Development of a Pneumatic-Driven Fiber-Shaped Robot Scaffold for Use as a Complex 3D Dynamic Culture System
Abstract
1. Introduction
2. Materials and Methods
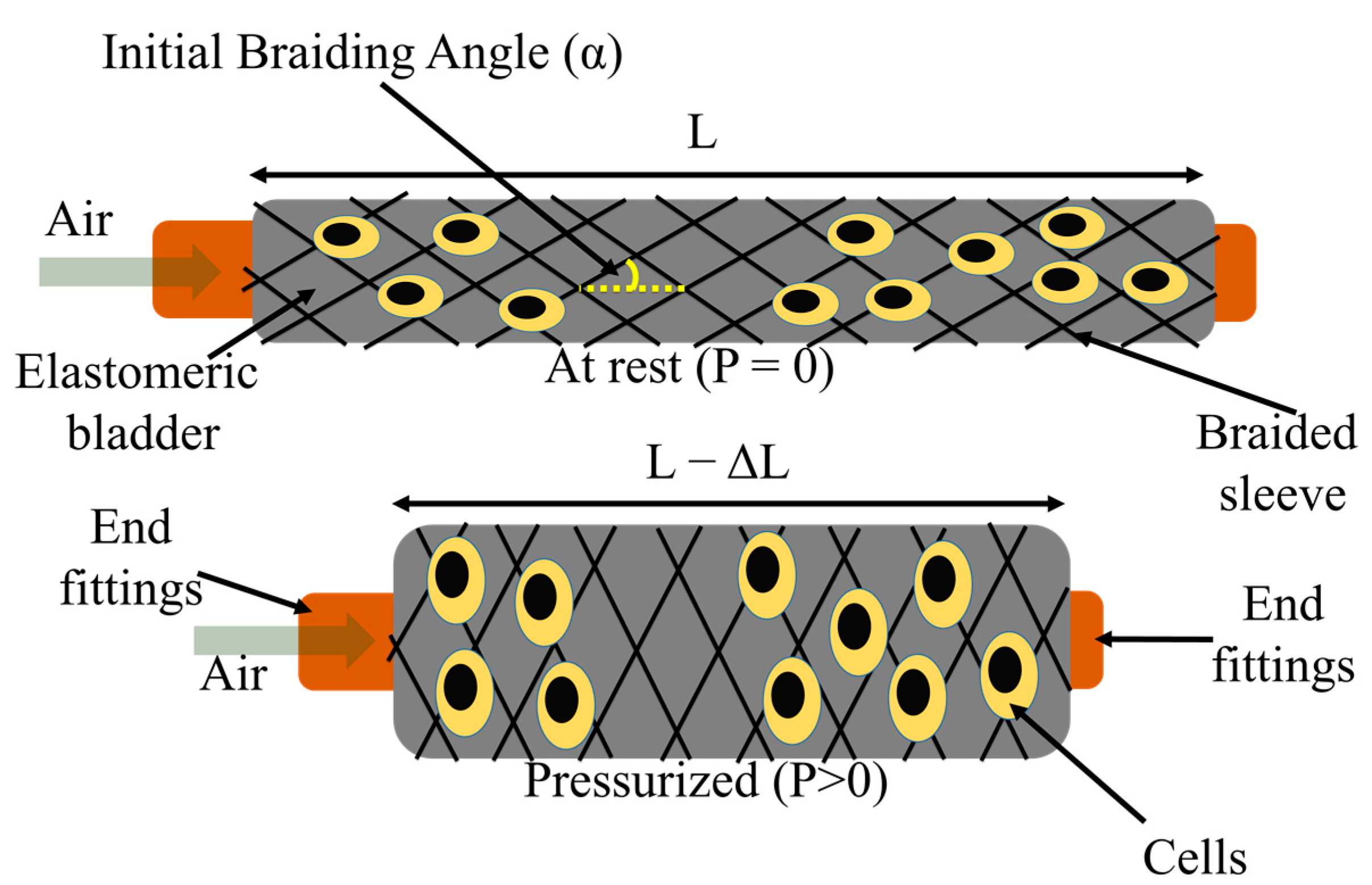
2.1. Fiber Robot Fabrication
2.2. Characterization of FR Scaffolds
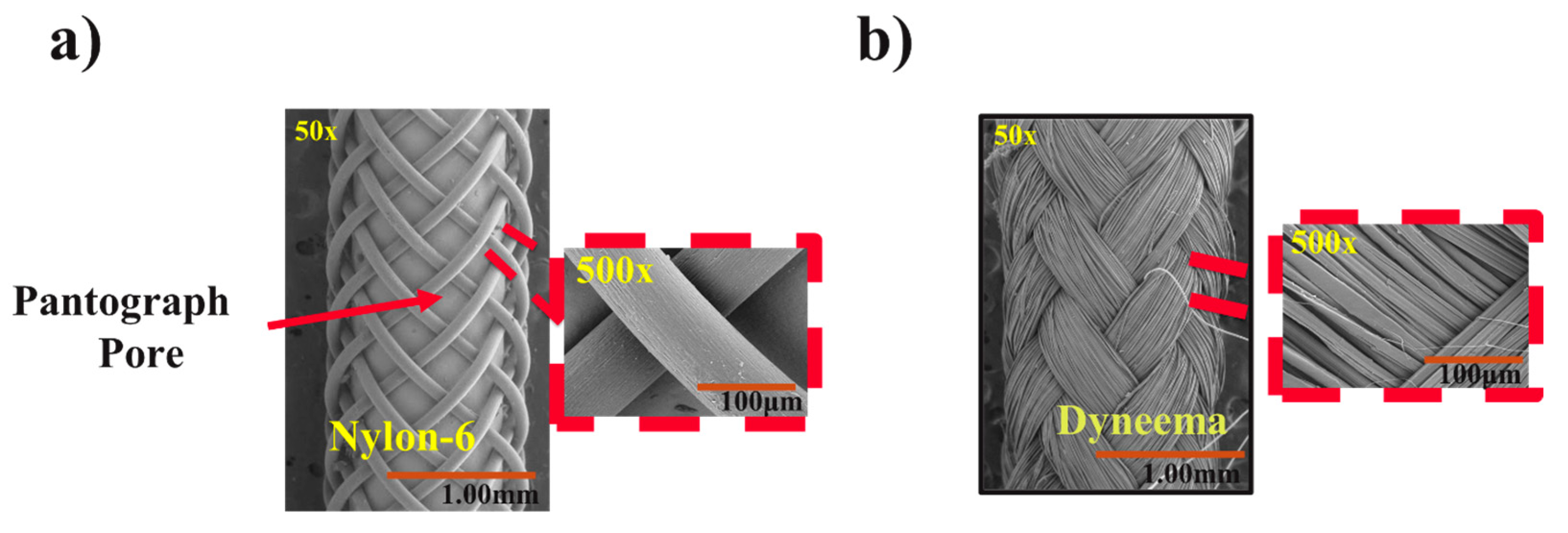
2.2.1. Scanning Electron Microscopy (SEM) Analysis
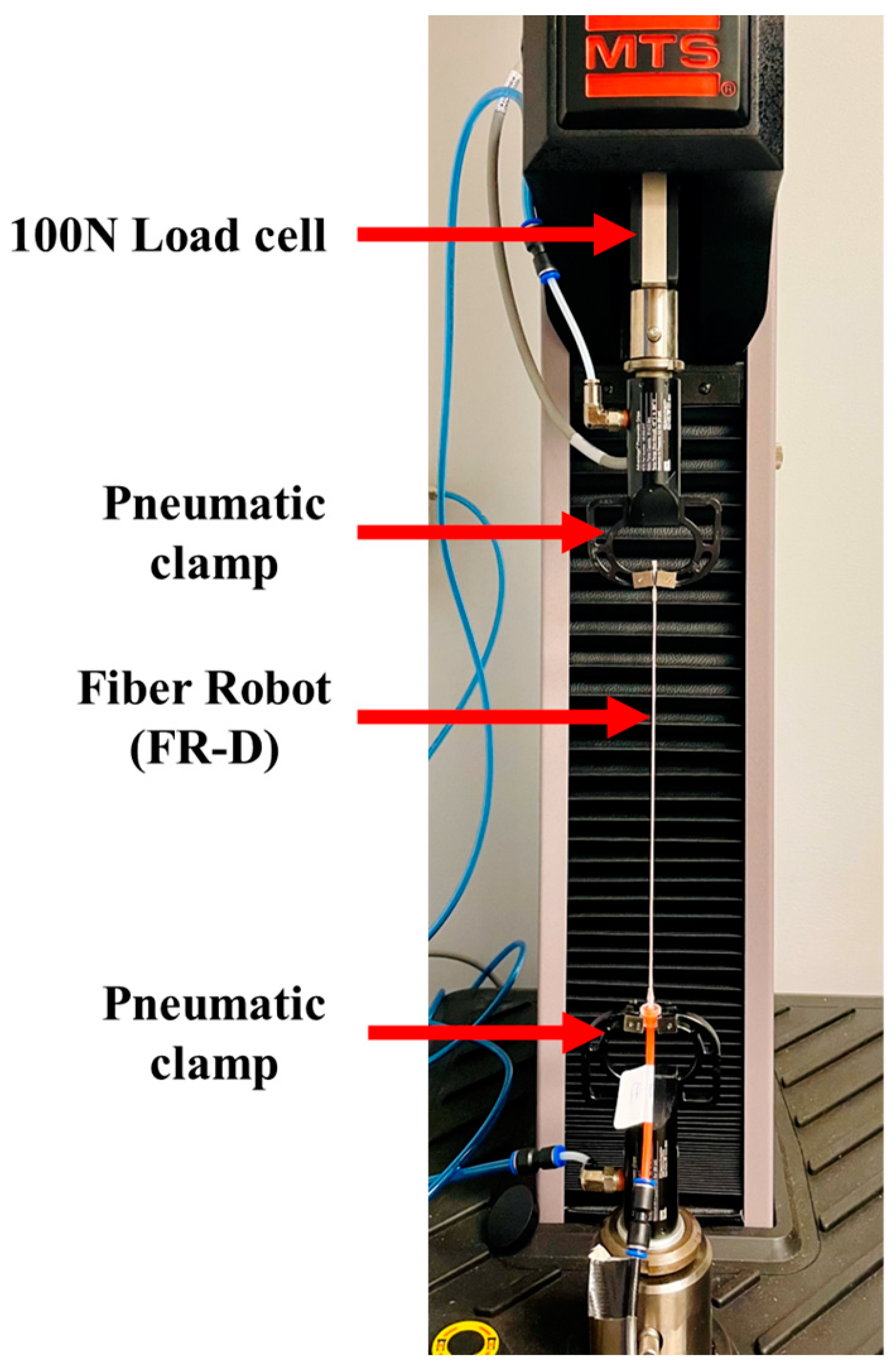
2.2.2. Actuation Property Testing
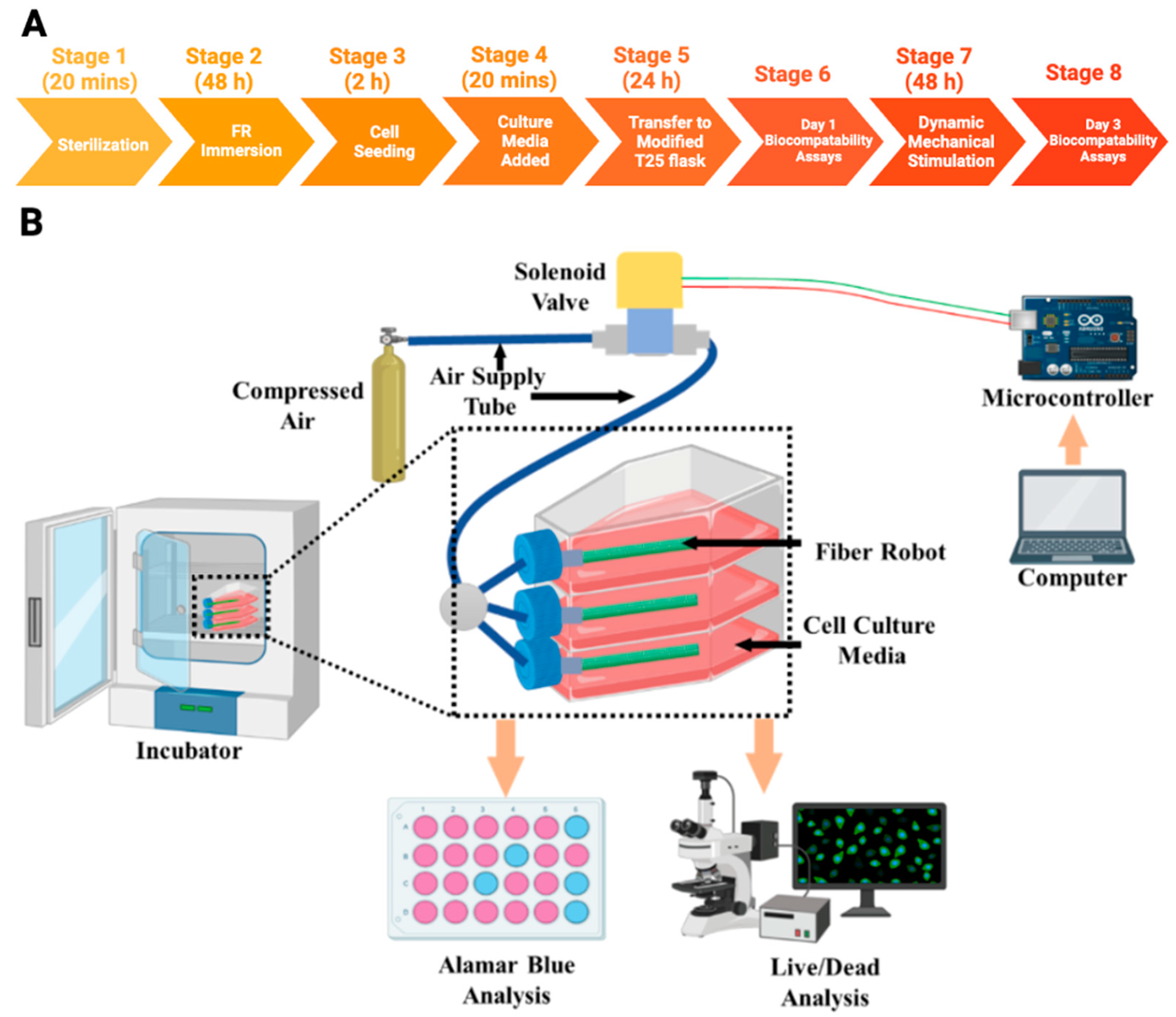
2.3. Cell Culture
2.3.1. Cell Seeding on Fiber Robots
2.3.2. Dynamic Mechanical Stimulations on Cells
2.3.3. Biocompatibility
2.4. Statistical Analysis
3. Results
3.1. Morphology of FRs
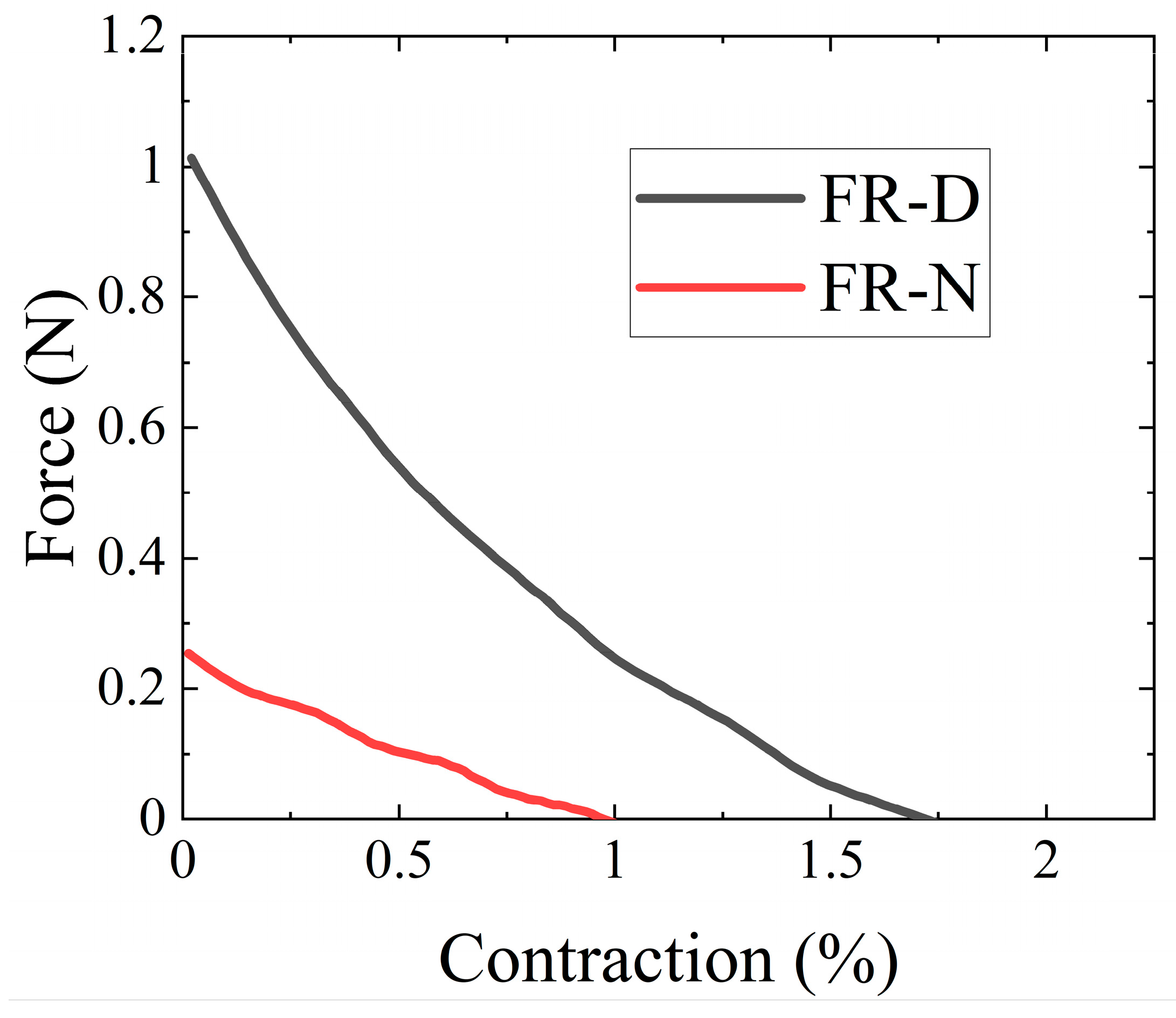
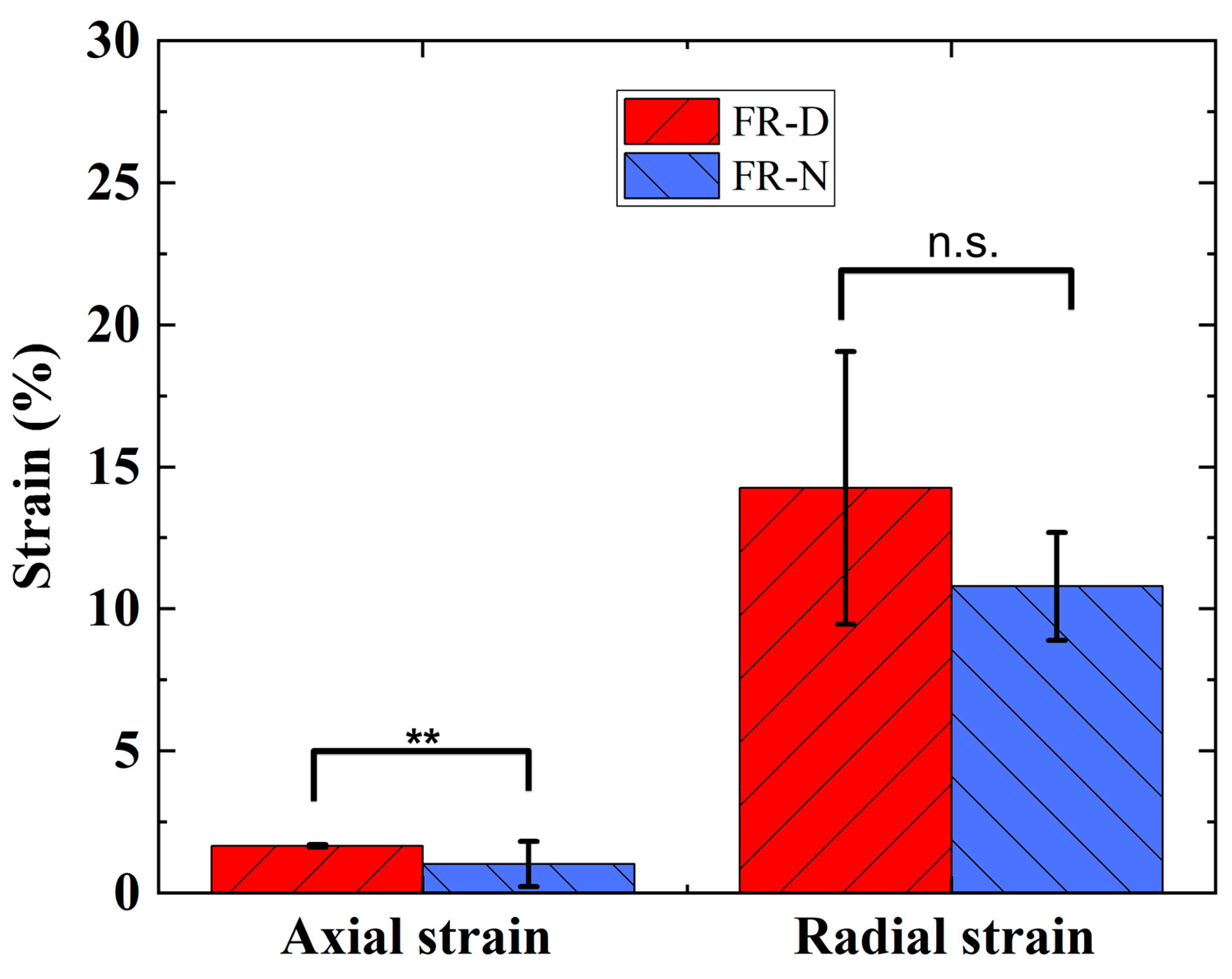
3.2. Actuation Characterization of FR Scaffolds
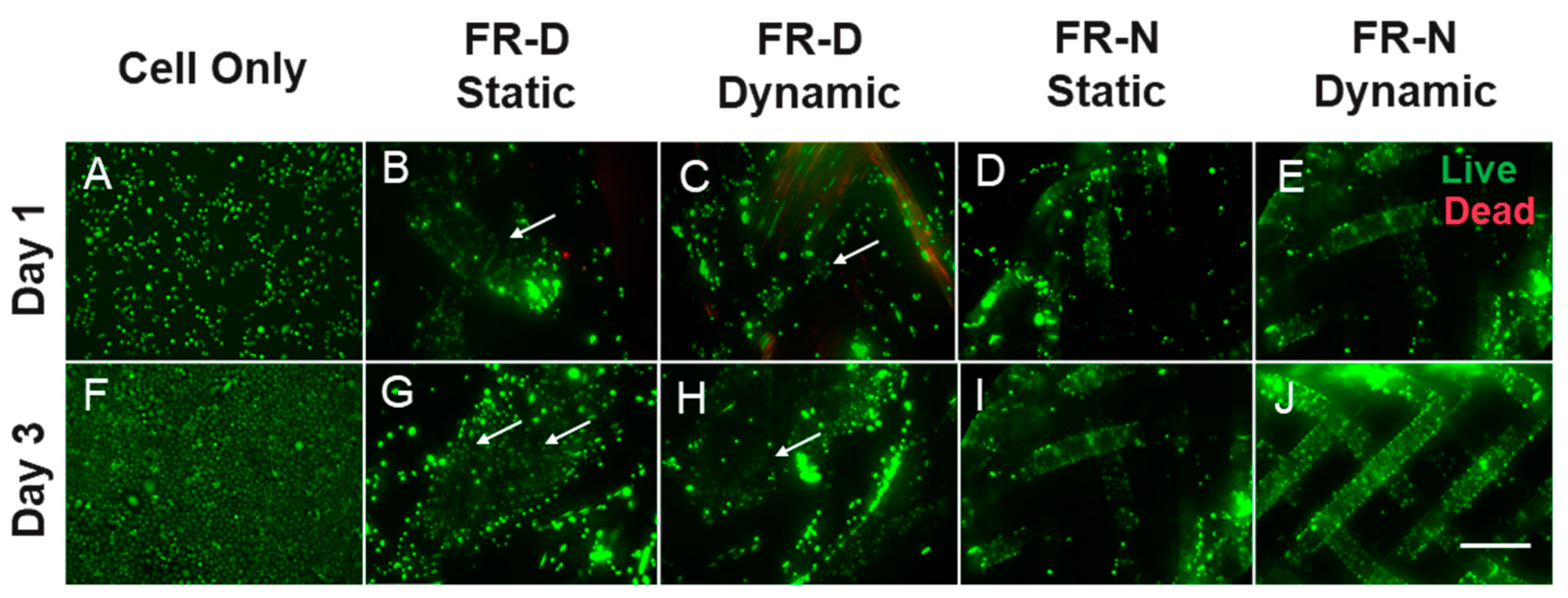
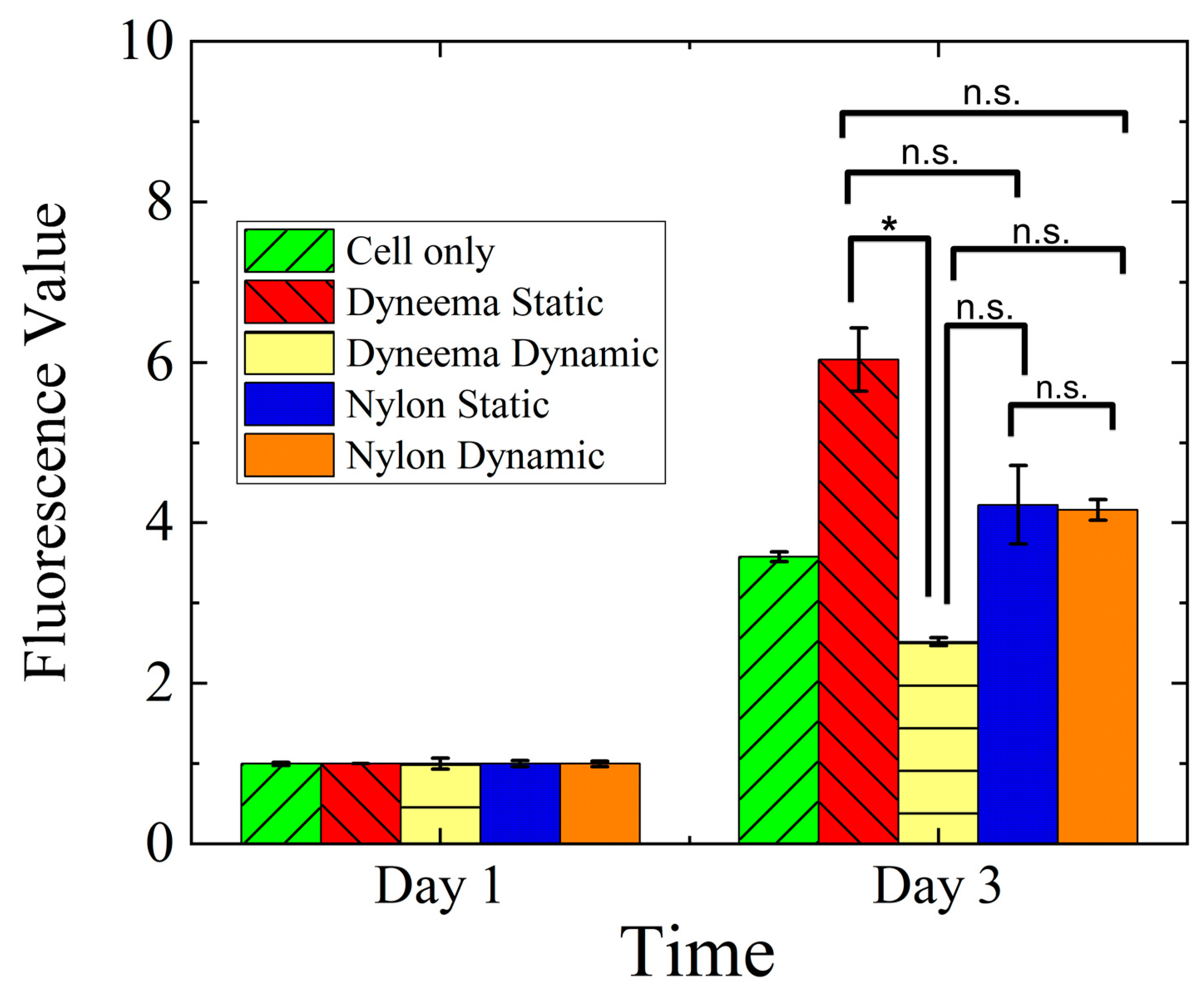
3.3. Cell Viability and Metabolic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; King, M.W. Biodegradable Polymers as the Pivotal Player in the Design of Tissue Engineering Scaffolds. Adv. Healthc. Mater. 2020, 9, e1901358. [Google Scholar] [CrossRef]
- Khademhosseini, A.; Langer, R. A decade of progress in tissue engineering. Nat. Protoc. 2016, 11, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Valdoz, J.C.; Johnson, B.C.; Jacobs, D.J.; Franks, N.A.; Dodson, E.L.; Sanders, C.; Cribbs, C.G.; Van Ry, P.M. The ECM: To Scaffold, or Not to Scaffold, That Is the Question. Int. J. Mol. Sci. 2021, 22, 12690. [Google Scholar] [CrossRef]
- Ghafar-Zadeh, E.; Waldeisen, J.R.; Lee, L.P. Engineered approaches to the stem cell microenvironment for cardiac tissue regeneration. Lab Chip 2011, 11, 3031–3048. [Google Scholar] [CrossRef]
- Dartsch, P.C.; Betz, E. Response of cultured endothelial cells to mechanical stimulation. Basic Res. Cardiol. 1989, 84, 268–281. [Google Scholar] [CrossRef]
- Altman, G.H.; Horan, R.L.; Martin, I.; Farhadi, J.; Stark, P.R.H.; Volloch, V.; Richmond, J.C.; Vunjak-Novakovic, G.; Kaplan, D.L. Cell differentiation by mechanical stress. FASEB J. 2002, 16, 1–13. [Google Scholar] [CrossRef]
