Reconstruction of Critical Sized Maxillofacial Defects Using Composite Allogeneic Tissue Engineering: Systematic Review of Current Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. PICO Question
2.3. Outcome
- The complication rates reported.
- The success rate measured as the amount of new bone volume gained (assessed either directly by percentage bone fill or assessed radiographically).
- Patient-centered outcomes: satisfaction rate.
2.4. Search Strategy
2.5. Inclusion Criteria
- Original studies, written in English, including randomized controlled trials (RCTs), Clinical trials, observational studies (cohorts and case series) as well as case reports on human patients who had been treated with composite allogeneic tissue engineering for immediate/delayed reconstruction of large maxillofacial defects with minimum/no bone harvesting site.
- The composite allogeneic tissue engineering was defined as a combination of allogenic bone (scaffolding), bone morphogenic aspirate (source of stem cells), rhBMP-2, and platelet-rich plasma/platelet-rich fibrin (cell signaling for the promotion of stem cell migration and differentiation into osteoblasts).
- No minimum follow-up was established.
- Studies must report on at least one of the outcomes of interest:
- The complication rates were reported. Either early post-surgical complications or long-term post-surgical complications.
- The success rate is measured as the amount of new bone volume gained (assessed either directly by gross observation or assessed radiographically).
- Patient-centered outcomes: satisfaction rate and esthetic and functional results.
- Nonhuman study and cadaver studies.
- Studies involving significant autogenous bone grafts from sites like the ilium, rib, fibula, or calvarium.
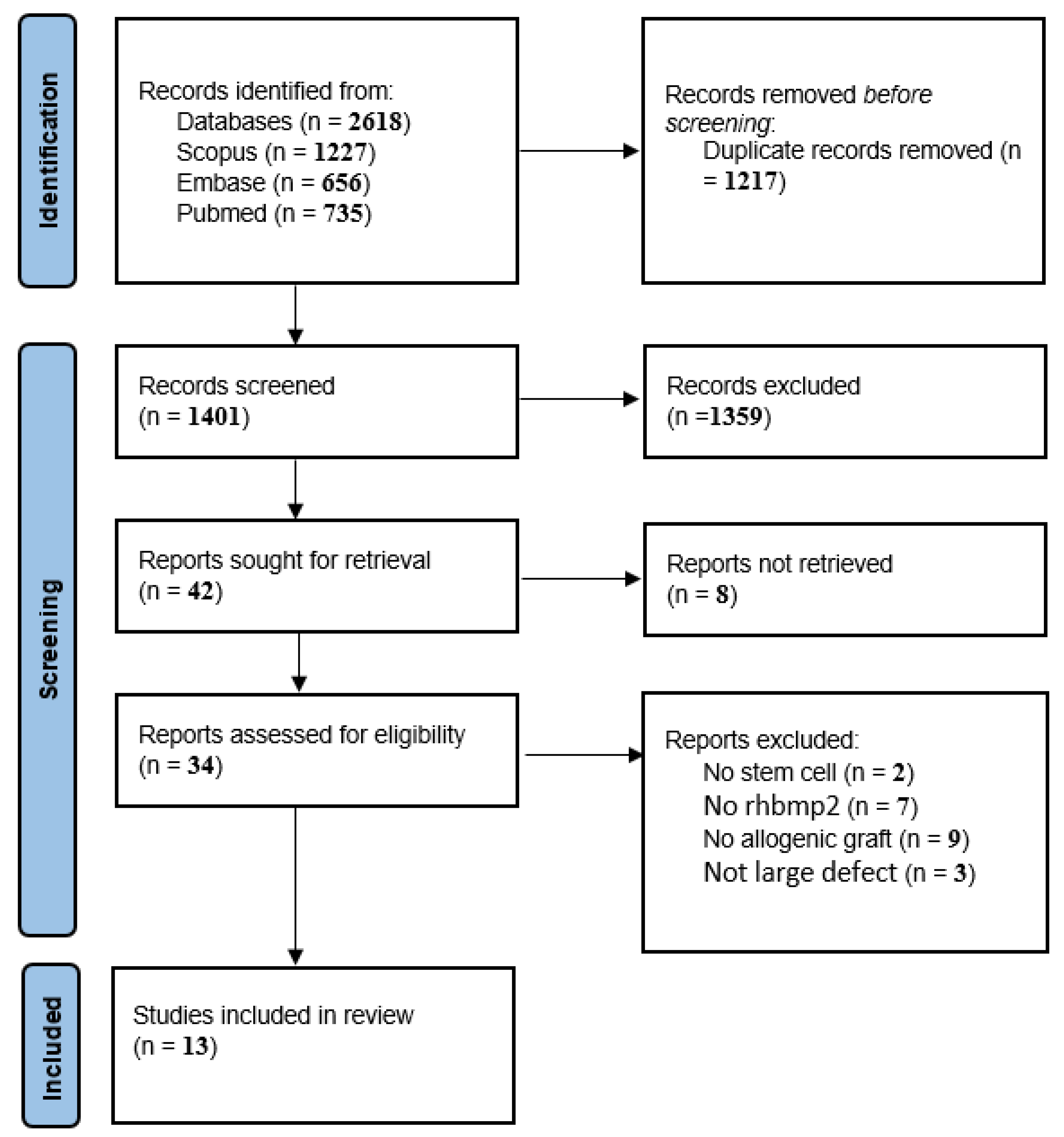
2.6. Study Selection Process
2.7. Data Extraction
2.8. Risk of Bias Assessment
| First Author (Year of Publication) | 1. Does the Patient(s) Represent(s) the Whole Experience of the Investigator (Centre) or Is the Selection Method UNCLEAR to the Extent That Other Patients with Similar Presentation May Not Have Been Reported? | 2. Was The Exposure Adequately Ascertained? | 3. Was the Outcome Adequately Ascertained? | 4. Were Other Alternative Causes that May Explain the Observation Ruled Out? | 5. Was There a Challenge/Rechallenge Phenomenon? | 6. Was There a Dose–Response Effect? | 7. Was Follow-up Long Enough for Outcomes to Occur? | 8. Is the Case(s) Described with Sufficient Details to Allow Other Investigators to Replicate the Research or to Allow Practitioners to Make Inferences Related to Their Own Practice? |
|---|---|---|---|---|---|---|---|---|
| James C. Melville, 2016, Houston, English [15] | N | Y | Y | N | Y | NA | Y | Y |
| James C. Melville, 2017, Houston, English [16] | N | Y | Y | N | Y | NA | Y | Y |
| J. C. Melville, 2014, Houston, English [17] | N | Y | Y | N | Y | NA | Y | Y |
| James C. Melville, 2019, Houston, English [18] | N | Y | Y | N | Y | NA | Y | Y |
| Matthias Schlund, 2019, Oman, English [19] | N | Y | Y | N | Y | NA | Y | N |
| Kamel Alraei, 2020, Saudi Arabia, English [20] | N | Y | Y | N | Y | NA | Y | Y |
| Jeanette Johnson, 2016, Texas, English [21] | N | Y | Y | N | Y | NA | Y | Y |
| N. Ali, 2018, Texas, English [22] | N | Y | Y | N | Y | NA | Y | Y |
| Todd G. Carter, 2008, Seattle, English [23] | N | Y | Y | Y | Y | NA | Y | Y |
| Weiss R., 2022, USA, English [24] | N | Y | Y | N | Y | NA | Y | Y |
| Melville et al., 2019, USA, English [25] | N | Y | Y | Y | Y | NA | Y | Y |
2.9. Data Analysis
3. Results
Study Characteristics
4. Discussion
Limitations of the Technique and Future Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Schimming, R.; Schmelzeisen, R. Tissue-engineered bone for maxillary sinus augmentation. J. Oral Maxillofac. Surg. 2004, 62, 724–729. [Google Scholar] [CrossRef]
