The Flexible Armor of Chinese Sturgeon: Potential Contribution of Fish Skin on Fracture Toughness and Flexural Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Morphology Observation
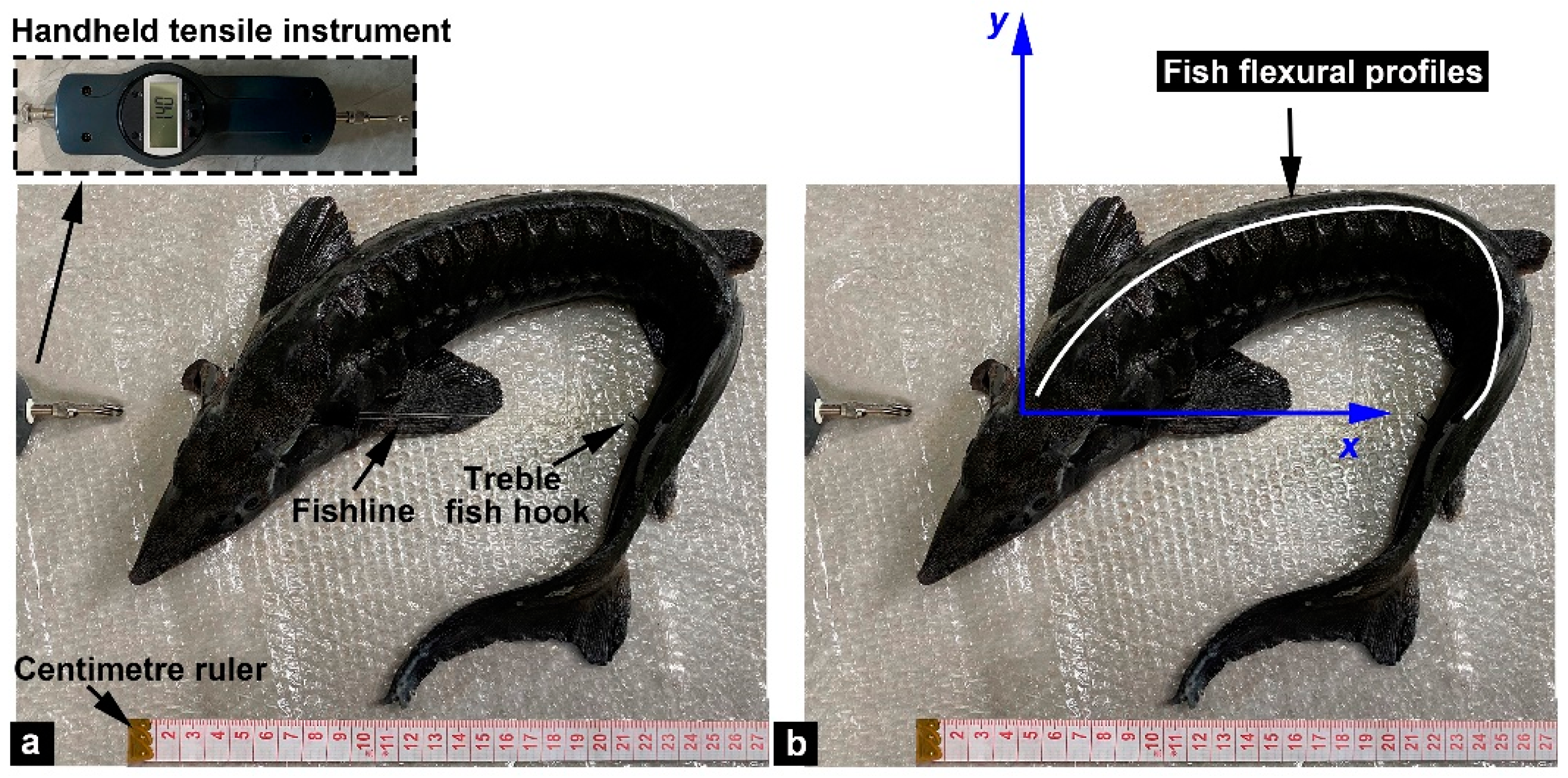
2.3. Tensile Fracture Experiments
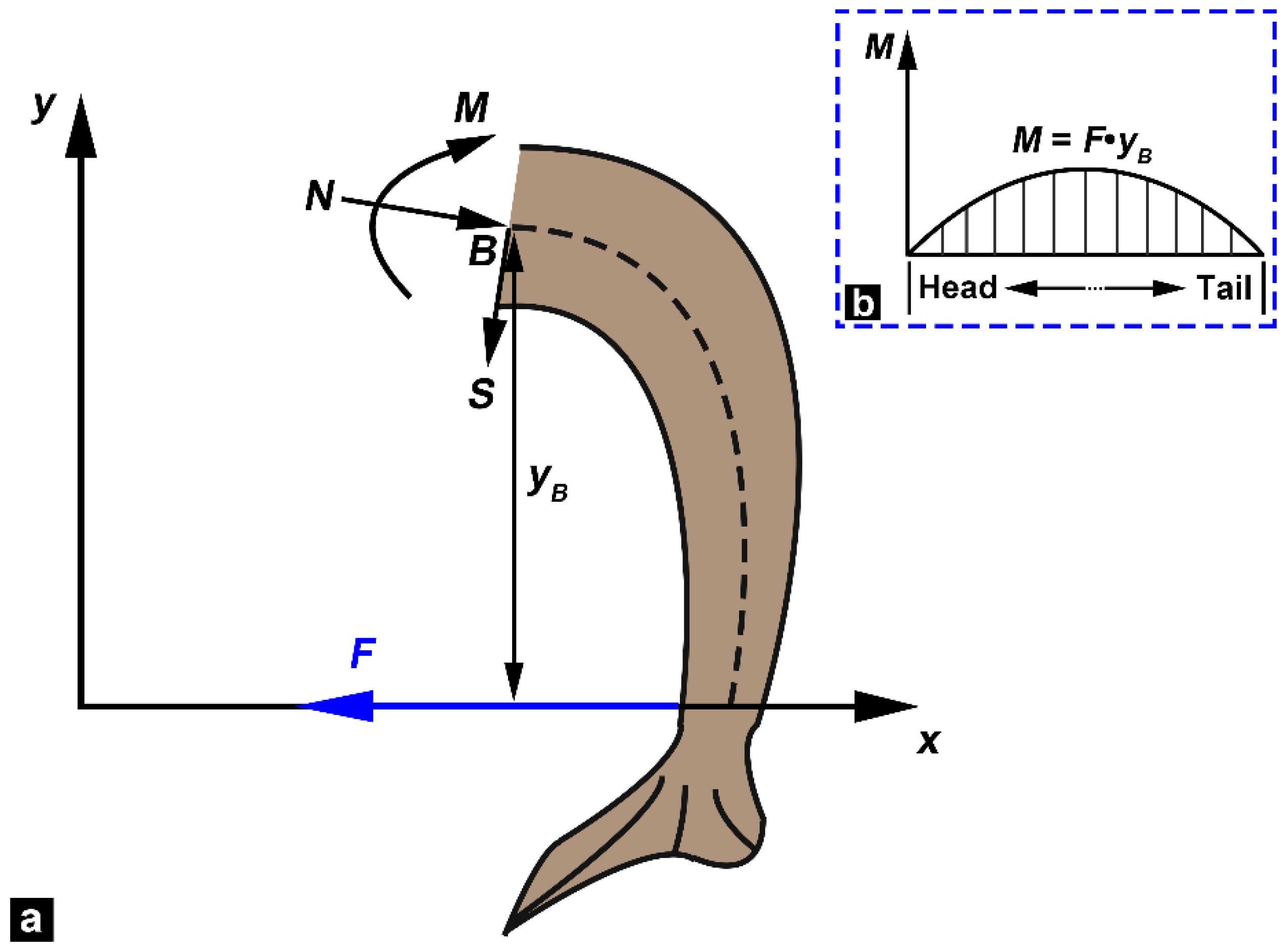
