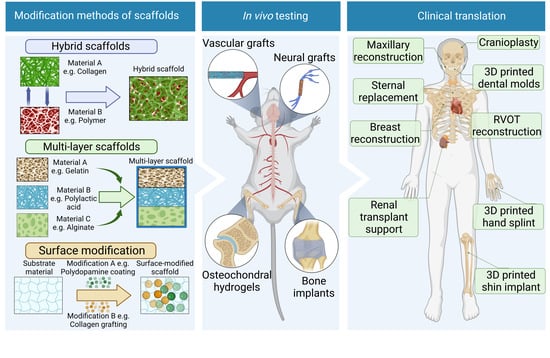
Recent Methods for Modifying Mechanical Properties of Tissue-Engineered Scaffolds for Clinical Applications
Abstract
1. Introduction
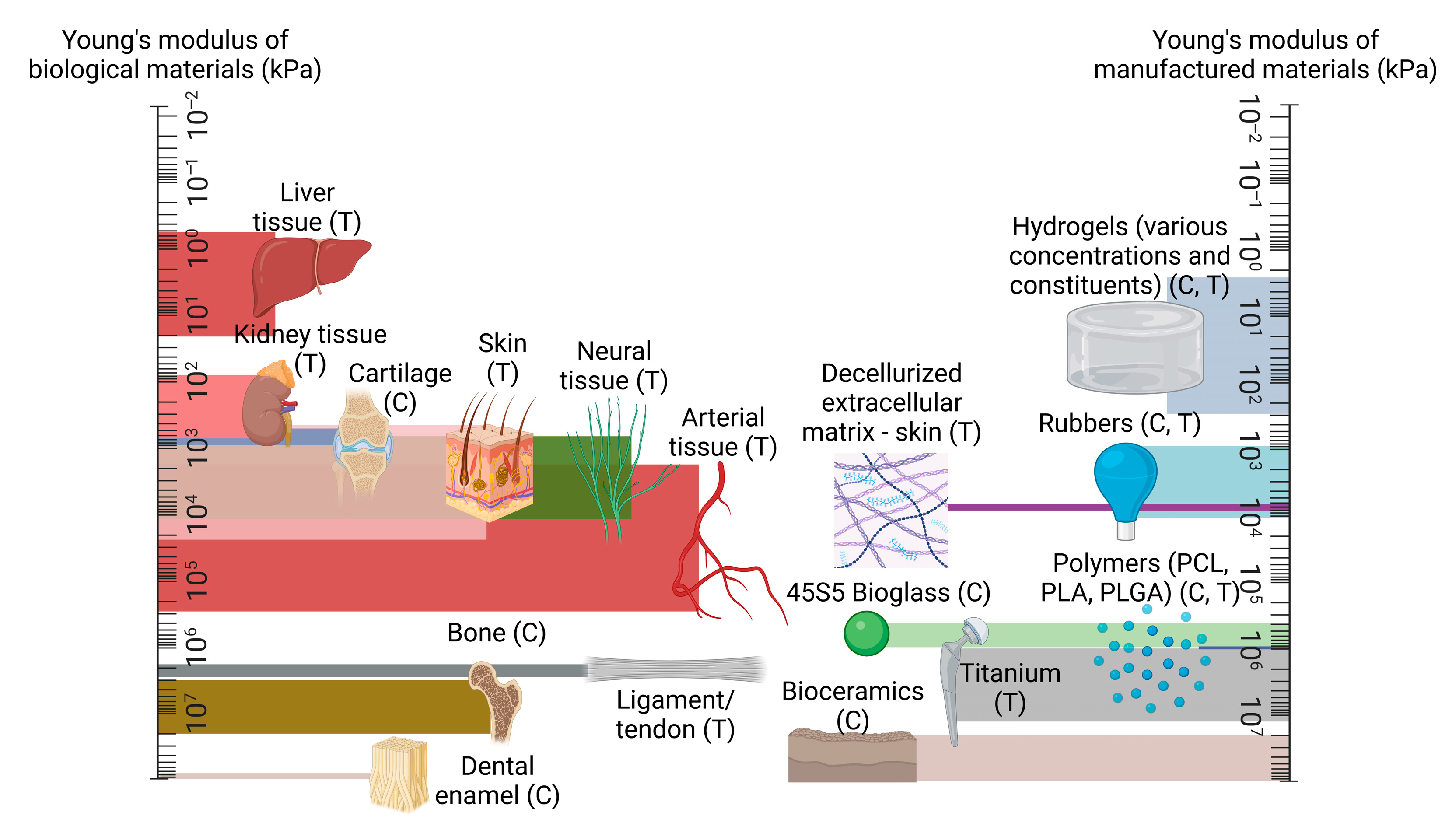
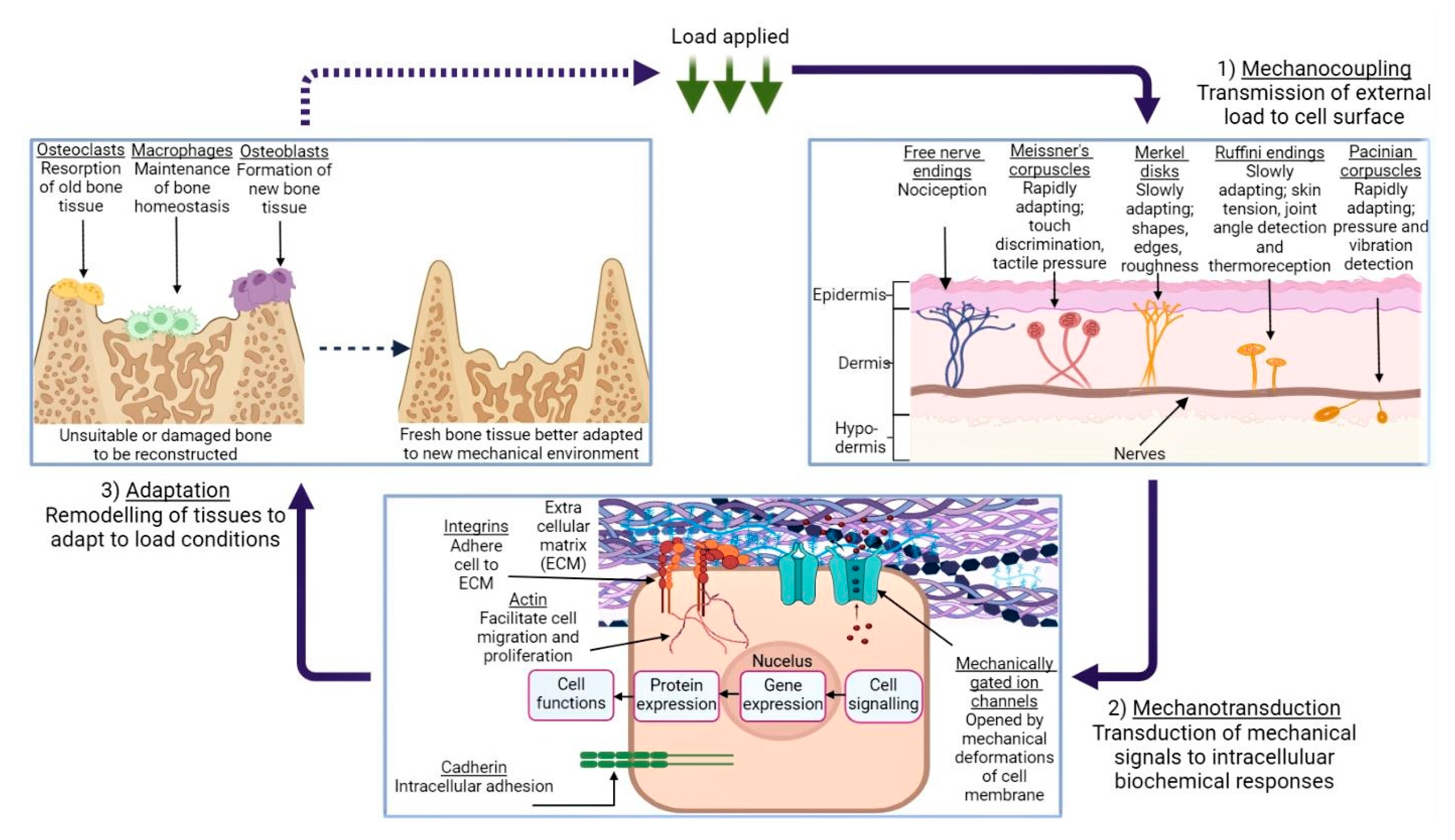
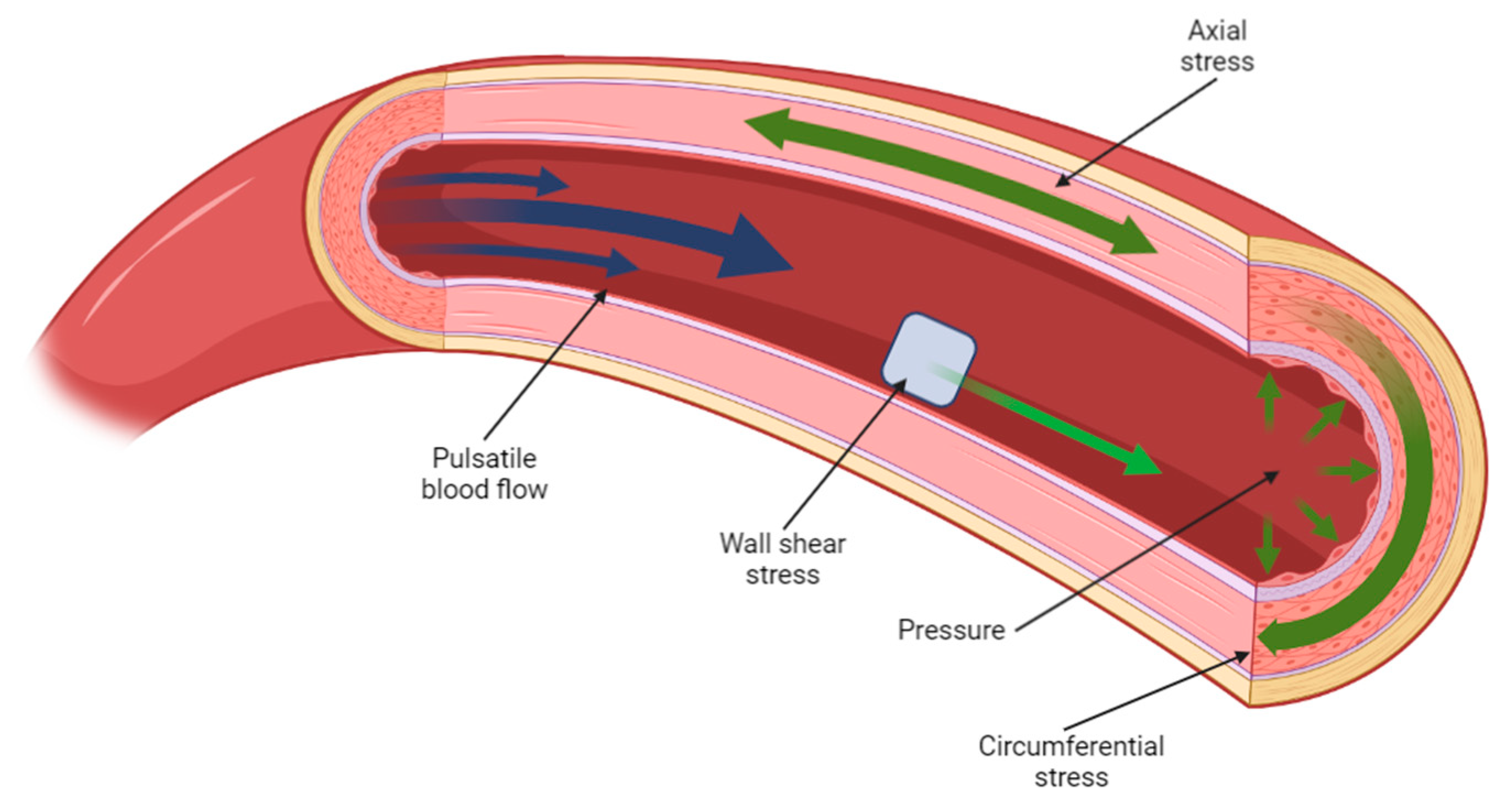
2. Mechanical Properties of Biological Tissues and Their Influence
3. Hybrid Materials
4. Multi-Layer Scaffolds
5. Surface Modification
6. In Vitro Limitations and Animal Research
7. Clinical Translation, Challenges, and Future Outlook
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sarasa-Renedo, A.; Chiquet, M. Mechanical signals regulating extracellular matrix gene expression in fibroblasts. Scand. J. Med. Sci. Sports 2005, 15, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Papanicolaou, M.; Cox, T.R. Extracellular Matrix (ECM). In Encyclopedia of Molecular Pharmacology; Springer: Cham, Switzerland, 2022; pp. 643–650. [Google Scholar]
- Yue, B. Biology of the extracellular matrix: An overview. J. Glaucoma 2014, 23 (Suppl. 1), S20–S23. [Google Scholar] [CrossRef] [PubMed]
- Chow, M.-J.; Turcotte, R.; Lin, C.P.; Zhang, Y. Arterial Extracellular Matrix: A Mechanobiological Study of the Contributions and Interactions of Elastin and Collagen. Biophys. J. 2014, 106, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L.; Bernstein, E.F.; Weiss, A.S.; Bates, D.; Humphrey, S.; Silberberg, M.; Daniels, R. Clinical Relevance of Elastin in the Structure and Function of Skin. Aesthetic Surg. J. Open Forum 2021, 3, ojab019. [Google Scholar] [CrossRef]
- Sinnott, M.D.; Cleary, P.W.; Harrison, S.M. Peristaltic transport of a particulate suspension in the small intestine. Appl. Math. Model. 2017, 44, 143–159. [Google Scholar] [CrossRef]
- Mecham, R. Elastin in lung development and disease pathogenesis. Matrix Biol. 2018, 73, 6–20. [Google Scholar] [CrossRef]
- Faury, G.; Ristori, M.; Verdetti, J.; Jacob, M.; Robert, L. Effect of Elastin Peptides on Vascular Tone. J. Vasc. Res. 1995, 32, 112–119. [Google Scholar] [CrossRef]
- Godinho, M.S.C.; Thorpe, C.T.; Greenwald, S.E.; Screen, H.R.C. Elastin is Localised to the Interfascicular Matrix of Energy Storing Tendons and Becomes Increasingly Disorganised with Ageing. Sci. Rep. 2017, 7, 9713. [Google Scholar] [CrossRef]
- De Brouwer, B.; Drent, M.; van den Ouweland, J.M.; Wijnen, P.A.; van Moorsel, C.H.; Bekers, O.; Grutters, J.C.; White, E.S.; Janssen, R. Increased circulating desmosine and age-dependent elastinolysis in idiopathic pulmonary fibrosis. Respir. Res. 2018, 19, 45. [Google Scholar] [CrossRef]
- Merla, G.; Brunetti-Pierri, N.; Piccolo, P.; Micale, L.; Loviglio, M.N. Supravalvular aortic stenosis: Elastin arteriopathy. Circ. Cardiovasc. Genet. 2012, 5, 692–696. [Google Scholar] [CrossRef]
- Heinz, A. Elastic fibers during aging and disease. Ageing Res. Rev. 2021, 66, 101255. [Google Scholar] [CrossRef] [PubMed]
- Rouchi, A.H.; Mahdavi-Mazdeh, M. Regenerative Medicine in Organ and Tissue Transplantation: Shortly and Practically Achievable? Int. J. Organ Transplant. Med. 2015, 6, 93–98. [Google Scholar]
- Chiarello, E.; Cadossi, M.; Tedesco, G.; Capra, P.; Calamelli, C.; Shehu, A.; Giannini, S. Autograft, allograft and bone substitutes in reconstructive orthopedic surgery. Aging Clin. Exp. Res. 2013, 25, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Tonk, G.; Yadav, P.K.; Agarwal, S.; Jamoh, K. Donor site morbidity in autologous bone grafting—A comparison between different techniques of anterior iliac crest bone harvesting: A prospective study. J. Orthop. Trauma Rehabil. 2022, 29, 22104917221092163. [Google Scholar] [CrossRef]
- Batten, P.; Rosenthal, N.; Yacoub, M.H. Immune response to stem cells and strategies to induce tolerance. Philos. Trans. R. Soc. B: Biol. Sci. 2007, 362, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Heim, M.; Nixon, I.J.; Emmerson, E.; Callanan, A. From hormone replacement therapy to regenerative scaffolds: A review of current and novel primary hypothyroidism therapeutics. Front. Endocrinol. 2022, 13, 2392. [Google Scholar] [CrossRef]
- Handley, E.; Callanan, A. Modulation of Tissue Microenvironment following Myocardial Infarction. Adv. NanoBiomed Res. 2022, 2, 2200005. [Google Scholar] [CrossRef]
- Sturtivant, A.; Callanan, A. The use of antifreeze proteins to modify pore structure in directionally frozen alginate sponges for cartilage tissue engineering. Biomed. Phys. Eng. Express 2020, 6, 055016. [Google Scholar] [CrossRef]
- Sofokleous, P.; Chin, M.H.; Day, R. Phase-separation technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds—Materials, Technologies and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 101–126. [Google Scholar]
- Xie, Z.; Gao, M.; Lobo, A.O.; Webster, T.J. 3D Bioprinting in Tissue Engineering for Medical Applications: The Classic and the Hybrid. Polymers 2020, 12, 1717. [Google Scholar] [CrossRef]
- Costantini, M.; Barbetta, A. Gas foaming technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds; Woodhead Publishing: Sawston, UK, 2018; pp. 127–149. [Google Scholar]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17 (Suppl. 4), 467–479. [Google Scholar] [CrossRef] [PubMed]
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Ghazali, F.A.; Syahrom, A.; Kamarul, T. The Effect of Tortuosity on Permeability of Porous Scaffold. Biomedicines 2023, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability 2023, 15, 823. [Google Scholar] [CrossRef]
- Hosseinkhani, M.; Mehrabani, D.; Karimfar, M.H.; Bakhtiyari, S.; Manafi, A.; Shirazi, R. Tissue Engineered Scaffolds in Regenerative Medicine. World J. Plast. Surg. 2014, 3, 3–7. [Google Scholar] [PubMed]
- Qiu, Y.; Chen, X.; Hou, Y.; Hou, Y.; Tian, S.; Chen, Y.; Yu, L.; Nie, M.; Liu, X. Characterization of different biodegradable scaffolds in tissue engineering. Mol. Med. Rep. 2019, 19, 4043–4056. [Google Scholar] [CrossRef] [PubMed]
- Rezania, N.; Asadi-Eydivand, M.; Abolfathi, N.; Bonakdar, S.; Mehrjoo, M.; Solati-Hashjin, M. Three-dimensional printing of polycaprolactone/hydroxyapatite bone tissue engineering scaffolds mechanical properties and biological behavior. J. Mater. Sci. Mater. Med. 2022, 33, 31. [Google Scholar] [CrossRef]
- De Pieri, A.; Rochev, Y.; Zeugolis, D.I. Scaffold-free cell-based tissue engineering therapies: Advances, shortfalls and forecast. Npj Regen. Med. 2021, 6, 18. [Google Scholar] [CrossRef]
- Jeong, W.-S.; Kim, Y.-C.; Min, J.-C.; Park, H.-J.; Lee, E.-J.; Shim, J.-H.; Choi, J.-W. Clinical Application of 3D-Printed Patient-Specific Polycaprolactone/Beta Tricalcium Phosphate Scaffold for Complex Zygomatico-Maxillary Defects. Polymers 2022, 14, 740. [Google Scholar] [CrossRef]
- Borschel, G.H.; Kia, K.F.; Kuzon, W.M.; Dennis, R.G. Mechanical properties of acellular peripheral nerve. J. Surg. Res. 2003, 114, 133–139. [Google Scholar] [CrossRef]
- Tomlins, P. 1—Material Types for Tissue Scaffolds, in Characterisation and Design of Tissue Scaffolds; Tomlins, P., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 1–21. [Google Scholar]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Polymer-Based Scaffolds for Soft-Tissue Engineering. Polymers 2020, 12, 1566. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2021, 10, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, D.M.; Neacsu, I.A.; Grumezescu, A.-M.; Andronescu, E. New Insights of Scaffolds Based on Hydrogels in Tissue Engineering. Polymers 2022, 14, 799. [Google Scholar] [CrossRef]
- Koushik, T.M.; Miller, C.M.; Antunes, E. Bone Tissue Engineering Scaffolds: Function of Multi-Material Hierarchically Structured Scaffolds. Adv. Healthc. Mater. 2023, 12, 2202766. [Google Scholar] [CrossRef]
- Salati, M.A.; Khazai, J.; Tahmuri, A.M.; Samadi, A.; Taghizadeh, A.; Taghizadeh, M.; Zarrintaj, P.; Ramsey, J.D.; Habibzadeh, S.; Seidi, F.; et al. Agarose-Based Biomaterials: Opportunities and Challenges in Cartilage Tissue Engineering. Polymers 2020, 12, 1150. [Google Scholar] [CrossRef] [PubMed]
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2020, 16, 280–306. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wu, D.; Wu, D.; Cui, Y.; Ren, G.; Wang, Y.; Wang, J.; Peng, C. Chitosan-Based Biomaterial Scaffolds for the Repair of Infected Bone Defects. Front. Bioeng. Biotechnol. 2022, 10, 755. [Google Scholar] [CrossRef]
- Gao, Y.; Callanan, A. Influence of surface topography on PCL electrospun scaffolds for liver tissue engineering. J. Mater. Chem. B 2021, 9, 8081–8093. [Google Scholar] [CrossRef]
- Dai, Y.; Lu, T.; Shao, M.; Lyu, F. Recent advances in PLLA-based biomaterial scaffolds for neural tissue engineering: Fabrication, modification, and applications. Front. Bioeng. Biotechnol. 2022, 10, 1011783. [Google Scholar] [CrossRef]
- Kotturi, H.; Abuabed, A.; Zafar, H.; Sawyer, E.; Pallipparambil, B.; Jamadagni, H.; Khandaker, M. Evaluation of Polyethylene Glycol Diacrylate-Polycaprolactone Scaffolds for Tissue Engineering Applications. J. Funct. Biomater. 2017, 8, 39. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Hartono, R.; Supriyono, T.; Santoso, G.; Sugiharto, S.; Permana, M.S. Polycrystalline Diamond as a Potential Material for the Hard-on-Hard Bearing of Total Hip Prosthesis: Von Mises Stress Analysis. Biomedicines 2023, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, E.; Iaquinta, M.R.; Lanzillotti, C.; Mazziotta, C.; Maritati, M.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Bioactive Materials for Soft Tissue Repair. Front. Bioeng. Biotechnol. 2021, 9, 61378. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.P.; da Silva, B.C.R.; Hamouda, A.E.I.; de Toledo, M.A.S.; Schalla, C.; Rütten, S.; Goetzke, R.; Mattoso, L.H.C.; Zenke, M.; Sechi, A. PLA/Hydroxyapatite scaffolds exhibit in vitro immunological inertness and promote robust osteogenic differentiation of human mesenchymal stem cells without osteogenic stimuli. Sci. Rep. 2022, 12, 2333. [Google Scholar] [CrossRef] [PubMed]