- Akinbami, B.O. Reconstruction of Continuity Defects of the Mandible with Non-vascularized Bone Grafts. Systematic Literature Review. Craniomaxillofac. Trauma Reconstr. 2016, 9, 195–205. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef] [PubMed]
- Spicer, P.; Young, S.; Kasper, F.K.; Athanasiou, K.A.; Mikos, A.G.; Wong, M.E.-K. Tissue engineering in oral and maxillofacial surgery. In Principles of Tissue Engineering; Elsevier: Tenta, Egypt, 2014; pp. 1487–1506. [Google Scholar]
- Jensen, O.T.; Sennerby, L. Histologic analysis of clinically retrieved titanium microimplants placed in conjunction with maxillary sinus floor augmentation. Int. J. Oral Maxillofac. Implant. 1998, 13, 513–521. [Google Scholar]
- Lorenzetti, M.; Mozzati, M.; Campanino, P.P.; Valente, G. Bone augmentation of the inferior floor of the maxillary sinus with autogenous bone or composite bone grafts: A histologic-histomorphometric preliminary report. Int. J. Oral Maxillofac. Implant. 1998, 13, 69–76. [Google Scholar]
- Vignesh, U.; Mehrotra, D.; Howlader, D.; Kumar, S.; Anand, V. Bone Marrow Aspirate in Cystic Maxillofacial Bony Defects. J. Craniofacial Surg. 2019, 30, e247–e251. [Google Scholar] [CrossRef]
- Viña, J.A.; El-Alami, M.; Gambini, J.; Borras, C.; Viña, J.; Peñarrocha, M.A. Application of mesenchymal stem cells in bone regenerative procedures in oral implantology. A literature review. J. Clin. Exp. Dent. 2014, 6, e60–e65. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Tariq, R.; Asiri, F.Y.; Abid, K.; Zafar, M.S. Literature search strategies in dental education. J. Taibah Univ. Med. Sci. 2021, 16, 799–806. [Google Scholar] [CrossRef]
- Wiltfang, J.; Rohnen, M.; Egberts, J.-H.; Lützen, U.; Wieker, H.; Açil, Y.; Naujokat, H. Man as a living bioreactor: Prefabrication of a custom vascularized bone graft in the gastrocolic omentum. Tissue Eng. Part C Methods 2016, 22, 740–746. [Google Scholar] [CrossRef]
- Deshmukh, A.; Kalra, R.; Chhadva, S.; Shetye, A. Bilateral maxillary sinus floor augmentation with tissue-engineered autologous osteoblasts and demineralized freeze-dried bone. Contemp. Clin. Dent. 2015, 6, 243. [Google Scholar] [CrossRef]
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological quality and synthesis of case series and case reports. BMJ Evid.-Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Melville, J.C.; Nassari, N.N.; Hanna, I.A.; Shum, J.W.; Wong, M.E.; Young, S. Immediate Transoral Allogeneic Bone Grafting for Large Mandibular Defects. Less Morbidity, More Bone. A Paradigm in Benign Tumor Mandibular Reconstruction? J. Oral Maxillofac. Surg. 2017, 75, 828–838. [Google Scholar] [CrossRef]
- Melville, J.C.; Tursun, R.; Green, J.M., III; Marx, R.E. Reconstruction of a post-traumatic maxillary ridge using a radial forearm free flap and immediate tissue engineering (bone morphogenetic protein, bone marrow aspirate concentrate, and cortical-cancellous bone): Case report. J. Oral Maxillofac. Surg. 2017, 75, 438.e1–438.e6. [Google Scholar] [CrossRef] [PubMed]
- Melville, J.; Marx, R.; Tursun, R.; Moody, M.; Hew, D.; Schacht, S.; Starley, E.; Broumand, V.; Peleg, M.; Sawatari, Y. The utilization of allogeneic bone, bone morphogenetic protein and bone marrow aspirate concentrate for immediate reconstruction of benign tumor continuity defects. J. Oral Maxillofac. Surg. 2014, 72, e204–e205. [Google Scholar] [CrossRef]
- Melville, J.C.; Tran, H.Q.; Bhatti, A.K.; Manon, V.; Young, S.; Wong, M.E. Is Reconstruction of Large Mandibular Defects Using Bioengineering Materials Effective? J. Oral Maxillofac. Surg. 2020, 78, 661.e1–661.e29. [Google Scholar] [CrossRef]
- Schlund, M.; Nicot, R.; Depeyre, A.; Alkasbi, J.; Ferri, J. Reconstruction of a large posttraumatic mandibular defect using bone tissue engineering with fresh-frozen humeral allograft seeded with autologous bone marrow aspirate and vascularized with a radial forearm flap. J. Craniofacial Surg. 2019, 30, 2085–2087. [Google Scholar] [CrossRef] [PubMed]
