States of Aggregation and Phase Transformation Behavior of Metallosurfactant Complexes by Hexacyanoferrate(II): Thermodynamic and Kinetic Investigation of ETR in Ionic Liquids and Liposome Vesicles
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Preparation of Reductant/Oxidant
2.2.2. Nature of the Reaction
2.2.3. Liposome Preparation
2.2.4. Kinetic Measurements
3. Results and Discussion
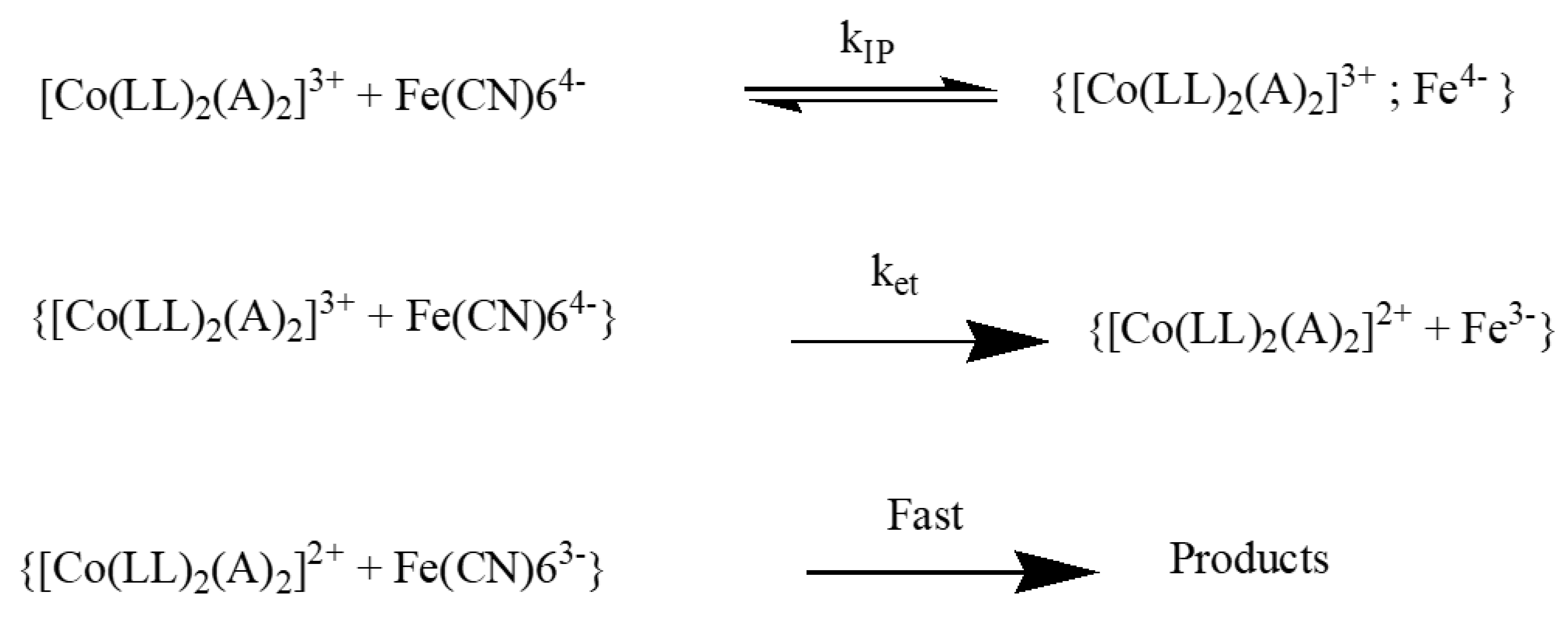
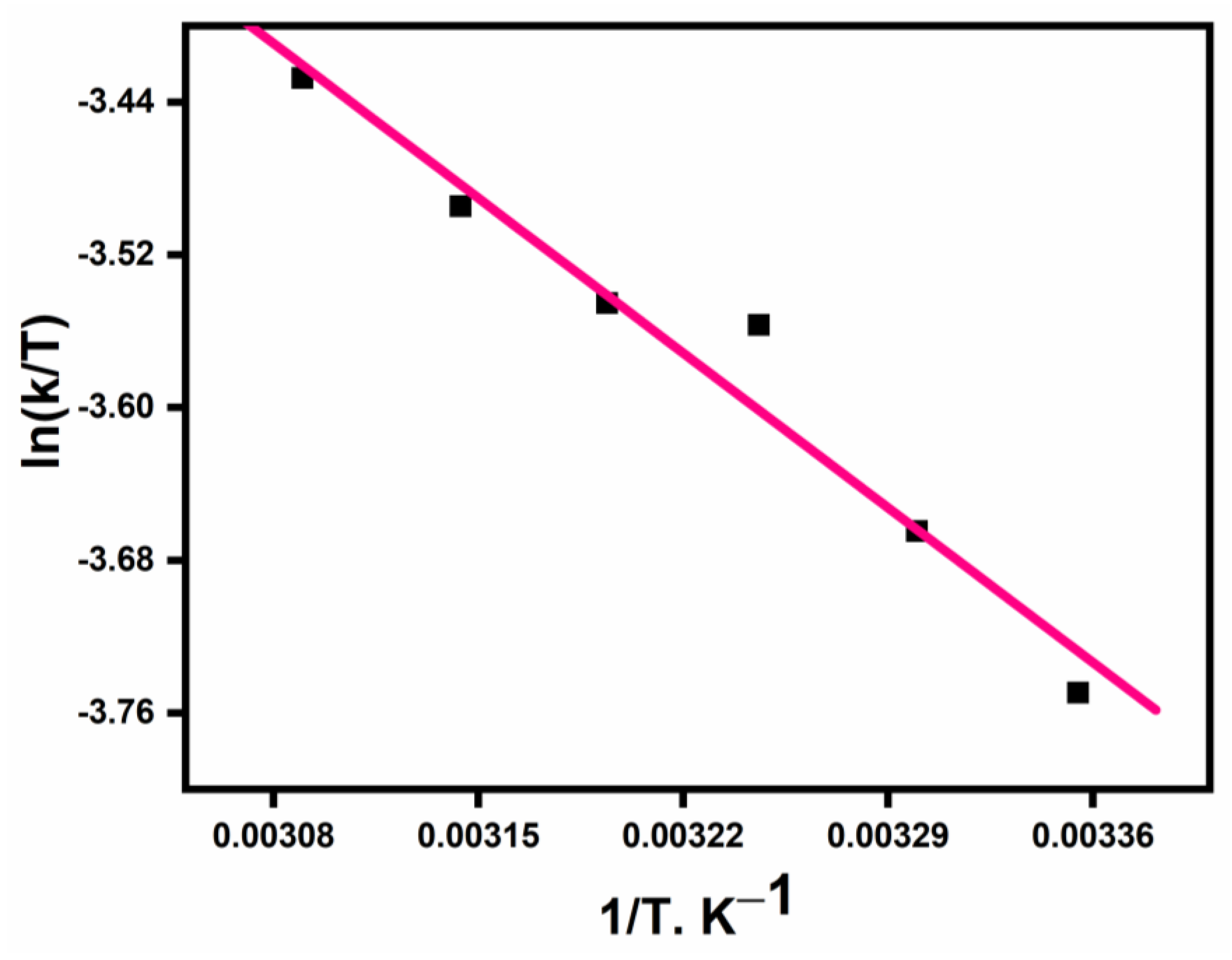
3.1. Kinetics of Outer-Sphere Electron Transfer Reaction
3.2. Effect of Electron Transfer Reaction in Vesicles and Ionic Liquid Media
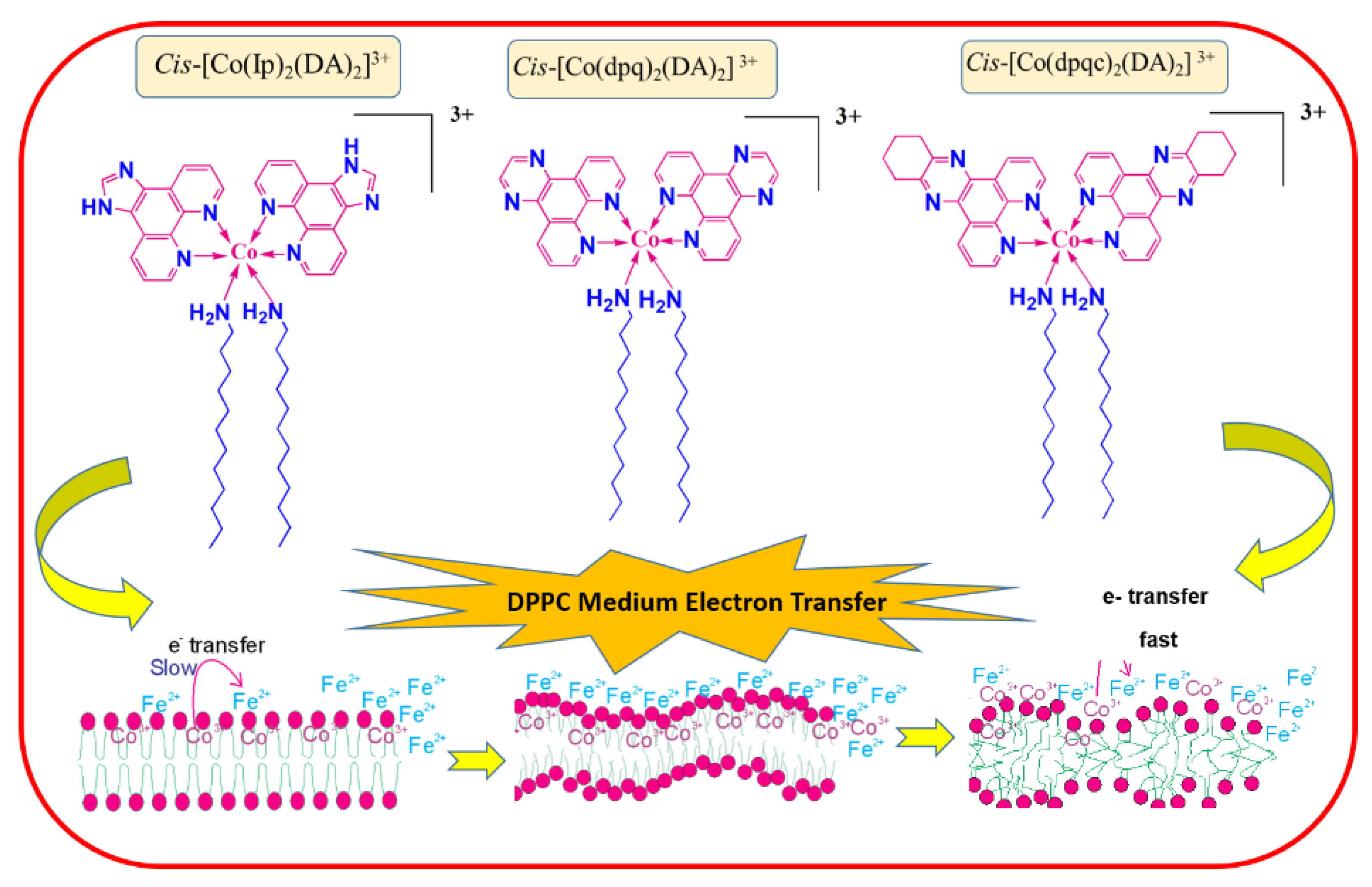
3.2.1. Effect of DPPC
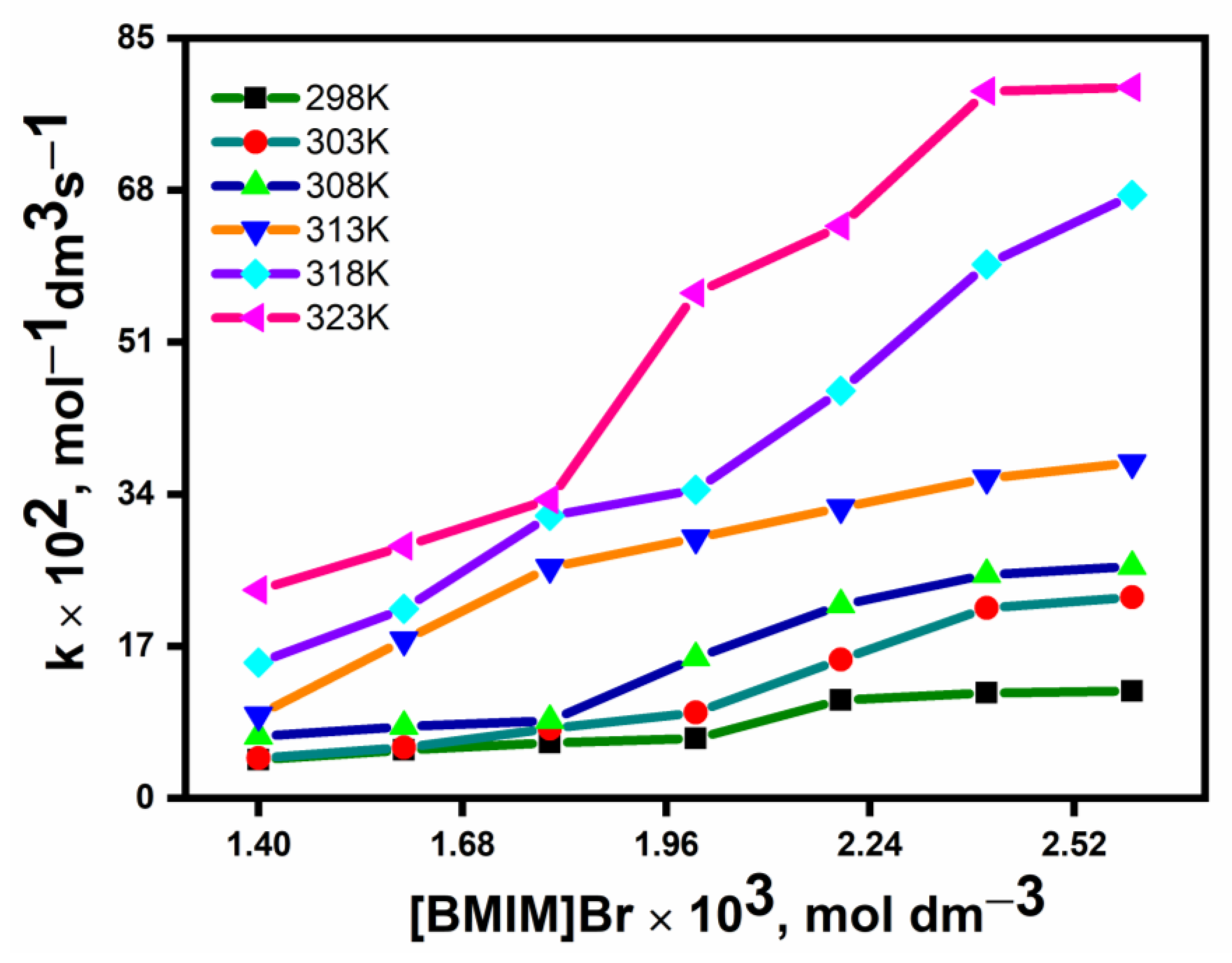
3.2.2. Effect of Ionic Liquids
3.3. Oxidants with Self-Aggregation-Forming Capacity
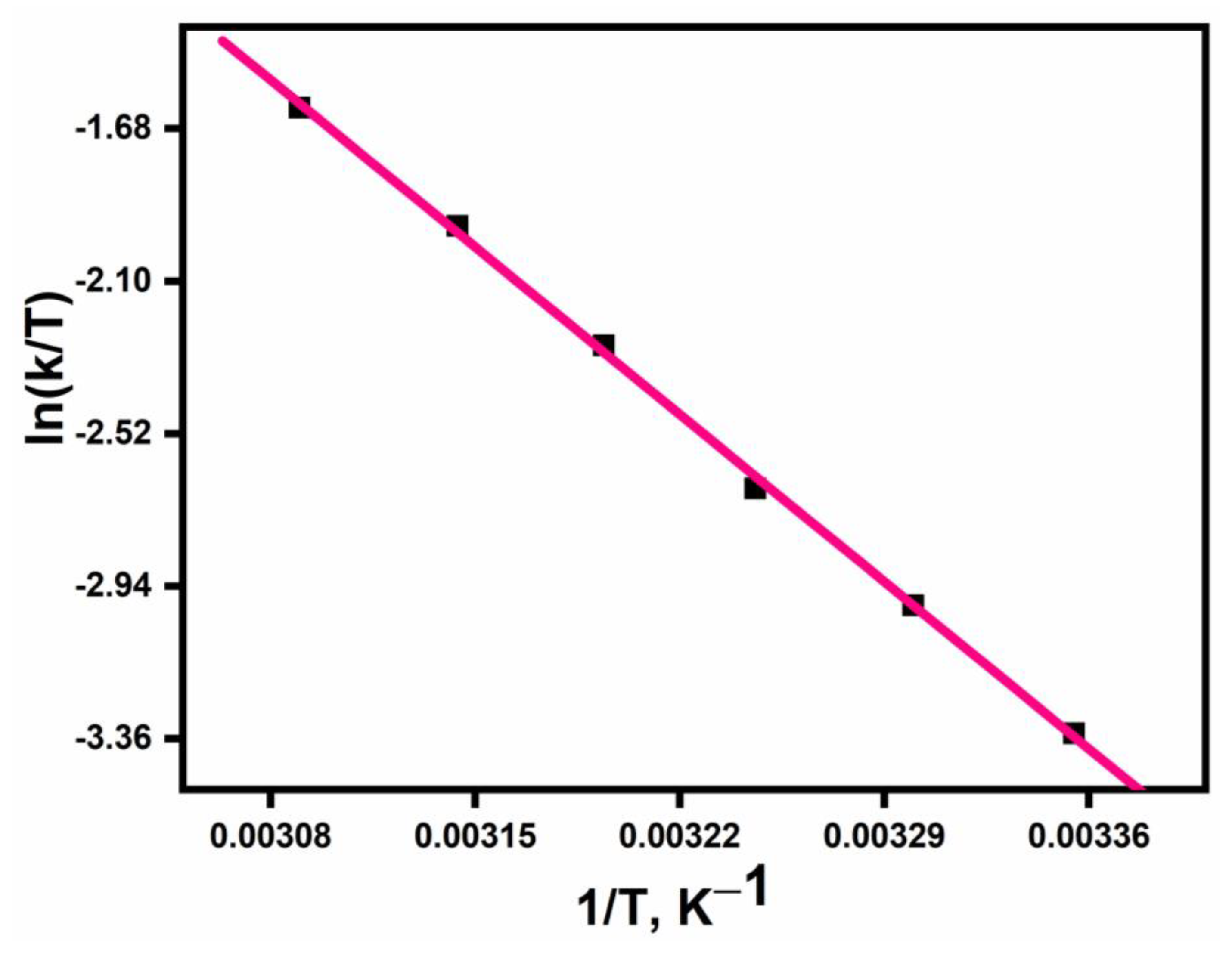
3.4. Activation Parameters
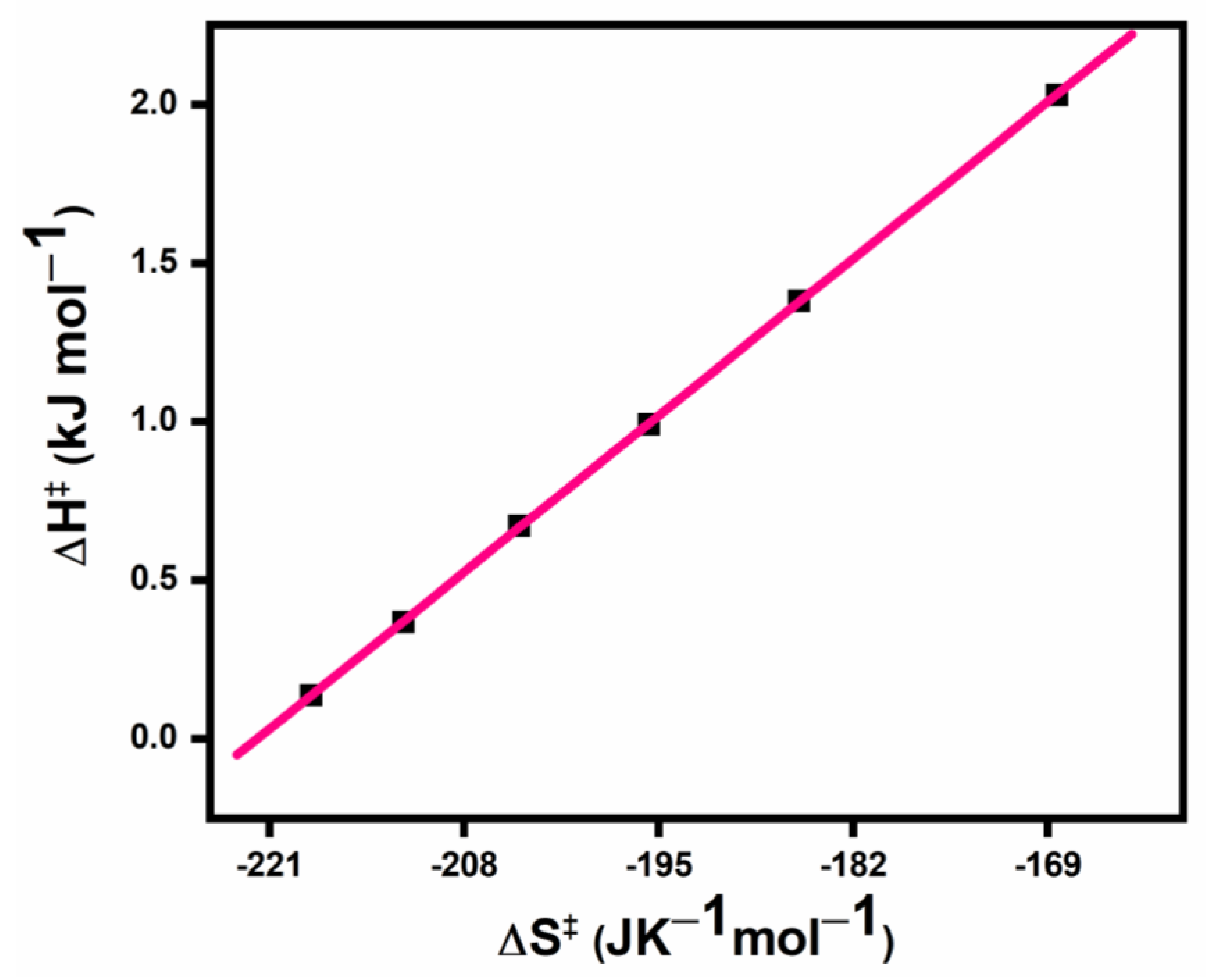
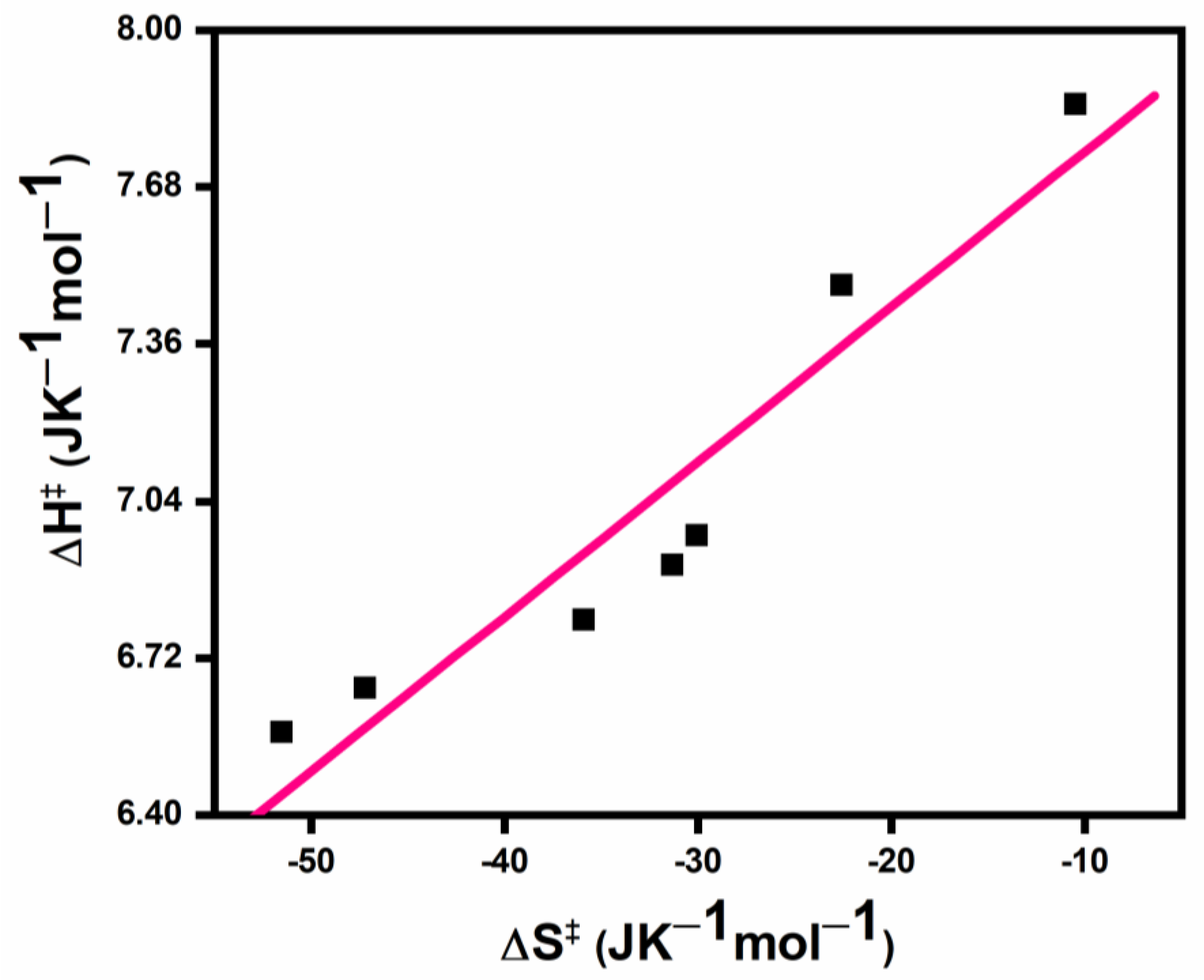
3.5. Isokinetic Plots
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosen, M.J. Surfactants and Interfacial Phenomena; Wiley: New York, NY, USA, 1978. [Google Scholar]
- McBain, J.W.; Heath, D.C. Handbook of Surface and Colloid Chemistry; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Tanford, C. The Hydrophobic Effect: Formation of Micelles and Biological Membranes; Wiley-Interscience: New York, NY, USA, 1973. [Google Scholar]
