Facile Fabrication of Methyl Gallate Encapsulated Folate ZIF-L Nanoframeworks as a pH Responsive Drug Delivery System for Anti-Biofilm and Anticancer Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals Used
2.2. Synthesis of MG@Folate ZIF-L Nanoframeworks
2.3. Quantification of Drug Loading Capacity
2.4. Physicochemical Characterization
2.5. Drug Release Study
2.6. Artemia salina Acute Toxicity Bioassay
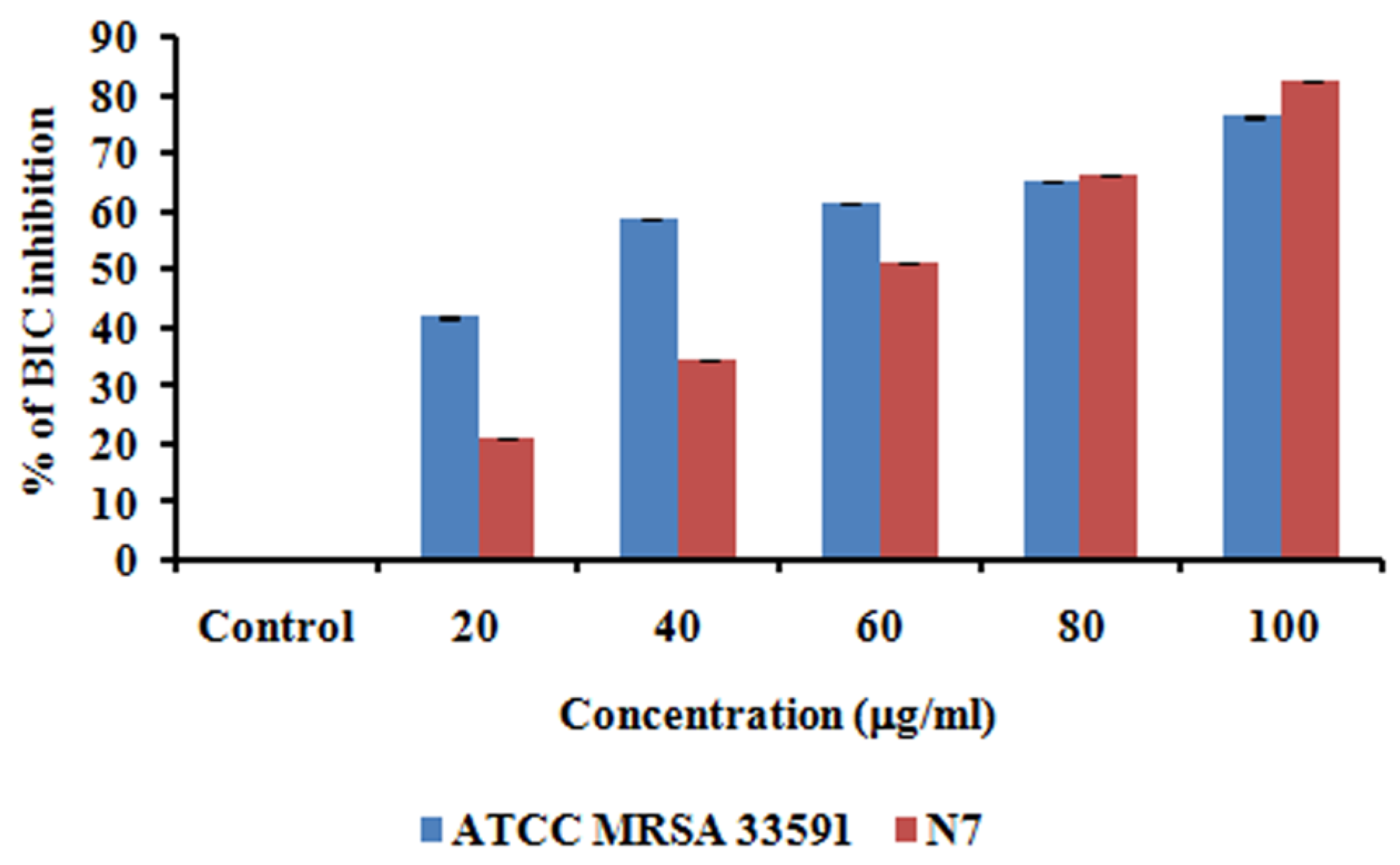
2.7. Anti-Biofilm Activity of MG@Folate ZIF-L Nanoframeworks
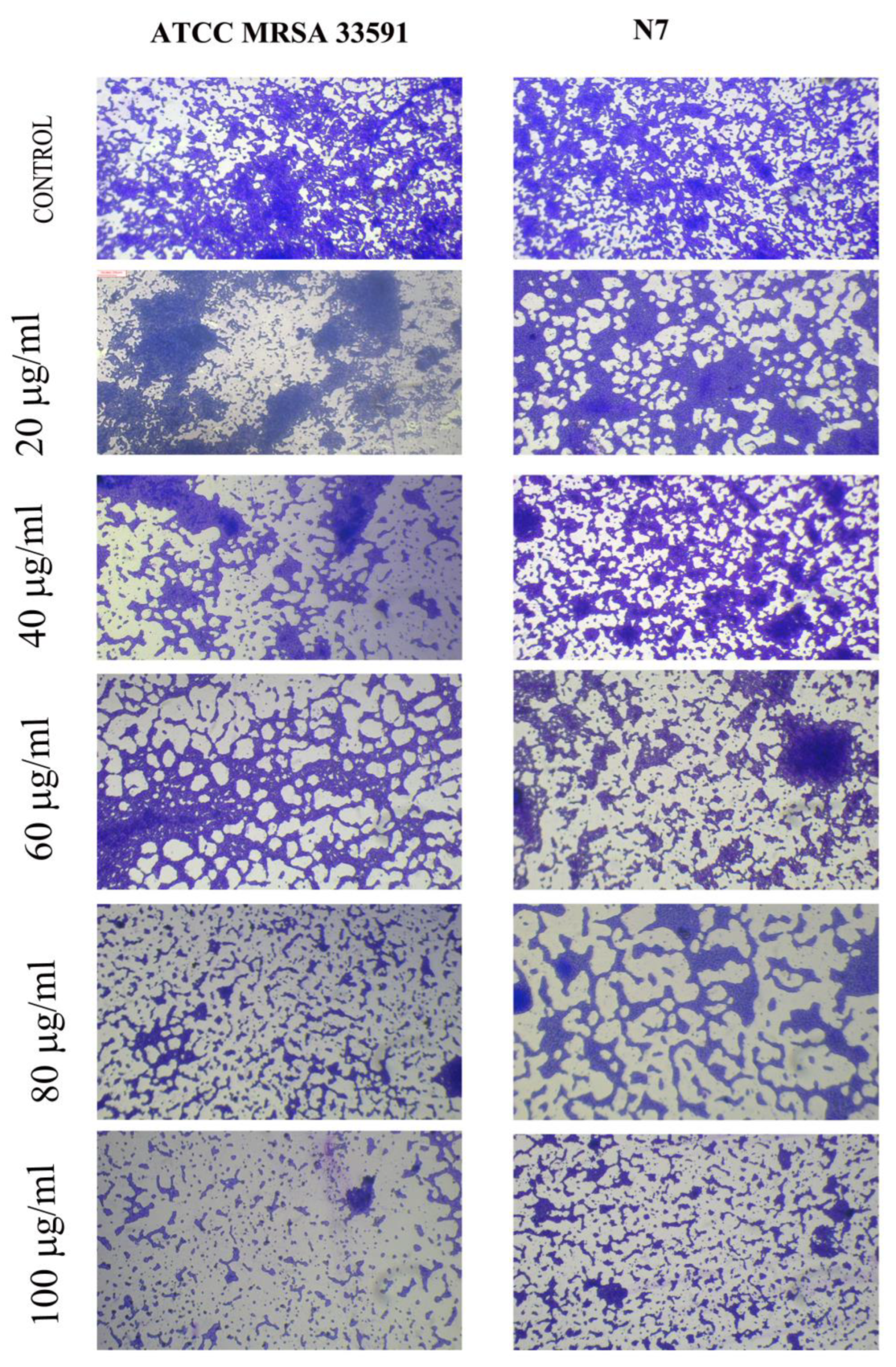
2.8. Microscopic Observation of Bio-Film Morphology
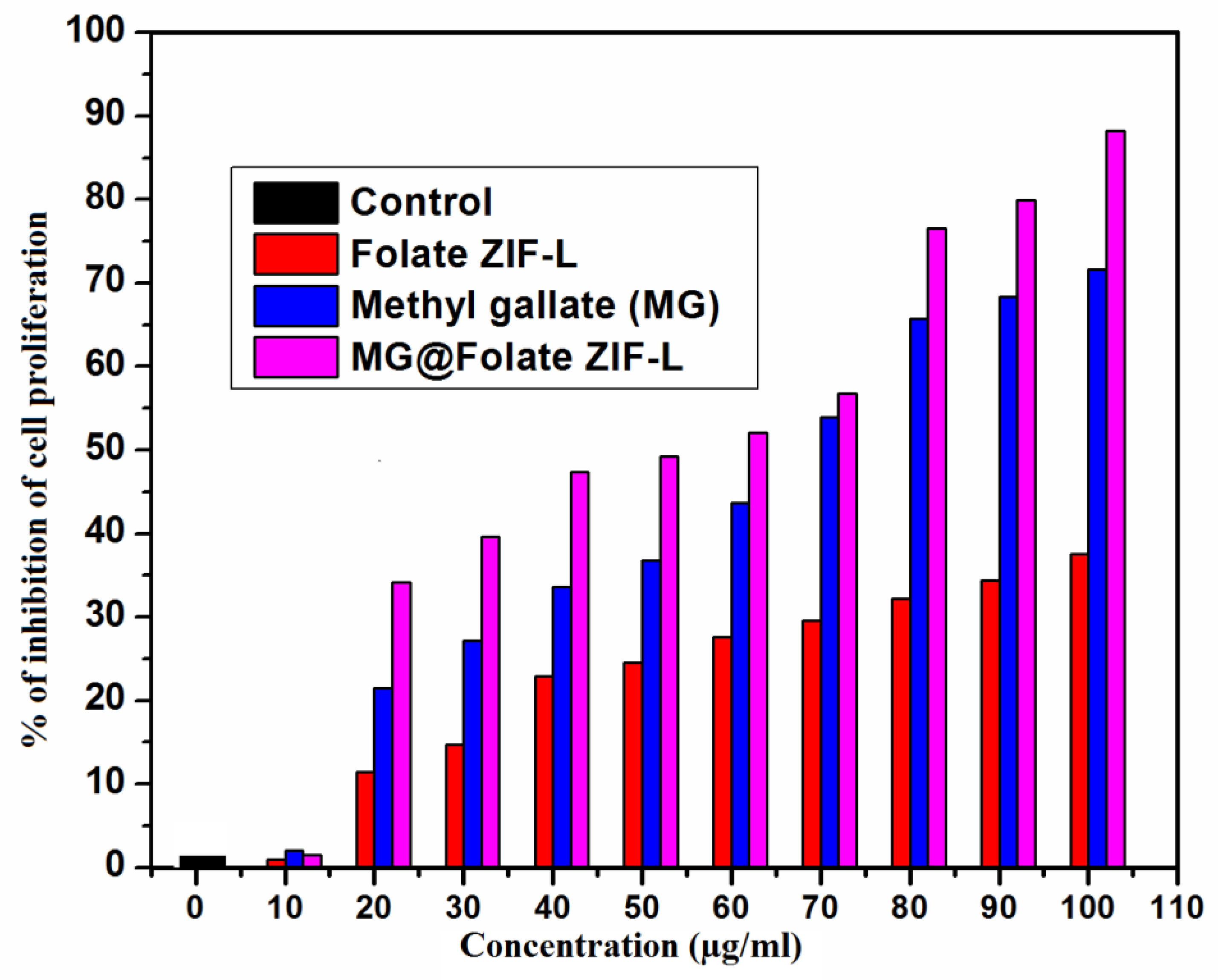
2.9. Assessment of Cytotoxic Effect against Lung Cancer Cells
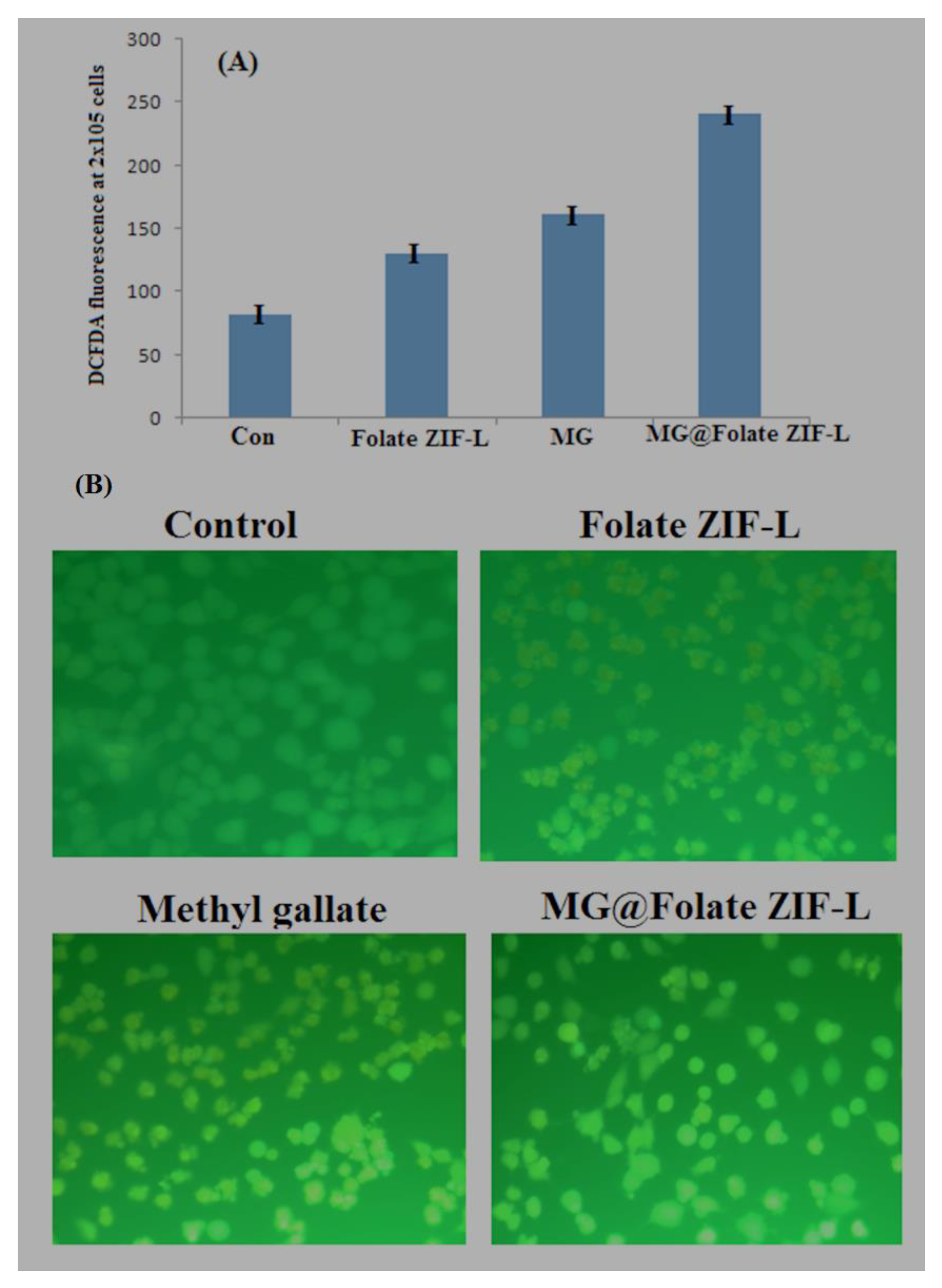
2.10. Assessment of ROS Generation by MG@Folate ZIF-L Treated Cells
2.11. Assessment of Nuclear Damage Using Hoechst Staining
2.12. Statistical Analysis
3. Results and Discussion
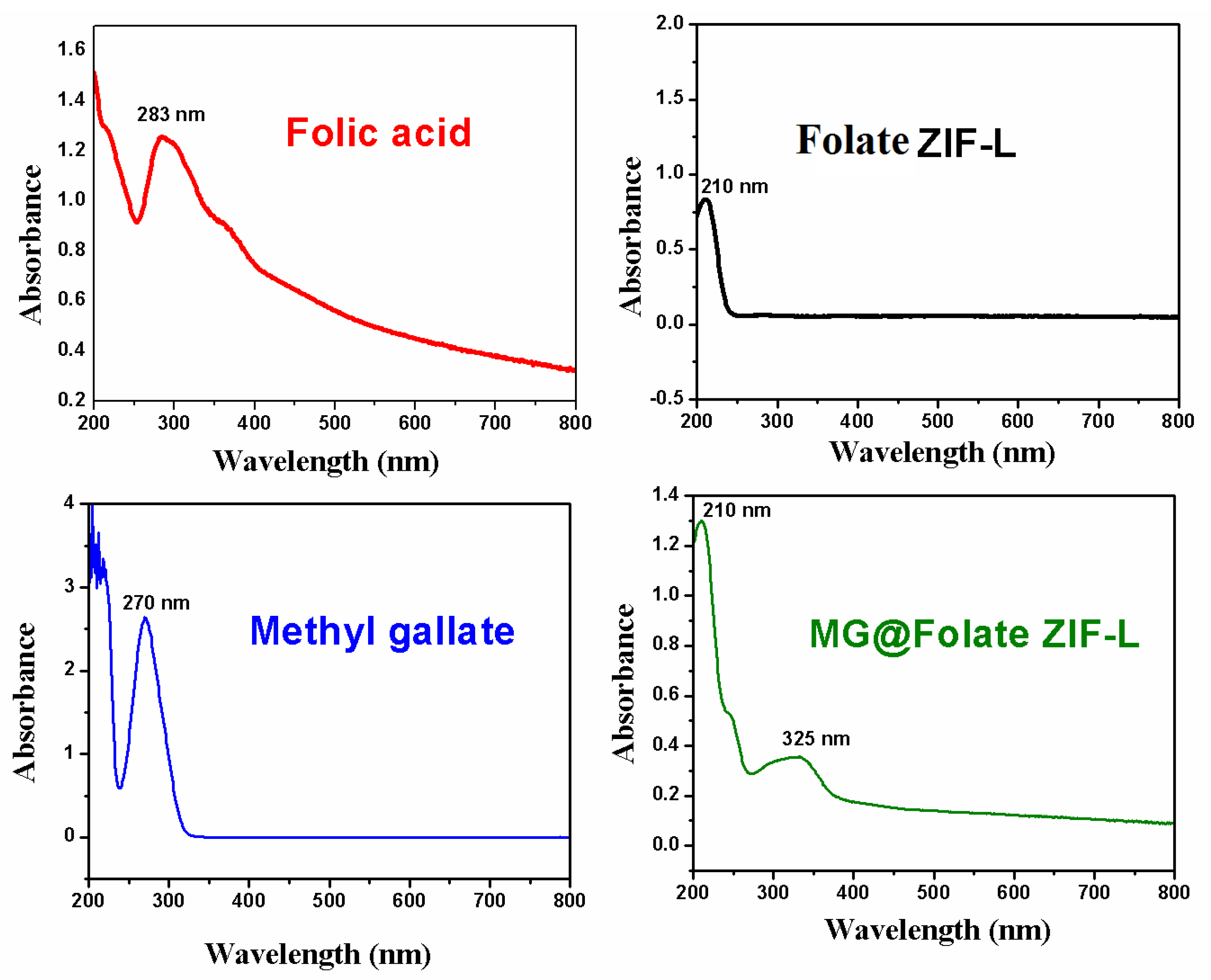
3.1. UV-Vis Spectroscopy Analysis
3.2. Powder XRD Analysis
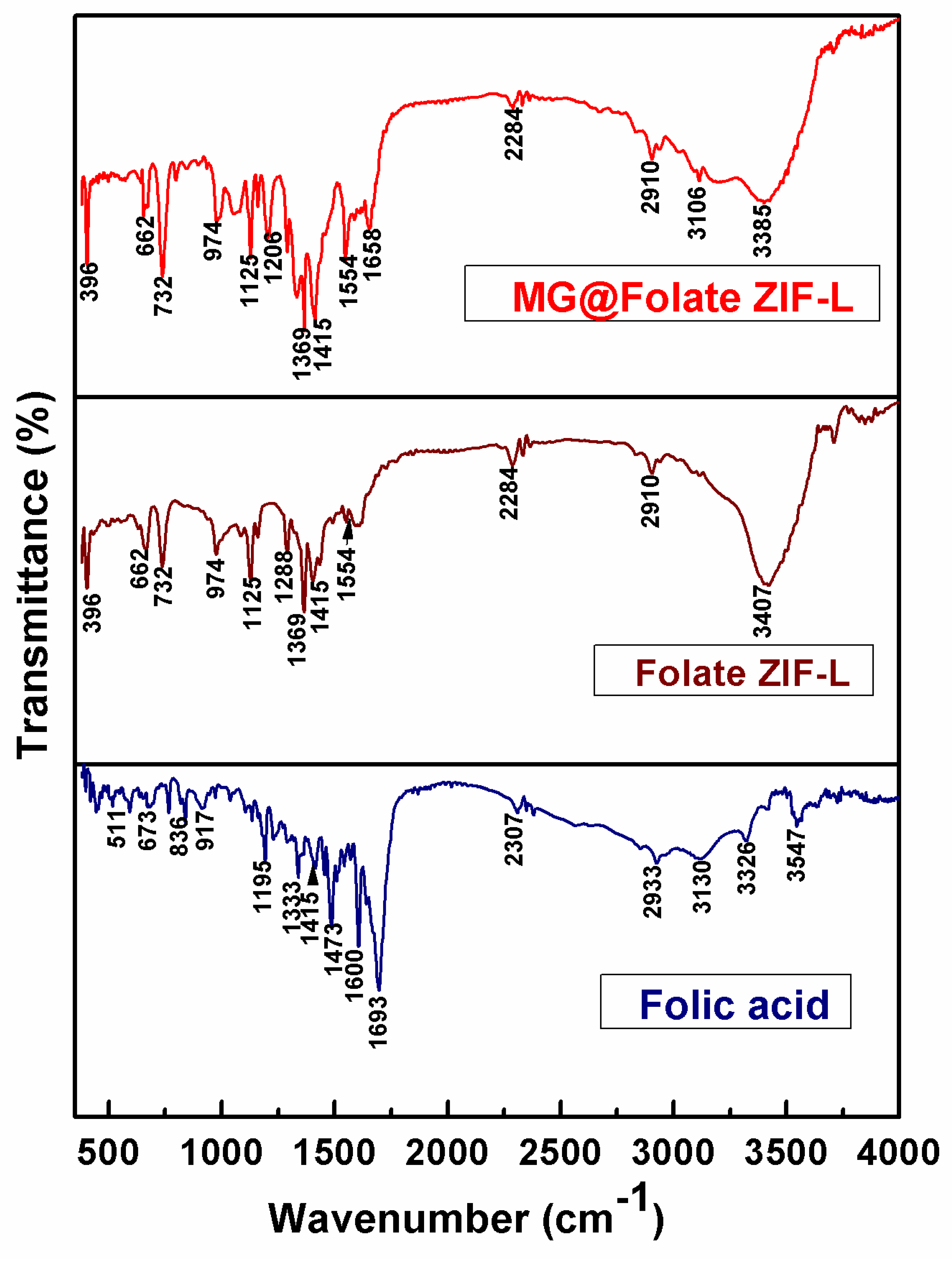
3.3. FTIR Analysis
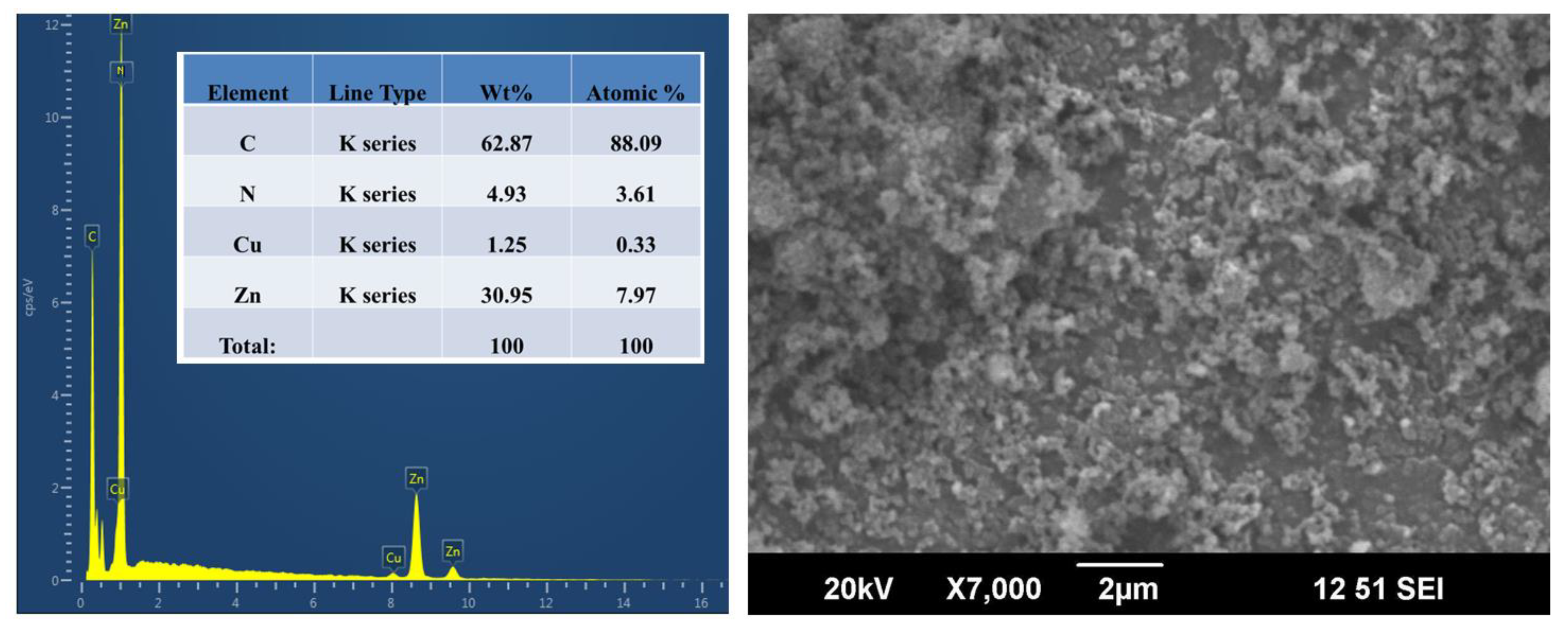
3.4. Chemical Composition and Morphological Analysis
3.5. TEM and DLS Analysis
3.6. pH-Responsive Drug Releasing Mechanism of MG@Folate ZIF-L Nanocomposite
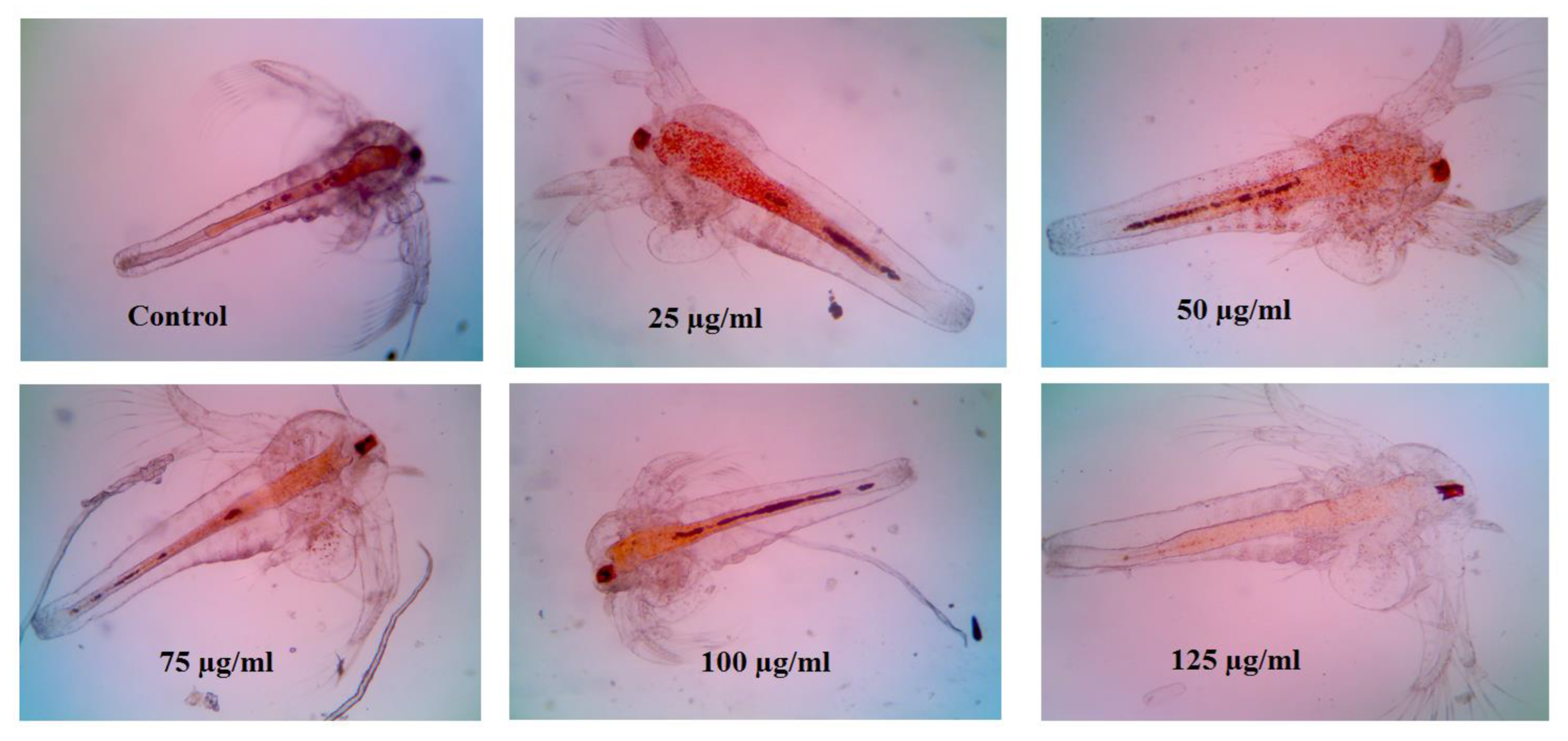
3.7. Cytotoxicity of Folate ZIF-L Nanocomposite on Artemia salina
3.8. Anti-Biofilm Activity of MG@Folate ZIF-L Nanoframeworks
3.9. Microscopic Visualization of MG@Folate ZIF-L Nanoframeworks Treated MRSA and N7 Bio-Films
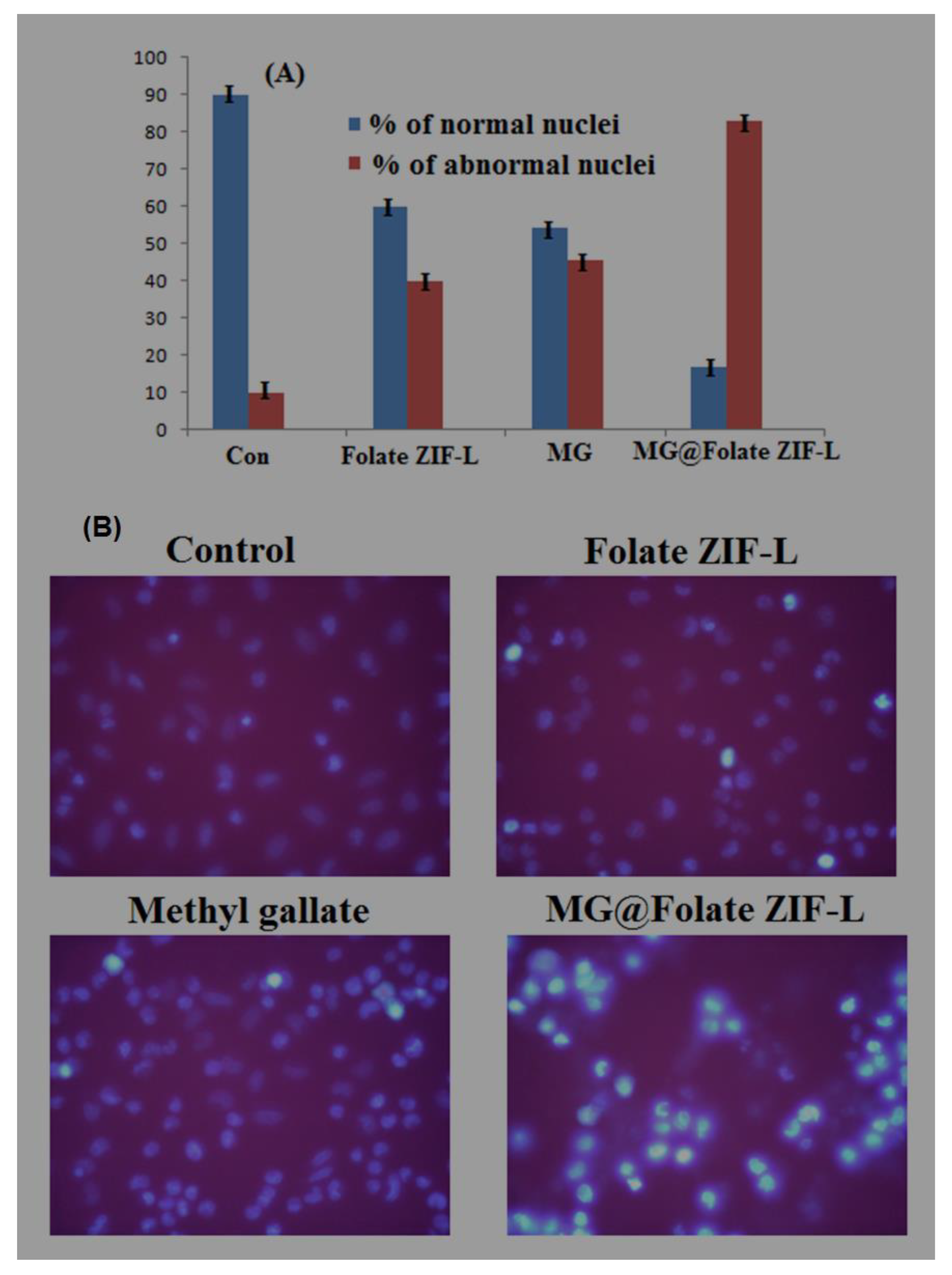
3.10. Anticancer Potential of MG@Folate ZIF-L Nanoframeworks