- Amirazad, H.; Dadashpour, M.; Zarghami, N. Application of decellularized bone matrix as a bioscaffold in bone tissue engineering. J. Biol. Eng. 2022, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Sarrigiannidis, S.O.; Rey, J.; Dobre, O.; González-García, C.; Dalby, M.; Salmeron-Sanchez, M. A tough act to follow: Collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater. Today Bio 2021, 10, 100098. [Google Scholar] [CrossRef]
- Andreazza, R.; Morales, A.; Pieniz, S.; Labidi, J. Gelatin-Based Hydrogels: Potential Biomaterials for Remediation. Polymers 2023, 15, 1026. [Google Scholar] [CrossRef]
- Chircov, C.; Grumezescu, A.M.; Bejenaru, L.E. Hyaluronic acid-based scaffolds for tissue engineering. Rom. J. Morphol. Embryol. 2018, 59, 71–76. [Google Scholar]
- Gao, H.; Yang, J.; Jin, X.; Qu, X.; Zhang, F.; Zhang, D.; Chen, H.; Wei, H.; Zhang, S.; Jia, W.; et al. Porous tantalum scaffolds: Fabrication, structure, properties, and orthopedic applications. Mater. Des. 2021, 210, 110095. [Google Scholar] [CrossRef]
- Hanawa, T. Titanium–Tissue Interface Reaction and Its Control With Surface Treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef]
- Sprague, A.L.; Awokuse, D.; Pohlig, R.T.; Cortes, D.H.; Silbernagel, K.G. Relationship between mechanical properties (shear modulus and viscosity), age, and sex in uninjured Achilles tendons. Transl. Sports Med. 2020, 3, 321–327. [Google Scholar] [CrossRef]
- LaCroix, A.S.; Duenwald-Kuehl, S.E.; Lakes, R.S.; Vanderby, R. Relationship between tendon stiffness and failure: A metaanalysis. J. Appl. Physiol. 2013, 115, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Maganaris, C.N.; Paul, J.P. In vivo human tendon mechanical properties. J. Physiol. 1999, 521 Pt 1, 307–313. [Google Scholar] [CrossRef]
- Urban, M.W.; Rule, A.D.; Atwell, T.D.; Chen, S. Novel Uses of Ultrasound to Assess Kidney Mechanical Properties. Kidney360 2021, 2, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Shojaei, A. Measurement of the Mechanical Properties of the Human Kidney. IRBM 2017, 38, 292–297. [Google Scholar] [CrossRef]
- Palmeri, M.L.; Wang, M.; Dahl, J.; Frinkley, K.; Nightingale, K. Quantifying Hepatic Shear Modulus In Vivo Using Acoustic Radiation Force. Ultrasound Med. Biol. 2008, 34, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Kemper, A.R.; Santago, A.C.; Stitzel, J.D.; Sparks, J.L.; Duma, S.M. Biomechanical response of human liver in tensile loading. Ann. Adv. Automot. Med. 2010, 54, 15–26. [Google Scholar]
- Yeh, W.-C.; Li, P.-C.; Jeng, Y.-M.; Hsu, H.-C.; Kuo, P.-L.; Li, M.-L.; Yang, P.-M.; Lee, P.H. Elastic modulus measurements of human liver and correlation with pathology. Ultrasound Med. Biol. 2002, 28, 467–474. [Google Scholar] [CrossRef]
- Arikawa, H. Dynamic Shear Modulus in Torsion of Human Dentin and Enamel. Dent. Mater. J. 1989, 8, 223–235,287. [Google Scholar] [CrossRef]
- Chun, K.; Choi, H.; Lee, J. Comparison of mechanical property and role between enamel and dentin in the human teeth. J. Dent. Biomech. 2014, 5, 1758736014520809. [Google Scholar] [CrossRef]
- Aghajanian, A.H.; Bigham, A.; Sanati, A.; Kefayat, A.; Salamat, M.R.; Sattary, M.; Rafienia, M. A 3D macroporous and magnetic Mg2SiO4-CuFe2O4 scaffold for bone tissue regeneration: Surface modification, in vitro and in vivo studies. Biomater. Adv. 2022, 137, 212809. [Google Scholar]
- Park, S.; Tao, J.; Sun, L.; Fan, C.-M.; Chen, Y. An Economic, Modular, and Portable Skin Viscoelasticity Measurement Device for In Situ Longitudinal Studies. Molecules 2019, 24, 907. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, R.; Attik, N.; Chevalier, C.; Salles, V.; Grosgogeat, B.; Gritsch, K.; Trunfio-Sfarghiu, A.-M. 3D Electrospun Polycaprolactone Scaffolds to Assess Human Periodontal Ligament Cells Mechanobiological Behaviour. Biomimetics 2023, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Mirzaali, M.J.; Schwiedrzik, J.J.; Thaiwichai, S.; Best, J.P.; Michler, J.; Zysset, P.K.; Wolfram, U. Mechanical properties of cortical bone and their relationships with age, gender, composition and microindentation properties in the elderly. Bone 2016, 93, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; Diaz-Rodriguez, P.; Balouch, B.; Paulsen, S.; Wu, S.; Miller, J.; Hahn, M.; Cosgriff-Hernandez, E. Elucidating the role of graft compliance mismatch on intimal hyperplasia using an ex vivo organ culture model. Acta Biomater. 2019, 89, 84–94. [Google Scholar] [CrossRef]
- Cao, T.; Jiang, Z.; Zhao, H.; Zhang, K.-Q.; Meng, K. Numerical simulation to study the impact of compliance mismatch between artificial and host blood vessel on hemodynamics. Med. Nov. Technol. Devices 2022, 15, 100152. [Google Scholar] [CrossRef]
- Teichtahl, A.J.; Wluka, A.E.; Wijethilake, P.; Wang, Y.; Ghasem-Zadeh, A.; Cicuttini, F.M. Wolff’s law in action: A mechanism for early knee osteoarthritis. Thromb. Haemost. 2015, 17, 207. [Google Scholar] [CrossRef]
- Ambrosi, D.; Ben Amar, M.; Cyron, C.J.; DeSimone, A.; Goriely, A.; Humphrey, J.D.; Kuhl, E. Growth and remodelling of living tissues: Perspectives, challenges and opportunities. J. R. Soc. Interface 2019, 16, 2019023. [Google Scholar] [CrossRef]
- Frost, H.M. A 2003 Update of Bone Physiology and Wolff’s Law for Clinicians. Angle Orthod. 2004, 74, 3–15. [Google Scholar]
- Raffa, M.L.; Nguyen, V.H.; Hernigou, P.; Flouzat-Lachaniette, C.H.; Haiat, G. Stress shielding at the bone-implant interface: Influence of surface roughness and of the bone-implant contact ratio. J. Orthop. Res. 2021, 39, 1174–1183. [Google Scholar] [CrossRef]
- Munir, N.; Callanan, A. Novel phase separated polycaprolactone/collagen scaffolds for cartilage tissue engineering. Biomed. Mater. 2018, 13, 051001. [Google Scholar] [CrossRef]
- Garrison, C.M.; Singh-Varma, A.; Pastino, A.K.; Steele, J.A.; Kohn, J.; Murthy, N.S.; Schwarzbauer, J.E. A multilayered scaffold for regeneration of smooth muscle and connective tissue layers. J. Biomed. Mater. Res. Part A 2021, 109, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Biazar, E.; Kamalvand, M.; Avani, F. Recent advances in surface modification of biopolymeric nanofibrous scaffolds. Int. J. Polym. Mater. Polym. Biomater. 2021, 71, 493–512. [Google Scholar] [CrossRef]
- Bailey, S.; Vashishth, D. Mechanical Characterization of Bone: State of the Art in Experimental Approaches-What Types of Experiments Do People Do and How Does One Interpret the Results? Curr. Osteoporos. Rep. 2018, 16, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Fan, S.; Zhou, G.; Ma, K.; Yao, X.; Zhang, Y. Effects of dynamic mechanical stimulations on the regeneration of in vitro and in vivo cartilage tissue based on silk fibroin scaffold. Compos. Part B Eng. 2022, 235, 109764. [Google Scholar] [CrossRef]
- Yang, Y.; Li, K.; Sommer, G.; Yung, K.-L.; A Holzapfel, G. Mechanical characterization of porcine liver properties for computational simulation of indentation on cancerous tissue. Math. Med. Biol. J. IMA 2020, 37, 469–490. [Google Scholar] [CrossRef]
- Wei, W.; Dai, H. Articular cartilage and osteochondral tissue engineering techniques: Recent advances and challenges. Bioact. Mater. 2021, 6, 4830–4855. [Google Scholar] [CrossRef]
- Rayudu, N.M.; Subburaj, K.; Mei, K.; Dieckmeyer, M.; Kirschke, J.S.; Noël, P.B.; Baum, T. Finite Element Analysis-Based Vertebral Bone Strength Prediction Using MDCT Data: How Low Can We Go? Front. Endocrinol. 2020, 11, 442. [Google Scholar] [CrossRef]
- Kurtaliaj, I.; Golman, M.; Abraham, A.C.; Thomopoulos, S. Biomechanical Testing of Murine Tendons. J. Vis. Exp. 2019, 152, e60280. [Google Scholar]
- Gough, A.; Stern, A.M.; Maier, J.; Lezon, T.; Shun, T.-Y.; Chennubhotla, C.; Schurdak, M.E.; Haney, S.A.; Taylor, D.L. Biologically Relevant Heterogeneity: Metrics and Practical Insights. SLAS Discov. Adv. Sci. Drug Discov. 2017, 22, 213–237. [Google Scholar] [CrossRef]
- Zhang, K.; Zhu, M.; Thomas, E.; Hopyan, S.; Sun, Y. Existing and Potential Applications of Elastography for Measuring the Viscoelasticity of Biological Tissues In Vivo. Front. Phys. 2021, 9, 670571. [Google Scholar] [CrossRef]
- Mitchell, G.R.; Tojeira, A. Role of Anisotropy in Tissue Engineering. Procedia Eng. 2013, 59, 117–125. [Google Scholar] [CrossRef]
- Karimi, A.; Navidbakhsh, M.; Shojaei, A.; Faghihi, S. Measurement of the uniaxial mechanical properties of healthy and atherosclerotic human coronary arteries. Mater. Sci. Eng. C 2013, 33, 2550–2554. [Google Scholar] [CrossRef] [PubMed]
- Claes, E.; Atienza, J.; Guinea, G.; Rojo, F.; Bernal, J.; Revuelta, J.; Elices, M. Mechanical properties of human coronary arteries. 2010, 2010, 3792–3795. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 3792–3795. [Google Scholar]
- A Vorp, D.; Schiro, B.J.; Ehrlich, M.P.; Juvonen, T.S.; Ergin, M.; Griffith, B.P. Effect of aneurysm on the tensile strength and biomechanical behavior of the ascending thoracic aorta. Ann. Thorac. Surg. 2003, 75, 1210–1214. [Google Scholar] [CrossRef]
- Gijsen, F.J.H.; Wentzel, J.J.; Thury, A.; Mastik, F.; Schaar, J.A.; Schuurbiers, J.C.H.; Slager, C.J.; Van Der Giessen, W.J.; De Feyter, P.J.; Van Der Steen, A.F.W.; et al. Strain distribution over plaques in human coronary arteries relates to shear stress. Am. J. Physiol. Circ. Physiol. 2008, 295, H1608–H1614. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yang, J.; Zhao, J.; Gregersen, H.; Kassab, G.S. Shear modulus of porcine coronary artery: Contributions of media and adventitia. Am. J. Physiol. Circ. Physiol. 2003, 285, H1966–H1975. [Google Scholar] [CrossRef] [PubMed]
- Eschweiler, J.; Horn, N.; Rath, B.; Betsch, M.; Baroncini, A.; Tingart, M.; Migliorini, F. The Biomechanics of Cartilage—An Overview. Life 2021, 11, 302. [Google Scholar] [CrossRef]
- Chizhik, S.A.; Wierzcholski, K.; Trushko, A.; Zhytkova, M.A.; Miszczak, A. Properties of Cartilage on Micro- and Nanolevel. Adv. Tribol. 2010, 2010, 8. [Google Scholar] [CrossRef]
- Wang, S.; Bao, Y.; Guan, Y.; Zhang, C.; Liu, H.; Yang, X.; Gao, L.; Guo, T.; Chen, Q. Strain distribution of repaired articular cartilage defects by tissue engineering under compression loading. J. Orthop. Surg. Res. 2018, 13, 19. [Google Scholar] [CrossRef]