- Fendler, J.H.; Fendler, E.J. Catalysis in Micellar and Macromolecular Systems; Academic Press: New York, NY, USA, 1975. [Google Scholar]
- Babich, O.A.; Gould, E.S. Electron transfer. Part 148. Reactions of corrin-bound cobalt(III) with s2 metal-ion reducing centers. Inorg. Chim. Acta 2002, 336, 80–86. [Google Scholar] [CrossRef]
- Walker, G.W.; Geue, R.J.; Sargeson, A.M.; Behm, C.A. Surface-active cobalt cage complexes: Synthesis, surface chemistry, biological activity, and redox properties. J. Chem. Soc. Dalton Trans. 2003, 15, 2992. [Google Scholar] [CrossRef]
- Szacilowski, K. Molecular Logic Gates Based on Pentacyanoferrate Complexes: From Simple Gates to Three-Dimensional Logic Systems. Eur. J. 2004, 10, 2520. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, J.; Kirchner, K.; Wherland, S. Extensive inhibition by ion pairing in a bimolecular, outer-sphere electron transfer reaction, reduction of a cobalt clathrochelate by ferrocene in methylene chloride. Inorg. Chim. Acta 2001, 313, 37. [Google Scholar] [CrossRef]
- Gaswick, D.; Haim, A. Direct measurement of a first-order rate constant for an elementary electron transfer step. J. Am. Chem. Soc. 1971, 93, 7347–7348. [Google Scholar] [CrossRef]