2.4. Bending Tests
3. Results
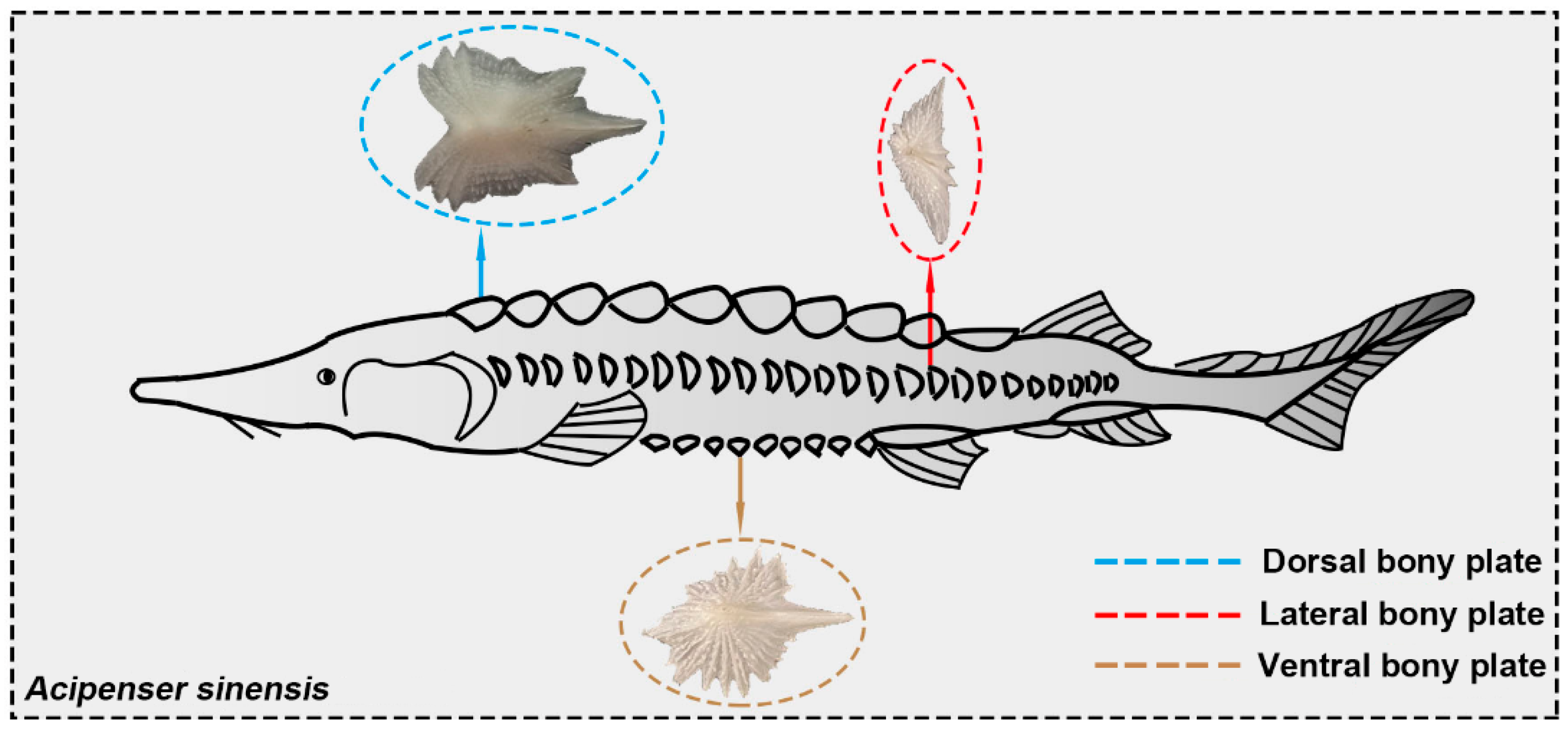
3.1. Microstructural Characterization
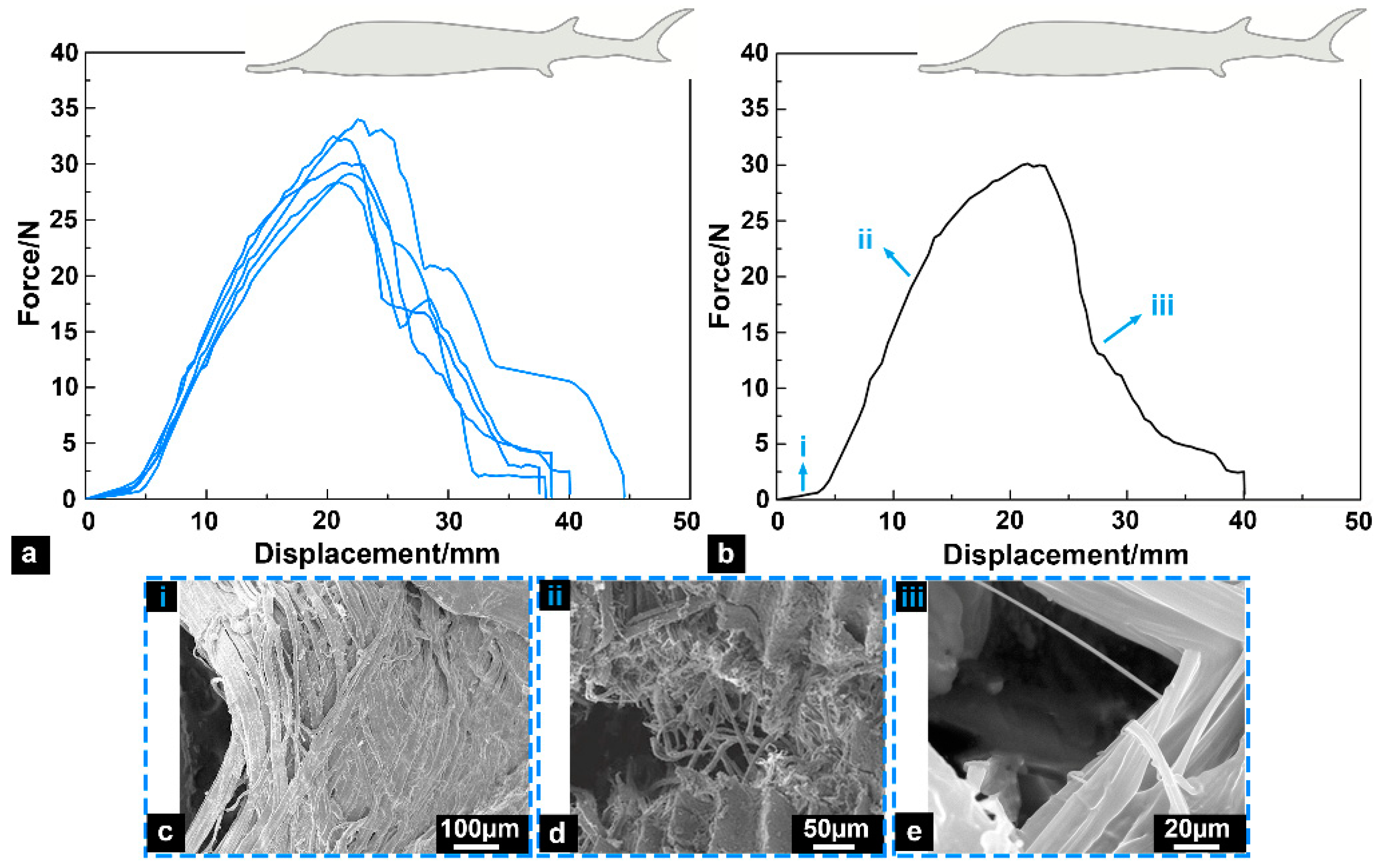
3.2. Tensile Fracture Response
- (i)
- In the early stage of stretching, the skin samples had significant fiber delamination characteristics;
- (ii)
- The tensile loads were significantly increased, and the skin samples mainly resisted the loads by fiber fracture;
- (iii)
- At the later stage of stretching, a small amount of remaining fibrous tissues fractured, and the tensile loads decreased significantly.