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, T.; Quan, G.; Niu, B.; Zhou, Y.; Zhao, Y.; Lu, C.; Pan, X.; Wu, C. Versatile nanoscale metal–organic frameworks (nMOFs): An emerging 3D nanoplatform for drug delivery and therapeutic applications. Small 2021, 17, 2005064. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, M.; Wang, J.; Zou, X.; Wang, H.; Wang, D.; Zhou, H.; Yang, L.; Gao, W.; Liang, C. MXene quantum dot/zeolitic imidazolate framework nanocarriers for dual stimulus triggered tumor chemo-phototherapy. Materials 2022, 15, 4543. [Google Scholar] [CrossRef]
- Arkaban, H.; Barani, M.; Akbarizadeh, M.R.; Pal Singh Chauhan, N.; Jadoun, S.; Dehghani Soltani, M.; Zarrintaj, P. Polyacrylic acid nanoplatforms: Antimicrobial, tissue engineering, and cancer theranostic applications. Polymers 2022, 14, 1259. [Google Scholar] [CrossRef] [PubMed]
- Soltani, B.; Nabipour, H.; Nasab, N.A. Efficient storage of gentamicin in nanoscale zeolitic imidazolate framework-8 nanocarrier for pH-responsive drug release. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1090–1097. [Google Scholar] [CrossRef]
- Ilango, K.B.; Gowthaman, S.; Seramaan, K.I.; Chidambaram, K.; Bayan, M.F.; Rahamathulla, M.; Balakumar, C. Mucilage of Coccinia grandis as an efficient natural polymer-based pharmaceutical excipient. Polymers 2022, 14, 215. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Chen, F. pH-responsive drug-delivery systems. Chem. Asian J. 2015, 10, 284–305. [Google Scholar] [CrossRef] [PubMed]