- Wong, B.L.; Bae, W.C.; Chun, J.; Gratz, K.R.; Lotz, M.; Robert, L.S. Biomechanics of Cartilage Articulation. Arthritis Rheum. 2008, 58, 2065–2074. [Google Scholar]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef]
- Ma, Z.; Qiang, Z.; Zhao, H.; Piao, H.; Ren, L. Mechanical properties of cortical bones related to temperature and orientation of Haversian canals. Mater. Res. Express 2020, 7, 015408. [Google Scholar] [CrossRef]
- Spatz, H.C.; Vincent, J.F. Young’s moduli and shear moduli in cortical bone. Proc. R. Soc. Lond. B 1996, 263, 287–294. [Google Scholar]
- Pawlaczyk, M.; Lelonkiewicz, M.; Wieczorowski, M. Age-dependent biomechanical properties of the skin. Adv. Dermatol. Allergol. 2013, 30, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Ottenio, M.; Tran, D.; Annaidh, A.N.; Gilchrist, M.D.; Bruyère, K. Strain rate and anisotropy effects on the tensile failure characteristics of human skin. J. Mech. Behav. Biomed. Mater. 2015, 41, 241–250. [Google Scholar] [CrossRef]
- Kwan, M.K.; Wall, E.J.; Massie, J.; Garfin, S.R. Strain, stress and stretch of peripheral nerve Rabbit experiments in vitro and in vivo. Acta Orthop. 1992, 63, 267–272. [Google Scholar] [CrossRef]
- Nicholson, K.J.; Winkelstein, B.A. Nerve and Nerve Root Biomechanics. Neural Tissue Biomech. 2010, 3, 203–229. [Google Scholar]
- Singh, A.; Magee, R. Mechanical Properties of Cervical Spinal Cord in Neonatal Piglet: In Vitro. Neurol. Neurobiol. 2020, 2, 3. [Google Scholar] [CrossRef]
- Durand, S.; Raffoul, W.; Christen, T.; Pedrazzi, N. Post-Operative Assessment of Ulnar Nerve Tension Using Shear-Wave Elastography. Neurol. Int. 2021, 13, 469–476. [Google Scholar] [CrossRef]
- Nicolle, S.; Palierne, J.-F. Dehydration effect on the mechanical behaviour of biological soft tissues: Observations on kidney tissues. J. Mech. Behav. Biomed. Mater. 2010, 3, 630–635. [Google Scholar] [CrossRef]
- Phillip, J.M.; Aifuwa, I.; Walston, J.; Wirtz, D. The Mechanobiology of Aging. Annu. Rev. Biomed. Eng. 2016, 17, 113–141. [Google Scholar] [CrossRef]
- Martino, F.; Perestrelo, A.R.; Vinarsky, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T.; Kollmannsberger, P.; Zitterbart, D.P.; Smith, J.; Fabry, B.; Goldmann, W.H. Mechano-Coupling and Regulation of Contractility by the Vinculin Tail Domain. Biophys. J. 2008, 94, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.M.; Scott, A. Mechanotherapy: How physical therapists’ prescription of exercise promotes tissue repair. Br. J. Sports Med. 2009, 43, 247–252. [Google Scholar] [CrossRef]
- Charkoudian, N. Mechanisms and modifiers of reflex induced cutaneous vasodilation and vasoconstriction in humans. J. Appl. Physiol. 2010, 109, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Yamako, G.; Janssen, D.; Hanada, S.; Anijs, T.; Ochiai, K.; Totoribe, K.; Chosa, E.; Verdonschot, N. Improving stress shielding following total hip arthroplasty by using a femoral stem made of β type Ti-33.6Nb-4Sn with a Young’s modulus gradation. J. Biomech. 2017, 63, 135–143. [Google Scholar] [CrossRef]
- Be’Ery-Lipperman, M.; Gefen, A. A method of quantification of stress shielding in the proximal femur using hierarchical computational modeling. Comput. Methods Biomech. Biomed. Eng. 2007, 9, 35–44. [Google Scholar] [CrossRef]
- Lei, B.; Guo, B.; Rambhia, K.J.; Ma, P.X. Hybrid polymer biomaterials for bone tissue regeneration. Front. Med. 2018, 13, 189–201. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Yu, H.; Lv, J.; Fan, M.; Wang, X.; Wang, X.; Liang, Y.; Mao, L.; Zhao, Z. The Biocompatibility of Multi-Source Stem Cells and Gelatin-Carboxymethyl Chitosan-Sodium Alginate Hybrid Biomaterials. Tissue Eng. Regen. Med. 2022, 19, 491–503. [Google Scholar] [CrossRef]
- Nokhbatolfoghahaei, H.; Paknejad, Z.; Bohlouli, M.; Rad, M.R.; Aminishakib, P.; Derakhshan, S.; Amirabad, L.M.; Nadjmi, N.; Khojasteh, A. Fabrication of Decellularized Engineered Extracellular Matrix through Bioreactor-Based Environment for Bone Tissue Engineering. ACS Omega 2020, 5, 31943–31956. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Hu, C.C.; Sakthivel, R.; Nabilla, S.C.; Huang, Y.W.; Yu, J.; Cheng, N.C.; Kuo, Y.J.; Chung, R.J. Preparation of gamma poly-glutamic acid/hydroxyapatite/collagen composite as the 3D-printing scaffold for bone tissue engineering. Biomater. Res. 2022, 26, 21. [Google Scholar] [CrossRef]
- Singh, B.N.; Nallakumarasamy, A.; Sinha, S.; Rastogi, A.; Mallick, S.P.; Divakar, S.; Srivastava, P. Generation of hybrid tissue engineered construct through embedding autologous chondrocyte loaded platelet rich plasma/alginate based hydrogel in porous scaffold for cartilage regeneration. Int. J. Biol. Macromol. 2022, 203, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.U.; Kim, D.; Jang, C.H.; Kim, G.H. Highly elastic 3D-printed gelatin/HA/placental-extract scaffolds for bone tissue engineering. Theranostics 2022, 12, 4051–4066. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, B.; Li, H.; Feng, G.; Pan, S.; Chen, Z.; Li, B.; Song, J. Biomimetic Mineralized Hydroxyapatite Nanofiber-Incorporated Methacrylated Gelatin Hydrogel with Improved Mechanical and Osteoinductive Performances for Bone Regeneration. Int. J. Nanomed. 2022, 17, 1511–1529. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaee, S.; Baheiraei, N.; Salehnia, M. Fabrication and characterization of PHEMA–gelatin scaffold enriched with graphene oxide for bone tissue engineering. J. Orthop. Surg. Res. 2022, 17, 216. [Google Scholar] [CrossRef]
- Min, Q.; Tian, D.; Zhang, Y.; Wang, C.; Wan, Y.; Wu, J. Strong and Elastic Chitosan/Silk Fibroin Hydrogels Incorporated with Growth-Factor-Loaded Microspheres for Cartilage Tissue Engineering. Biomimetics 2022, 7, 41. [Google Scholar] [CrossRef]
- Dorishetty, P.; Balu, R.; Gelmi, A.; Mata, J.P.; Quigley, A.; Dutta, N.K.; Choudhury, N.R. Microporosity engineered printable silk/graphene hydrogels and their cytocompatibility evaluations. Mater. Today Adv. 2022, 14, 100233. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Xiong, X.; Cui, R.; Zhang, G.; Wang, C.; Xiao, D.; Qu, S.; Weng, J. Hybridizing gellan/alginate and thixotropic magnesium phosphate-based hydrogel scaffolds for enhanced osteochondral repair. Mater. Today Bio 2022, 14, 100261. [Google Scholar] [CrossRef]
- Baskapan, B.; Callanan, A. Electrospinning Fabrication Methods to Incorporate Laminin in Polycaprolactone for Kidney Tissue Engineering. Tissue Eng. Regen. Med. 2022, 19, 73–82. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, X.; Dai, X. Properties of Electrospun Aligned Poly(lactic acid)/Collagen Fibers With Nanoporous Surface for Peripheral Nerve Tissue Engineering. Macromol. Mater. Eng. 2022, 307, 2200256. [Google Scholar] [CrossRef]
- Salehi, R.; Mohammadzadeh, L.; Mahkam, M.; Jafarizad, A.; Rahbarghazi, R. Electrospun gelatin/methylcellulose hybrid nanofibers promoted the maturation of human cutaneous tissue progenitor cells toward keratinocyte-like cells. Cellulose 2022, 29, 7837–7848. [Google Scholar] [CrossRef]
- Li, P.; Ruan, L.; Wang, R.; Liu, T.; Song, G.; Gao, X.; Jiang, G.; Liu, X. Electrospun Scaffold of Collagen and Polycaprolactone Containing ZnO Quantum Dots for Skin Wound Regeneration. J. Bionic Eng. 2021, 18, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Cheng, R.; Cao, Y.; Yan, Y.; Shen, Z.; Zhao, Y.; Han, Y. Biocompatible chitosan/polyethylene glycol/multi-walled carbon nanotube composite scaffolds for neural tissue engineering. J. Zhejiang Univ. B 2022, 23, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.S.; Kaliannagounder, V.K.; Rahaman, A.; Park, C.H.; Kim, C.S.; Kim, B. Synergistic Effect of Reinforced Multiwalled Carbon Nanotubes and Boron Nitride Nanosheet-Based Hybrid Piezoelectric PLLA Scaffold for Efficient Bone Tissue Regeneration. ACS Biomater. Sci. Eng. 2022, 8, 3542–3556. [Google Scholar] [CrossRef] [PubMed]
- Jiawei, S.; Xuemeng, B.; Yahui, Z.; Jianfei, C.; Yinghong, X. Sugarcane Stem Derived Hybrid Scaffold for Bone Tissue Engineering via Top-down Approach. Compos. Interfaces 2022, 30, 323–340. [Google Scholar] [CrossRef]
- Karšaj, I.; Humphrey, J.D. A multilayered wall model of arterial growth and remodeling. Mech. Mater. 2013, 44, 110–119. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, D.; Wang, T. Hierarchical Structures of Bone and Bioinspired Bone Tissue Engineering. Small 2016, 12, 4611–4632. [Google Scholar] [CrossRef]
- Totonelli, G.; Maghsoudlou, P.; Fishman, J.M.; Orlando, G.; Ansari, T.; Sibbons, P.; A Birchall, M.; Pierro, A.; Eaton, S.; De Coppi, P. Esophageal tissue engineering: A new approach for esophageal replacement. World J. Gastroenterol. 2012, 18, 6900–6907. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, M.; Jiang, F.; Yin, S.; Lin, S.; Yang, G.; Lu, Y.; Zhang, W.; Jiang, X. Marginal sealing around integral bilayer scaffolds for repairing osteochondral defects based on photocurable silk hydrogels. Bioact. Mater. 2021, 6, 3976–3986. [Google Scholar] [CrossRef]
- Dargoush, S.A.; Hanaee-Ahvaz, H.; Irani, S.; Soleimani, M.; Khatami, S.M.; Sohi, A.N. A composite bilayer scaffold functionalized for osteochondral tissue regeneration in rat animal model. J. Tissue Eng. Regen. Med. 2022, 16, 559–574. [Google Scholar] [CrossRef]
- Yang, T.; Tamaddon, M.; Jiang, L.; Wang, J.; Liu, Z.; Liu, Z.; Meng, H.; Hu, Y.; Gao, J.; Yang, X.; et al. Bilayered scaffold with 3D printed stiff subchondral bony compartment to provide constant mechanical support for long-term cartilage regeneration. J. Orthop. Transl. 2021, 30, 112–121. [Google Scholar] [CrossRef]
- Tevlek, A.; Aydin, H.M. Multi-layered in vitro 3D-bone model via combination of osteogenic cell sheets with electrospun membrane interlayer. J. Biomater. Appl. 2021, 36, 818–833. [Google Scholar] [CrossRef] [PubMed]
- Semitela, Â.; Leal Pereira, A.; Sousa, C.; Mendes, A.F.; Marques, P.A.; Completo, A. Multi-layered electrospinning and electrospraying approach: Effect of polymeric supplements on chondrocyte suspension. J. Biomater. Appl. 2021, 36, 1629–1640. [Google Scholar] [CrossRef] [PubMed]