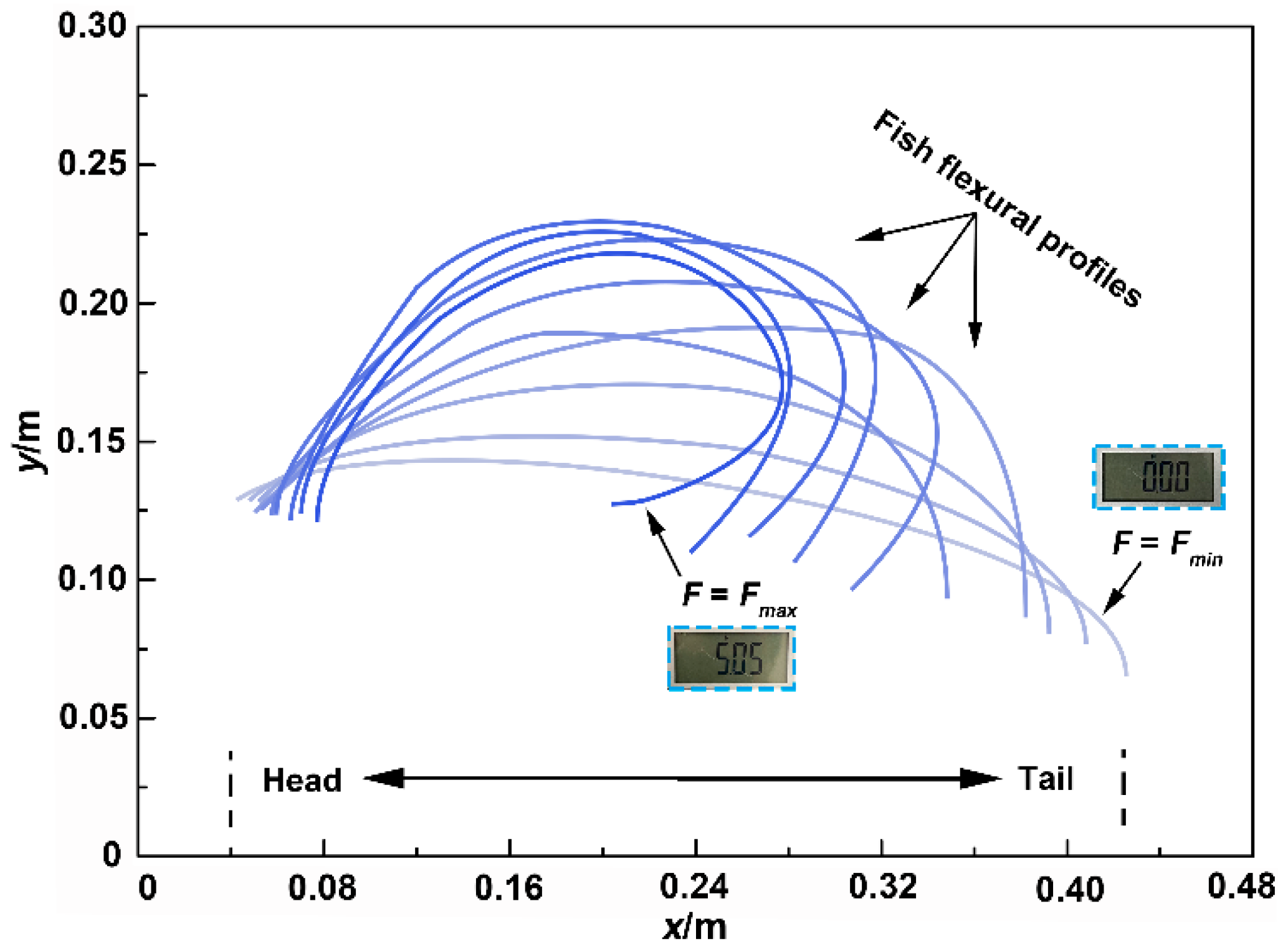
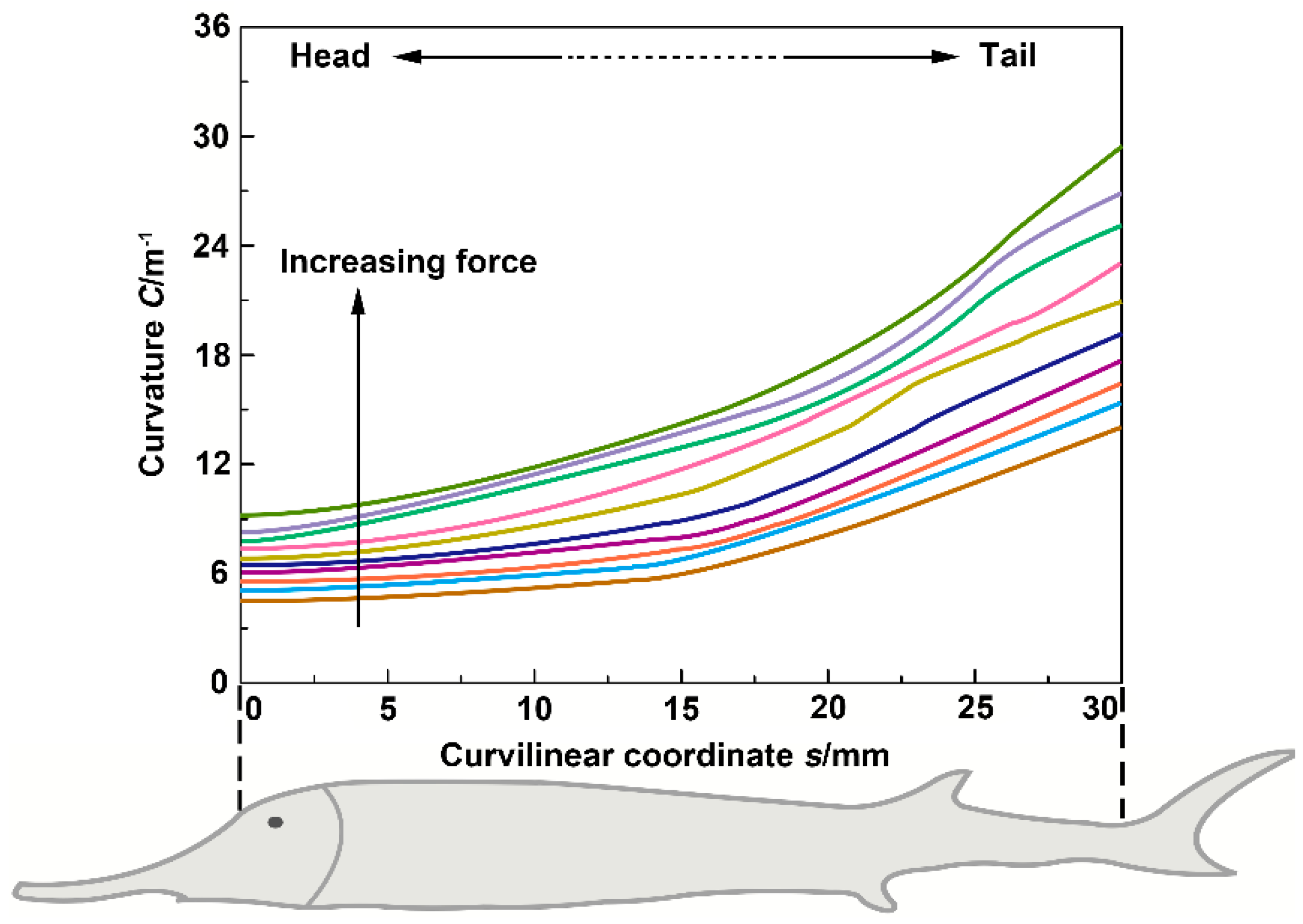
3.3. Flexural Profile Analysis
3.4. Bending Moment versus Curvature
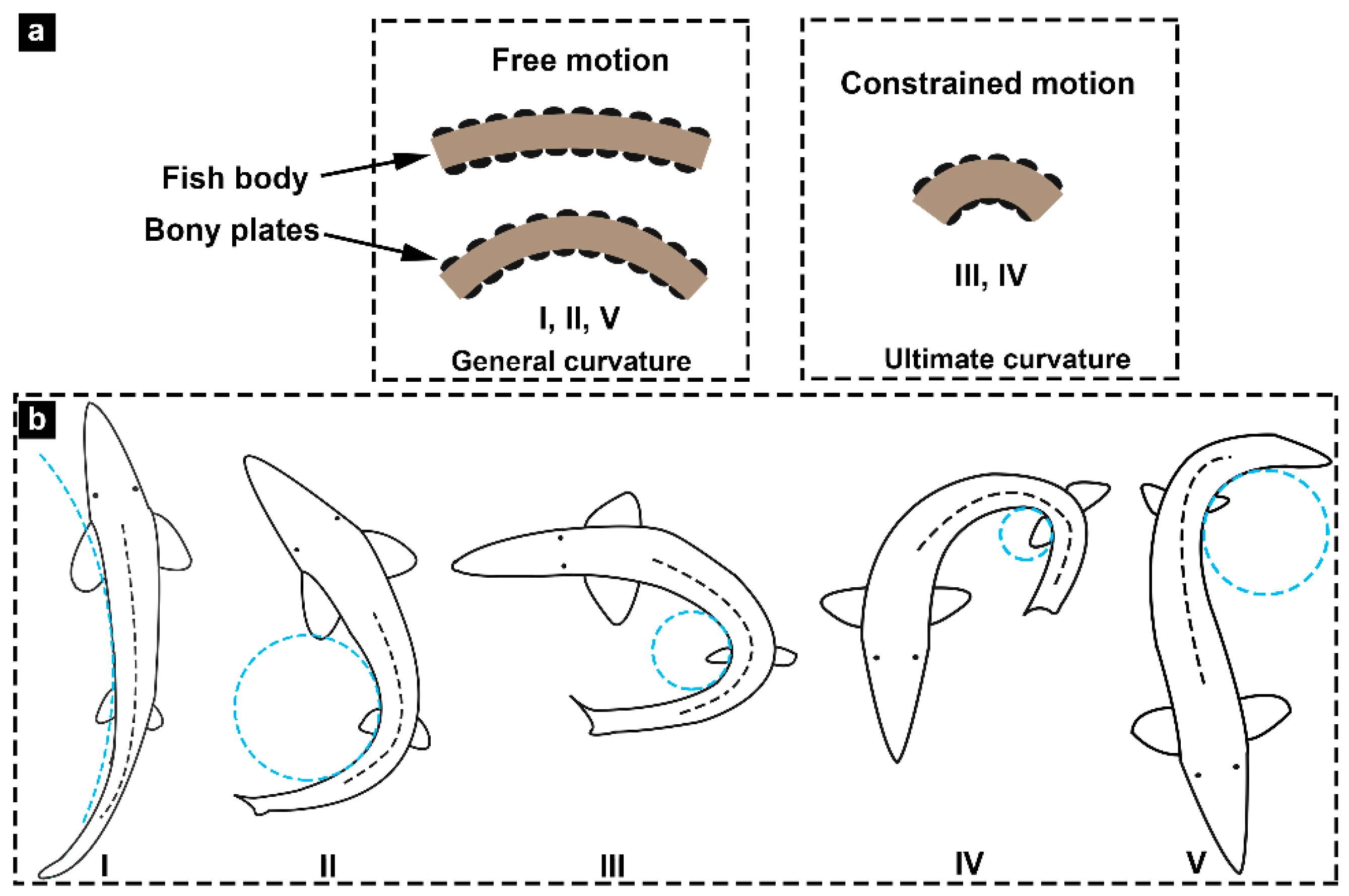
3.5. Flexural Response Characteristics
4. Discussion
5. Conclusions
- (1)
- Some tiny placoid scales are distributed on the skin surface of Chinese sturgeon. These scales are irregularly arranged and show considerably different structural morphology, indicating that they have degenerated tissues.
