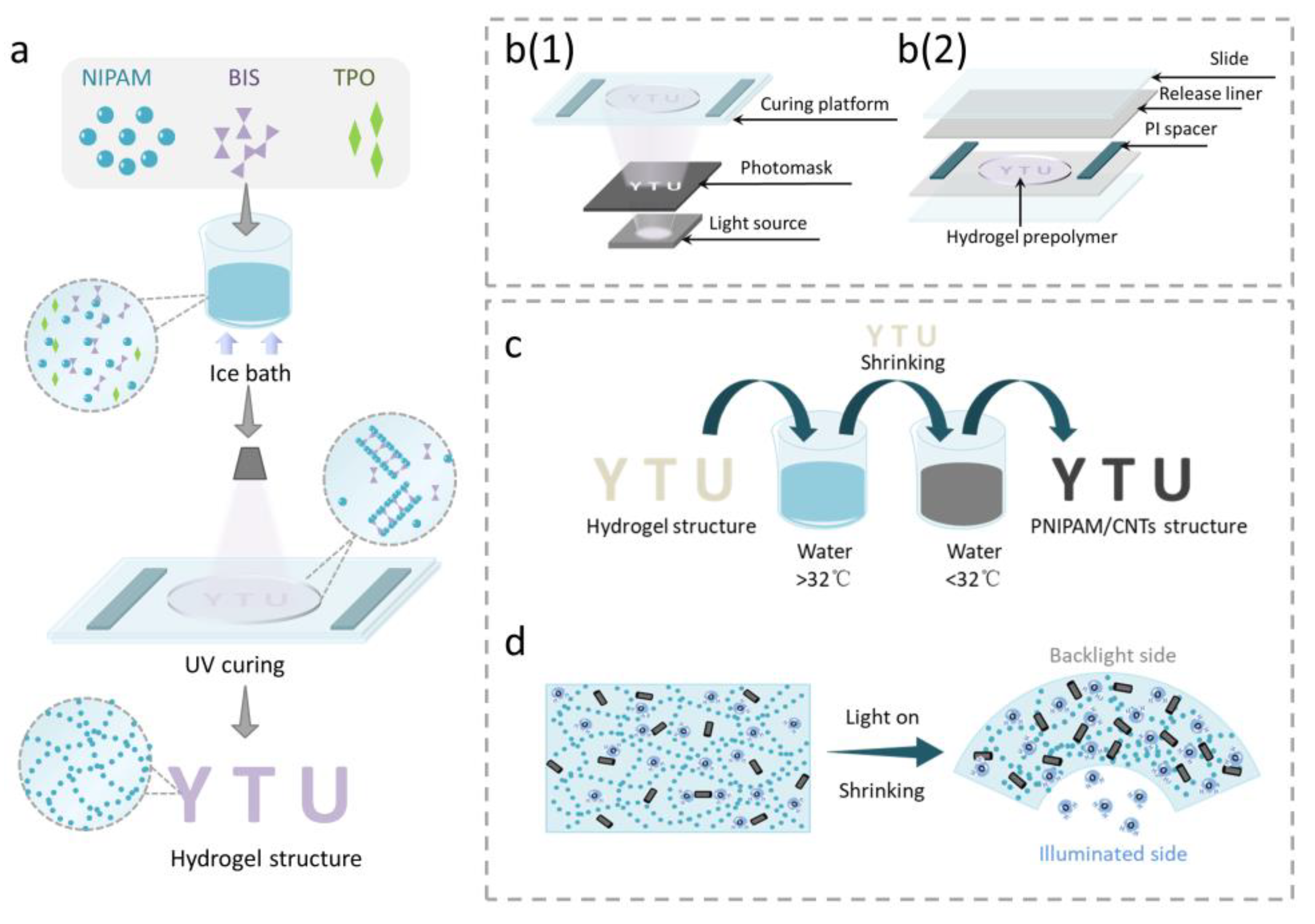
2.2. Preparation of PEGDA Prepolymer
2.3. Preparation of PNIPAM Prepolymer
2.4. Fabrication of Bilayer Structure
3. Results
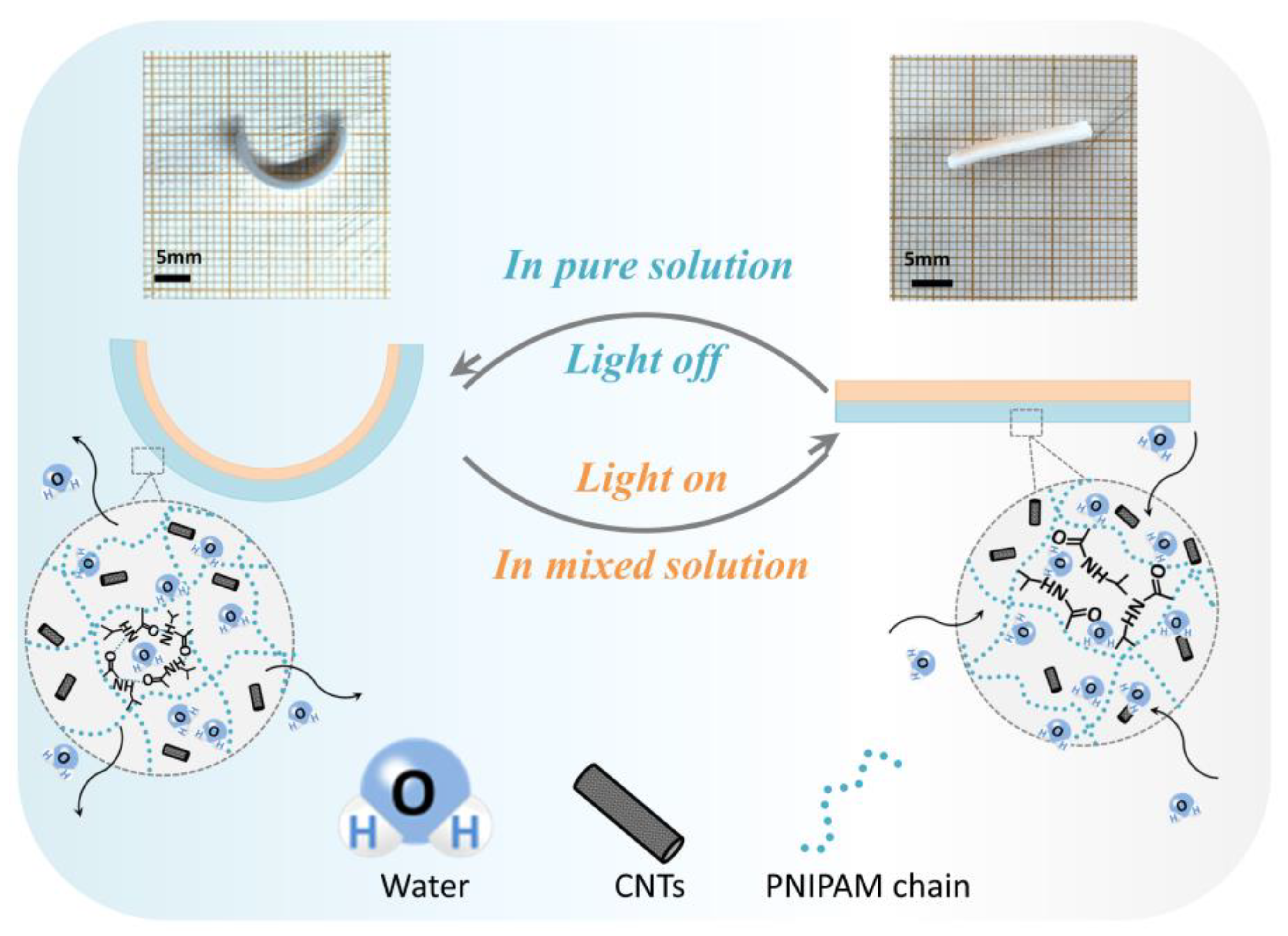
3.1. Fabrication of Light-Driven PNIPAM Sheet
3.2. Deformation Properties of PNIPAM Sheet
3.3. Research on the Swelling Ratio of PNIPAM–PEGDA Bilayer Structure
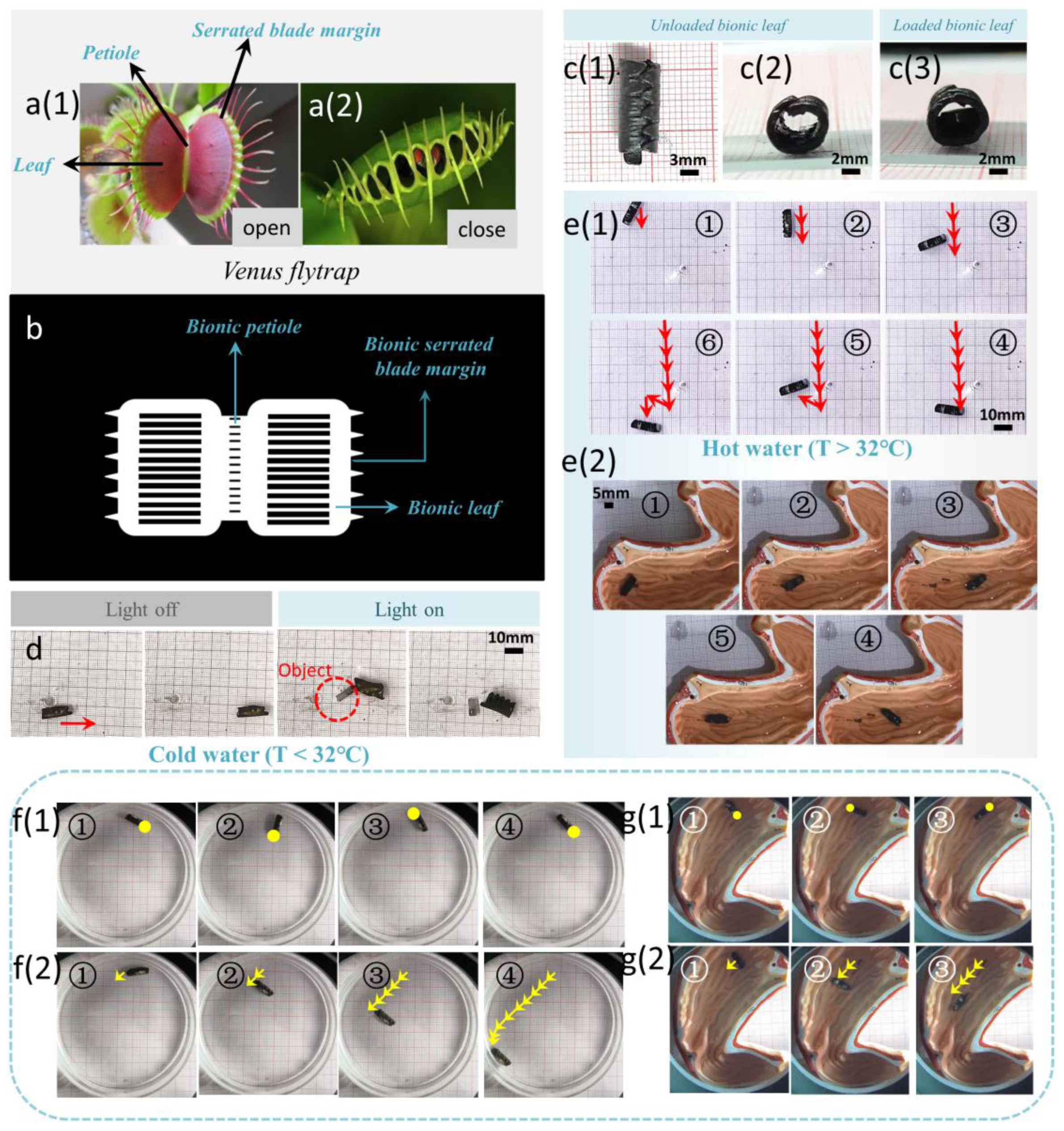
3.4. Opening and Closing of Dual Responsive Bionic Flower
3.5. Intelligent Transportation Based on the Bionic Venus Flytrap Soft Microrobot
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palacín, J.; Clotet, E.; Martínez, D.; Moreno, J.; Tresanchez, M. Automatic Supervision of Temperature, Humidity, and Luminance with an Assistant Personal Robot. J. Sens. 2017, 2017, 1480401. [Google Scholar] [CrossRef]
- Medina-Sánchez, M.; Magdanz, V.; Guix, M.; Fomin, V.M.; Schmidt, O.G. Swimming Microrobots: Soft, Reconfigurable, and Smart. Adv. Funct. Mater. 2018, 28, 1707228. [Google Scholar] [CrossRef]
- Cao, Y.; Tan, Y.J.; Li, S.; Lee, W.W.; Guo, H.; Cai, Y.; Wang, C.; Tee, B.C.K. Self-healing electronic skins for aquatic environments. Nat. Electron. 2019, 2, 75–82. [Google Scholar] [CrossRef]
- Liang, S.; Tu, Y.; Chen, Q.; Jia, W.; Wang, W.; Zhang, L. Microscopic hollow hydrogel springs, necklaces and ladders: A tubular robot as a potential vascular scavenger. Mater. Horiz. 2019, 6, 2135–2142. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.; Yang, Y.; Wang, L.; Shen, Y. Micro-rocket robot with all-optic actuating and tracking in blood. Light Sci. Appl. 2020, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Soto, F.; Wang, J.; Ahmed, R.; Demirci, U. Medical Micro/Nanorobots in Precision Medicine. Adv. Sci. 2020, 7, 2002203. [Google Scholar] [CrossRef]