- Bayan, M.F.; Marji, S.M.; Salem, M.S.; Begum, M.Y.; Chidambaram, K.; Chandrasekaran, B. Development of Polymeric-Based Formulation as Potential Smart Colonic Drug Delivery System. Polymers 2022, 14, 3697. [Google Scholar] [CrossRef]
- Prabhu, R.; Anjali, R.; Archunan, G.; Prabhu, N.M.; Pugazhendhi, A.; Suganthy, N. Ecofriendly one pot fabrication of methyl gallate@ ZIF-L nanoscale hybrid as pH responsive drug delivery system for lung cancer therapy. Process Biochem. 2019, 84, 39–52. [Google Scholar]
- Bayan, M.F.; Salem, M.S.; Bayan, R.F. Development and In Vitro Evaluation of a Large-Intestinal Drug Delivery System. Res. J. Pharm. Technol. 2022, 15, 35–39. [Google Scholar] [CrossRef]
- Raju, P.; Natarajan, S. Investigation of Pesticidal and Anti-biofilm Potential of Calotropis gigantea Latex Encapsulated Zeolitic Imidazole Nanoframeworks. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2771–2780. [Google Scholar] [CrossRef]
- Raju, P.; Natarajan, S. Anticancer, anti-biofilm and antimicrobial activity of fucoidan-loaded zeolitic imidazole framework fabricated by one-pot synthesis method. Appl. Nanosci. 2021, 11, 1–19. [Google Scholar] [CrossRef]
- Wolski, P.; Nieszporek, K.; Panczyk, T. Pegylated and folic acid functionalized carbon nanotubes as pH-controlled carriers of doxorubicin. Molecular dynamics analysis of the stability and drug release mechanism. Phys. Chem. Chem. Phys. 2017, 19, 9300–9312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Newell, B.B.; Irudayaraj, J. Folic acid protected silver nanocarriers for targeted drug delivery. J. Biomed. Nanotechnol. 2012, 8, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Narmani, A.; Rezvani, M.; Farhood, B.; Darkhor, P.; Mohammadnejad, J.; Amini, B.; Abdi Goushbolagh, N. Folic acid functionalized nanoparticles as pharmaceutical carriers in drug delivery systems. Drug Dev. Res. 2019, 80, 404–424. [Google Scholar] [CrossRef]