- (2)
- The results of tensile fracture tests revealed that the skin of the Chinese sturgeon is a biological material with significant toughness characteristics, and its average fracture toughness is 22.67 ± 1.23 kJ/m2, which is even tougher than that of the general cortical bone. The superior mechanical properties of sturgeon fish skin can effectively help the Chinese sturgeon resist predator attacks and avoid damage to the fish body caused by large bending movements.
- (3)
- The bending tests showed that flexural stiffness decreased gradually from the anterior region to the posterior region of the sturgeon fish body, which indicated that the posterior region of the fish body had higher flexibility. In addition, compared with the intact samples, the flexural stiffness of the samples (with scales removed) decreased slightly, and the decline in the posterior region of the fish body was more significant than in the anterior and middle regions. Compared with the intact samples and the samples with scales removed, the flexural stiffness of the dermis-cut samples was significantly reduced, indicating that the fish skin played a vital role in the flexural response of the fish body.
- (4)
- Unlike the scales of most fish species, for Chinese sturgeon, there was no overlap between the lateral and ventral bony plates. The bending tests showed that when the posterior region of the fish body was significantly bent, the lateral bony plates would hinder the bending deformation of the fish body to a certain extent. The bony plates showed higher mineralization and deformation resistance compared to most fish scales. This means that the non-overlapping bony plates could effectively decrease the difficulty of fish deformation, thus reducing the energy consumed during bending deformation. This work provides a novel bionic template for exploring and designing flexible, protective, and locomotory systems.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ahn, H.; Da, J.G.; Lee, H.H.; Seo, J.Y.; Yeo, S.Y. Mechanical properties of porcine and fish skin-based collagen and conjugated collagen fibers. Polymers 2021, 13, 2151. [Google Scholar] [CrossRef]
- Santos, J.P.; Vanessa, M.E.; Catarine, C.M.; Pinto, L. Crosslinking agents effect on gelatins from carp and tilapia skins and in their biopolymeric films. Collods Surf. 2018, 539, 184–191. [Google Scholar] [CrossRef]
- Theerawitayaart, W.; Prodpran, T.; Benjakul, S. Enhancement of hydrophobicity of fish skin gelatin via molecular modification with oxidized linoleic acid. J. Chem. 2019, 6, 1–11. [Google Scholar] [CrossRef]
- Getachew, A.T.; Ahmad, R.; Park, J.S.; Chun, B.S. Fish skin gelatin-based packaging films functionalized by subcritical water extract from the spent coffee ground. Food. Packag. Shelf. 2021, 29, 100735. [Google Scholar] [CrossRef]
- Benjamin, D.C.; Hynes, R.O. Intravital imaging of metastasis in adult zebrafish. BMC. Cancer 2017, 17, 660–672. [Google Scholar] [CrossRef]
- Sarasamma, S.; Lai, Y.H.; Liang, S.T.; Liu, K.C.; Hsiao, C.D. The power of fish models to elucidate skin cancer pathogenesis and impact the discovery of new therapeutic opportunities. Int. J. Mol. Sci. 2018, 19, 3929. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Ye, P.; Ge, P.X.; Ma, W.L.; Li, G. Principle and method of measuring the tensile strength of grass carp skin. In Proceedings of the International Conference on Intelligent Computation Technology and Automation (ICICTA), Changsha, China, 22–23 September 2018; Volume 9, pp. 48–52. [Google Scholar]
