Preparation, Characterization, and Drug Delivery of Hexagonal Boron Nitride-Borate Bioactive Glass Biomimetic Scaffolds for Bone Tissue Engineering
Abstract
1. Introduction
2. Experimental Studies
2.1. Materials
2.2. Porous Biomimetic Scaffold Manufacture
2.3. Instrumentation
2.4. In Vitro Mineralization
2.5. Drug Delivery Studies
2.5.1. Gentamicin
2.5.2. Fluorouracil (5-FU)
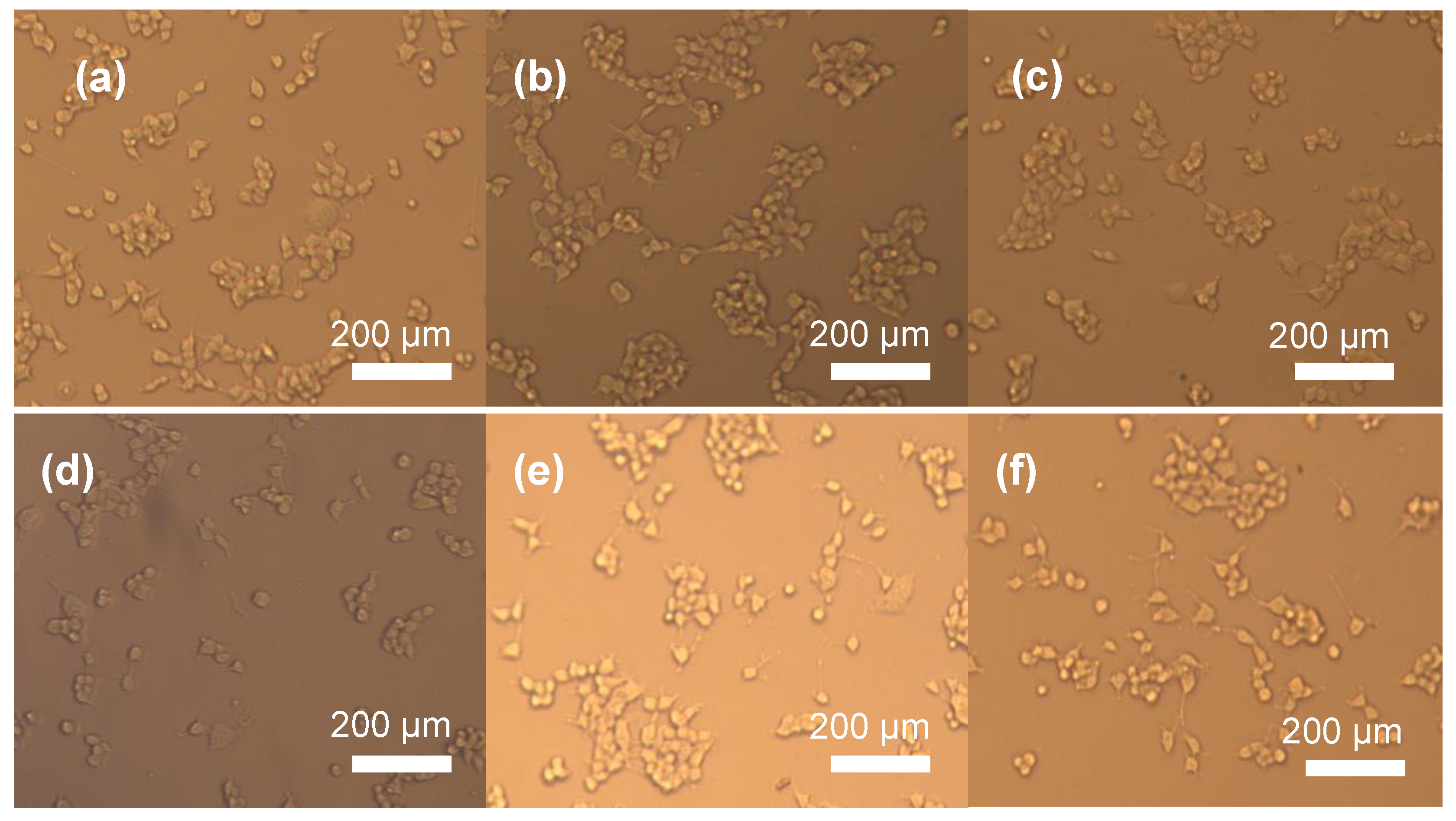
2.6. Cytotoxicity
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, E.T.; Zhao, M. Regulation of tissue repair and regeneration by electric fields. Chin. J. Traumatol. 2010, 13, 55–61. [Google Scholar] [PubMed]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 1998, 81, 1705–1728. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Biactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef]
- Fu, Q.; Rahaman, M.N.; Fu, H.; Liu, X. Silicate, borosilicate, and borate bioactive glass scaffolds with controllable degradation rate for bone tissue engineering applications. I. Preparation and in vitro degradation. J. Biomed. Mater. Res. Part A 2010, 95, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Brink, M.; Turunen, T.; Happonen, R.P.; Yli-Urpo, A. Compositional dependence of bioactivity of glasses in the system Na2O-K2O-MgO-CaO-B2O3-P2O5-SiO2. J. Biomed. Mater. Res. 1997, 37, 114–121. [Google Scholar] [CrossRef]
- Fu, H.; Fu, Q.; Zhou, N.; Huang, W.; Rahaman, M.N.; Wang, D.; Liu, X. In vitro evaluation of borate-based bioactive glass scaffolds prepared by a polymer foam replication method. Mater. Sci. Eng. C 2009, 29, 2275–2281. [Google Scholar] [CrossRef]
- Bi, L.; Rahaman, M.N.; Day, D.E.; Brown, Z.; Samujh, C.; Liu, X.; Mohammadkhah, A.; Dusevich, V.; Eick, J.D.; Bonewald, L.F. Effect of bioactive borate glass microstructure on bone regeneration, angiogenesis, and hydroxyapatite conversion in a rat calvarial defect model. Acta Biomater. 2013, 9, 8015–8026. [Google Scholar] [CrossRef]
- Porwal, H.; Grasso, S.; Cordero-Arias, L.; Li, C.; Boccaccini, A.R.; Reece, M.J. Processing and bioactivity of 45S5 Bioglass®-graphene nanoplatelets composites. J. Mater. Sci. Mater. Med. 2014, 25, 1403–1413. [Google Scholar] [CrossRef]
- Gao, C.; Liu, T.; Shuai, C.; Peng, S. Enhancement mechanisms of graphene in nano-58S bioactive glass scaffold: Mechanical and biological performance. Sci. Rep. 2014, 4, 4712. [Google Scholar] [CrossRef]
- Turk, M.; Deliormanlı, A.M. Electrically conductive borate-based bioactive glass scaffolds for bone tissue engineering applications. J. Biomater. Appl. 2017, 32, 28–39. [Google Scholar] [CrossRef]
- Türk, M.; Deliormanlı, A.M. Graphene-containing PCL- coated Porous 13-93B3 Bioactive Glass Scaffolds for Bone Regeneration. Mater. Res. Express 2018, 5, 045406. [Google Scholar] [CrossRef]
- Ilyas, K.; Zahid, S.; Batool, M.; Chaudhry, A.A.; Jamal, A.; Iqbal, F.; Nawaz, M.H.; Goerke, O.; Gurlo, A.; Shah, A.T.; et al. In-vitro investigation of graphene oxide reinforced bioactive glass ceramics composites. J. Non-Cryst. Solids 2019, 505, 122–130. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, I.R.; Kim, Y.; Kim, D.H.; Park, S.B.; Park, B.S.; Bae, M.K.; Kim, Y.I. The Effect of Mesoporous Bioactive Glass Nanoparticles/Graphene Oxide Composites on the Differentiation and Mineralization of Human Dental Pulp Stem Cells. Nanomaterials 2020, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Deliormanlı, A.M.; Ensoylu, M.; Issa, S.A.; Elshami, W.; Al-Baradi, A.M.; Al-Buriahi, M.S.; Tekin, H.O. WS2/bioactive glass composites: Fabrication, structural, mechanical and radiation attenuation properties. Ceram. Int. 2021, 47, 29739–29747. [Google Scholar] [CrossRef]
- Ensoylu, M.; Atmaca, H.; Deliormanlı, A.M. Fabrication and in vitro characterization of macroporous WS2/ bioactive glass scaffolds for biomedical applications. J. Aust. Ceram Soc. 2022, 58, 397–409. [Google Scholar] [CrossRef]
- Wang, J.; Ma, F.; Sun, M. Graphene, hexagonal boron nitride, and their heterostructures: Properties and applications. RSC Adv. 2017, 7, 16801–16822. [Google Scholar] [CrossRef]
- Yankowitz, M.; Ma, Q.; Jarillo-Herrero, P.; LeRoy, B.J. van der Waals heterostructures combining graphene and hexagonal boron nitride. Nat. Rev. Phys. 2019, 1, 112–125. [Google Scholar] [CrossRef]