- Janarthanan, G.; Kim, J.H.; Kim, I.; Lee, C.; Chung, E.-J.; Noh, I. Manufacturing of self-standing multi-layered 3D-bioprinted alginate-hyaluronate constructs by controlling the cross-linking mechanisms for tissue engineering applications. Biofabrication 2022, 14, 035013. [Google Scholar] [CrossRef]
- Tamaddon, M.; Blunn, G.; Tan, R.; Yang, P.; Sun, X.; Chen, S.-M.; Luo, J.; Liu, Z.; Wang, L.; Li, D.; et al. In vivo evaluation of additively manufactured multi-layered scaffold for the repair of large osteochondral defects. Bio-Design Manuf. 2022, 5, 481–496. [Google Scholar] [CrossRef]
- Rashidi, N.; Tamaddon, M.; Liu, C.; Brand, D.D.; Czernuszka, J. A Bilayer Osteochondral Scaffold with Self-Assembled Monomeric Collagen Type-I, Type-II, and Polymerized Chondroitin Sulfate Promotes Chondrogenic and Osteogenic Differentiation of Mesenchymal Stem Cells. Adv. NanoBiomed Res. 2021, 2, 2100089. [Google Scholar] [CrossRef]
- Li, M.; Song, P.; Wang, W.; Xu, Y.; Li, J.; Wu, L.; Gui, X.; Zeng, Z.; Zhou, Z.; Liu, M.; et al. Preparation and characterization of biomimetic gradient multi-layer cell-laden scaffolds for osteochondral integrated repair. J. Mater. Chem. B 2022, 10, 4172–4188. [Google Scholar] [CrossRef]
- Nejad, Z.M.; Zamanian, A.; Saeidifar, M.; Vanaei, H.R.; Amoli, M.S. 3D Bioprinting of Polycaprolactone-Based Scaffolds for Pulp-Dentin Regeneration: Investigation of Physicochemical and Biological Behavior. Polymers 2021, 13, 4442. [Google Scholar] [CrossRef]
- Bazgir, M.; Zhang, W.; Zhang, X.; Elies, J.; Saeinasab, M.; Coates, P.; Youseffi, M.; Sefat, F. Fabrication and Characterization of PCL/PLGA Coaxial and Bilayer Fibrous Scaffolds for Tissue Engineering. Materials 2021, 14, 6295. [Google Scholar] [CrossRef]
- Li, M.X.; Li, L.; Zhou, S.Y.; Cao, J.H.; Liang, W.H.; Tian, Y.; Shi, X.T.; Yang, X.B.; Wu, D.Y. A biomimetic orthogonal-bilayer tubular scaffold for the co-culture of endothelial cells and smooth muscle cells. RSC Adv. 2021, 11, 31783–31790. [Google Scholar] [CrossRef]
- Do, T.M.; Yang, Y.; Deng, A. Porous Bilayer Vascular Grafts Fabricated from Electrospinning of the Recombinant Human Collagen (RHC) Peptide-Based Blend. Polymers 2021, 13, 4042. [Google Scholar] [CrossRef]
- Smith, M.J.; Dempsey, S.G.; Veale, R.W.; Duston-Fursman, C.G.; Rayner, C.A.F.; Javanapong, C.; Gerneke, D.; Dowling, S.G.; A Bosque, B.; Karnik, T.; et al. Further structural characterization of ovine forestomach matrix and multi-layered extracellular matrix composites for soft tissue repair. J. Biomater. Appl. 2021, 36, 996–1010. [Google Scholar] [CrossRef] [PubMed]
- Kilian, D.; von Witzleben, M.; Lanaro, M.; Wong, C.S.; Vater, C.; Lode, A.; Allenby, M.C.; Woodruff, M.A.; Gelinsky, M. 3D Plotting of Calcium Phosphate Cement and Melt Electrowriting of Polycaprolactone Microfibers in One Scaffold: A Hybrid Additive Manufacturing Process. J. Funct. Biomater. 2022, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zou, Q.; Wang, C.; Lin, M.; Li, Y.; Zhang, R.; Li, Y. Electrospinning and 3D printed hybrid bi-layer scaffold for guided bone regeneration. Mater. Des. 2021, 210, 110047. [Google Scholar] [CrossRef]
- Schoenfeld, C.M.; Lautenschlager, E.P.; Meyer, P.R. Mechanical properties of human cancellous bone in the femoral head. Med. Biol. Eng. Comput. 1974, 12, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Chiba, D.; Yamada, N.; Mori, Y.; Oyama, M.; Ohtsu, S.; Kuwahara, Y.; Baba, K.; Tanaka, H.; Aizawa, T.; Hanada, S.; et al. Mid-term results of a new femoral prosthesis using Ti-Nb-Sn alloy with low Young’s modulus. BMC Musculoskelet. Disord. 2021, 22, 987. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; He, N. A mini-review of embedded 3D printing: Supporting media and strategies. J. Mater. Chem. B 2020, 8, 10474–10486. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, T.E.; Thomson, S.L. Embedded 3D printing of multi-layer, self-oscillating vocal fold models. J. Biomech. 2021, 121, 110388. [Google Scholar] [CrossRef]
- Alipour, F.; Vigmostad, S. Measurement of Vocal Folds Elastic Properties for Continuum Modeling. J. Voice 2012, 26, 816.e21–816.e29. [Google Scholar] [CrossRef]
- Peng, Y.-Y.; Srinivas, S.; Narain, R. Chapter 5—Modification of Polymers, in Polymer Science and Nanotechnology; Narain, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 95–104. [Google Scholar]
- Sengupta, P.; Prasad, B.L.V. Surface Modification of Polymers for Tissue Engineering Applications: Arginine Acts as a Sticky Protein Equivalent for Viable Cell Accommodation. ACS Omega 2018, 3, 4242–4251. [Google Scholar] [CrossRef]
- Browe, D.C.; Díaz-Payno, P.J.; Freeman, F.E.; Schipani, R.; Burdis, R.; Ahern, D.P.; Nulty, J.M.; Guler, S.; Randall, L.D.; Buckley, C.T.; et al. Bilayered extracellular matrix derived scaffolds with anisotropic pore architecture guide tissue organization during osteochondral defect repair. Acta Biomater. 2022, 143, 266–281. [Google Scholar] [CrossRef]
- DiCerbo, M.; Benmassaoud, M.M.; Vega, S.L. Porous Scaffold-Hydrogel Composites Spatially Regulate 3D Cellular Mechanosensing. Front. Med. Technol. 2022, 4, 884314. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Wang, L.; Deng, J.; Zheng, Y.; Ke, Q.; Yang, X.; Zhang, X.; Jia, W.; Huang, C. Enhanced cellular infiltration of tissue-engineered scaffolds fabricated by PLLA nanogrooved microfibers. Nano Res. 2022, 16, 1614–1625. [Google Scholar] [CrossRef]
- Sheikhi, F.; Khorram, M.; Hashemi, S.-S.; Mohammadi, A.; Peyrovedin, H. Preparation, Characterization, and Surface Modification of Polycaprolactone-Based Nanofibrous Scaffold by Grafting with Collagen for Skin Tissue Engineering. Regen. Eng. Transl. Med. 2022, 8, 545–562. [Google Scholar] [CrossRef]
- Chi, M.; Li, N.; Cui, J.; Karlin, S.; Rohr, N.; Sharma, N.; Thieringer, F.M. Biomimetic, mussel-inspired surface modification of 3D-printed biodegradable polylactic acid scaffolds with nano-hydroxyapatite for bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 989729. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Shu, Z.; Zhang, C.; Chen, X.; Wang, M.; Fan, L. Surface modification of silk fibroin composite bone scaffold with polydopamine coating to enhance mineralization ability and biological activity for bone tissue engineering. J. Appl. Polym. Sci. 2022, 139, e52900. [Google Scholar] [CrossRef]
- Habibizadeh, M.; Nadri, S.; Fattahi, A.; Rostamizadeh, K.; Mohammadi, P.; Andalib, S.; Hamidi, M.; Forouzideh, N. Surface modification of neurotrophin-3 loaded PCL/chitosan nanofiber/net by alginate hydrogel microlayer for enhanced biocompatibility in neural tissue engineering. J. Biomed. Mater. Res. Part A 2021, 109, 2237–2254. [Google Scholar] [CrossRef]
- Zhong, H.; Li, Z.; Zhao, T.; Chen, Y. Surface Modification of Nanofibers by Physical Adsorption of Fiber-Homologous Amphiphilic Copolymers and Nanofiber-Reinforced Hydrogels with Excellent Tissue Adhesion. ACS Biomater. Sci. Eng. 2021, 7, 4828–4837. [Google Scholar] [CrossRef]
- Wang, D.; Yu, X.; Xu, Y.; Wang, X.; Wang, H.; Zhang, Y.; Li, Q.; Turng, L.-S. Physical shish-kebab modification vs. chemical surface coating on expanded polytetrafluoroethylene vascular grafts for enhanced endothelial cell adhesion. Mater. Des. 2022, 220, 110889. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, W.; Wang, Y.; Zhang, C.; Zhang, X.; Zhang, S.; Wu, W. Recombinant DTβ4-inspired porous 3D vascular graft enhanced antithrombogenicity and recruited circulating CD93+/CD34+ cells for endothelialization. Sci. Adv. 2022, 8, eabn1958. [Google Scholar] [CrossRef]
- Mahendiran, B.; Muthusamy, S.; Janani, G.; Mandal, B.B.; Rajendran, S.; Krishnakumar, G.S. Surface Modification of Decellularized Natural Cellulose Scaffolds with Organosilanes for Bone Tissue Regeneration. ACS Biomater. Sci. Eng. 2022, 8, 2000–2015. [Google Scholar] [CrossRef]
- Zareidoost, A.; Yousefpour, M.; Ghaseme, B.; Amanzadeh, A. The relationship of surface roughness and cell response of chemical surface modification of titanium. J. Mater. Sci. Mater. Med. 2012, 23, 1479–1488. [Google Scholar] [CrossRef]
- Wang, T.; Feng, Z.-Q.; Leach, M.K.; Wu, J.; Jiang, Q. Nanoporous fibers of type-I collagen coated poly(l-lactic acid) for enhancing primary hepatocyte growth and function. J. Mater. Chem. B 2012, 1, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Zamani, F.; Tehran, M.A.; Latifi, M.; Shokrgozar, M.A. The influence of surface nanoroughness of electrospun PLGA nanofibrous scaffold on nerve cell adhesion and proliferation. J. Mater. Sci. Mater. Med. 2013, 24, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Boschetto, F.; Yagi, S.; Marin, E.; Adachi, T.; Chen, X.; Pezzotti, G.; Sakurai, S.; Sasaki, S.; Aoki, T.; et al. Enhancing the bioactivity of melt electrowritten PLLA scaffold by convenient, green, and effective hydrophilic surface modification. Biomater. Adv. 2022, 135, 112686. [Google Scholar] [CrossRef] [PubMed]
- Dokos, S.; LeGrice, I.J.; Smaill, B.H.; Kar, J.; Young, A. A Triaxial-Measurement Shear-Test Device for Soft Biological Tissues. J. Biomech. Eng. 2000, 122, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Ajayan, P.M.; Narayanan, T.N. Dynamic mechanical analysis in materials science: The Novice’s Tale. Oxf. Open Mater. Sci. 2020, 1, itaa001. [Google Scholar] [CrossRef]
- Villalona, G.A.; Udelsman, B.; Duncan, D.R.; McGillicuddy, E.; Sawh-Martinez, R.F.; Hibino, N.; Painter, C.; Mirensky, T.; Erickson, B.; Shinoka, T.; et al. Cell-Seeding Techniques in Vascular Tissue Engineering. Tissue Eng. Part B Rev. 2010, 16, 341–350. [Google Scholar] [CrossRef]
- Keong, L.C.; Halim, A.S. In Vitro Models in Biocompatibility Assessment for Biomedical-Grade Chitosan Derivatives in Wound Management. Int. J. Mol. Sci. 2009, 10, 1300–1313. [Google Scholar] [CrossRef]
- Buffinton, C.M.; Tong, K.J.; Blaho, R.A.; Buffinton, E.M.; Ebenstein, D.M. Comparison of mechanical testing methods for biomaterials: Pipette aspiration, nanoindentation, and macroscale testing. J. Mech. Behav. Biomed. Mater. 2015, 51, 367–379. [Google Scholar] [CrossRef]
- Wałdoch, A.; Sabiniewicz, R.; Meyer-Szary, J. Interventional treatment using a 3D model of a right pulmonary artery to left atrial fistula in an infant. Adv. Interv. Cardiol. 2022, 18, 170–172. [Google Scholar] [CrossRef]