- Xin, C.; Jin, D.; Hu, Y.; Yang, L.; Li, R.; Wang, L.; Ren, Z.; Wang, D.; Ji, S.; Hu, K.J.A.N. Environmentally adaptive shape-morphing microrobots for localized cancer cell treatment. ACS Nano 2021, 15, 18048–18059. [Google Scholar] [CrossRef]
- Rogóż, M.; Zeng, H.; Xuan, C.; Wiersma, D.S.; Wasylczyk, P. Light-Driven Soft Robot Mimics Caterpillar Locomotion in Natural Scale. Adv. Opt. Mater. 2016, 4, 1689–1694. [Google Scholar] [CrossRef]
- Chen, L.; Weng, M.; Zhou, P.; Zhang, L.; Huang, Z.; Zhang, W. Multi-responsive actuators based on a graphene oxide composite: Intelligent robot and bioinspired applications. Nanoscale 2017, 9, 9825–9833. [Google Scholar] [CrossRef]
- Kurumaya, S.; Phillips, B.T.; Becker, K.P.; Rosen, M.H.; Gruber, D.F.; Galloway, K.C.; Suzumori, K.; Wood, R.J. A Modular Soft Robotic Wrist for Underwater Manipulation. Soft Robot. 2018, 5, 399–409. [Google Scholar] [CrossRef]
- Du, X.; Cui, H.; Xu, T.; Huang, C.; Wang, Y.; Zhao, Q.; Xu, Y.; Wu, X. Reconfiguration, Camouflage, and Color-Shifting for Bioinspired Adaptive Hydrogel-Based Millirobots. Adv. Funct. Mater. 2020, 30, 1909202. [Google Scholar]
- Li, C.; Lau, G.C.; Yuan, H.; Aggarwal, A.; Dominguez, V.L.; Liu, S.; Sai, H.; Palmer, L.C.; Sather, N.A. Fast and programmable locomotion of hydrogel-metal hybrids under light and magnetic fields. Sci. Robot. 2020, 5, eabb9822. [Google Scholar] [CrossRef] [PubMed]