- Miralles, A.J.; Szecsy, A.P.; Haim, A. Electron-transfer reactions of ion pairs: Reductions of various substituted pyridinepentaamminecobalt(III) complexes by hexacyanoferrate(II). Inorg. Chem. 1982, 21, 697–699. [Google Scholar] [CrossRef]
- Rillema, P.; Endicott, J.F.; Patel, R.C. Outer-sphere electron-transfer reactions of macrocyclic complexes of cobalt(III). Critical assessment of linear free energy relations. J. Am. Chem. Soc. 1972, 94, 394–401. [Google Scholar] [CrossRef]
- van Eldik, R.; Kelm, H. Concerning the pressure dependence of outer-sphere electron-transfer reactions: The reduction of Co(NH3)5OH3+2 by Fe(CN)4−6 in aqueous acidic solution. Inorg. Chim. Acta. 1982, 73, 91. [Google Scholar] [CrossRef]
- Kustin, K.; Epstein, I.R.; Simoyi, R.H. Systematic design of chemical oscillators. Part 56. Kinetics and mechanism of the oxidation of hexacyanoferrate(II) by aqueous bromine. J. Chem Soc. Dalton Trans. 1990, 971–975. [Google Scholar] [CrossRef]
- Larrson, R. Classification of glyceride crystal forms. Acta. Chem. Scand. 1967, 21, 257. [Google Scholar] [CrossRef]
- Mustafina, A.R.; Shtyrin, V.G.; Zakharova, L.Y.; Skripacheva, V.V.; Zairov, R.R.; Soloreva, S.E.; Antipen, I.S.; Konovalov, A.I. The outer-sphere association of p-sulfonatothiacalix[4]arene with some Co(III) complexes: The effect on their redox activity in aqueous solutions. J. Incl. Phenom. Macrocycl. Chem. 2007, 29, 25. [Google Scholar] [CrossRef]