- Chen, W.; Ma, L.; Shao, J.; Bi, C.; Xie, Y.; Zhao, S. Morphological specificity analysis of an image-based 3D model of airway filling in a difficult airway. BMC Anesthesiol. 2022, 22, 336. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.; Prather, R.; Divo, E.; Kassab, A.; Nykanen, D.; Farias, M.; DeCampli, W.M. Computational fluid dynamics investigation of the novel hybrid comprehensive stage II operation. JTCVS Open 2021, 7, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Rossi, T.; Querzoli, G.; Badas, M.G.; Angius, F.; Telani, S.; Ripandelli, G. Computational Fluid Dynamics of Intraocular Silicone Oil Tamponade. Transl. Vis. Sci. Technol. 2021, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Schollenberger, J.; Osborne, N.H.; Hernandez-Garcia, L.; Figueroa, C.A. A Combined Computational Fluid Dynamics and Arterial Spin Labeling MRI Modeling Strategy to Quantify Patient-Specific Cerebral Hemodynamics in Cerebrovascular Occlusive Disease. Front. Bioeng. Biotechnol. 2021, 9, 722445. [Google Scholar] [CrossRef] [PubMed]
- Eltes, P.E.; Bartos, M.; Hajnal, B.; Pokorni, A.J.; Kiss, L.; Lacroix, D.; Varga, P.P.; Lazary, A. Development of a Computer-Aided Design and Finite Element Analysis Combined Method for Affordable Spine Surgical Navigation with 3D-Printed Customized Template. Front. Surg. 2021, 7, 583386. [Google Scholar] [CrossRef]
- Irarrázaval, S.; Ramos-Grez, J.A.; Pérez, L.I.; Besa, P.; Ibáñez, A. Finite element modeling of multiple density materials of bone specimens for biomechanical behavior evaluation. SN Appl. Sci. 2021, 3, 776. [Google Scholar] [CrossRef]
- Jahangir, S.; Mohammadi, A.; Mononen, M.E.; Hirvasniemi, J.; Suomalainen, J.-S.; Saarakkala, S.; Korhonen, R.K.; Tanska, P. Rapid X-ray-Based 3-D Finite Element Modeling of Medial Knee Joint Cartilage Biomechanics during Walking. Ann. Biomed. Eng. 2022, 50, 666–679. [Google Scholar] [CrossRef]
- Jing, M.; Cui, Z.; Fu, H.; Chen, X. Real-Time Deformation Simulation of Kidney Surgery Based on Virtual Reality. J. Shanghai Jiaotong Univ. (Sci.) 2021, 26, 290–297. [Google Scholar] [CrossRef]
- Fujimoto, K.; Shiinoki, T.; Yuasa, Y.; Tanaka, H. Estimation of liver elasticity using the finite element method and four-dimensional computed tomography images as a biomarker of liver fibrosis. Med. Phys. 2021, 48, 1286–1298. [Google Scholar] [CrossRef]
- Peshin, S.; Karakulova, Y.; Kuchumov, A.G. Finite Element Modeling of the Fingers and Wrist Flexion/Extension Effect on Median Nerve Compression. Appl. Sci. 2023, 13, 1219. [Google Scholar] [CrossRef]
- Hislop, B.D.; Heveran, C.M.; June, R.K. Development and analytical validation of a finite element model of fluid transport through osteochondral tissue. J. Biomech. 2021, 123, 110497. [Google Scholar] [CrossRef] [PubMed]
- Jobanputra, R.D.; Hayes, J.; Royyuru, S.; Masen, M.A. A numerical analysis of skin–PPE interaction to prevent facial tissue injury. Sci. Rep. 2021, 11, 16248. [Google Scholar] [CrossRef]
- Helou, B.; Bel-Brunon, A.; Dupont, C.; Ye, W.; Silvestro, C.; Rochette, M.; Lucas, A.; Kaladji, A.; Haigron, P. Patient-specific finite element simulation of peripheral artery percutaneous transluminal angioplasty to evaluate the procedure outcome without stent implantation. Int. J. Numer. Methods Biomed. Eng. 2023, 39, e3685. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Zhang, M.; Qin, C.; Zhai, D.; Wang, Y.; Zhou, Y.; Chang, J.; Zhu, Y.; Wu, C. 3D printing of conch-like scaffolds for guiding cell migration and directional bone growth. Bioact. Mater. 2022, 22, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Leenaars, C.H.C.; Kouwenaar, C.; Stafleu, F.R.; Bleich, A.; Ritskes-Hoitinga, M.; De Vries, R.B.M.; Meijboom, F.L.B. Animal to human translation: A systematic scoping review of reported concordance rates. J. Transl. Med. 2019, 17, 223. [Google Scholar] [CrossRef] [PubMed]
- Pound, P.; Ritskes-Hoitinga, M. Can prospective systematic reviews of animal studies improve clinical translation? J. Transl. Med. 2020, 18, 15. [Google Scholar] [CrossRef]
- Bas-Cristóbal Menéndez, A.; Du, Z.; van den Bosch, T.P.; Othman, A.; Gaio, N.; Silvestri, C.; Quirós, W.; Lin, H.; Korevaar, S.; Merino, A.; et al. Creating a kidney organoid-vasculature interaction model using a novel organ-on-chip system. Sci. Rep. 2022, 12, 20699. [Google Scholar] [CrossRef]
- Dhwaj, A.; Roy, N.; Jaiswar, A.; Prabhakar, A.; Verma, D. 3D-Printed Impedance Micropump for Continuous Perfusion of the Sample and Nutrient Medium Integrated with a Liver-On-Chip Prototype. ACS Omega 2022, 7, 40900–40910. [Google Scholar] [CrossRef]
- Li, P.; Cui, F.; Chen, H.; Yang, Y.; Li, G.; Mao, H.; Lyu, X. A Microfluidic Cell Co-Culture Chip for the Monitoring of Interactions between Macrophages and Fibroblasts. Biosensors 2022, 13, 70. [Google Scholar] [CrossRef]
- Xu, C.; Wang, K.; Huang, P.; Liu, D.; Guan, Y. Single-Cell Isolation Microfluidic Chip Based on Thermal Bubble Micropump Technology. Sensors 2023, 23, 3623. [Google Scholar] [CrossRef]
- Yang, J.; Hirai, Y.; Iida, K.; Ito, S.; Trumm, M.; Terada, S.; Sakai, R.; Tsuchiya, T.; Tabata, O.; Kamei, K.-I. Integrated-gut-liver-on-a-chip platform as an in vitro human model of non-alcoholic fatty liver disease. Commun. Biol. 2023, 6, 310. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Ohk, K.; Won, J.; Choi, D.-H.; Jung, Y.H.; Yang, J.H.; Jun, Y.; Kim, J.-A.; Chung, S.; Lee, S.-H. Modeling of three-dimensional innervated epidermal like-layer in a microfluidic chip-based coculture system. Nat. Commun. 2023, 14, 1488. [Google Scholar] [CrossRef] [PubMed]
- Özkan, A.; Stolley, D.L.; Cressman, E.N.K.; McMillin, M.; Yankeelov, T.E.; Rylander, M.N. Vascularized Hepatocellular Carcinoma on a Chip to Control Chemoresistance through Cirrhosis, Inflammation and Metabolic Activity. Small Struct. 2023, 2200403. [Google Scholar] [CrossRef]
- Das, S.L.; Sutherland, B.P.; Lejeune, E.; Eyckmans, J.; Chen, C.S. Mechanical response of cardiac microtissues to acute localized injury. Am. J. Physiol. Circ. Physiol. 2022, 323, H738–H748. [Google Scholar] [CrossRef]
- Podunavac, I.; Djocos, M.; Vejin, M.; Birgermajer, S.; Pavlovic, Z.; Kojic, S.; Petrovic, B.; Radonic, V. 3D-Printed Microfluidic Chip for Real-Time Glucose Monitoring in Liquid Analytes. Micromachines 2023, 14, 503. [Google Scholar] [CrossRef]
- Waldrop, T.I.; Graham, C.; Gard, W.; Ingle, K.; Ptacek, T.; Nguyen, N.; Lose, B.; Sethu, P.; Lee, T. Biomimetic cardiac tissue chip and murine arteriovenous fistula models for recapitulating clinically relevant cardiac remodeling under volume overload conditions. Front. Bioeng. Biotechnol. 2023, 11, 1101622. [Google Scholar] [CrossRef]
- Boot, R.C.; Roscani, A.; van Buren, L.; Maity, S.; Koenderink, G.H.; Boukany, P.E. High-throughput mechanophenotyping of multicellular spheroids using a microfluidic micropipette aspiration chip. Lab Chip 2023, 23, 1768–1778. [Google Scholar] [CrossRef]
- Ferrari, D.; Sengupta, A.; Heo, L.; Pethö, L.; Michler, J.; Geiser, T.; Perez, V.A.D.J.; Kuebler, W.M.; Zeinali, S.; Guenat, O.T. Effects of biomechanical and biochemical stimuli on angio- and vasculogenesis in a complex microvasculature-on-chip. iScience 2023, 26, 106198. [Google Scholar] [CrossRef]
- Hart, D.C.t.; Yildiz, D.; Palacio-Castañeda, V.; Li, L.; Gumuscu, B.; Brock, R.; Verdurmen, W.P.R.; van der Vlag, J.; Nijenhuis, T. Co-Culture of Glomerular Endothelial Cells and Podocytes in a Custom-Designed Glomerulus-on-a-Chip Model Improves the Filtration Barrier Integrity and Affects the Glomerular Cell Phenotype. Biosensors 2023, 13, 339. [Google Scholar] [CrossRef]
- Wu, J.; Chen, C.; Qin, C.; Li, Y.; Jiang, N.; Yuan, Q.; Duan, Y.; Liu, M.; Wei, X.; Yu, Y.; et al. Mimicking the Biological Sense of Taste In Vitro Using a Taste Organoids-on-a-Chip System. Adv. Sci. 2023, 10, e2206101. [Google Scholar] [CrossRef]
- Erbay, I.H.; Polatli, E.; Koc, A.C.; Özbilgiç, R.; Karaman, O.; Güven, S. Bioengineering Bone-on-a-Chip Model Harnessing Osteoblastic and Osteoclastic Resolution. Adv. Eng. Mater. 2023, 25, 2201063. [Google Scholar] [CrossRef]
- Kim, D.; Hwang, K.S.; Seo, E.U.; Seo, S.; Lee, B.C.; Choi, N.; Choi, J.; Kim, H.N. Vascularized Lung Cancer Model for Evaluating the Promoted Transport of Anticancer Drugs and Immune Cells in an Engineered Tumor Microenvironment. Adv. Healthc. Mater. 2022, 11, 2102581. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, W.E.; Pawate, A.S.; Larry, T.A.; Schieferstein, J.M.; Whittenberg, J.J.; Leckband, D.E.; Kenis, P.J.A. Development of microfluidic platform that enables ‘on-chip’ imaging of cells exposed to shear stress and cyclic stretch. Microfluid. Nanofluidics 2023, 27, 11. [Google Scholar] [CrossRef]
- Knight, S.R.; Aujla, R.; Biswas, S.P. Total Hip Arthroplasty—Over 100 years of operative history. Orthop. Rev. 2011, 3, e16. [Google Scholar]
- Oberweis, C.V.; Marchal, J.A.; López-Ruiz, E.; Galvez-Martin, M.P. A Worldwide Overview of Regulatory Frameworks for Tissue-Based Products. Tissue Eng. Part B Rev. 2020, 26, 181–196. [Google Scholar] [CrossRef]
- Waldburger, L.; Schaller, R.; Furthmüller, C.; Schrepfer, L.; Schaefer, D.J.; Kaempfen, A. 3D-Printed Hand Splints versus Thermoplastic Splints: A Randomized Controlled Pilot Feasibility Trial. Int. J. Bioprinting 2021, 8, 474. [Google Scholar] [CrossRef]
- Laubach, M.; Suresh, S.; Herath, B.; Wille, M.-L.; Delbrück, H.; Alabdulrahman, H.; Hutmacher, D.W.; Hildebrand, F. Clinical translation of a patient-specific scaffold-guided bone regeneration concept in four cases with large long bone defects. J. Orthop. Transl. 2022, 34, 73–84. [Google Scholar] [CrossRef]
- Hee, M.; Greasley, S.; Whiting, G.; Harkin, C.; Oliver, G.; Marsden, D.; Andrews, R.; Sireau, S.; Price, R.D.; Anwar, F.; et al. 3D printed customised external cranial plate in a patient with syndrome of trephined: ‘A case report’. 3D Print Med. 2021, 7, 35. [Google Scholar]
- Sharma, U.; Dabadi, S.; Dhungel, R.R.; Shrestha, D.; Gurung, P.; Shrestha, R.; Pant, B. Customized cost-effective polymethyl-methacrylate cranioplasty implant using three-dimensional printer. Asian J. Neurosurg. 2021, 16, 150–154. [Google Scholar]