- Dong, S.; Cho, H.J.; Lee, Y.W.; Roman, M. Synthesis and cellular uptake of folic acid-conjugated cellulose nanocrystals for cancer targeting. Biomacromolecules 2014, 15, 1560–1567. [Google Scholar] [CrossRef]
- Bamidele, O.P.; Emmambux, M.N. Encapsulation of bioactive compounds by “extrusion” technologies: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 3100–3118. [Google Scholar] [CrossRef]
- Chin, S.F.; Iyer, K.S.; Saunders, M.; St Pierre, T.G.; Buckley, C.; Paskevicius, M.; Raston, C.L. Encapsulation and sustained release of curcumin using super paramagnetic silica reservoirs. Chem.-A Eur. J. 2009, 15, 5661–5665. [Google Scholar] [CrossRef]
- Vincekovic, M.; Viskic, M.; Juric, S.; Giacometti, J.; Kovacevic, D.B.; Putnik, P.; Jambrak, A.R. Innovative technologies for encapsulation of Mediterranean plants extracts. Trends Food Sci. Technol. 2017, 69, 1–12. [Google Scholar] [CrossRef]
- Kacergius, T.; Abu Lafi, S.; Kirkliauskiene, A.; Gabe, V.; Adawi, A.; Rayan, M.; Rayan, A. Inhibitory capacity of Rhus coriaria L. extract and its major component methyl gallate on Streptococcus mutans biofilm formation by optical profilometry: Potential applications for oral health. Mol. Med. Rep. 2017, 16, 949–956. [Google Scholar] [CrossRef]
- Rodriguez-Perez, C.; Quirantes-Pine, R.; Amessis-Ouchemoukh, N.; Madani, K.; Segura-Carretero, A.; Fernandez-Gutierrez, A. A metabolite-profiling approach allows the identification of new compounds from Pistacia lentiscus leaves. J. Pharm. Biomed. Anal. 2013, 77, 167–174. [Google Scholar] [CrossRef]