- Miralles, A.J.; Armstrong, R.E.; Haim, A. The outer-sphere reductions of pyridinepentaamminecobalt(III) and pyridinepentaammineruthenium(III) by hexacyanoferrate(II). J. Am. Chem. Soc. 1977, 99, 1416–1420. [Google Scholar] [CrossRef]
- Holder, A.A.; Dasgupta, T.P. Kinetics and mechanism of the reduction of the molybdatopentaamminecobalt(III) ion by aqueous sulfite and aqueous potassium hexacyanoferrate(II). Inorg. Chim. Acta 2002, 331, 279. [Google Scholar] [CrossRef]
- Szecsy, A.P.; Haim, A.J. Intramolecular electron transfer from pentacyanoferrate(II) to pentaamminecobalt(III) via an imidazolate bridge. The role of distance in inner-sphere reactions. Am. Chem. Soc. 1981, 103, 1679. [Google Scholar]
- Jwo, J.J.; Gaus, P.L.; Haim, A. Intramolecular electron transfer from pentacyanoferrate(II) to pentaamminecobalt(III) mediated by various 4,4′-bipyridines. J. Am. Chem. Soc. 1979, 101, 6189–6197. [Google Scholar] [CrossRef]
- Martinez, M.; Pitarque, M.A.; Eldik, R.V. Outer-sphere redox reactions of (N)5-macrocyclic cobalt(III) complexes. A temperature and pressure dependence kinetic study on the influence of size and geometry of different macrocycles. Inorg. Chim. Acta 1997, 256, 51. [Google Scholar] [CrossRef]