- Alraei, K.; Sharqawi, J.; Harcher, S.; Ghita, I. Efficacy of the Combination of rhBMP-2 with Bone Marrow Aspirate Concentrate in Mandibular Defect Reconstruction after a Pindborg Tumor Resection. Case Rep. Dent. 2020, 2020, 8281741. [Google Scholar] [CrossRef]
- Johnson, J.; Jundt, J.; Hanna, I.; Shum, J.W.; Badger, G.; Melville, J.C. Resection of an ameloblastoma in a pediatric patient and immediate reconstruction using a combination of tissue engineering and costochondral rib graft: A case report. J. Am. Dent. Assoc. 2017, 148, 40–43. [Google Scholar] [CrossRef]
- Ali, N.; Young, S.; Shum, J.W.; Hanna, I.; Wong, M.E.; Melville, J.C. The Efficacy of Bioengineering (Stem Cells, Allogeneic Bone, and rhBMP-2) for Reconstruction of Large Mandibular Continuity Defects: A Retrospective Study of 24 Patients over a 3-Year Period. J. Oral Maxillofac. Surg. 2018, 76, e75. [Google Scholar] [CrossRef]
- Carter, T.G.; Brar, P.S.; Tolas, A.; Beirne, O.R. Off-label use of recombinant human bone morphogenetic protein-2 (rhBMP-2) for reconstruction of mandibular bone defects in humans. J. Oral Maxillofac. Surg. 2008, 66, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Melville, J.C.; Mañón, V.A.; Blackburn, C.; Young, S. Current Methods of Maxillofacial Tissue Engineering. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 579–591. [Google Scholar] [CrossRef]
- Bauermeister, A.J.; Zuriarrain, A.; Newman, M.I. Three-Dimensional Printing in Plastic and Reconstructive Surgery: A Systematic Review. Ann. Plast. Surg. 2016, 77, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, C.K.; Ayad, M.; Lechtholz-Zey, E.; Chen, Y.; Lieberman, J.R. 3D-Printing for Critical Sized Bone Defects: Current Concepts and Future Directions. Bioengineering 2022, 9, 680. [Google Scholar] [CrossRef]
- Marx, R.E.; Harrell, D.B. Translational research: The CD34+ cell is crucial for large-volume bone regeneration from the milieu of bone marrow progenitor cells in craniomandibular reconstruction. Int. J. Oral Maxillofac. Implant. 2014, 29, e201–e209. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E.; Armentano, L.; Olavarria, A.; Samaniego, J. rhBMP-2/ACS grafts versus autogenous cancellous marrow grafts in large vertical defects of the maxilla: An unsponsored randomized open-label clinical trial. Int. J. Oral Maxillofac. Implant. 2013, 28, e243-51. [Google Scholar] [CrossRef]
- Weiss, R.O.; Wong, P.E.; Reddy, L.V. (Eds.) Immediate Reconstruction of Segmental Mandibular Defects via Tissue Engineering; Baylor University Medical Center Proceedings; Taylor & Francis: Montgomery, TX, USA, 2022. [Google Scholar]
- Melville, J.C.; Tran, H.Q.; Shum, J.W.; Tursun, R.; Marx, R.E. Reconstruction of Post-Traumatic Maxillary Ridges Using a Radial Forearm Free Flap and Allogeneic Tissue-Engineered Bone Grafts. In Regenerative Medicine and Plastic Surgery: Elements, Research Concepts and Emerging Technologies; Springer: Cham, Switzerland, 2019; pp. 349–355. [Google Scholar]
- Nerem, R.M.; Seliktar, D. Vascular tissue engineering. Annu. Rev. Biomed. Eng. 2001, 3, 225–243. [Google Scholar] [CrossRef]
- Hertrampf, K.; Wenz, H.J.; Lehmann, K.M.; Lorenz, W.; Koller, M. Quality of life of patients with maxillofacial defects after treatment for malignancy. Int. J. Prosthodont. 2004, 17, 657–665. [Google Scholar]
- Elsalanty, M.E.; Genecov, D.G. Bone grafts in craniofacial surgery. Craniomaxillofac. Trauma Reconstr. 2009, 2, 125–134. [Google Scholar] [CrossRef]
- Misch, C.M.; Jensen, O.T.; Pikos, M.A.; Malmquist, J.P. Vertical bone augmentation using recombinant bone morphogenetic protein, mineralized bone allograft, and titanium mesh: A retrospective cone beam computed tomography study. Int. J. Oral Maxillofac. Implant. 2015, 30, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.D.; Anthony, J.P.; Sharma, A.; Pogrel, M.A. Vascularized bone flaps versus nonvascularized bone grafts for mandibular reconstruction: An outcome analysis of primary bony union and endosseous implant success. Head Neck 1999, 21, 66–71. [Google Scholar] [CrossRef]