- Mainard, N.; Sharma, D.; Fron, D.; Mezel, A.; Canavese, F.; Bonnevalle, M.; Nectoux, E. Porous Ceramic Sternal Prosthesis Implantation in a 13-Year-Old Patient Presenting with Metastatic Ewing’s Sarcoma. Eur. J. Pediatr. Surg. Rep. 2022, 10, e1–e5. [Google Scholar] [CrossRef]
- Morales, D.L.; Herrington, C.; Bacha, E.A.; Morell, V.O.; Prodán, Z.; Mroczek, T.; Sivalingam, S.; Cox, M.; Bennink, G.; Asch, F.M. A Novel Restorative Pulmonary Valve Conduit: Early Outcomes of Two Clinical Trials. Front. Cardiovasc. Med. 2021, 7, 583360. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, G.; Zheng, Y.; Gao, J.; Fu, Y.; Wang, Q.; Huang, L.; Pan, X.; Ding, J. ‘Invisible’ orthodontics by polymeric ‘clear’ aligners molded on 3D-printed personalized dental models. Regen. Biomater. 2022, 9, rbac007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Y.; Ding, S.; Liang, J.; Kuang, J.; Mao, Q.; Ying, W.; Shu, Y.; Li, J.; Jiang, C. A clinical trial to compare a 3D-printed bolus with a conventional bolus with the aim of reducing cardiopulmonary exposure in postmastectomy patients with volumetric modulated arc therapy. Cancer Med. 2022, 11, 1037–1047. [Google Scholar] [CrossRef]
- Kalaskar, R.; Bhaje, P.; Balasubramanian, S.; Kalaskar, A. Effectiveness of the novel impression tray “cleftray” for infants with cleft lip and palate: A randomized controlled clinical trial. J. Korean Assoc. Oral Maxillofac. Surg. 2021, 47, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Şenayli, A.; Çankaya, G.; Öztorun, C.I.; Oflaz, H.; Şenel, E. Clinical trials of 3D printing splints to avoid contracture development in burned children. Turk. J. Med. Sci. 2021, 51, 2543–2553. [Google Scholar] [PubMed]
- Cui, D.; Wu, B.; He, D.; Wang, Y.; Jiao, Y.; Zhang, B. 3D-Printed Cold Preservation Device in Renal Autotransplantation for the Treatment of a Patient with Renal Artery Stenosis. Front. Bioeng. Biotechnol. 2022, 9, 1312. [Google Scholar] [CrossRef] [PubMed]
- Desvigne, M.N.; Bauer, K.; Holifield, K.; Day, K.; Gilmore, D.; Wardman, A.L. Case Report: Surgical Closure of Chronic Soft Tissue Defects Using Extracellular Matrix Graft Augmented Tissue Flaps. Front. Surg. 2021, 7, 559450. [Google Scholar] [CrossRef]
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthc. Eng. 2019, 5340616. [Google Scholar] [CrossRef]
| Tissue Type | Failure Strain | Yield Stress (MPa) | Young’s Modulus (MPa) | Shear Modulus (MPa) | Reference |
|---|---|---|---|---|---|
| Coronary Artery | 0.45 | 0.39–1.8 | 1.55 ± 0.26 | 0.3 | [86,87,88,89,90] |
| Cartilage | 0.183 | 4.58 ± 2.04 | 0.5–0.9 | 0.26–0.32 | [91,92,93,94] |
| Bone | 0.25–0.67 | 71.56 | 17,900–19,080 | 3600 | [95,96,97] |
| Skin | 1.5 | 17–21 | 60–70 | 0.002–0.008 | [65,98,99] |
| Spine/ Sciatic/Ulnar Nerve | 0.293–0.73 | 11.7 | 0.7–10 | 0.02–0.054 | [100,101,102,103] |
| Tissue Type | Material | Fabrication Method | Mechanical Properties Assessed | Author, Year, Reference | |||
|---|---|---|---|---|---|---|---|
| Tensile/Compressive Modulus | Failure Strain | Ultimate Tensile Strength | Storage/Loss Modulus | ||||
| Bone | PCL + HA | 3D printing | ✓ | ✓ | Rezania et al. (2022) [29] | ||
| Bone | Gelatine + HA + hPE | 3D printing | ✓ | ✓ | Lee et al. (2022) [117] | ||
| Bone | Col and Col + PGA and Col + PGA/HA + PGA and Col + PGA/HA + PGA (2-layer membrane) | 3D printing | ✓ | ✓ | ✓ | Nguyen et al. (2022) [115] | |
| Bone | HA-NFs + GelMA | Hydrogel solution | ✓ | ✓ | ✓ | Wang et al. (2022) [118] | |
| Bone | Gelatine + GO + PHEMA | Freeze-drying | ✓ | Tabatabaee et al. (2022) [119] | |||
| Cartilage | Alginate (Core) + Chitosan (Shell and gel) + SF(gel) | Core-shell microspheres | ✓ | ✓ | ✓ | Min et al. (2022) [120] | |
| Cartilage | GelMA + HyAMA + chondrospheroids | Hydrogel solution | ✓ | Wang et al. (2022) [118] | |||
| Cartilage | PEC + SF + SA + PrP | Phase separation | ✓ | Singha et al. (2022) [18] | |||
| Cartilage | rSF and rSF + rGO | Electrospinning | ✓ | ✓ | Dorishettya et al. (2022) [121] | ||
| Cartilage/Bone | Gellan gum + Alginate sodium and Gellan gum + Alginate sodium + TMP-BG | 3D Printing | ✓ | ✓ | ✓ | ✓ | Chen et al. (2022) [122] |
| Kidney | PCL + Laminin + Span80™ emulsion | Electrospinning | ✓ | ✓ | ✓ | Baskapan et al. (2022) [123] | |
| Nerve | PLA + Col | Electrospinning | ✓ | ✓ | ✓ | Xu et al. (2022) [124] | |
| Skin | PCL + SF + SESM + Gelatine and PCL + SF + SESM + MC | Electrospinning | ✓ | ✓ | ✓ | Salehi et al. (2022) [125] | |
| Skin | PCL+ Col and PCL + Col + ZnO and PCL + Col + ZnO + VEGF | Electrospinning | ✓ | ✓ | ✓ | Li et al. (2021) [126] | |
| Tissue Type | Material | Fabrication Method | Elastic Modulus Range Attained in Tension (T) or Compression (C) | Author, Year, Reference | |||||
|---|---|---|---|---|---|---|---|---|---|
| <1 kPa | 1–100 kPa | 100–1000 kPa | 1–100 MPa | 100–1000 MPa | >1 GPa | ||||
| Bone | PCL + HA | 3D printing | ✓ (C) | ✓ (T) | Rezania et al. (2022) [29] | ||||
| Bone | Gelatine + HA + hPE | 3D printing | ✓ (C) | Lee et al. (2022) [117] | |||||
| Bone | Col and Col + PGA and Col + PGA/HA + PGA and Col + PGA/HA + PGA (2-layer membrane) | 3D printing | ✓ (C) | ✓ (C) | Nguyen et al. (2022) [115] | ||||
| Bone | HA-NFs + GelMA | Hydrogel solution | ✓ (C) | Wang et al. (2022) [118] | |||||
| Bone | Gelatine + GO + PHEMA | Freeze-drying | ✓ (C) | Tabatabaee et al. (2022) [119] | |||||
| Cartilage | Alginate (Core) + Chitosan (Shell and gel) + SF(gel) | Core-shell microspheres | ✓ (C) | Min et al. (2022) [120] | |||||
| Cartilage | GelMA + HyAMA + chondrospheroids | Hydrogel | ✓ (C) | Wang et al. (2022) [118] | |||||
| Cartilage | PEC + SF + SA + PrP | Phase separation | ✓ (C) | Singha et al. (2022) [18] | |||||
| Cartilage | rSF and rSF + rGO | Electrospinning | ✓ (C) | ✓ (C) | Dorishettya et al. (2022) [121] | ||||
| Cartilage/Bone | Gellan gum + Alginate sodium and Gellan gum + Alginate sodium + TMP-BG | 3D Printing | ✓ (C) | ✓ (C) | ✓ (C) | Chen et al. (2022) [122] | |||
| Kidney | PCL + Laminin + Span80™ emulsion | Electrospinning | ✓ (T) | Baskapan et al. (2022) [123] | |||||
| Nerve | PLA + Col | Electrospinning | ✓ (T) | ✓ (T) | Xu et al. (2022) [124] | ||||
| Skin | PCL + SF + SESM + Gelatine and PCL + SF + SESM + MC | Electrospinning | ✓ (T) | Salehi et al. (2022) [125] | |||||
| Skin | PCL+ Col and PCL + Col + ZnO and PCL + Col + ZnO + VEGF | Electrospinning | ✓ (T) | ✓ (T) | Li et al. (2021) [126] | ||||
| Tissue Type | Material per Layer | Fabrication Method | Mechanical Properties Modified and Assessed | Author, Year, Reference | |||
|---|---|---|---|---|---|---|---|
| Tensile/ Compressive Modulus | Failure Strain | Ultimate Tensile Strength | Storage/Loss Modulus | ||||
| Bone | PLLA/Cell sheet | Electrospinning | ✓ | ✓ | Tevlek et al. (2021) [136] | ||
| Cartilage | PCL + Gelatine/Gelatine + Alginate | Electrospinning | ✓ | Semitela et al. (2021) [137] | |||
| Cartilage | Alg + HA 50:50 (~70 layers) and Alg + HA 70:30 (~70 layers) | Bioprinting | ✓ | ✓ | Janarthanan et al. (2022) [138] | ||
| Osteochondral | Col + PLGA and Ti/Col + PLGA | 3D printing/freeze-drying | ✓ | Yang et al. (2021) [135] | |||
| Osteochondral | Ti/PLA/ Col + PLGA and Col + HA | 3D printing | ✓ | Tamaddon et al. (2022) [139] | |||
| Osteochondral | PCS/Col + HA | Freeze-drying | ✓ | Rashidi et al. (2021) [140] | |||
| Osteochondral | PCL + PEO + PES and PCL + PEO/rGO + HA-Sr + PES-BH0.5% | Electrospinning | ✓ | Dargoush et al. (2022) [134] | |||
| Osteochondral | Silk and BMP-2 + Silk and Silk/Silk (bilayer) and BMP-2 + bilayer and BMP-2 + bilayer/TGF-β1 + SilMA | Hydrogel solution | ✓ | ✓ | ✓ | Wu et al. (2021) [133] | |
| Osteochondral | GelMA + PEO + HA/GelMA + PEO + HA/GelMA + PEO | Hydrogel solution | ✓ | ✓ | Li et al. (2022) [141] | ||
| Periodontal | PCL/BG and PCL/HyA | 3D printing | ✓ | ✓ | Nejad et al. (2021) [142] | ||
| Vascular | PLGA/PCL | Electrospinning | ✓ | Bazgir et al. (2021) [143] | |||
| Vascular | PCL/PCL | Electrospinning | ✓ | ✓ | ✓ | Li et al. (2021) [144] | |
| Vascular | RHC + PCL/PEO + PCL | Electrospinning | ✓ | ✓ | ✓ | Do et al. (2021) [145] | |
| Vascular | dECM/GAGF/dECM/dECM | Decellurizing and gas foaming | ✓ | ✓ | ✓ | Smith et al. (2022) [146] | |
| Tissue Type | Material per Layer | Fabrication Method | Elastic Modulus Range Attained in Tension (T) or Compression (C) | Author, Year, Reference | |||||
|---|---|---|---|---|---|---|---|---|---|
| <1 kPa | 1–100 kPa | 100–1000 kPa | 1–100 MPa | 100–1000 MPa | >1 GPa | ||||
| Bone | PLLA/Cell sheet | Electrospinning | ✓ (T) | Tevlek et al. (2021) [136] | |||||
| Cartilage | PCL + gelatine/Gelatine + Alginate | Electrospinning | ✓ (T) | ✓ (T) | Semitela et al. (2021) [137] | ||||
| Cartilage | Alg + HA 50:50 (~70 layers) and Alg + HA 70:30 (~70 layers) | Bioprinting | ✓ (C) | Janarthanan et al. (2022) [138] | |||||
| Osteochondral | Col + PLGA and Ti/Col + PLGA | 3D printing/freeze-drying | ✓ (C) | Yang et al. (2021) [135] | |||||
| Osteochondral | Ti/PLA/ Col + PLGA and Col + HA | 3D printing | ✓ (C) | ✓ (C) | ✓ (C) | Tamaddon et al. (2022) [139] | |||
| Osteochondral | PCS/Col + HA | Freeze-drying | ✓ (C) | Rashidi et al. (2021) [140] | |||||
| Osteochondral | PCL + PEO + PES and PCL + PEO/rGO + HA-Sr + PES-BH0.5% | Electrospinning | ✓ (T) | Dargoush et al. (2022) [134] | |||||
| Osteochondral | Silk and BMP-2 + Silk and silk/silk (bilayer) and BMP-2 + bilayer and BMP-2 + bilayer/TGF-β1 + SilMA | Hydrogel solution | ✓ (C) | ✓ (C) | Wu et al. (2021) [133] | ||||
| Osteochondral | GelMA + PEO + HA/GelMA + PEO + HA/GelMA + PEO | Hydrogel solution | ✓ (C) | Li et al. (2022) [141] | |||||
| Periodontal | PCL/BG and PCL/HyA | 3D printing | ✓ (C) | ✓ (C) | Nejad et al. (2021) [142] | ||||
| Vascular | PLGA/PCL | Electrospinning | ✓ (T) | Bazgir et al. (2021) [143] | |||||
| Vascular | PCL/PCL | Electrospinning | ✓ (T) | Li et al. (2021) [144] | |||||
| Vascular | RHC + PCL/PEO + PCL | Electrospinning | ✓ (T) | Do et al. (2021) [145] | |||||
| Vascular | dECM/GAGF/dECM/dECM | Decellurizing and gas foaming | ✓ (T) | Smith et al. (2022) [146] | |||||