- Rehor, I.; Maslen, C.; Moerman, P.G.; van Ravensteijn, B.G.P.; van Alst, R.; Groenewold, J.; Eral, H.B.; Kegel, W.K. Photoresponsive Hydrogel Microcrawlers Exploit Friction Hysteresis to Crawl by Reciprocal Actuation. Soft Robot. 2020, 8, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.G.; Gwac, H.; Jang, Y.; Richards, C.; Warren, H.; Spinks, G.; Kim, S.J.J.M. Poly (N-isopropylacrylamide) hydrogel for diving/surfacing device. Micromachine 2021, 12, 210. [Google Scholar] [CrossRef]
- Pan, D.; Wu, D.; Li, P.-J.; Ji, S.-Y.; Nie, X.; Fan, S.-Y.; Chen, G.-Y.; Zhang, C.-C.; Xin, C.; Xu, B.; et al. Transparent Light-Driven Hydrogel Actuator Based on Photothermal Marangoni Effect and Buoyancy Flow for Three-Dimensional Motion. Adv. Funct. Mater. 2021, 31, 2009386. [Google Scholar] [CrossRef]
- Giorgio-Serchi, F.; Arienti, A.; Corucci, F.; Giorelli, M.; Laschi, C. Hybrid parameter identification of a multi-modal underwater soft robot. Bioinspiration Biomim. 2017, 12, 025007. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, J.; Chang, L.; Yang, L.; Xu, A.; Qi, K.; Lu, P.; Wu, G.; Chen, W.; Wu, Y. Electrically and Sunlight-Driven Actuator with Versatile Biomimetic Motions Based on Rolled Carbon Nanotube Bilayer Composite. Adv. Funct. Mater. 2017, 27, 1704388. [Google Scholar] [CrossRef]
- Peng, X.; Liu, T.-Q.; Shang, C.; Jiao, C.; Wang, H.-l. Mechanically strong Janus poly(N-isopropylacrylamide)/graphene oxide hydrogels as thermo-responsive soft robots. Chin. J. Polym. Sci. 2017, 35, 1268–1275. [Google Scholar] [CrossRef]