- Lorrette, C.; Weisbecker, P.; Jacques, S.; Pailler, R.; Goyhénèche, J.M. Deposition and characterization of hex-BN coating on carbon fibres using tris (dimethylamino) borane precursor. J. Eur. Ceram. Soc. 2007, 27, 2737–2743. [Google Scholar] [CrossRef]
- Jedrzejczak-Silicka, M.; Trukawka, M.; Dudziak, M.; Piotrowska, K.; Mijowska, E. Hexagonal Boron Nitride Functionalized with Au Nanoparticles—Properties and Potential Biological Applications. Nanomaterials 2018, 8, 605. [Google Scholar] [CrossRef]
- Lu, T.; Wang, L.; Jiang, Y.; Huang, C. Hexagonal boron nitride nanoplates as emerging biological nanovectors and their potential applications in biomedicine. J. Mater. Chem. B 2016, 4, 6103–6110. [Google Scholar] [CrossRef]
- Horvath, L.; Magrez, A.; Golberg, D.; Zhi, C.; Bando, Y.; Smajda, R.; Horvath, E.; Forro, L.; Schwaller, B. In Vitro Investigation of the Cellular Toxicity of Boron Nitride Nanotubes. ACS Nano 2011, 5, 3800–3810. [Google Scholar] [CrossRef] [PubMed]
- Kartal, İ.; Boztoprak, Y. Bor nitrür partikülleriyle takviye edilmiş vinil ester matrisli kompozitlerin mekanik özelliklerinin incelenmesi. El-Cezerî J. Sci. Eng. 2019, 6, 43–50. [Google Scholar]
- Lahiri, D.; Singh, V.; Benaduce, A.P.; Seal, S.; Kos, L.; Agarwal, A. Boron nitride nanotube reinforced hydroxyapatite composite: Mechanical and tribological performance and in-vitro biocompatibility to osteoblasts. J. Mech. Behav. Biomed. Mater. 2011, 4, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Wang, F.; Cao, L.; Kong, C.Y.; Huang, X. Hexagonal Boron Nitride Nanomaterials: Advances towards Bioapplications. Nanosci. Nanotechnol. Lett. 2012, 4, 949–961. [Google Scholar] [CrossRef]
- Ensoylu, M.; Deliormanlı, A.M.; Atmaca, H. Hexagonal Boron Nitride/PCL/PLG Coatings on Borate Bioactive Glass Scaffolds for Bone Regeneration. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1551–1566. [Google Scholar] [CrossRef]
- Saggar, R.; Porwal, H.; Tatarko, P.; Dlouhý, I.; Reece, M.J. Boron nitride nanosheets reinforced glass matrix composites. Adv. Appl. Ceram. 2015, 114 (Suppl. 1), S26–S33. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How Useful is SBF in Predicting in vivo Bone Bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Wojcik-Pastuszka, D.; Krzak, J.; Macikowski, B.; Berkowski, R.; Osiński, B.; Musiał, W. Evaluation of the Release Kinetics of a Pharmacologically Active Substance from Model Intra-Articular Implants Replacing the Cruciate Ligaments of the Knee. Materials 2019, 12, 1202. [Google Scholar] [CrossRef]
- Songfeng, E.; Ye, X.; Wang, M.; Huang, J.; Ma, Q.; Jin, Z.; Ning, D.; Lu, Z. Enhancing the tribological properties of boron nitride by bioinspired polydopamine modification. Appl. Surf. Sci. 2020, 529, 147054. [Google Scholar]
- Wang, L.; Hang, R.; Xu, Y.; Guo, C.; Qian, Y. From ultrathin nanosheets, triangular plates to nanocrystals with exposed (102) facets, a morphology and phase transformation of sp2 hybrid BN nanomaterials. RSC Adv. 2014, 4, 14233–14240. [Google Scholar] [CrossRef]
- Ojha, P.K.; Maji, R.; Karmakar, S. Effect of crystallinity on droplet regression and disruptive burning characteristics of nanofuel droplets containing amorphous and crystalline boron nanoparticles. Combust. Flame 2018, 188, 412–427. [Google Scholar] [CrossRef]
- Balachander, L.; Ramadevudu, G.; Shareefuddin; Sayanna, R.; Venudhar, Y.C. IR analysis of borate glasses containing three alkali oxides. ScienceAsia 2013, 39, 278–283. [Google Scholar] [CrossRef]
- Sudeep, P.M.; Vinod, S.; Ozden, S.; Sruthi, R.; Kukovecz, A.; Konya, Z.; Vajtai, R.; Anantharaman, M.R.; Ajayan, P.M.; Narayanan, T.N. Functionalized boron nitride porous solids. RSC Adv. 2015, 5, 93964. [Google Scholar] [CrossRef]
- Geick, R.; Perry, C.H.; Rupprecht, G. Normal modes in hexagonal boron nitride. Phys. Rev. 1966, 146, 543–547. [Google Scholar] [CrossRef]
- Pierson, H.O. Handbook of Carbon, Graphite, Diamond, and Fullerenes: Properties, Processing, and Applications; Noyes Publications: Devon, UK, 1993. [Google Scholar]
- Riaz, I. Graphene and Boron Nitride: Members of Two Dimensional Material Family. Doctor’s Thesis, School of Physics and Astronomy, Manchester Üniversitesi, Manchester, UK, 2012, 144p.