- Chen, K.; Henn, D.; Sivaraj, D.; Bonham, C.A.; Griffin, M.; Kussie, H.C.; Padmanabhan, J.; Trotsyuk, A.A.; Wan, D.C.; Januszyk, M.; et al. Mechanical Strain Drives Myeloid Cell Differentiation Toward Proinflammatory Subpopulations. Adv. Wound Care 2022, 11, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; Trujillo-Miranda, M.; Maier, M.; Heath, D.E.; O’Connor, A.J.; Salehi, S. Effects of External Stimulators on Engineered Skeletal Muscle Tissue Maturation. Adv. Mater. Interfaces 2021, 8, 2001167. [Google Scholar] [CrossRef]
- Hoffman, B.D.; Crocker, J.C. Cell Mechanics: Dissecting the Physical Responses of Cells to Force. Annu. Rev. Biomed. Eng. 2009, 11, 259–288. [Google Scholar] [CrossRef]
- Aragona, M.; Panciera, T.; Manfrin, A.; Giulitti, S.; Michielin, F.; Elvassore, N.; Dupont, S.; Piccolo, S. A Mechanical Checkpoint Controls Multicellular Growth through YAP/TAZ Regulation by Actin-Processing Factors. Cell 2013, 154, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Happe, C.; Engler, A. Mechanical Forces Reshape Differentiation Cues That Guide Cardiomyogenesis. Circ. Res. 2016, 118, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.H.; Siperko, L.M. Mechanosensing and Mechanochemical Transduction: How Is Mechanical Energy Sensed and Converted Into Chemical Energy in an Extracellular Matrix? Crit. Rev. Biomed. Eng. 2003, 31, 255–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Thampatty, B.P. An Introductory Review of Cell Mechanobiology. Biomech. Model. Mechanobiol. 2006, 5, 1–16. [Google Scholar] [CrossRef]
- Juliano, R.L.; Haskill, S. Signal Transduction from the Extracellular Matrix. J. Cell Biol. 1993, 120, 577–585. [Google Scholar] [CrossRef]
- Maniotis, A.J.; Chen, C.S.; Ingber, D.E. Demonstration of Mechanical Connections between Integrins, Cytoskeletal Filaments, and Nucleoplasm that Stabilize Nuclear Structure. Proc. Natl. Acad. Sci. USA 1997, 94, 849–854. [Google Scholar] [CrossRef]