- Pogrel, M.A.; Podlesh, S.; Anthony, J.P.; Alexander, J. A comparison of vascularized and nonvascularized bone grafts for reconstruction of mandibular continuity defects. J. Oral Maxillofac. Surg. 1997, 55, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.P.; Mikos, A.G.; Bronzino, J.D.; Peterson, D.R. Tissue Engineering: Principles and Practices; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Nelms, L.; Palmer, W.J. Tissue engineering in mandibular reconstruction: Osteogenesis-inducing scaffolds. Plast. Aesthetic Res. 2019, 6, 21. [Google Scholar] [CrossRef]
- Stevens, B.; Yang, Y.; Mohandas, A.; Stucker, B.; Nguyen, K.T. A review of materials, fabrication methods, and strategies used to enhance bone regeneration in engineered bone tissues. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 85, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Forriol, F.; Denaro, L.; Longo, U.G.; Taira, H.; Maffulli, N.; Denaro, V. Bone lengthening osteogenesis, a combination of intramembranous and endochondral ossification: An experimental study in sheep. Strateg. Trauma Limb Reconstr. 2010, 5, 71–78. [Google Scholar] [CrossRef]
- Gianakos, A.L.; Sun, L.; Patel, J.N.; Adams, D.M.; Liporace, F.A. Clinical application of concentrated bone marrow aspirate in orthopaedics: A systematic review. World J. Orthop. 2017, 8, 491–506. [Google Scholar] [CrossRef]
- Molinari, R.W.; Molinari, C. The Use of Bone Morphogenetic Protein in Pediatric Cervical Spine Fusion Surgery: Case Reports and Review of the Literature. Glob. Spine J. 2016, 6, e41–e46. [Google Scholar] [CrossRef]
- Kengelbach-Weigand, A.; Thielen, C.; Bäuerle, T.; Götzl, R.; Gerber, T.; Körner, C.; Beier, J.P.; Horch, R.E.; Boos, A.M. Personalized medicine for reconstruction of critical-size bone defects—A translational approach with customizable vascularized bone tissue. NPJ Regen. Med. 2021, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Javaid, M.A.; Hamdan, N.; Hashmi, R. Bone Regeneration Using Bone Morphogenetic Proteins and Various Biomaterial Carriers. Materials 2015, 8, 1778–1816. [Google Scholar] [CrossRef]
| PubMed | ||
|---|---|---|
| 1 | (“Bioengineering” [Mesh]) OR (“Bioengineering material” [Title/Abstract]) OR (“osteogenic scaffold” [Title/Abstract]) OR (“tissue engineering” [Title/Abstract]) OR (“Tissue Engineering” [Mesh]) OR (“Bone Morphogenetic Proteins” [Mesh]) OR (“Bone Morphogenetic Proteins” [Title/Abstract]) OR (“Mesenchymal Stem Cells” [Mesh]) OR (“Bone Mesenchymal Stem Cells” [Title/Abstract]) OR (“beta-tri calcium phosphate” [Title/Abstract]) OR (“Bone Morphogenetic Protein 2” [Mesh]) OR (rhBPM2) OR (rhBPM-2) | 149,201 |
| 2 | (Mandible[Title/Abstract]) OR (Mandibular[Title/Abstract]) OR (Maxilla[Title/Abstract]) OR (Maxillary[Title/Abstract]) OR (Maxillofacial[Title/Abstract]) | 175,429 |
| 3 | (“Reconstructive Surgical Procedures” [Mesh]) OR (Reconstruct[Title/Abstract]) OR (Augment[Title/Abstract]) | 558,576 |
| 1 AND 2 AND 3 | (TITLE-ABS-KEY (bioengineering) OR TITLE-ABS-KEY (“osteogenic scaffold”) OR TITLE-ABS-KEY (“osteogenic scaffolds”) OR TITLE-ABS-KEY (“tissue engineering”) OR TITLE-ABS-KEY (“Bone Morphogenetic Proteins”) OR TITLE-ABS-KEY (“Bone Morphogenetic Protein”) OR TITLE-ABS-KEY (“Mesenchymal Stem Cells”) OR TITLE-ABS-KEY (“Bone Mesenchymal Stem Cells”) OR TITLE-ABS-KEY (“beta-tri calcium phosphate”) OR TITLE-ABS-KEY (“Bone Morphogenetic Protein 2”) OR TITLE-ABS-KEY (rhbpm2) OR TITLE-ABS-KEY (rhbpm-2)) AND (TITLE-ABS-KEY (mandible) OR TITLE-ABS-KEY (mandibular) OR TITLE-ABS-KEY (maxilla) OR TITLE-ABS-KEY (maxilla) OR TITLE-ABS-KEY (maxillary) OR TITLE-ABS-KEY (maxillofacial)) AND (TITLE-ABS-KEY (“Reconstructive Surgical Procedures”) OR TITLE-ABS-KEY (reconstruct) OR TITLE-ABS-KEY (augment) OR TITLE-ABS-KEY (reconstruction) OR TITLE-ABS-KEY (augmentation)) | 735 |