- Shi, Q.; Xia, H.; Li, P.; Wang, Y.-S.; Wang, L.; Li, S.-X.; Wang, G.; Lv, C.; Niu, L.-G.; Sun, H.-B. Photothermal Surface Plasmon Resonance and Interband Transition-Enhanced Nanocomposite Hydrogel Actuators with Hand-Like Dynamic Manipulation. Adv. Opt. Mater. 2017, 5, 1700442. [Google Scholar] [CrossRef]
- Zeng, H.; Wani, O.M.; Wasylczyk, P.; Kaczmarek, R.; Priimagi, A. Self-Regulating Iris Based on Light-Actuated Liquid Crystal Elastomer. Adv. Mater. 2017, 29, 1701814. [Google Scholar] [CrossRef]
- Pilz da Cunha, M.; Foelen, Y.; van Raak, R.J.H.; Murphy, J.N.; Engels, T.A.P.; Debije, M.G.; Schenning, A.P.H.J. An Untethered Magnetic- and Light-Responsive Rotary Gripper: Shedding Light on Photoresponsive Liquid Crystal Actuators. Adv. Opt. Mater. 2019, 7, 1801643. [Google Scholar] [CrossRef]
- Wani, O.M.; Verpaalen, R.; Zeng, H.; Priimagi, A.; Schenning, A.P.H.J. An Artificial Nocturnal Flower via Humidity-Gated Photoactuation in Liquid Crystal Networks. Adv. Mater. 2019, 31, 1805985. [Google Scholar] [CrossRef]
- Wang, M.; Hu, X.-B.; Zuo, B.; Huang, S.; Chen, X.-M.; Yang, H. Liquid crystal elastomer actuator with serpentine locomotion. Chem. Commun. 2020, 56, 7597–7600. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, M.; Li, D.; Shen, Y. Graphene-Based Light-Driven Soft Robot with Snake-Inspired Concertina and Serpentine Locomotion. Adv. Mater. Technol. 2019, 4, 1800366. [Google Scholar] [CrossRef]