- Kosuru, R.Y.; Roy, A.; Das, S.K.; Bera, S. Gallic acid and gallates in human health and disease: Do mitochondria hold the key to success. Mol. Nutr. Food Res. 2018, 62, 1700699. [Google Scholar] [CrossRef]
- Nagle, D.G.; Ferreira, D.; Zhou, Y.D. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef]
- Chaudhuri, D.; Ghate, N.B.; Singh, S.S.; Mandal, N. Methyl gallate isolated from Spondias pinnata exhibits anticancer activity against human glioblastoma by induction of apoptosis and sustained extracellular signal-regulated kinase 1/2 activation. Pharmacogn. Mag. 2015, 11, 269. [Google Scholar] [PubMed]
- Lee, H.; Lee, H.; Kwon, Y.; Lee, J.H.; Kim, J.; Shin, M.K.; Kin, S.H.; Bae, H. Methyl gallate exhibits potent antitumor activities by inhibiting tumor infiltration of CD4+ CD25+ regulatory T cells. J. Immunol. 2010, 185, 6698–6705. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, G.; Sohn, S.H.; Lee, C.; Kwak, J.W.; Bae, H. Immunotherapy with methyl gallate, an inhibitor of Treg cell migration, enhances the anti-cancer effect of cisplatin therapy. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2016, 20, 261. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.Z.; Satyam, S.M.; Shetty, P.; DSouza, M.R. Methyl gallate attenuates doxorubicin-induced cardiotoxicity in rats by suppressing oxidative stress. Scientifica 2021, 2021, 6694340. [Google Scholar] [CrossRef] [PubMed]
- Raju, P.; Ramalingam, T.; Nooruddin, T.; Natarajan, S. In vitro assessment of antimicrobial, antibiofilm and larvicidal activities of bioactive nickel metal organic framework. J. Drug Deliv. Sci. Technol. 2020, 56, 101560. [Google Scholar] [CrossRef]