- Wei, X.; Meng, Z.; Ruiz, L.; Xia, W.; Lee, C.; Kysar, J.W.; Hone, J.C.; Keten, S.; Espinosa, H.D. Recoverable slippage mechanism in multilayer graphene leads to repeatable energy dissipation. ACS Nano 2016, 10, 1820–1828. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeong, J.H.; Kim, N.; Joshi, R.; Lee, G.H. Mechanical properties of two-dimensional materials and their applications. J. Phys. D Appl. Phys. 2018, 52, 083001. [Google Scholar] [CrossRef]
- Golberg, D.; Bando, Y.; Huang, Y.; Terao, T.; Mitome, M.; Tang, C.; Zhi, C. Boron Nitride Nanotubes and Nanosheets. ACS Nano 2010, 4, 2979–2993. [Google Scholar] [CrossRef]
- Shuai, C.; Han, Z.; Feng, P.; Gao, C.; Xiao, T.; Peng, S. Akermanite scaffolds reinforced with boron nitride nanosheets in bone tissue engineering. J. Mater. Sci. Mater. Med. 2015, 26, 188. [Google Scholar] [CrossRef] [PubMed]
- Berzina-Cimdina, L.; Borodajenko, N. Research of calcium phosphates using Fourier transform infrared spectroscopy. Infrared Spectrosc.-Mater. Sci. Eng. Technol. 2012, 12, 251–263. [Google Scholar]