- Zhang, R.; Sherehiy, A.; Yang, Z.; Wei, D.; Harnett, C.K.; Popa, D.O. Chevbot–an untethered microrobot powered by laser for microfactory applications. In Proceedings of the 2019 International Conference on Robotics and Automation (ICRA), Montreal, QC, Canada, 20–24 May 2019; IEEE: Piscataway, NJ, USA. [Google Scholar]
- Pilz da Cunha, M.; Ambergen, S.; Debije, M.G.; Homburg, E.F.G.A.; den Toonder, J.M.J.; Schenning, A.P.H.J. A Soft Transporter Robot Fueled by Light. Adv. Sci. 2020, 7, 1902842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Klotz, J.F.; Wei, D.; Yang, Z.; Sherehiy, A.; Saadatzi, M.N.; Popa, D.O. SolarPede: A stick-and-slip, light-powered, Mobile micro-crawler. J. Micro-Bio Robot. 2020, 16, 1–12. [Google Scholar] [CrossRef]
- Bunea, A.-I.; Martella, D.; Nocentini, S.; Parmeggiani, C.; Taboryski, R.; Wiersma, D.S. Light-Powered Microrobots: Challenges and Opportunities for Hard and Soft Responsive Microswimmers. Adv. Intell. Syst. 2021, 3, 2000256. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, L.; Wang, S.; Yang, T.; Li, H. Soft Untethered Robots and Grippers Based on Humidity-Gated Magnetic-Responsive Film Actuators. ACS Appl. Polym. Mater. 2021, 3, 4726–4734. [Google Scholar] [CrossRef]
- Ju, Y.; Hu, R.; Xie, Y.; Yao, J.; Li, X.; Lv, Y.; Han, X.; Cao, Q.; Li, L.J.N.E. Reconfigurable magnetic soft robots with multimodal locomotion. Nano Energy 2021, 87, 106169. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, B.; Li, B.; Bao, L.; Sheng, W.; Ma, Y.; Ma, S.; Yu, B.; Zhou, F. Toward a Multifunctional Light-Driven Biomimetic Mudskipper-Like Robot for Various Application Scenarios. ACS Appl. Mater. Interface 2022, 14, 20291–20302. [Google Scholar] [CrossRef]