| Scopus | ||
| 1 | TITLE-ABS-KEY (bioengineering) OR TITLE-ABS-KEY (“osteogenic scaffold”) OR TITLE-ABS-KEY (“osteogenic scaffolds”) OR TITLE-ABS-KEY (“tissue engineering”) OR TITLE-ABS-KEY (“Bone Morphogenetic Proteins”) OR TITLE-ABS-KEY (“Bone Morphogenetic Protein”) OR TITLE-ABS-KEY (“Mesenchymal Stem Cells”) OR TITLE-ABS-KEY (“Bone Mesenchymal Stem Cells”) OR TITLE-ABS-KEY (“beta-tri calcium phosphate”) OR TITLE-ABS-KEY (“Bone Morphogenetic Protein 2”) OR TITLE-ABS-KEY (rhbpm2) OR TITLE-ABS-KEY (rhbpm-2) | 273,893 |
| 2 | TITLE-ABS-KEY (mandible) OR TITLE-ABS-KEY (mandibular) OR TITLE-ABS-KEY (maxilla) OR TITLE-ABS-KEY (maxillary) OR TITLE-ABS-KEY (maxillofacial) | 275,089 |
| 3 | TITLE-ABS-KEY (“Reconstructive Surgical Procedures”) OR TITLE-ABS-KEY (reconstruct) OR TITLE-ABS-KEY (augment) OR TITLE-ABS-KEY (reconstruction) OR TITLE-ABS-KEY (augmentation) | 1,114,947 |
| 1 AND 2 AND 3 | (TITLE-ABS-KEY (bioengineering) OR TITLE-ABS-KEY (“osteogenic scaffold”) OR TITLE-ABS-KEY (“osteogenic scaffolds”) OR TITLE-ABS-KEY (“tissue engineering”) OR TITLE-ABS-KEY (“Bone Morphogenetic Proteins”) OR TITLE-ABS-KEY (“Bone Morphogenetic Protein”) OR TITLE-ABS-KEY (“Mesenchymal Stem Cells”) OR TITLE-ABS-KEY (“Bone Mesenchymal Stem Cells”) OR TITLE-ABS-KEY (“beta-tri calcium phosphate”) OR TITLE-ABS-KEY (“Bone Morphogenetic Protein 2”) OR TITLE-ABS-KEY (rhbpm2) OR TITLE-ABS-KEY (rhbpm-2)) AND (TITLE-ABS-KEY (mandible) OR TITLE-ABS-KEY (mandibular) OR TITLE-ABS-KEY (maxilla) OR TITLE-ABS-KEY (maxilla) OR TITLE-ABS-KEY (maxillary) OR TITLE-ABS-KEY (maxillofacial)) AND (TITLE-ABS-KEY (“Reconstructive Surgical Procedures”) OR TITLE-ABS-KEY (reconstruct) OR TITLE-ABS-KEY (augment) OR TITLE-ABS-KEY (reconstruction) OR TITLE-ABS-KEY (augmentation)) | 1227 |
| Embase | ||
| 1 | bioengineering:ti,ab,kw OR ‘osteogenic scaffold’:ti,ab,kw OR ‘tissue engineering’:ti,ab,kw OR ‘bone morphogenetic protein’:ti,ab,kw OR ‘mesenchymal stem cell’:ti,ab,kw OR ‘beta-tri calcium phosphate’:ti,ab,kw OR ‘bone morphogenetic protein 2′:ti,ab,kw OR rhbpm2:ti,ab,kw | 115,437 |
| 2 | mandible:ti,ab,kw OR ‘jaw disease’:ti,ab,kw OR mandibular:ti,ab,kw OR maxilla:ti,ab,kw OR maxillary:ti,ab,kw OR ‘maxillofacial disorder’:ti,ab,kw OR maxillofacial:ti,ab,kw | 202,168 |
| 3 | ‘reconstructive surgery’:ti,ab,kw OR reconstruct:ti,ab,kw OR reconstruction:ti,ab,kw OR augment:ti,ab,kw OR augmentation:ti,ab,kw | 473,086 |
| 1 AND 2 AND 3 | 656 |
| Articles (First Author, Year, Title) | Reason for Exclusion |
|---|---|
| N.M.A. Lopes, 2012, Use of rhBMP-2 to reconstruct a severely atrophic mandible: A modified approach | tricalcium phosphate instead of allogenic bone |
| Schuckert KH, 2009, Mandibular Defect Reconstruction Using Three-Dimensional Polycaprolactone Scaffold in Combination with Platelet-Rich Plasma and Recombinant Human Bone Morphogenetic Protein-2: De Novo Synthesis of Bone in a Single Case | de novo not allogenic |
| Jörg Wiltfang, 2016, man as a Living Bioreactor: Prefabrication of a Custom Vascularized Bone Graft in the Gastrocolic Omentum | bovine bone not allograft |
| Rômulo Maciel Lustosa, 2014, Mandible reconstruction using rhBMP-2: case report and literature review | bovine bone xenograft not allograft |
| G. K. Sándor,2013, Adipose stem cell tissue-engineered construct used to treat large anterior mandibular defect: a case report and review of the clinical application of good manufacturing practice-level adipose stem cells for bone regeneration (β-tricalcium phosphate) | (β-TCP) granules not allogenic |
| K. Mesimäki, 2009, Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells | beta-tricalcium phosphate not allogenic |
| B. Zamiri, 2013, Reconstruction of human mandibular continuity defects with allogenic scaffold and autologous marrow mesenchymal stem cells | ex-vivo MSC |
| L. M. S. Zanettini, 2018, use of Recombinant Human Bone Morphogenetic Protein-2 Associated With Lyophilized Bovine Bone in Reconstruction of Atrophic Maxilla | bovine not allograft |