- Lahiri, D.; Singh, V.; Keshri, A.K.; Seal, S.; Agarwal, A. Apatite formability of boron nitride nanotubes. Nanotechnology 2011, 22, 205601. [Google Scholar] [CrossRef]
- Schuhladen, K.; Pantulap, U.; Engel, K.; Jeleń, P.; Olejniczak, Z.; Hupa, L.; Sitarz, M.; Boccaccini, A.R. Influence of the replacement of silica by boron trioxide on the properties of bioactive glass scaffolds. Int J Appl Glass Sci. 2021, 12, 293–312. [Google Scholar] [CrossRef]
- El-Batal, F.; El-Kheshen, A.A.; El-Bassyouni, G.T.; Abd El Aty, A.A. In Vitro Bioactivity Behavior of some Borate Glasses and their Glass-Ceramic Derivatives Containing Zn2+, Ag+ or Cu2+ by Immersion in Phosphate Solution and their Anti-Microbial Activity. Silicon 2018, 10, 943–957. [Google Scholar] [CrossRef]
- Ksouri, D.; Khireddine, H.; Aksas, A.; Valente, T.; Bir, F.; Slimani, N.; Cabal, B.; Torrecillas, R.; Santos, J.D. Synthesis of ternary bioactive glass derived aerogel and xerogel: Study of their structure and bioactivity. Nova Biotechnol. Chim. 2018, 17, 150–159. [Google Scholar] [CrossRef]