- Tavernier, H.L.; Barzykin, A.V.; Tachiya, M.; Fayer, M.D. Solvent reorganization energy and free energy change for donor/acceptor electron transfer at micelle surfaces: Theory and experiment. J. Phys. Chem. B 1998, 102, 6078–6088. [Google Scholar] [CrossRef]
- Hammarstrom, L.; Norrby, T.; Stenhangen, G.; Martensson, J.; Akermark, B.; Almgren, M.J. Two-dimensional emission quenching and charge separation using a Ru(II)-photosensitizer assembled with membrane-bound acceptors. Phys. Chem. B. 1997, 101, 7494. [Google Scholar] [CrossRef]
- Wang, X.L.; Chao, H.; Li, H.; Hong, X.L.; Liu, Y.J.; Tan, L.F.; Ji, L.N. DNA interactions of cobalt(III) mixed-polypyridyl complexes containing asymmetric ligands. J. Inorg. Biochem. 2004, 98, 1143. [Google Scholar] [CrossRef]
- Ji, L.N.; Zou, X.H.; Liu, J.G. Shape and enantioselective interaction of Ru(II)/Co(III) polypyridyl complexes with DNA. Coord. Chem. Rev. 2001, 513, 216–217. [Google Scholar] [CrossRef]
- Srinivasan, S.; Annaraj, J.; Athappan, P.R. Spectral and redox studies on mixed ligand complexes of cobalt(III) phenanthroline/bipyridyl and benzoylhydrazones, their DNA binding and antimicrobial activity. J. Inorg. Biochem. 2005, 99, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Predo-Gotor, R.; Jiminez, R.; Lopez, P.; Perez, C.; Gomez-Herrera, F.; Sanchez, F. Micellar effects upon the reaction between acetonitrile pentacyanoferrate(II) and Bi s(ethylenediammine)(2-pyrazinecarboxylato)cobalt(III). Langmuir 1998, 14, 1539. [Google Scholar] [CrossRef]