- Nurilmala, M.; Suryamarevita, H.; Hizbullah, H.H.; Jacoeb, A.M.; Ochiai, Y. Fish skin as a biomaterial for halal collagen and gelatin. Saudi. J. Biol. Sci. 2022, 29, 1100–1110. [Google Scholar] [CrossRef]
- Vernerey, F.J.; Barthelat, F. On the mechanics of fish scale structures. Int. J. Solids Struct. 2010, 47, 2268–2275. [Google Scholar] [CrossRef]
- Szewciw, L.; Barthelat, F. Mechanical properties of striped bass fish skin: Evidence of an exotendon function of the stratum compactum. J. Mech. Behav. Biomed. Mater. 2017, 73, 28–37. [Google Scholar] [CrossRef]
- Kenaley, C.P.; Andres, S.; Jeanelle, A.; John, Y.; Anudeep, A. Skin stiffness in ray-finned fishes: Contrasting material properties between species and body regions. J. Morphol. 2018, 279, 1419–1431. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, X.; Guo, C. Unique structures and material properties of the skin in different body regions of three species of fish. Microsc. Res. Tech. 2023, 86, 516–528. [Google Scholar] [CrossRef]
- Donatelli, C.M.; Summers, A.P.; Tytell, E.D. Long-axis twisting during locomotion of elongate fishes. J. Exp. Biol. 2017, 220, 3632–3640. [Google Scholar] [CrossRef]
- Clark, A.J.; Crawford, C.H.; King, B.D.; Demas, A.M.; Uyeno, T.A. Material properties of hagfish skin with insights into knotting behaviors. Biol. Bull. 2016, 230, 243–256. [Google Scholar] [CrossRef]
- Lailvaux, S.P.; Leifer, J.; Kircher, B.K.; Johnson, M.A. The incredible shrinking dewlap: Signal size, skin elasticity, and mechanical design in the green anole lizard (Anolis carolinensis). Ecol. Evol. 2015, 5, 4400–4409. [Google Scholar] [CrossRef]
- Zhu, D.J.; Szewciw, L.; Vernerey, F.; Barthelat, F. Puncture resistance of the scaled skin from striped bass: Collective mechanisms and inspiration for new flexible armor designs. J. Mech. Behav. Biomed. Mater. 2013, 24, 30–40. [Google Scholar] [CrossRef]
- Zheng, Y.; Guo, C.; Li, L.H.; Ma, Y.P. Morphology and mechanical properties of the dorsal bony plates in the Chinese sturgeon (Acipenser sinensis). Microsc. Res. Tech. 2019, 82, 1083–1091. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, X.; Liu, P.; Chen, Y.; Guo, C. The armor of the Chinese sturgeon: A study of the microstructure and mechanical properties of the ventral bony plates. Micromachines 2023, 14, 256. [Google Scholar] [CrossRef]
- Domenici, P.; Norin, T.; Bushnell, P.G.; Johansen, J.L.; Skov, P.V.; Svendsen, M.B.S.; Steffensen, J.F.; Abe, A.S. Fast-starting after a breath: Air-breathing motions are kinematically similar to escape responses in the catfish Hoplosternum littorale. Biol. Open 2015, 4, 79–85. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y.; Ma, Y.; Jin, T.; Zhang, Z. DEM simulation of bionic subsoilers (tillage depth > 40 cm) with drag reduction and lower soil disturbance characteristics. Adv. Eng. Softw. 2018, 119, 30–37. [Google Scholar] [CrossRef]
- Palmer, C.; Young, M.T. Surface drag reduction and flow separation control in pelagic vertebrates, with implications for interpreting scale morphologies in fossil taxa. R. Soc. Open Sci. 2015, 2, 140163. [Google Scholar] [CrossRef]