- Kaunas, R.; Nguyen, P.; Usami, S.; Chien, S. Cooperative Effects of Rho and Mechanical Stretch on Stress Fiber Organization. Proc. Natl. Acad. Sci. USA 2005, 102, 15895–15900. [Google Scholar] [CrossRef]
- Collinsworth, A.M.; Torgan, C.E.; Nagda, S.N.; Rajalingam, R.J.; Kraus, W.E.; Truskey, G.A. Orientation and length of mammalian skeletal myocytes in response to a unidirectional stretch. Cell Tissue Res. 2000, 302, 243–251. [Google Scholar] [CrossRef]
- Young, J.L.; Kretchmer, K.; Ondeck, M.G.; Zambon, A.C.; Engler, A.J. Mechanosensitive Kinases Regulate Stiffness-Induced Cardiomyocyte Maturation. Sci. Rep. 2014, 4, 6425. [Google Scholar] [CrossRef]
- Heo, J.S.; Lee, J. β-catenin mediates cyclic strain-stimulated cardiomyogenesis in mouse embryonic stem cells through ROS-dependent and integrin-mediated PI3K/Akt pathways. J. Cell. Biochem. 2011, 112, 1880–1889. [Google Scholar] [CrossRef]
- Jufri, N.F.; Mohamedali, A.; Avolio, A.; Baker, M.S. Mechanical stretch: Physiological and pathological implications for human vascular endothelial cells. Vasc. Cell 2015, 7, 8. [Google Scholar] [CrossRef]
- Paek, J.; Song, J.W.; Ban, E.; Morimitsu, Y.; Osuji, C.O.; Shenoy, V.B.; Huh, D.D. Soft robotic constrictor for in vitro modeling of dynamic tissue compression. Sci. Rep. 2021, 11, 16478. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, J.; Zhou, Y.; Thampatty, B.P.; Wang, J.H. Tendon Stem/Progenitor Cells and Their Interactions with Extracellular Matrix and Mechanical Loading. Stem Cells Int. 2019, 2019, 3674647. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.H. Mechanobiological response of tendon stem cells: Implications of tendon homeostasis and pathogenesis of tendinopathy. J. Orthop. Res. 2010, 28, 639–643. [Google Scholar] [CrossRef]
- Ciardulli, M.C.; Lovecchio, J.; Scala, P.; Lamparelli, E.P.; Dale, T.P.; Giudice, V.; Giordano, E.; Selleri, C.; Forsyth, N.R.; Maffulli, N.; et al. 3D Biomimetic Scaffold for Growth Factor Controlled Delivery: An In-Vitro Study of Tenogenic Events on Wharton’s Jelly Mesenchymal Stem Cells. Pharmaceutics 2021, 13, 1448. [Google Scholar] [CrossRef]