- Cameron, P.J.; Peter, L.M.; Zakeeruddin, S.M.; Gratzel, M. Electrochemical studies of the Co(III)/Co(II)(dbbip) 2 redox couple as a mediator for dye-sensitized nanocrystalline solar cells. Coord. Chem. Rev. 2004, 248, 1447–1453. [Google Scholar] [CrossRef]
- Chonn, A.; Cullis, P.R. Recent advances in liposomal drug-delivery systems. Cur. Opi. Biotech. 1995, 6, 698–708. [Google Scholar] [CrossRef]
- Pignatello, R.; Musumeci, T.; Basile, L.; Carbone, C.; Puglisi, G.J. Biomembrane models and drug-biomembrane interaction studies: Involvement in drug design and development. Pharm. Bioallied Sci. 2011, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Paternostre, M.T.; Roux, M.; Rigaud, J.L. Mechanisms of Membrane Protein Insertion into Liposomes during Reconstitution Procedures Involving the Use of Detergents. 1. Solubilization of Large Unilamellar Liposomes (Prepared by Reverse-Phase Evaporation) by Triton X-100, Octyl Glucoside, and Sodium Cholate. Biochemistry 1998, 27, 2667–2668. [Google Scholar]
- Almog, S.; Litman, B.J.; Wimley, W.; Cohen, J.; Wachtel, E.J.; Barenholz, Y.; Ben-Shaul, A.; Lichtenberg, D. States of Aggregation and Phase Transformations in Mixtures of Phosphatidylcholine and Octyl Glucoside. Biochemistry 1990, 29, 4582–4592. [Google Scholar] [CrossRef]