- Nawaz, A.; Bano, S.; Yasir, M.; Wadood, A.; Rehman, M.A.U. Ag and Mn-doped mesoporous bioactive glass nanoparticles incorporated into the chitosan/gelatin coatings deposited on PEEK/bioactive glass layers for favorable osteogenic differentiation and antibacterial activity. Mater. Adv. 2020, 1, 1273–1284. [Google Scholar] [CrossRef]
- Bruschi, M.L. Chapter 5—Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Sawston, UK, 2015; pp. 63–86. [Google Scholar] [CrossRef]
- Longley, D.; Harkin, D.; Johnston, P. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhu, H.; Zhou, S.; Wu, T.; Wu, H.; Yang, H.; Mao, H.; SekharKathera, C.; Janardhan, A.; Edick, A.M.; et al. Wnt pathway is involved in 5-FU drug resistance of colorectal cancer cells. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sara, J.D.; Kaur, J.; Khodadadi, R.; Rehman, M.; Lobo, R.; Chakrabarti, S.; Herrmann, J.; Lerman, A.; Grothey, A. 5-fluorouracil and cardiotoxicity: A review. Ther. Adv. Med. Oncol. 2018, 10, 1758835918780140. [Google Scholar] [CrossRef]
- Zarghami Dehaghani, M.; Yousefi, F.; Sajadi, S.M.; Tajammal Munir, M.; Abida, O.; Habibzadeh, S.; Mashhadzadeh, A.H.; Rabiee, N.; Mostafavi, E.; Saeb, M.R. Theoretical Encapsulation of Fluorouracil (5-FU) Anti-Cancer Chemotherapy Drug into Carbon Nanotubes (CNT) and Boron Nitride Nanotubes (BNNT). Molecules 2021, 26, 4920. [Google Scholar] [CrossRef]