- Wahlsten, A.; Rütsche, D.; Nanni, M.; Giampietro, C.; Biedermann, T.; Reichmann, E.; Mazza, E. Mechanical stimulation induces rapid fibroblast proliferation and accelerates the early maturation of human skin substitutes. Biomaterials 2021, 273, 120779. [Google Scholar] [CrossRef]
- Riehl, B.D.; Park, J.; Kwon, I.K.; Lim, J.Y. Mechanical Stretching for Tissue Engineering: Two-Dimensional and Three-Dimensional Constructs. Tissue Eng. Part B 2012, 18, 288–300. [Google Scholar] [CrossRef]
- Higgins, S.; Lee, J.S.; Ha, L.; Lim, J.Y. Inducing Neurite Outgrowth by Mechanical Cell Stretch. BioResearch Open Access 2013, 2, 212–216. [Google Scholar] [CrossRef]
- Dhein, S.; Schreiber, A.; Steinbach, S.; Apel, D.; Salameh, A.; Schlegel, F.; Kostelka, M.; Dohmen, P.M.; Mohr, F.W. Mechanical control of cell biology. Effects of cyclic mechanical stretch on cardiomyocyte cellular organization. Prog. Biophys. Mol. Biol. 2014, 115, 93–102. [Google Scholar] [CrossRef]
- Kamble, H.; Barton, M.J.; Jun, M.; Park, S.; Nguyen, N. Cell stretching devices as research tools: Engineering and biological considerations. Lab Chip 2016, 16, 3193–3323. [Google Scholar] [CrossRef]
- Kang, M.S.; Lee, S.H.; Park, W.J.; Lee, J.E.; Kim, B.; Han, D. Advanced Techniques for Skeletal Muscle Tissue Engineering and Regeneration. Bioengineering 2020, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Zhou, Z.; Tan, G.; Zhu, Y.; Mao, C. Electroactive polymers for tissue regeneration: Developments and perspectives. Prog. Polym. Sci. 2018, 81, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Sraj, I.; Eggleton, C.D.; Jimenez, R.; Hoover, E.E.; Squier, J.A.; Chichester, J.; Marr, D.W. Cell deformation cytometry using diode-bar optical stretchers. J. Biomed. Opt. 2010, 15, 047010. [Google Scholar] [CrossRef] [PubMed]