- Javadian, S.; Ruhi, V.; Heydari, A.; Shahir, A.A.; Yousefi, A.; Akbari, J. Self-Assembled CTAB Nanostructures in Aqueous/Ionic Liquid Systems: Effects of Hydrogen Bonding. Ind. Eng. Chem. Res. 2013, 52, 4517–4526. [Google Scholar] [CrossRef]
- Blesic, M.; Marques, M.H.; Plechkova, N.V.; Seddon, K.R.; Rebelo, L.P.N.; Lopes, A. Self-aggregation of ionic liquids: Micelle formation in aqueous solution. Green Chem. 2007, 9, 481–490. [Google Scholar] [CrossRef]
- Kristin, A.; Siddharth, F.; Pandey, M. Surfactant Aggregation within Room-Temperature Ionic Liquid 1-Ethyl-3-methylimidazolium Bis (trifluoromethylsulfonyl) imide. Langmuir 2004, 20, 33–36. [Google Scholar]
- Nagaraj, K.; Arunachalam, S. Studies on outer-sphere electron transfer reactions of surfactant cobalt(III) complexes with iron(II) in liposome (dipalmitoylphosphotidylcholine) vesicles. Trans. Met. Chem. 2012, 37, 423–429. [Google Scholar] [CrossRef]
- Nagaraj, K.; Arunachalam, S. Synthesis and electron transfer kinetics of a surfactant cobalt(III) complex: Effects of micelles, b-cyclodextrin, and ionic liquids. Trans. Met. Chem. 2013, 38, 649–657. [Google Scholar] [CrossRef]
- Nagaraj, K.; Arunachalam, S. Synthesis, CMC Determination, and Outer Sphere Electron Transfer Reaction of the Surfactant] Complex Ion, cis-[Co(en)2(4CNP)(DA)]3+ with [Fe(CN)6]4− in Micelles, b-cyclodextrin, and Liposome (Dipalmidoylphosphotidylcholine) Vesicles. Aust. J. Chem. 2013, 66, 930–937. [Google Scholar] [CrossRef]