- Raju, P.; Balakrishnan, K.; Mishra, M.; Ramasamy, T.; Natarajan, S. Fabrication of pH responsive FU@ Eu-MOF nanoscale metal organic frameworks for lung cancer therapy. J. Drug Deliv. Sci. Technol. 2022, 70, 103223. [Google Scholar] [CrossRef]
- Zheng, H.; Xing, L.; Cao, Y.; Che, S. Coordination bonding-based pH-responsive drug delivery systems. Coord. Chem. Rev. 2013, 257, 1933–1944. [Google Scholar] [CrossRef]
- Milivojevic, D.; Sumonja, N.; Medić, S.; Pavic, A.; Moric, I.; Vasiljevic, B.; Nikodinovic-Runic, J. Biofilm-forming ability and infection potential of Pseudomonas aeruginosa strains isolated from animals and humans. Pathog. Dis. 2018, 76, fty041. [Google Scholar] [CrossRef]
- Rybtke, M.; Hultqvist, L.D.; Givskov, M.; Tolker-Nielsen, T. Pseudomonas aeruginosa biofilm infections: Community structure, antimicrobial tolerance and immune response. J. Mol. Biol. 2015, 427, 3628–3645. [Google Scholar] [CrossRef] [PubMed]
- Vuotto, C.; Donelli, G. Novel treatment strategies for biofilm-based infections. Drugs 2019, 79, 1635–1655. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Shivapriya, P.M.; Gautam, P.K.; Misra, K.; Sahoo, A.K.; Samanta, S.K. A review on basic biology of bacterial biofilm infections and their treatments by nanotechnology-based approaches. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 243–259. [Google Scholar] [CrossRef]
- Banerjee, A.; Qi, J.; Gogoi, R.; Wong, J.; Mitragotri, S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J. Control. Release 2016, 238, 176–185. [Google Scholar] [CrossRef]