- Wang, C.; Shen, L.; Wu, L. Adsorption and sensing of an anticancer drug on the boron nitride nanocones; a computational inspection. Comput. Methods Biomech. Biomed. Eng. 2021, 24, 151–160. [Google Scholar] [CrossRef]
- El-Kady, A.; Farag, M. Bioactive Glass Nanoparticles as a New Delivery System for Sustained 5-Fluorouracil Release: Characterization and Evaluation of Drug Release Mechanism. J. Nanomater. 2015, 16, 399. [Google Scholar] [CrossRef]
- Khalid, A.; Ahmad, P.; Khan, A.; Khandaker, M.U.; Kebaili, I.; Alam, M.M.; Din, I.U.; Muhammad, S.; Razzaq, Z.; Rehman, I.U.; et al. Cytotoxic and photocatalytic studies of hexagonal boron nitride nanotubes: A potential candidate for wastewater and air treatment. RSC Adv. 2022, 12, 6592. [Google Scholar] [CrossRef] [PubMed]

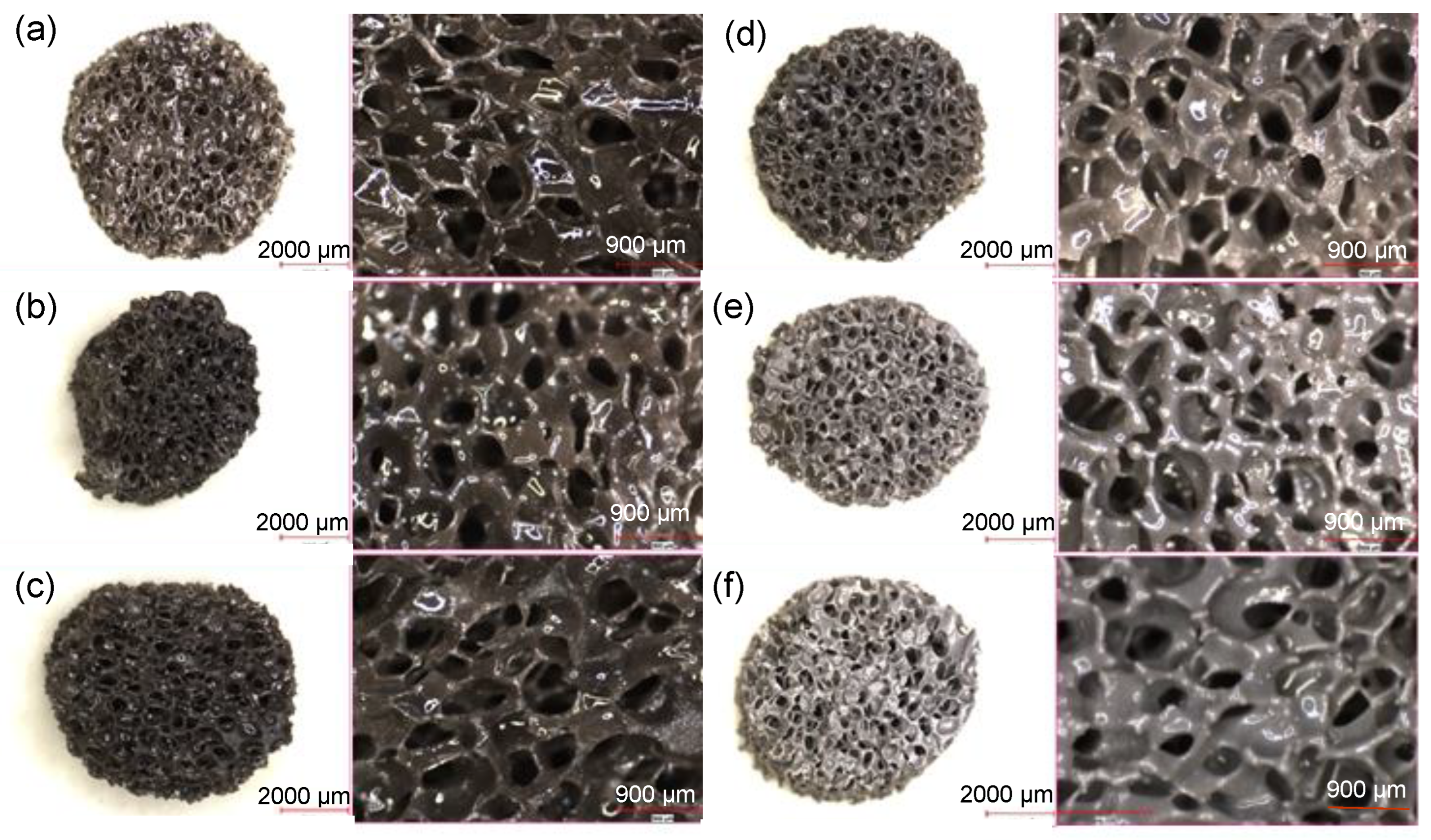
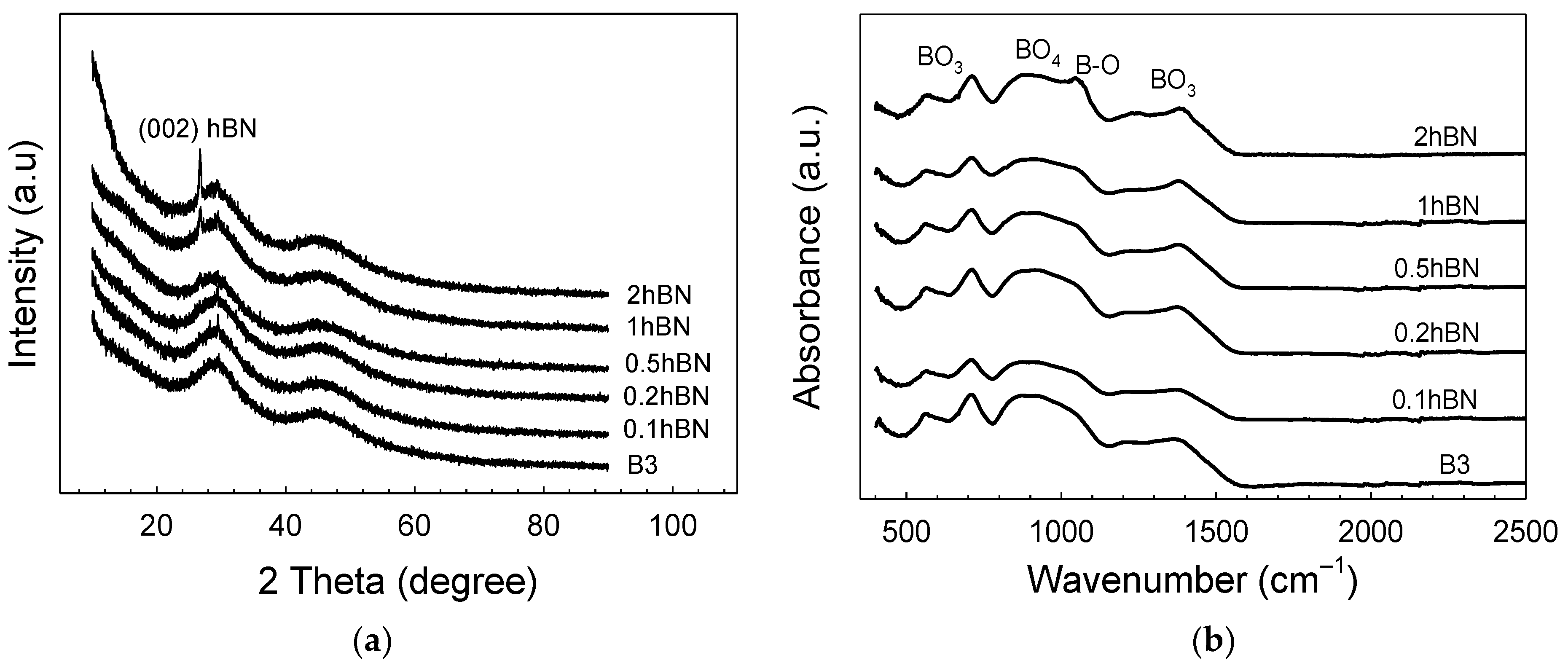
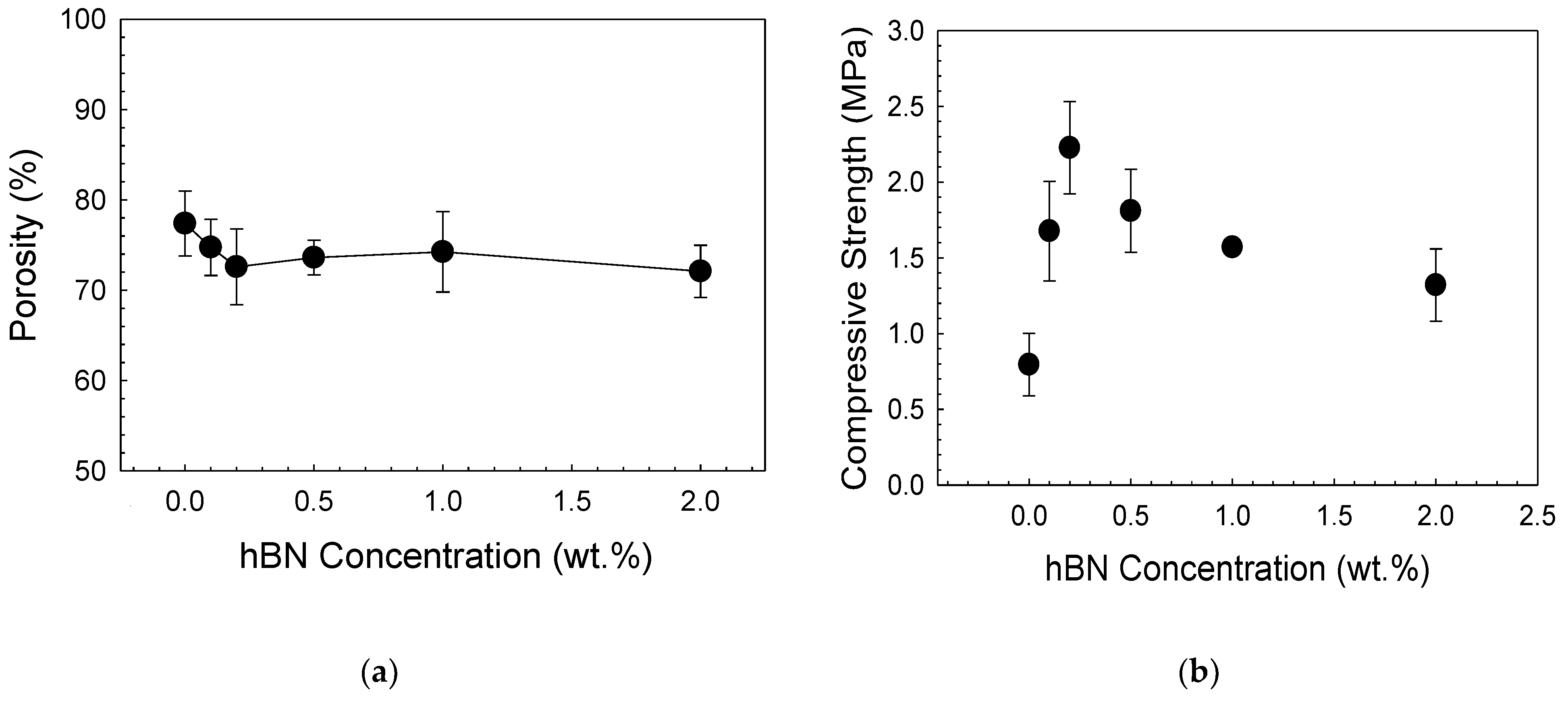
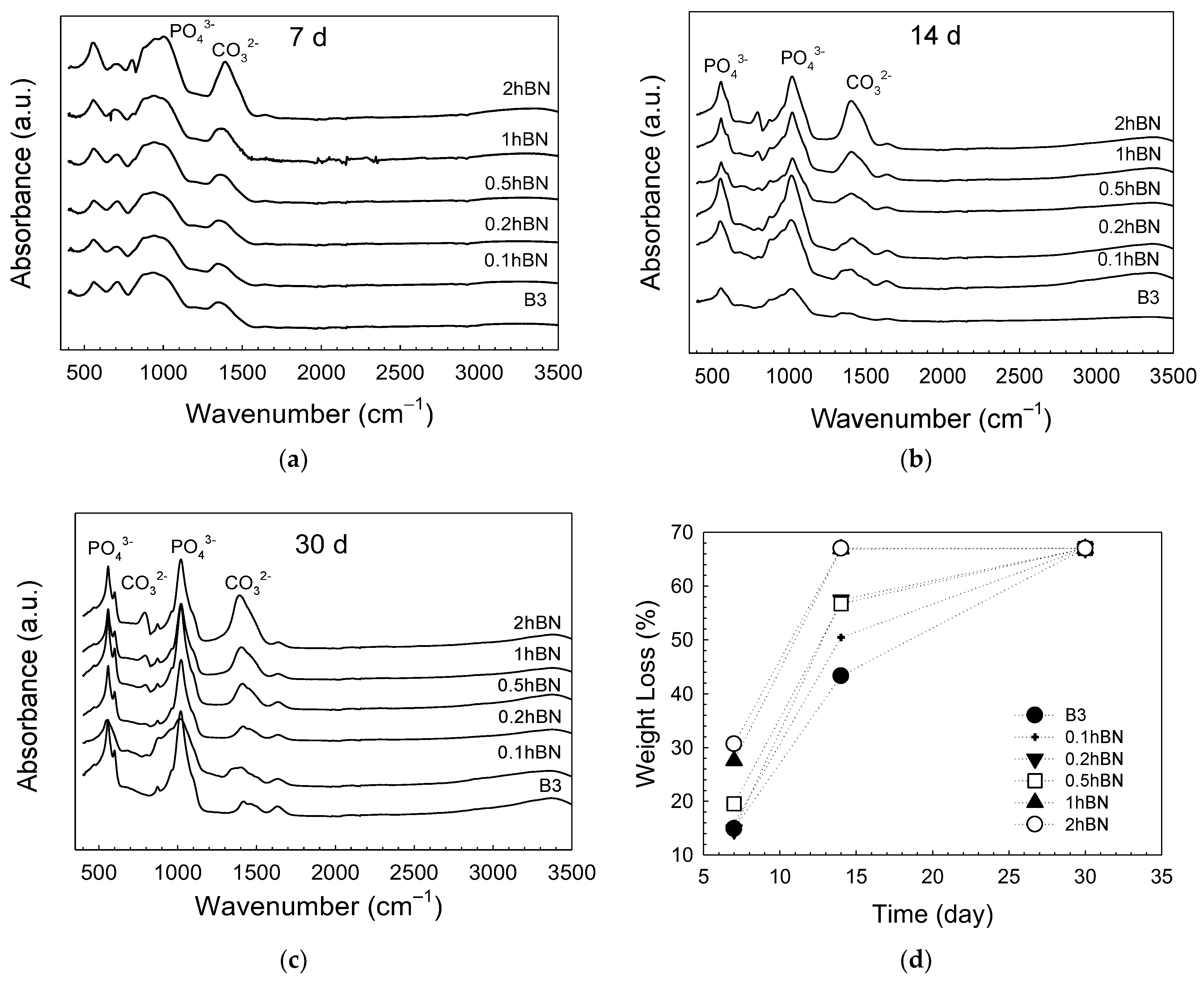
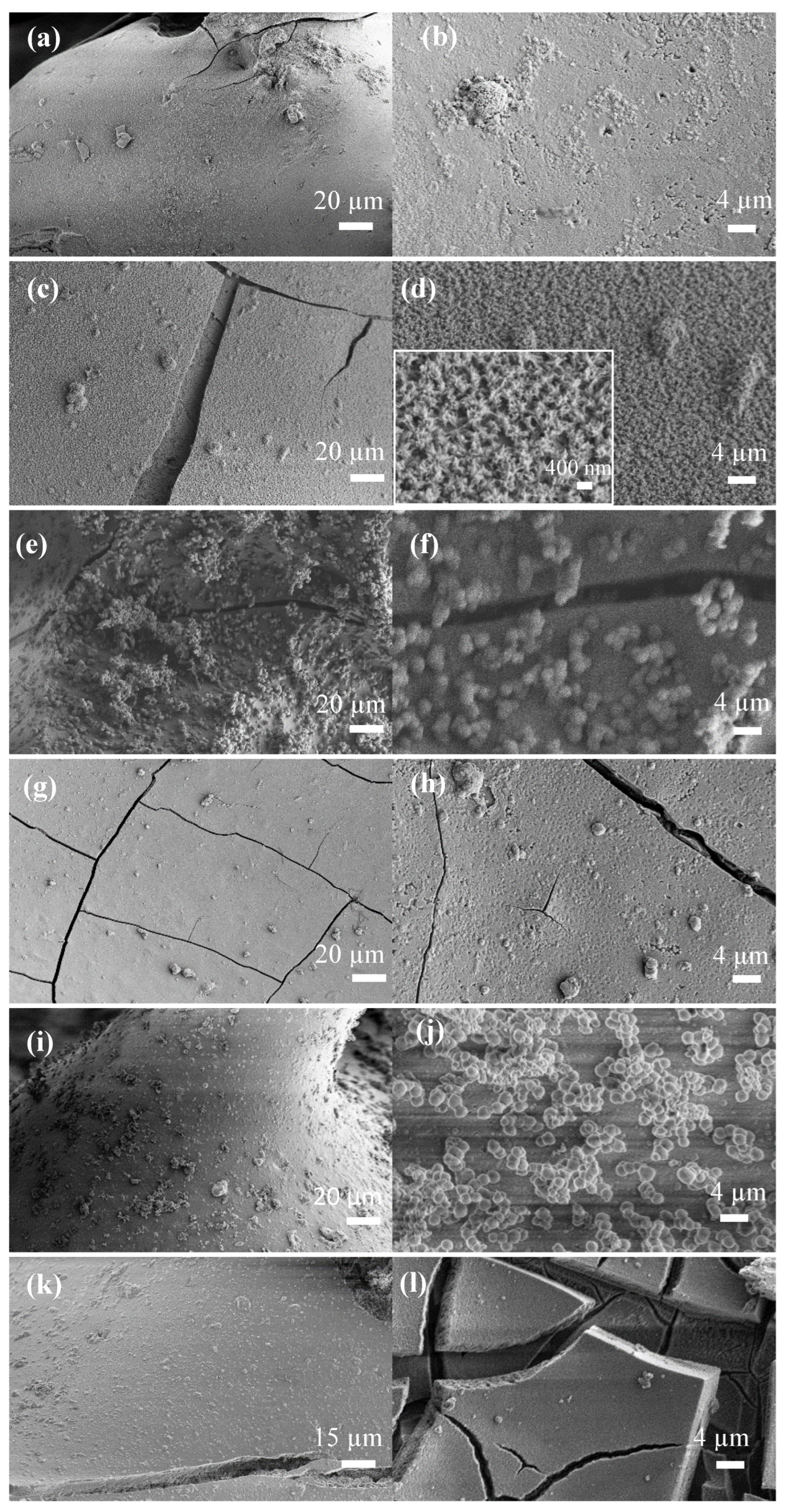

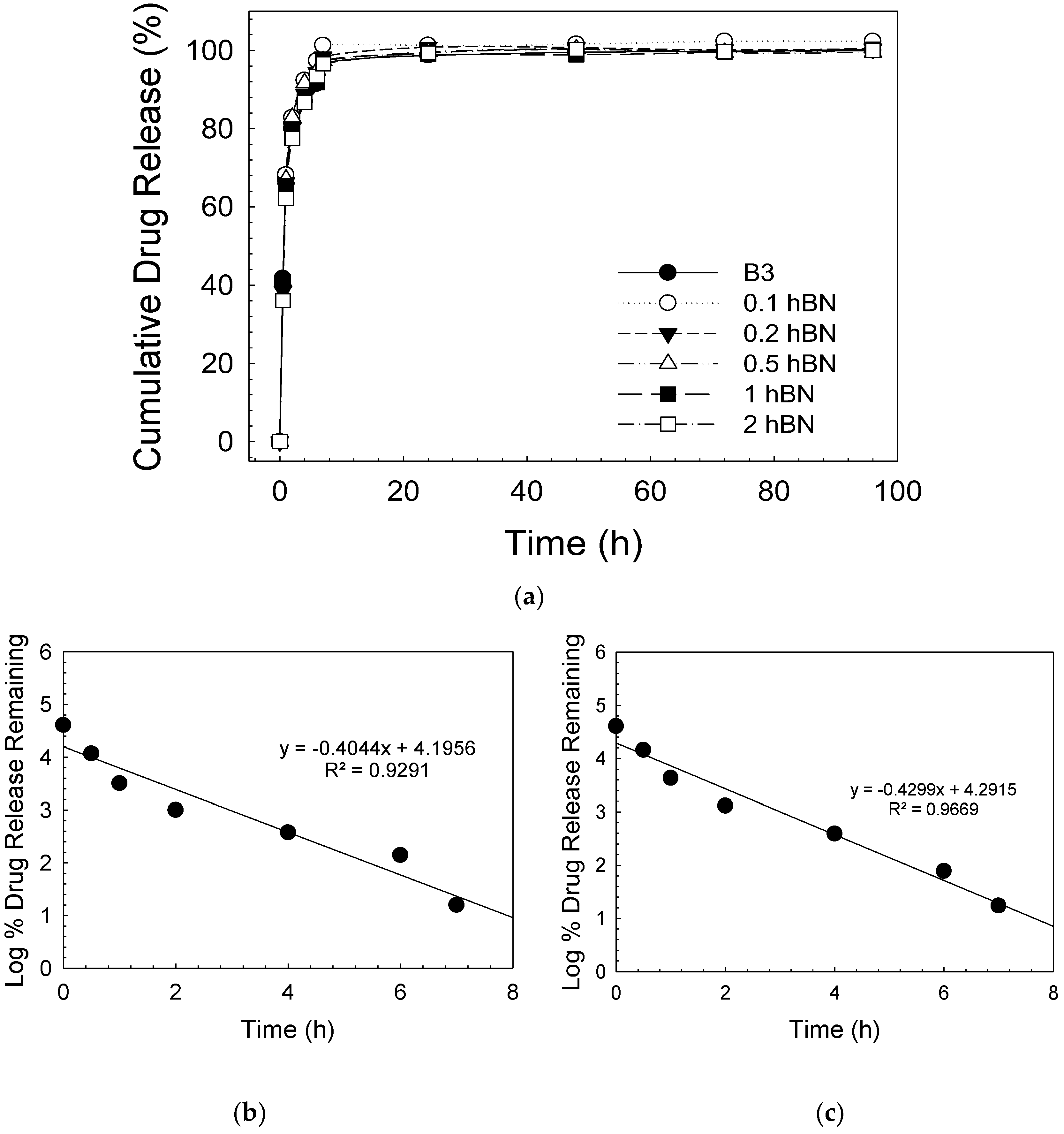