| M. Albanese, 2012, Fresh-frozen human bone graft to repair defect after mandibular giant follicular cyst removal: A case report. Cell and Tissue Banking. | no rhBMP |
| R. Bertolai, 2015, Bone graft and mesenchimal stem cells: Clinical observations and histological analysis. Clinical Cases in Mineral and Bone Metabolism. | mesenchymal stem cells engineered freeze-dried bone allografts/no rhBMP |
| C. M. Clokie, 2008, Reconstruction of 10 major mandibular defects using bioimplants containing BMP-7 | no rhBMP |
| M. Cicciù, 2012,Protein-Signaled Guided Bone Regeneration Using Titanium Mesh and Rh-BMP2 in Oral Surgery: A Case Report Involving Left Mandibular Reconstruction after Tumor Resection | no allograft |
| S. C. Desai, 2013, Use of Recombinant Human Bone Morphogenetic Protein 2 for Mandible Reconstruction | no BMA |
| C. M. Misch, 2015, Vertical Bone Augmentation Using Recombinant Bone Morphogenetic Protein, Mineralized Bone Allograft, and Titanium Mesh: A Retrospective Cone Beam Computed Tomography Study | not large defect |
| B. B. Kim, 2014, Hybrid mandibular reconstruction technique: Preliminary case series of prosthetically-driven vascularized fibula free flap combined with tissue engineering and virtual surgical planning | no rhBMP2 |
| Mark C. Fagan, 2008, Simultaneous hard and soft tissue augmentation for implants in the esthetic zone: Report of 37 consecutive cases. | no rhBMP2 |
| B. Haj Yahya, 2020, Non-Autogenous Innovative Reconstruction Method Following Mandibulectomy | defect size is small |
| H. I. Canter, 2007, Reconstruction of mandibular defects using autografts combined with demineralized bone matrix and cancellous allograft | bone harvest included |
| C. Loperfido, 2014, Severe mandibular atrophy treated with a subperiosteal implant and simultaneous graft with rhBMP-2 and mineralized allograft: a case report | no stem cell |
| A. Deshmukh, 2015, Bilateral maxillary sinus floor augmentation with tissue-engineered autologous osteoblasts and demineralized freeze-dried bone [2] | defect size is small |
| M. S. Block, 2010, Use of Living Cell Construct to Enhance Bone Reconstruction: Preliminary Results | no rhBMP |
| First Author/Year/Country of Origin/Language | Type of Study | Number of Cases/Duration of Follow Up | Mean Age/Sex | Summary of Method | cause of Defect/Size of Defect/Filling Rate of Defect (Bone Gain) | Mesenchymal Stem Cells Harvesting Site | Success Rate |
|---|---|---|---|---|---|---|---|
| James C. Melville, 2016, Houston, English [15] | Case report | 5 cases/12–14 months | 18–66 years old/3 men and 2 women | the freeze-dried cortical-cancellous bone in combination with large rhBMP-2 (12 mg)/absorbable collagen sponge (ACS) and 120 mL of BMAC obtained from anterior or posterior hip were mixed homogeneously. 10 mL of crushed cortical cancellous bone for each 1 cm length of the defect. | benign mandibular tumors with no history of chemotherapy or radiation to the mandible (Ossifying fibroma, Desmoplastic, Juvenile ossifying fibroma)/3.5 to 8.0 cm/10–14.5 mm and regenerated bone height was in the range of 22–26 mm | BMAC was harvested from either the bilateral anterior iliac crest or unilateral posterior iliac crest | 100% success |
| James C. Melville, 2017, Houston, English [16] | Case report | 1 case/6 months | 45 years old/woman | radial forearm fasciocutaneous flap combined with a tissue-engineered bone graft consisting of allogeneic bone, rhBMP-2, and BMAC. | trauma (deficient projection of the left malar region, loss of left maxillary ridge alveolar bone, loss of dentition and upper eyelid ptosis, and lower eyelid ectropion) | BMAC form the iliac crest | 100% success |