| Tissue Type | Materials | Fabrication Method | Modification Method | Mechanical Properties Modified and Assessed | Author, Year, Reference | |||
|---|---|---|---|---|---|---|---|---|
| Tensile/ Compressive Modulus | Failure Strain | Ultimate Tensile strength | Storage/Loss Modulus | |||||
| Bone | PLA/HA/PDA | 3D printing | PDA coating | ✓ | Chi et al. (2022) [160] | |||
| Bone | SF/OcPh/PDA | Freeze-drying | PDA coating | ✓ | Peng et al. (2022) [161] | |||
| Bone | Forsterite/Copper ferrite/P3HB | Sol–gel combustion | P3HB coating | ✓ | Aghajanian et al. (2022) [64] | |||
| Neural | PCL + Chitosan and PCL + Chitosan + Alginate | Electrospinning | Alginate coating | ✓ | ✓ | ✓ | Habibizadeh et al. (2022) [162] | |
| Osteochondral | AC-dECM/ Bone-dECM/ | Freeze-drying | Annealing | ✓ | Browe et al. (2022) [156] | |||
| Osteochondral | PCL/GelMA | PDMS mould UV curing | Salt leaching | ✓ | DiCerbo et al. (2022) [157] | |||
| Skin | PCL/PEG/ PCEC/Hydrogel | Electrospinning | Copolymer/hydrogel adsorption | ✓ | ✓ | Zhong et al. (2022) [163] | ||
| Skin | PCL/Chitosan/Gelatine | Electrospinning | Collagen grafting | ✓ | ✓ | ✓ | Sheikhi et al. (2022) [159] | |
| Vascular | PCL/ ePTFE/RGD | Material/solvent solution | Induced crystallisation and RGD coating | ✓ | ✓ | ✓ | Wang et al. (2022) [164] | |
| Vascular | PCL + PGS + DTβ4 | Electrospinning and 3D printing | Molecular modification | ✓ | ✓ | Xiao et al. (2022) [165] | ||
| Tissue Type | Materials | Fabrication Method | Modification Method | Elastic Modulus Range Attained in Tension (T) or Compression (C) | Author, Year, Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| <1 kPa | 1–100 kPa | 100–1000 kPa | 1–100 MPa | 100–1000 MPa | >1 GPa | |||||
| Bone | PLA/HA/PDA | 3D printing | PDA coating | ✓ (C) | ✓ (T) | Chi et al. (2022) [160] | ||||
| Bone | SF/OcPh/PDA | Freeze-drying | PDA coating | ✓ (C) | Peng et al. (2022) [161] | |||||
| Bone | Forsterite/Copper ferrite/P3HB | Sol–gel combustion | P3HB coating | ✓ (C) | Aghajanian et al. (2022) [64] | |||||
| Neural | PCL + Chitosan and PCL + Chitosan + Alginate | Electrospinning | Alginate coating | ✓ (T) | Habibizadeh et al. (2022) [162] | |||||
| Osteochondral | PCL/ GelMA | PDMS mould UV curing | Salt leaching | ✓ (C) | ✓ (C) | Browe et al. (2022) [156] | ||||
| Osteochondral | AC-dECM/Bone-dECM/ | Freeze-drying | Annealing | ✓ (C) | DiCerbo et al. (2022) [157] | |||||
| Skin | PCL/PEG/ PCEC/Hydrogel | Electrospinning | Copolymer/hydrogel adsorption | ✓ (C) | Zhong et al. (2022) [163] | |||||
| Skin | PCL/Chitosan/Gelatine | Electrospinning | Collagen grafting | ✓ (T) | Sheikhi et al. (2022) [159] | |||||
| Vascular | PCL/ ePTFE/RGD | Material/solvent solution | Induced crystallisation and RGD coating | ✓ (T) | ✓ (T) | Wang et al. (2022) [164] | ||||
| Vascular | PCL + PGS + DTβ4 | Electrospinning and 3D printing | Molecular modification | ✓ (C) | Xiao et al. (2022) [165] | |||||
| Tissue Type | Model Type | Based on Native Tissue Assessment? | Author, Year, Reference |
|---|---|---|---|
| Bone | Linear | ✓ | Irarrázaval et al. (2021) [182] |
| Cartilage | Non-linear | ✗ | Jahangir et al. (2022) [183] |
| Kidney | Linear | ✗ | Jing et al. (2021) [184] |
| Liver | Linear | ✓ | Fujimoto (2021) [185] |
| Neural | Non-linear | ✓ | Peshin (2023) [186] |
| Osteochondral | Non-linear | ✗ | Hislop et al. (2021) [187] |
| Skin | Linear | ✓ | Jobanputra et al. (2021) [188] |
| Vascular | Linear | ✓ | Helou et al. (2023) [189] |
| Tissue Type | Material Type | Fabrication Method | Translation Species | Length of Study | Minimised Immune Response? | Study Summary | Author, Year, Reference |
|---|---|---|---|---|---|---|---|
| Bone | Gelatine + HA and gelatine + HA + hPE | 3D printing | Sprague Dawley rats | 12 weeks | ✓ | The loading of hPE into the gelatine/HA scaffold induced a superior osteogenic response compared to that of the unmodified scaffold. | Lee et al. (2022) [117] |
| Bone | Col and Col + PGA and Col + PGA/HA + PGA and Col + PGA/HA + PGA (2-layer membrane) | 3D printing | Sprague Dawley rats/Nude mice | 4 weeks/1, 2, and 4 weeks | ✓ | The Col + PGA/HA + PGA scaffold indicated the highest cell proliferation and osteogenesis. The next highest cell viability was found in the Col + PGA scaffold. For the membrane-based scaffold, the cell core appeared on the surface of the membrane, with the ECM inside it. The Col scaffold showed the lowest cell viability. | Nguyen et al. (2022) [115] |
| Bone | PCL + Gel + HA and PCL + Gel + Hep/PCL + Gel + HA | Electrospinning and 3D printing | New Zealand white rabbits | 5 and 20 weeks | ✓ | The composite scaffold showed good integrative and regenerative properties, and displayed no cytotoxicity, while also acting as a barrier to prevent infiltration by fibrous connective tissue. The bilayer scaffold demonstrated much greater new bone tissue formation, however. | Liu et al. (2021) [148] |
| Bone | Forsterite + copper ferrite and Forsterite + copper ferrite/P3HB | Sol–gel combustion | Wistar rats | 8 weeks | ✓ | Both scaffolds induced a positive response in terms of new tissue formation and trabecular thickness when compared to the control study. However, the P3HB was observed to slightly increase these properties further. Additionally, neither specimen seemed to induce an immune response. | Aghajanian et al. (2022) [64] |
| Bone | Palm dECM + silicon (OTS-modified) and Palm dECM + silicon (APTES-modified) | Decellurizing | Wistar rats | 2 and 4 weeks | ✓ | Neither scaffold presented signs of inflammation nor infection. Both scaffolds exhibited neovascularisation, the presence of endothelial cells, and collagen network fibres. The quantification of these differences was not presented. | Mahendiran et al. (2022) [166] |
| Cartilage | HA-NFs + GelMA | Hydrogel | Sprague Dawley rats | 12 weeks | ✓ | Increasing quantities of HA-NFs in the GelMA promoted a stronger osteogenic response, with 15 and 25 wt/wt% HA-NFs showing new bone deposition and blood tissue formation, compared to 0 and 5 wt/wt% which showed little new tissue formation. | Wang et al. (2022) [118] |
| Cartilage | GelMA + HAMA + chondrospheroids | Hydrogel | Nude mice | 1 and 2 months | ✓ | The chondro-spheroids maintained their morphology during the study. Genes COL 2, SOX 9, and HIF-1a were upregulated in comparison to the positive control (natural cartilage), while COL 10 was downregulated in comparison. | Wang et al. (2021) [113] |
| Cartilage | Alg + HA 50:50 (~70 layers) and Alg + HA 70:30 (~70 layers) | Bioprinting | C57BL/6 mice | 1 and 4 weeks | ✓ | No significant differences were found, in terms of integration, between 50:50 and 70:30 ratios of Alg + HA; however, both scaffolds demonstrated high expression rates of macrophage F4/80 and angiogenesis protein CD31 compared to the control solution. | Janarthanan et al. (2022) [138] |
| Neural | PCL + Chitosan and PCL + Chitosan + Alginate | Electrospinning | Wistar rats | 2, 4, and 8 weeks | ✓ | The PCL + Chitosan sheet showed a moderate inflammatory response and rapid degradation in comparison to the PCL + Chitosan + Alginate construct, which induced a mild inflammatory response and featured a slower degradation rate. | Habibizadeh et al. (2022) [162] |
| Osteochondral | Gellan gum + Alginate sodium and Gellan gum + Alginate sodium + TMP-BG | 3D printing | New Zealand white rabbits | 6 and 12 weeks | ✓ | After 6 weeks of implantation, subchondral bone growth was slightly diminished in the alginate + gellan growth, and significantly higher in the TMP-BG group, compared to the control. By week 12, the alginate + gellan group showed improved subchondral bone growth compared to the control, and the TMP group showed further enhanced proliferation. | Chen et al. (2022) [122] |
| Osteochondral | Col + PLGA and Ti/Col + PLGA | 3D printing/freeze-drying | New Zealand white rabbits | 4, 12, and 24 weeks | ✓ | For the Col-PLGA group, while it indicated superior cell proliferation at the defect site after 24 weeks, this was mostly just fibrous tissue. In comparison, the bilayered scaffold showed more new bone tissue and better integration with the host. The defect did not fully heal after 24 weeks for either construct. | Yang et al. (2021) [135] |
| Osteochondral | Ti/PLA/Col + PLGA and Col + HA | 3D printing | Ovine condyle model | 12 weeks | ✓ | The multi-layer scaffold provided a more homogenous response in terms of ‘filling in’ the defect. In addition, the Col + HA scaffold was rougher than the multi-layer, featuring cracks and fissures. In general, the multi-layer scaffold offered a more complete repair response. | Tamaddon et al. (2022) [139] |
| Osteochondral | PCL + PEO + PES and PCL + PEO/rGO + HAP-Sr + PES-BH0.5% | Electrospinning | Wistar rats | 2 months | ✓ | The nanocomposite scaffold showed larger upregulation in the COL II, COL X, SOX 9, ALP, and Osteocalcin genes and protein compared to the hybrid scaffold. The hybrid scaffold may have induced an immune response due to degradation. | Dargoush et al. (2022) [134] |
| Osteochondral | AC-dECM/ Bone-dECM/ | Freeze-drying | Caprine models | 24 weeks | ✓ | Broad variation in defect repair quality was found. Generally, however, the bilayered scaffold promoted zonally defined tissue, and was able to return the mechanical properties of the region close to that of the surrounding osseous region. Bone repair was more consistent than that of the natural healing process. | Browe et al. (2022) [156] |
| Osteochondral | Silk and BMP-2 + silk and silk/silk (bilayer) and BMP-2 + bilayer and BMP-2 + bilayer/TGF-β1 + SilMA | Hydrogel solution | New Zealand white rabbits | 0, 3, and 8 weeks | ✓ | The silk and BMP-2 + silk integrated poorly; however, the latter of these did promote large volumes of new bone tissue. Comparatively, the bilayer scaffold alone showed very little new tissue formation. The BMP-2 + bilayer integrated and promoted new tissue growth well, superseded only by the silk + SilMA composite scaffold. | Wu et al. (2021) [133] |