- Munther, M.; Palma, T.; Angeron, I.; Salari, S.; Ghassemi, H.; Vasefi, M.; Beheshti, A.; Davami, K. Microfabricated Biomimetic placoid Scale-Inspired surfaces for antifouling applications. Appl. Surf. Sci. 2018, 453, 166–172. [Google Scholar] [CrossRef]
- Zhu, D.J.; Ortega, C.F.; Motamedi, R.; Szewciw, L.; Vernerey, F.; Barthelat, F. Structure and mechanical performance of a “modern” fish scale. Adv. Eng. Mater. 2012, 14, B185–B194. [Google Scholar] [CrossRef]
- Koester, K.J.; Ager, J.W.; Ritchie, R.O. The true toughness of human cortical bone measured with realistically short cracks. Nat. Mater. 2008, 7, 672–677. [Google Scholar] [CrossRef]
- Dastjerdi, A.K.; Barthelat, F. Teleost fish scales amongst the toughest collagenous materials. J. Mech. Behav. Biomed. Mater. 2015, 52, 95–107. [Google Scholar] [CrossRef]
- Zheng, Y.; Guo, C.; Li, L.H.; Ma, Y.P. Unique morphology and mechanical property of Chinese sturgeon (Acipenser sinensis) fish skin. IET Nanobiotechnol. 2020, 14, 281–288. [Google Scholar] [CrossRef]
- Lin, Y.S.; Wei, C.T.; Olevsky, E.A. Mechanical properties and the laminate structure of Arapaima gigas scales. J. Mech. Behav. Biomed. Mater. 2011, 4, 1145–1156. [Google Scholar] [CrossRef]
- Yang, W.; Sherman, V.R.; Gludovatz, B.; Mackey, M.; Zimmermann, E.A.; Chang, E.H.; Schaible, E.; Qin, Z.; Buehler, M.J. Protective role of Arapaima gigas fish scales: Structure and mechanical behavior. Acta Biomater. 2014, 10, 3599–3614. [Google Scholar] [CrossRef]
- Szewciw, L.; Zhu, D.J.; Barthelat, F. The nonlinear flexural response of a whole teleost fish: Contribution of scales and skin. J. Mech. Behav. Biomed. Mater. 2017, 76, 97–103. [Google Scholar] [CrossRef]
- Long, J.H.; Hale, M.E.; McHenry, M.J.; Westneat, M. Functions of fish skin: Flexural stiffness and steady swimming of longnose gar, Lepisosteus osseus. J. Exp. Biol. 1996, 199, 2139–2151. [Google Scholar] [CrossRef]


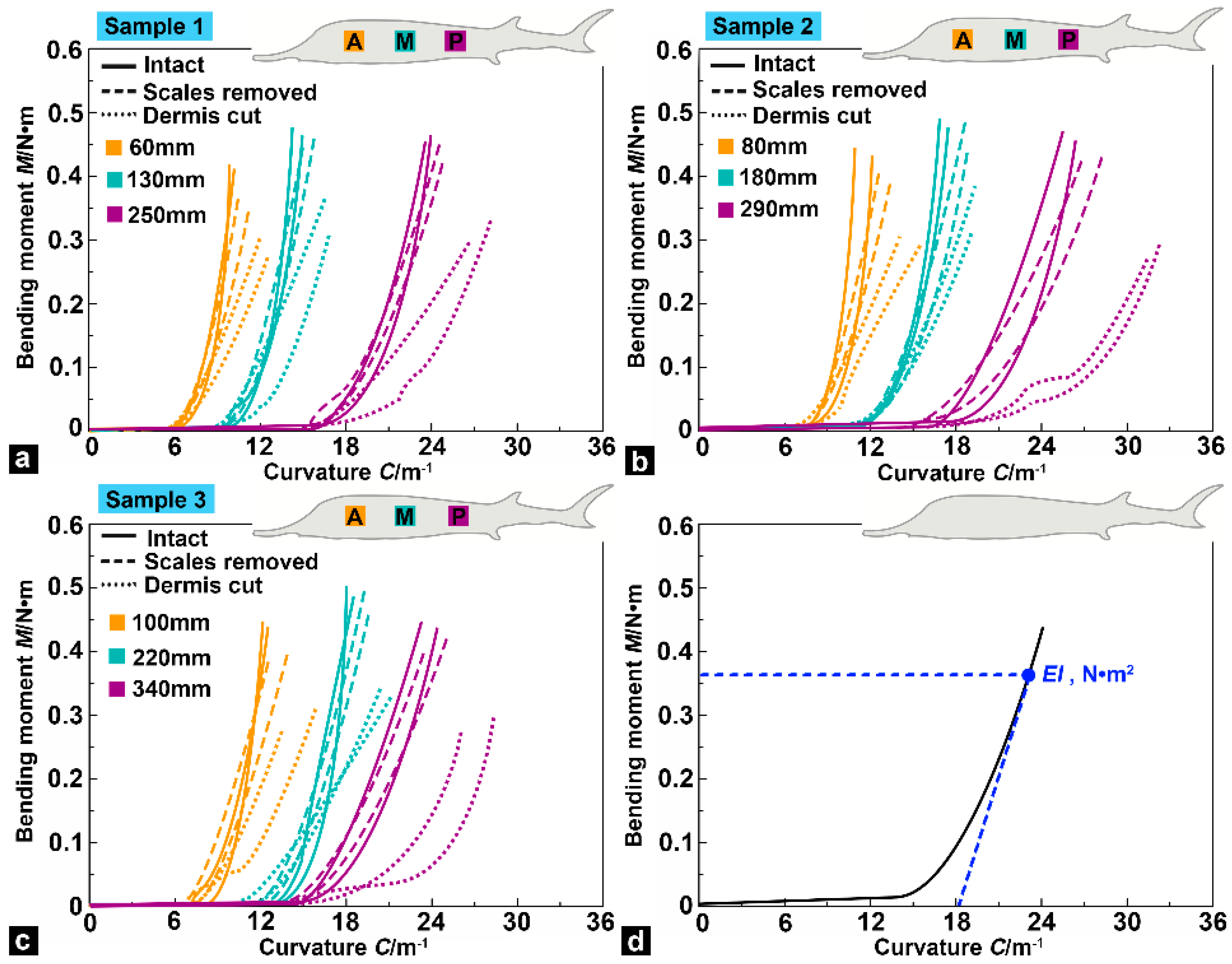
| Sample | Locations/mm | Flexural Stiffness EI/N·m2 | ||
|---|---|---|---|---|
| Intact | Scales Removed | Dermis Cut | ||
| Sample 1 | 60 | 0.047 | 0.044 | 0.028 |
| 130 | 0.036 | 0.035 | 0.019 | |
| 250 | 0.021 | 0.018 | 0.007 | |
| Sample 2 | 80 | 0.045 | 0.043 | 0.026 |
| 180 | 0.033 | 0.031 | 0.016 | |
| 290 | 0.016 | 0.013 | 0.003 | |
| Sample 3 | 100 | 0.044 | 0.042 | 0.023 |
| 220 | 0.031 | 0.030 | 0.014 | |
| 340 | 0.017 | 0.014 | 0.004 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Li, X.; Liu, P.; Chen, Y.; Guo, C. The Flexible Armor of Chinese Sturgeon: Potential Contribution of Fish Skin on Fracture Toughness and Flexural Response. Biomimetics 2023, 8, 232. https://doi.org/10.3390/biomimetics8020232
Zheng Y, Li X, Liu P, Chen Y, Guo C. The Flexible Armor of Chinese Sturgeon: Potential Contribution of Fish Skin on Fracture Toughness and Flexural Response. Biomimetics. 2023; 8(2):232. https://doi.org/10.3390/biomimetics8020232
Chicago/Turabian StyleZheng, Yu, Xin Li, Ping Liu, Ying Chen, and Ce Guo. 2023. "The Flexible Armor of Chinese Sturgeon: Potential Contribution of Fish Skin on Fracture Toughness and Flexural Response" Biomimetics 8, no. 2: 232. https://doi.org/10.3390/biomimetics8020232
APA StyleZheng, Y., Li, X., Liu, P., Chen, Y., & Guo, C. (2023). The Flexible Armor of Chinese Sturgeon: Potential Contribution of Fish Skin on Fracture Toughness and Flexural Response. Biomimetics, 8(2), 232. https://doi.org/10.3390/biomimetics8020232