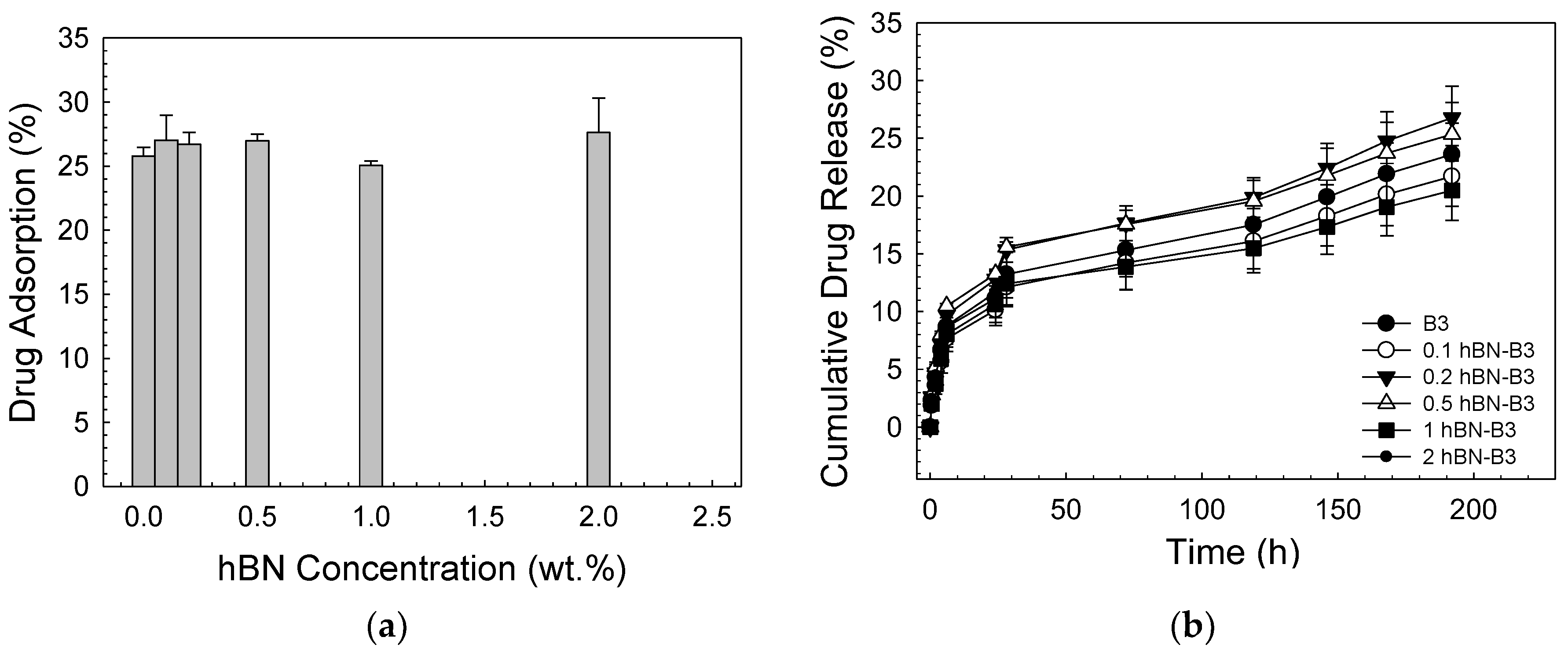
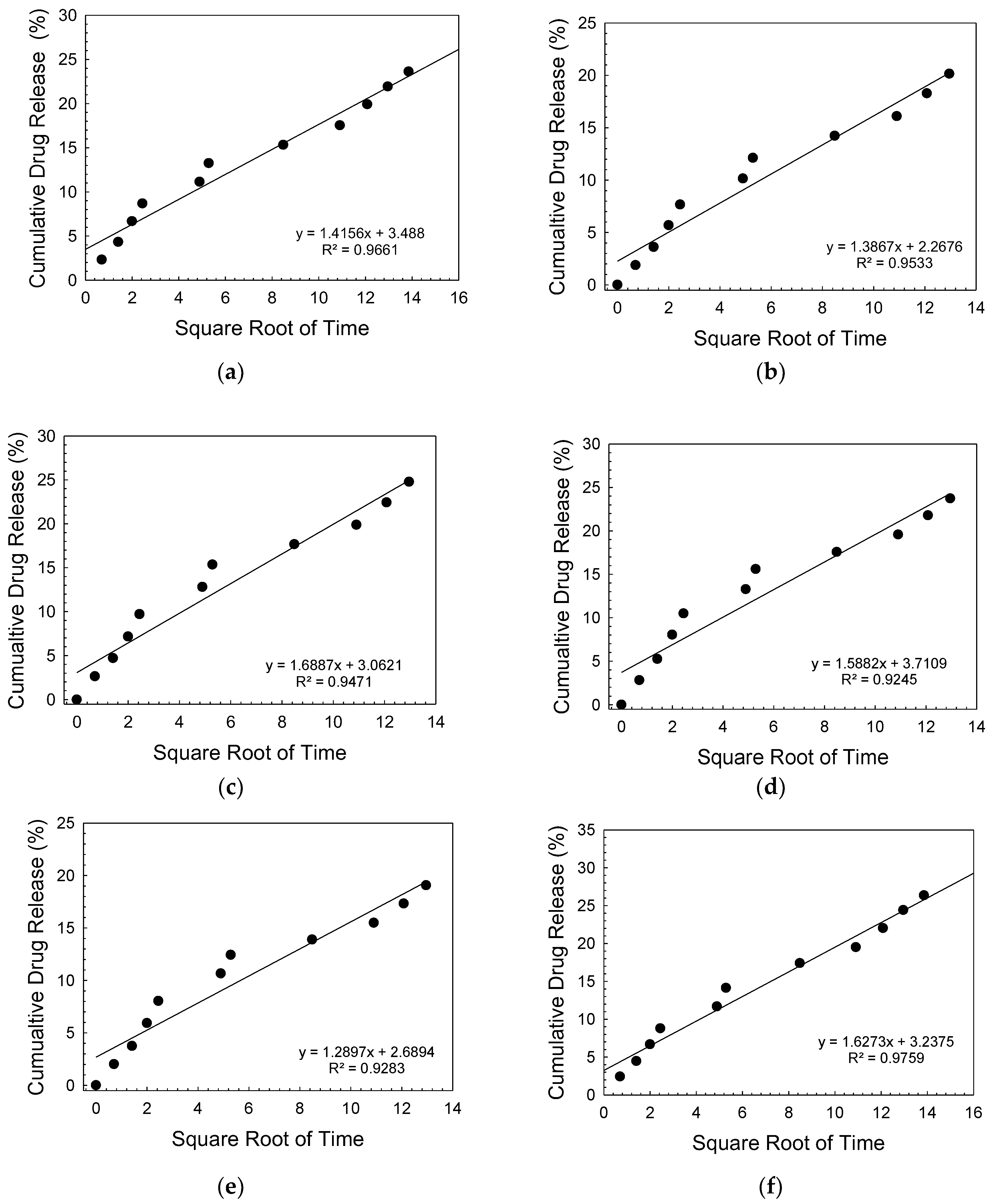

| Sample | Zero Order | First Order | Higuchi | |||
|---|---|---|---|---|---|---|
| R2 | K0 | R2 | K1 | R2 | KH | |
| B3 | 0.6478 | 10.024 | 0.9291 | 0.4044 | 0.8682 | 33.372 |
| 0.1 hBN-B3 | 0.6729 | 10.901 | 0.9809 | 0.5964 | 0.8855 | 35.956 |
| 0.2 hBN-B3 | 0.6754 | 10.595 | 0.9526 | 0.5013 | 0.886 | 34.895 |
| 0.5 hBN-B3 | 0.6462 | 10.389 | 0.9501 | 0.4641 | 0.8681 | 34.626 |
| 1 hBN-B3 | 0.6671 | 10.229 | 0.9357 | 0.4301 | 0.8819 | 33.82 |
| 2 hBN-B3 | 0.7027 | 10.676 | 0.9669 | 0.4299 | 0.9041 | 34.824 |
| Sample | Zero Order | First Order | Higuchi | |||
|---|---|---|---|---|---|---|
| R2 | K0 | R2 | K1 | R2 | KH | |
| B3 | 0.896 | 0.0923 | 0.9138 | 0.0011 | 0.9661 | 1.415 |
| 0.1 hBN-B3 | 0.893 | 0.0866 | 0.9097 | 0.001 | 0.9669 | 1.331 |
| 0.2 hBN-B3 | 0.887 | 0.1048 | 0.9079 | 0.0012 | 0.9632 | 1.613 |
| 0.5 hBN-B3 | 0.59 | 0.0951 | 0.8814 | 0.0011 | 0.9487 | 1.476 |
| 1 hBN-B3 | 0.859 | 0.782 | 0.8767 | 0.0009 | 0.948 | 1.214 |