- Tondu, B. Modelling of the McKibben artificial muscle: A review. J. Intell. Mater. Syst. Struct. 2012, 23, 225–253. [Google Scholar] [CrossRef]
- Kilic Afsar, O.; Shtarbanov, A.; Mor, H.; Nakagaki, K.; Forman, J.; Modrei, K.; Jeong, S.H.; Hjort, K.; Höök, K.; Ishii, H. OmniFiber: Integrated Fluidic Fiber Actuators for Weaving Movement Based Interactions into the ‘Fabric of Everyday Life’. In Proceedings of the 34th Annual ACM Symposium on User Interface Software and Technology, Virtual, 10–14 October 2021; pp. 1010–1026. [Google Scholar]
- Koizumi, S.; Chang, T.; Nabae, H.; Endo, G.; Suzumori, K.; Mita, M.; Saitoh, K.; Hatakeyama, K.; Chida, S.; Shimada, Y. Soft Robotic Gloves with Thin McKibben Muscles for Hand Assist and Rehabilitation. In Proceedings of the 2020 IEEE/SICE International Symposium on System Integration (SII), Honolulu, HI, USA, 12–15 January 2020; pp. 93–98. [Google Scholar]
- Kurumaya, S.; Suzumori, K.; Nabae, H.; Wakimoto, S. Musculoskeletal lower-limb robot driven by multifilament muscles. Robomech. J. 2016, 3, 18. [Google Scholar] [CrossRef]
- Russo, M.A.; Santarelli, D.M.; O’Rourke, D. The physiological effects of slow breathing in the healthy human. Breathe 2017, 13, 298–309. [Google Scholar] [CrossRef]
- Tremblay, D.; Chagnon-Lessard, S.; Mirzaei, M.; Pelling, A.E.; Godin, M. A microscale anisotropic biaxial cell stretching device for applications in mechanobiology. Biotechnol. Lett. 2014, 36, 657–665. [Google Scholar] [CrossRef]
- Boccafoschi, F.; Bosetti, M.; Gatti, S.; Cannas, M. Dynamic Fibroblast Cultures. Cell Adhes. Migr. 2007, 1, 124–128. [Google Scholar] [CrossRef]
- Guyader, G.; Gabor, A.; Hamelin, P. Analysis of 2D and 3D circular braiding processes: Modeling the interaction between the process parameters and the pre-form architecture. Mech. Mach. Theory 2013, 69, 90–104. [Google Scholar] [CrossRef]
- Hoque, M.A.; Petersen, E.; Fang, X. Effect of Material Properties on Fiber-Shaped Pneumatic Actuators Performance. Actuators 2023, 12, 129. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef]