| Osteochondral | GelMA + PEO + HA/GelMA + PEO + HA/GelMA + PEO | Hydrogel solution | New Zealand white rabbits | 12 weeks | ✓ | It was stated and illustrated that the tri-layer scaffold demonstrated good capacity for regenerating cartilage, subchondral bone, and trabecular bone. These properties were not quantified or examined further, however. | Li et al. (2022) [141] |
| Skin | PCL+ Col and PCL + Col + ZnO and PCL + Col + ZnO + VEGF | Electrospinning | Sprague Dawley rats | 6 and 12 days | ✓ | Gross imaging and linked diagrams indicated that the wound healing rate was enhanced by the use of PCL + Col, and further enhanced by the inclusion of ZnO, before reaching its highest rate with the inclusion of Zno + VEGF. A similar trend was noted during Col and TGF-β1 expression analysis. | Li et al. (2021) [126] |
| Vascular | PCL + PGS + DTβ4 | Electrospinning and 3D printing | New Zealand white rabbits | 2 and 12 weeks | ✗ | Patency rate for scaffold was maintained at 80% across test animals, showing no signs of dilation or thrombosis. However, the grafts degraded before native tissue could remodel around the grafts. The slight generation of cross-linked elastin was noted, as well as rapid endothelialisation. | Xiao et al. (2022) [165] |
| Cell/Molecule Type | Chip Material Type/System | Fabrication Method | Study Purpose | Mechanical Properties Considered? | Author, Year, Reference |
|---|---|---|---|---|---|
| RAW264.7 macrophages and NIH-3T3 fibroblasts | Polydimethylsiloxane (PDMS) | Soft lithography | Skin wound healing | ✗ | Li et al. (2023) [195] |
| Chinese hamster ovary cells | Silicon | Micro-electro-mechanical system | Single cell analysis | ✗ | Xu et al. (2023) [196] |
| Caco-2 and HepG2 cells | PDMS | Soft lithography | Disease modelling (non-alcoholic fatty liver disease) | ✗ | Yang et al. (2023) [197] |
| Keratinocytes | PDMS | Soft lithography | Skin reconstruction | ✗ | Ahn et al. (2023) [198] |
| Hepatocytes, stellate cells, Kupffer-like macrophages, and endothelial cells | PDMS | Soft lithography | Studying effects of inflammation and cirrhosis on drug metabolism during hepatocellular carcinoma | ✓ | Özkan et al. (2023) [199] |
| Cardiomyocytes and cardiac fibroblasts | PDMS | Casting | Examining effects on cardiac tissue mechanical response following infarction | ✓ | Das et al. (2022) [200] |
| Glucose molecules | Polymethyl methacrylate | Stereolithography | Glucose sensing | ✗ | Podunavac et al. (2023) [201] |
| H9c2 rat cardiac myoblasts and adult human dermal fibroblasts | Agarose | Casting | Cardiac remodelling following arteriovenous fistula | ✗ | Waldrop et al. (2023) [202] |
| Human embryonic kidney 293 cells, NIH3T3 embryonic mouse fibroblasts, and human mammary MCF10A cells | PDMS | Soft lithography | Cell spheroid viscoelasticity quantification | ✓ | Boot et al. (2023) [203] |
| Human umbilical vein endothelial cells | PDMS | Stereolithography | Effect of biomechanical/biochemical stimuli on angio- and vasculogenesis | ✗ | Ferrari et al. (2023) [204] |
| Mouse podocytes and mouse glomerular endothelial cells | PDMS | Casting | Investigating crosstalk between glomerular endothelial cells and glomerular epithelial cells | ✗ | Hart et al. (2023) [205] |
| Taste receptor cells | MEA2100-System | Unstated | Ex vivo sense of taste simulation | ✗ | Wu et al. (2023) [206] |
| Bone marrow mesenchymal stem cells | PDMS | Casting | Cross-cellular interactions in osseous tissue | ✓ | Erbay et al. (2022) [207] |
| Human lung fibroblasts and human umbilical vein endothelial cells | PDMS | Casting | Controlling angiogenesis in lung cancer spheroids | ✗ | Kim et al. (2022) [208] |
| Bovine aortic endothelial cells and human pulmonary artery endothelial cells | PDMS | Spin coating and dual-layer lithography | Cell shear stress analysis and imaging | ✓ | Sinclair et al. (2023) [209] |
| Tissue Type (Region) | Material Type | Fabrication Method | Procedure | Study Length | Post-Operative Positive Outcome (Patient Number/Total Cases) | Study Summary | Author, Year, Reference |
|---|---|---|---|---|---|---|---|
| Bone (hand) | PLA | 3D printing | Splint fitting | 4–6 weeks | 10/10 | Compared to the control (thermoplastic) splint, patients reported a similar level of comfort, with the cost-effectiveness of the design potentially outperforming current designs. Two patients reported splint breakage after heavy use. | Waldburger et al. (2021) [212] |
| Bone (jaw) | PCL + β-TCP | 3D printing | Maxillary reconstruction | 6 months | 8/8 | While one patient suffered post-operative wound dehiscence (which was subsequently covered with a local flap), all cases showed signs of bone regeneration and scaffold integration. However, the group concluded that definitive parameters, such as implant efficacy and degradation, could not be accurately assessed. | Jeong et al. (2022) [31] |
| Bone (leg) | PCL + TCP | 3D printing | Scaffold implantation | 9–23 months | 4/4 | The scaffold, designed as a mesh which wraps around the bone defect site, was tested in four cases. In all cases, load-bearing functionality of the affected bone area was eventually restored, with bone development into the scaffold noted in three of the four cases. | Laubach et al. (2022) [213] |
| Bone (skull) | Surgical guide resin | 3D printing | Cranioplasty | 3 days | 0/1 | Patient mobility and cognition improved during the period of study. Patient symptoms began to redevelop, but not to the same extent as prior to the procedure, before passing away due to complications as a result of their initial condition. | Mee et al. (2021) [214] |
| Bone (skull) | PMMA | 3D printing | Cranioplasty | 10 days | 3/3 | The first case initially showed signs of fluid collection between the implant and dura; however, this was resolved shortly thereafter. The other two cases progressed well as expected. | Dabadi et al. (2021) [215] |
| Bone (sternum) | Aluminium oxide | Unspecified—company trademarked | Sternal replacement | 28 months | 1/1 | The patient did not present any physiological complaints at the 28 month follow-up appointment, and was able to take part in physical activities without displacement or irritation of the prosthesis. This particular construct has a history of prior use with paediatric patients. | Mainard et al. (2022) [216] |
| Cardiac | UPy | Supramolecular chemistry | RVOT reconstruction | 12 months | 12/18 | Overall, the conduit performed well across patients; however, several outlier complicated cases developed. These were either a result of immune response and thrombi formation, failure of the valve leaflets, or poor integration times. These were resolved using alternative measures. | Morales et al. (2021) [217] |
| Dental | Undefined resin—company trademarked | 3D printing | Aligner fitting | 76 weeks | 120/120 | Patients presented dental problems such as crowding, over-, under-, and cross-bite. All 120 cases were corrected using 3D-printed clear aligners and resulted in well-aligned teeth. | Yu et al. (2022) [218] |
| Dermal (face) | Unspecified thermoplastic | 3D printing | Volumetric modulated arc therapy | 12 weeks | 23/35 | Twelve of the patients treated with the 3D-printed bolus suffered from grade ≥2 radiation dermatitis, while seventeen did with the conventional bolus. Additionally, none of the patients suffered from radiation pneumonitis, which is significant compared to the three which did using the conventional bolus. | Zhang et al. (2021) [219] |
| Oral tissue | Acrylic | CAD/Casting | Impression of cleft palate tray | Procedure length (~16 min) | Not stated | In comparison to the standard ‘finger technique’ of the impression of cleft palate trays, the 3D-modelled acrylic tray recorded greater detail, showed zero distortions, took less time to obtain the impression, and induced a lower heart rate in the infants who were tested. | Kalaskar et al. (2021) [220] |
| Oral tissue/Limb | PLA, PLU-branded elastomer, TPU | 3D printing | Splint fitting | - | 1/18 | Three sets of constructs were tested: oral, upper-extremity, and lower-extremity splints. The oral splints could not be tested fully as patients resisted their use. Only one patient tolerated the upper-extremity splint, which did remove the risk of contracture during its use. The lower-extremity splints caused discomfort due to tightness and incongruity. The group recommend the usage of dynamic splints as a remedial measure. | Şenayli et al. (2021) [221] |
| Renal | PLA | 3D printing | Renal autotransplantation | 12 months | 1/1 | This construct took the form of a 3D-printed cold jacket, for use during kidney transplantation. One year after the operation, an ultrasound scan indicated normal kidney size and shape, and that ordinary blood flow had resumed. However, random comparison to existing methods and/or extending this procedure to other patients were not considered. | Cui et al. (2022) [222] |
| Soft tissue | dECM | Cell sheet | Flap reconstruction | 6 months | 7/9 | Integration of the latter cases was complicated by wound dehiscence. This was later resolved via secondary healing. Additionally, five patients received incisional negative pressure wound therapy, but the impact of which could not be fully assessed. | Desvigne et al. (2021) [223] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnston, A.; Callanan, A. Recent Methods for Modifying Mechanical Properties of Tissue-Engineered Scaffolds for Clinical Applications. Biomimetics 2023, 8, 205. https://doi.org/10.3390/biomimetics8020205
Johnston A, Callanan A. Recent Methods for Modifying Mechanical Properties of Tissue-Engineered Scaffolds for Clinical Applications. Biomimetics. 2023; 8(2):205. https://doi.org/10.3390/biomimetics8020205
Chicago/Turabian StyleJohnston, Andrew, and Anthony Callanan. 2023. "Recent Methods for Modifying Mechanical Properties of Tissue-Engineered Scaffolds for Clinical Applications" Biomimetics 8, no. 2: 205. https://doi.org/10.3390/biomimetics8020205
APA StyleJohnston, A., & Callanan, A. (2023). Recent Methods for Modifying Mechanical Properties of Tissue-Engineered Scaffolds for Clinical Applications. Biomimetics, 8(2), 205. https://doi.org/10.3390/biomimetics8020205