| 2 hBN-B3 | 0.908 | 0.1063 | 0.9268 | 0.0013 | 0.9759 | 1.627 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ensoylu, M.; Deliormanlı, A.M.; Atmaca, H. Preparation, Characterization, and Drug Delivery of Hexagonal Boron Nitride-Borate Bioactive Glass Biomimetic Scaffolds for Bone Tissue Engineering. Biomimetics 2023, 8, 10. https://doi.org/10.3390/biomimetics8010010
Ensoylu M, Deliormanlı AM, Atmaca H. Preparation, Characterization, and Drug Delivery of Hexagonal Boron Nitride-Borate Bioactive Glass Biomimetic Scaffolds for Bone Tissue Engineering. Biomimetics. 2023; 8(1):10. https://doi.org/10.3390/biomimetics8010010
Chicago/Turabian StyleEnsoylu, Mertcan, Aylin M. Deliormanlı, and Harika Atmaca. 2023. "Preparation, Characterization, and Drug Delivery of Hexagonal Boron Nitride-Borate Bioactive Glass Biomimetic Scaffolds for Bone Tissue Engineering" Biomimetics 8, no. 1: 10. https://doi.org/10.3390/biomimetics8010010
APA StyleEnsoylu, M., Deliormanlı, A. M., & Atmaca, H. (2023). Preparation, Characterization, and Drug Delivery of Hexagonal Boron Nitride-Borate Bioactive Glass Biomimetic Scaffolds for Bone Tissue Engineering. Biomimetics, 8(1), 10. https://doi.org/10.3390/biomimetics8010010