- Huang, Y.; Nguyen, N. A polymeric cell stretching device for real-time imaging with optical microscopy. Biomed. Microdevices 2013, 15, 1043–1054. [Google Scholar] [CrossRef]
- Mann, J.M.; Lam, R.H.W.; Weng, S.; Sun, Y.; Fu, J. A silicone-based stretchable micropost array membrane for monitoring live-cell subcellular cytoskeletal response. Lab Chip 2012, 12, 731–740. [Google Scholar] [CrossRef]
- Moraes, C.; Chen, J.; Sun, Y.; Simmons, C.A. Microfabricated arrays for high-throughput screening of cellular response to cyclic substrate deformation. Lab Chip 2010, 1, 227–234. [Google Scholar] [CrossRef]
- Simmons, C.S.; Sim, J.Y.; Baechtold, P.; Gonzalez, A.; Chung, C.; Borghi, N.; Pruitt, B.L. Integrated strain array for cellular mechanobiology studies. J. Micromech. Microeng. 2011, 21, 54016. [Google Scholar] [CrossRef]
- Shimizu, K.; Shunori, A.; Morimoto, K.; Hashida, M.; Konishi, S. Development of a biochip with serially connected pneumatic balloons for cell-stretching culture. Sens. Actuators B Chem. 2011, 156, 486–493. [Google Scholar] [CrossRef]
- Heo, Y.J.; Kan, T.; Iwase, E.; Matsumoto, K.; Shimoyama, I. Stretchable cell culture platforms using micropneumatic actuators. Nano-Micro Lett. 2013, 8, 865–868. [Google Scholar] [CrossRef]
- Kreutzer, J.; Ikonen, L.; Hirvonen, J.; Pekkanen-Mattila, M.; Aalto-Setälä, K.; Kallio, P. Pneumatic cell stretching system for cardiac differentiation and culture. Med. Eng. Phys. 2014, 36, 496–501. [Google Scholar] [CrossRef]
- Dong, Y.; Li, Y. A review on the mechanical property evaluation and optimization design of fabric rubber composite structure. Compos. Part C Open Access 2022, 8, 100289. [Google Scholar] [CrossRef]
- Man, K.; Liu, J.; Phan, K.M.; Wang, K.; Lee, J.Y.; Sun, X.; Story, M.; Saha, D.; Liao, J.; Sadat, H.; et al. Dimensionality-Dependent Mechanical Stretch Regulation of Cell Behavior. ACS Appl. Mater. Interfaces 2022, 14, 17081–17092. [Google Scholar] [CrossRef]