| Robert E Marx, 2014, USA, English [26,27] | Case report | 40 case/6 months | mean age 57 years (19–78 years)/22 men and 12 women | in situ tissue-engineered graft containing 54 ± 38 CD34+ cells/mL along with 54 ± 38 CD44+, CD90+, and CD105+ cells/mL together with rhBMP-2 in an absorbable collagen sponge (1 mg/cm of defect) and crushed cancellous allogeneic bone. | /6- to 8-cm continuity defects/trabecular bone area of 36 ± 10%, versus 67 ± 13% for group B | four puncture sites in the bilateral anterior iliac crest | Group A: 40% success rate Group B: 100% success rate |
| J. C. Melville, 2014, Houston, English [17] | retrospective study | 9 cases/4 years | mean age 23.7-year-old (3 patients under the age of 17)/5 men, 4 women | freeze-dried cortical cancellous bone was obtained used in combination with 12 mg of rhBMP-2/ACS and 120 cc of Bone Marrow Aspirate Concentrate | ameloblastoma, OKC, Myxoma, Ossifying Fibroma and Central Giant Cell Tumor/4 cm to 12 cm | anterior hip | 100% success rate |
| Robert E Marx, 2013, USA, English [28] | clinical trial | 20 cases/6 months | Mean age 58 and 62/5 men, 15 women | two types of grafts in large vertical maxillary defects: a composite graft of recombinant human bone morphogenetic protein-2/acellular collagen sponge (rhBMP-2/ACS), crushed cancellous freeze-dried allogeneic bone (CCFDAB), and platelet-rich plasma (PRP); and size-matched 100% autogenous grafts | horizontal defects/1 cm vertical deficiency and 1 cm horizontal deficiency spanning at least a four-tooth length/2-mm-diameter bone core (bone area 59 ± 12% and 54 ± 10%) | tibia plateau or anterior ilium | composite graft: 97.4% success rate autogenous grafts: 100% success rate |
| James C. Melville, 2019, Houston, English [18] | retrospective case | 34 cases/5 years | mean age 37.79 ± 20.4 (9–89 years old)/19 men, 15 women | first, BMAC was obtained from the patient’s anterior or posterior hip using the Harvest Bone Marrow Aspirate Concentrate System. Second, a medium to large rhBMP-2 kit was used, according to size of the defect (a large kit was used for defects greater than 6 cm). Third, corticocancellous bone (MTF Biologics. Edison, NJ, USA) was milled down to a 1.0- to 2.0-mm particulate graft. Finally, a non-resorbable titanium mesh or resorbable poly (L-lactide) (PLLA) or poly (D, L-lactide) (PDLLA) membrane was used as the containment system for the graft. | ablative tumor surgery or traumatic accidents (ameloblastoma, ossifying fibroma, odontogenic keratocyst (OKC), and sclerosing osteomyelitis, odontogenic myxoma, giant cell tumor associated with hyperparathyroidism, and central giant cell granuloma)/continuity defect 5.61 ± 2.92 and Noncontinuity defect 4.77 ± 3.33/mean height 2.12 ± 0.44 Mean width 1.53 ± 0.55 | anterior or posterior hip | Continuity defect 90% Noncontinuity defect 100% |
| Matthias Schlund, 2019, Oman, English [19] | clinical report | 1 cases/1 years | 33-year-old/men | using a fresh-frozen humeral allograft as scaffold seeded with progenitor cells collected through iliac bone marrow aspirate and vascularized with a radial forearm free flap | severe craniofacial trauma resulting in several fractures of the facial skeleton including a comminuted mandibular fracture from left parasymphysis to left angle | iliac bone marrow aspirate | 100% success rate |
| Kamel Alraei, 2020, Saudi Arabia, English [20] | case report | 1 case/4 years | 27 years old/female | reconstruction of the mandibular defect using rhBMP-2 combined with bone marrow aspirate concentrate (BMAC), and an allograft with a titanium mesh. placed a total of 12 mg of rhBMP-2 in four absorbable collagen sponges | a calcifying, cystic, odontogenic tumor (a Pindborg tumor)/2.7 mm | 60 cc of bone marrow from left iliac crest | Success rate 100% |
| Jeanette Johnson, 2016, Texas, English [21] | Case report | 1 case/1 years | 11 years old/female | reconstruction using a costochondral rib graft, allogeneic bone, bone marrow aspirate concentrate, and recombinant human morphogenetic protein-2. | unilocular radiolucent lesion of the posterior left mandible/3.4 × 4.2 × 3.1 cm | bone marrow aspirate concentrate | Success rate 100% |