- Shen, H.; Cai, S.; Wang, Z.; Yuan, Z.; Yu, H.; Yang, W.J.J.O.B.E. A Programmable Inchworm-Inspired Soft Robot Powered by a Rotating Magnetic Field. J. Bionic Eng. 2023, 20, 506–514. [Google Scholar] [CrossRef]
- Plunkett, K.N.; Zhu, X.; Moore, J.S.; Leckband, D.E. PNIPAM Chain Collapse Depends on the Molecular Weight and Grafting Density. Langmuir 2006, 22, 4259–4266. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, S.; Zhang, Y.; Liu, J.; Yu, M.; Yu, H. Hydrogen Bond Enhances Photomechanical Swing of Liquid-Crystalline Polymer Bilayer Films. ACS Appl. Mater. Interface 2021, 13, 6585–6596. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Gao, Y.; Ma, X.; Li, W.; Yang, W. A Bionic Venus Flytrap Soft Microrobot Driven by Multiphysics for Intelligent Transportation. Biomimetics 2023, 8, 429. https://doi.org/10.3390/biomimetics8050429
Wang X, Gao Y, Ma X, Li W, Yang W. A Bionic Venus Flytrap Soft Microrobot Driven by Multiphysics for Intelligent Transportation. Biomimetics. 2023; 8(5):429. https://doi.org/10.3390/biomimetics8050429
Chicago/Turabian StyleWang, Xiaowen, Yingnan Gao, Xiaoyang Ma, Weiqiang Li, and Wenguang Yang. 2023. "A Bionic Venus Flytrap Soft Microrobot Driven by Multiphysics for Intelligent Transportation" Biomimetics 8, no. 5: 429. https://doi.org/10.3390/biomimetics8050429
APA StyleWang, X., Gao, Y., Ma, X., Li, W., & Yang, W. (2023). A Bionic Venus Flytrap Soft Microrobot Driven by Multiphysics for Intelligent Transportation. Biomimetics, 8(5), 429. https://doi.org/10.3390/biomimetics8050429