- Cui, Y.; Hameed, F.M.; Yang, B.; Lee, K.; Pan, C.Q.; Park, S.; Sheetz, M. Cyclic stretching of soft substrates induces spreading and growth. Nat. Commun. 2015, 6, 6333. [Google Scholar] [CrossRef]
- Klute, G.K.; Czerniecki, J.M.; Hannaford, B. McKibben artificial muscles: Pneumatic actuators with biomechanical intelligence. In Proceedings of the 1999 IEEE/ASME International Conference on Advanced Intelligent Mechatronics (Cat. No.99TH8399), Atlanta, GA, USA, 19–23 September 1999; pp. 221–226. [Google Scholar]
- Klute, G.K.; Hannaford, B. Fatigue characteristics of McKibben artificial muscle actuators. In Proceedings of the 1998 IEEE/RSJ International Conference on Intelligent Robots and Systems. Innovations in Theory, Practice and Applications (Cat. No.98CH36190), Victoria, BC, Canada, 17 October 1998; Volume 3, pp. 1776–1781. [Google Scholar]
- Kingsley, D.; Quinn, R. Fatigue life and frequency response of braided pneumatic actuators. In Proceedings of the IEEE International Conference on Robotics and Automation, Washington, DC, USA, 11–15 May 2002; Volume 3, pp. 2830–2835. [Google Scholar]
- Iwadate, Y.; Yumura, S. Cyclic stretch of the substratum using a shape-memory alloy induces directional migration in Dictyostelium cells. Biotechniques 2009, 47, 757–767. [Google Scholar] [CrossRef]
- Zheng, W.; Jiang, B.; Wang, D.; Zhang, W.; Wang, Z.; Jiang, X. A microfluidic flow-stretch chip for investigating blood vessel biomechanics. Lab Chip 2012, 12, 3441–3450. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Howard, P.S.; Kucich, U.; Taliwal, R.; Korostoff, J.M. Mechanical forces alter extracellular matrix synthesis by human periodontal ligament fibroblasts. J. Periodontal Res. 1998, 33, 500–508. [Google Scholar] [CrossRef]
- Joung, I.S.; Iwamoto, M.N.; Shiu, Y.; Quam, C.T. Cyclic strain modulates tubulogenesis of endothelial cells in a 3D tissue culture model. Microvasc. Res. 2006, 71, 1–11. [Google Scholar] [CrossRef]
- Wang, J.H.; Grood, E.S. The Strain Magnitude and Contact Guidance Determine Orientation Response of Fibroblasts to Cyclic Substrate Strains. Connect. Tissue Res. 2000, 41, 29–36. [Google Scholar] [CrossRef]