| N. Ali, 2018, Texas, English [22] | Retrospective study | 24 cases/3 years | Mean age 32.1 years old (11–65 years old)/ 14 men and 10 women | freeze-dried cancellous bone was obtained from MTF and used in combination with 12 mg of rhBMP-2/ACS, and 120 cc of BMAC was obtained from the patients | ablative tumor and trauma/ 5–17 cm/ 3.0 cm bone height and >1.0 cm bone width | anterior or posterior hip | 88% success rate |
| Todd G. Carter, 2008, Seattle, English [23] | Case report | 5 cases/22 months | 42,43, 41, 81/2 men, 3 women | reconstruction with rhBMP-2. Because, in case 1, rhBMP-2 absorbed on a collagen sponge alone failed to regenerate bone, autogenous bone marrow, and allogenic bone were combined with rhBMP-2-impregnated collagen sponges to increase the osteogenic. | open and displaced left mandibular angle fracture, with an infection that required incision and drainage, multiple facial lacerations and a comminuted mandible fracture, osteomyelitis of the right mandible, radiolucent lesions in the mandible/4 cm, 4.5 cm | bone marrow from left iliac crest, bone marrow cells from the patient’s right anterior iliac crest. | - |
| Weiss R., 2022, USA, English [29] | Case report | 2 cases/4 months | 62-year-old woman/24-year-old woman | reconstruction with (1) corticocancellous bone chips (2) bone marrow aspirate, (3) rhBMP-2 A combined intraoral and extraoral approach was used to allow access. Cadaveric rib allograft was secured to the inferior aspect of the reconstruction plate. Nerve allografts were secured to the inferior alveolar nerve stumps and a water-tight closure | excisional biopsy of ameloblastoma 8 years earlier, segmental defect 6.0 × 5.0 × 3.7 cm/no past medical history, 4.4 × 2.1 × 2.1 cm | bone marrow was harvested via trochar and cannula from the anterior iliac crest | 100% |
| Melville et al., 2019, USA, English [30] | Case report | 1 case/6 months | 45-year-old female | exposing and preparing the alveolar defect as well as harvesting the BMAC from the iliac crest and harvesting the radial forearm. crushed corticocancellous bone and rhBMP-2 were mixed with BMAC. The tissue-engineered graft was placed and packed onto the defect. The radial forearm was then sutured around and over the graft. | post-traumatic maxillary alveolar ridge defect/size not mentioned | bone marrow can be aspirated from the anterior ilium | 100% success |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramezanzade, S.; Aeinehvand, M.; Ziaei, H.; Khurshid, Z.; Keyhan, S.O.; Fallahi, H.R.; Melville, J.C.; Saeinasab, M.; Sefat, F. Reconstruction of Critical Sized Maxillofacial Defects Using Composite Allogeneic Tissue Engineering: Systematic Review of Current Literature. Biomimetics 2023, 8, 142. https://doi.org/10.3390/biomimetics8020142
Ramezanzade S, Aeinehvand M, Ziaei H, Khurshid Z, Keyhan SO, Fallahi HR, Melville JC, Saeinasab M, Sefat F. Reconstruction of Critical Sized Maxillofacial Defects Using Composite Allogeneic Tissue Engineering: Systematic Review of Current Literature. Biomimetics. 2023; 8(2):142. https://doi.org/10.3390/biomimetics8020142
Chicago/Turabian StyleRamezanzade, Shaqayeq, Mahsa Aeinehvand, Heliya Ziaei, Zohaib Khurshid, Seied Omid Keyhan, Hamid R. Fallahi, James C. Melville, Morvarid Saeinasab, and Farshid Sefat. 2023. "Reconstruction of Critical Sized Maxillofacial Defects Using Composite Allogeneic Tissue Engineering: Systematic Review of Current Literature" Biomimetics 8, no. 2: 142. https://doi.org/10.3390/biomimetics8020142
APA StyleRamezanzade, S., Aeinehvand, M., Ziaei, H., Khurshid, Z., Keyhan, S. O., Fallahi, H. R., Melville, J. C., Saeinasab, M., & Sefat, F. (2023). Reconstruction of Critical Sized Maxillofacial Defects Using Composite Allogeneic Tissue Engineering: Systematic Review of Current Literature. Biomimetics, 8(2), 142. https://doi.org/10.3390/biomimetics8020142