- Malhotra, J.; Malvezzi, M.; Negri, E.; La Vecchia, C.; Boffetta, P. Risk factors for lung cancer worldwide. Eur. Respir. J. 2016, 48, 889–902. [Google Scholar] [CrossRef]
- Stapelfeld, C.; Dammann, C.; Maser, E. Sex-specificity in lung cancer risk. Int. J. Cancer 2020, 146, 2376–2382. [Google Scholar] [CrossRef]
- Bayan, M.F. Drug Release Control and Enhancement Using Carriers with Different Concentrations of Capmul® MCM C8. Int. J. Appl. Pharm. 2021, 13, 249–252. [Google Scholar] [CrossRef]
- Torre, L.A.; Siegel, R.L.; Jemal, A. Lung cancer statistics. Lung Cancer Pers. Med. 2016, 893, 1–19. [Google Scholar]
- Chen, P.H.; Bendris, N.; Hsiao, Y.J.; Reis, C.R.; Mettlen, M.; Chen, H.Y.; Schmid, S.L. Crosstalk between CLCb/Dyn1-mediated adaptive clathrin-mediated endocytosis and epidermal growth factor receptor signaling increases metastasis. Dev. Cell 2017, 40, 278–288. [Google Scholar] [CrossRef]
- Bayan, M.F.; Bayan, R.F. Recent advances in mesalamine colonic delivery systems. Future J. Pharm. Sci. 2020, 6, 43. [Google Scholar] [CrossRef]
- Zhang, W.; Pang, Q.; Yan, C.; Wang, Q.; Yang, J.; Yu, S.; Xiao, Z. Induction of PD-L1 expression by epidermal growth factor receptor–mediated signaling in esophageal squamous cell carcinoma. OncoTargets Ther. 2017, 10, 763. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Munoz, J.L.; Rodriguez-Cruz, V.; Greco, S.J.; Ramkissoon, S.H.; Ligon, K.L.; Rameshwar, P. Temozolomide resistance in glioblastoma cells occurs partly through epidermal growth factor receptor-mediated induction of connexin 43. Cell Death Dis. 2014, 5, e1145. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Ko, H.; You, D.G.; Kataoka, K.; Park, J.H. Nanomedicines for reactive oxygen species mediated approach: An emerging paradigm for cancer treatment. Acc. Chem. Res. 2019, 52, 1771–1782. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.K.; Chang, W.T.; Lin, I.L.; Chen, Y.F.; Padalwar, N.B.; Cheng, K.C.; Chiu, C.C. The role of necroptosis in ROS-mediated cancer therapies and its promising applications. Cancers 2020, 12, 2185. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marji, S.M.; Bayan, M.F.; Jaradat, A. Facile Fabrication of Methyl Gallate Encapsulated Folate ZIF-L Nanoframeworks as a pH Responsive Drug Delivery System for Anti-Biofilm and Anticancer Therapy. Biomimetics 2022, 7, 242. https://doi.org/10.3390/biomimetics7040242
Marji SM, Bayan MF, Jaradat A. Facile Fabrication of Methyl Gallate Encapsulated Folate ZIF-L Nanoframeworks as a pH Responsive Drug Delivery System for Anti-Biofilm and Anticancer Therapy. Biomimetics. 2022; 7(4):242. https://doi.org/10.3390/biomimetics7040242
Chicago/Turabian StyleMarji, Saeed M., Mohammad F. Bayan, and Abdolelah Jaradat. 2022. "Facile Fabrication of Methyl Gallate Encapsulated Folate ZIF-L Nanoframeworks as a pH Responsive Drug Delivery System for Anti-Biofilm and Anticancer Therapy" Biomimetics 7, no. 4: 242. https://doi.org/10.3390/biomimetics7040242
APA StyleMarji, S. M., Bayan, M. F., & Jaradat, A. (2022). Facile Fabrication of Methyl Gallate Encapsulated Folate ZIF-L Nanoframeworks as a pH Responsive Drug Delivery System for Anti-Biofilm and Anticancer Therapy. Biomimetics, 7(4), 242. https://doi.org/10.3390/biomimetics7040242