| Fiber Robot | Bladder Outer Diameter | Bladder Wall Thickness | Bladder Material | Bladder Shore Hardness | Yarn Materials | Yarn Linear Density | Yarns’ Initial Modulus |
|---|---|---|---|---|---|---|---|
| FR-D | 0.94 mm | 0.2 mm | Silicone | 53A | UHMWPE (Dyneema®) | 11.1 tex | 136.4 N/tex |
| FR-N | Nylon® | 5.6 tex | 6.7 N/tex |
| Fiber Robot | FR Scaffold Diameter (mm) | Braided Yarn Diameter (mm) | Porosity (%) | Braiding Angle (o) |
|---|---|---|---|---|
| FR-D (Dyneema) | 1.50 ± 0.04 | 0.43 ± 0.03 * | Too small to be measured | 37.56 ± 1.31 |
| FR-N (Nylon) | 1.33 ± 0.03 | 0.08 ± 0.01 | 43.30 ± 0.82 | 43.96 ± 1.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoque, M.A.; Mahmood, N.; Ali, K.M.; Sefat, E.; Huang, Y.; Petersen, E.; Harrington, S.; Fang, X.; Gluck, J.M. Development of a Pneumatic-Driven Fiber-Shaped Robot Scaffold for Use as a Complex 3D Dynamic Culture System. Biomimetics 2023, 8, 170. https://doi.org/10.3390/biomimetics8020170
Hoque MA, Mahmood N, Ali KM, Sefat E, Huang Y, Petersen E, Harrington S, Fang X, Gluck JM. Development of a Pneumatic-Driven Fiber-Shaped Robot Scaffold for Use as a Complex 3D Dynamic Culture System. Biomimetics. 2023; 8(2):170. https://doi.org/10.3390/biomimetics8020170
Chicago/Turabian StyleHoque, Muh Amdadul, Nasif Mahmood, Kiran M. Ali, Eelya Sefat, Yihan Huang, Emily Petersen, Shane Harrington, Xiaomeng Fang, and Jessica M. Gluck. 2023. "Development of a Pneumatic-Driven Fiber-Shaped Robot Scaffold for Use as a Complex 3D Dynamic Culture System" Biomimetics 8, no. 2: 170. https://doi.org/10.3390/biomimetics8020170
APA StyleHoque, M. A., Mahmood, N., Ali, K. M., Sefat, E., Huang, Y., Petersen, E., Harrington, S., Fang, X., & Gluck, J. M. (2023). Development of a Pneumatic-Driven Fiber-Shaped Robot Scaffold for Use as a Complex 3D Dynamic Culture System. Biomimetics, 8(2), 170. https://doi.org/10.3390/biomimetics8020170