- Nagaraj, K.; Arunachalam, S. Kinetics of reduction of cis-bis(dodecylamine)bis(1,10-phenanthroline)cobalt(III) perchlorate and cis-bis(dodecylamine)bis(2,2′-bipyridine)cobalt(III) perchlorate by Fe(II) in dipalmitoylphosphatidylcholine vesicle. Monatsh. Chem. 2014, 145, 427–433. [Google Scholar] [CrossRef]
- Cannon, R.D.; Gardiner, J.J. Kinetics of electron transfer: The reaction of acetatopenta-amminecobalt(III) with Nmethyliminodiacetatoiron(II). J. Chem. Soc. Dalton. Trans. 1972, 89, 887–890. [Google Scholar] [CrossRef]
- Influence of self-assembly on intercalative DNA binding interaction of double-chain surfactant Co(III) complexes containing imidazo[4,5-f][1,10]phenanthroline and dipyrido [3,2-d:2′-3′-f]quinoxaline ligands: Experimental and theoretical study. Dalton Trans. 2014, 43, 18074–18086. [CrossRef]
- Kipp, E.B.; Haines, R.A. Infrared studies of cis- and trans-bis(halogenoacetato)bis(ethylenediamine)-cobalt(III) complexes. Can. J. Chem. 1969, 47, 1073–1078. [Google Scholar] [CrossRef]
- Morris, M.L.; Busch, D.H. Infrared Spectra Studies on the cis and trans Isomers of Diacidobis-(ethylenediamine)-cobalt(III) Complexes. J. Am. Chem. Soc. 1960, 82, 1521–1526. [Google Scholar] [CrossRef]
- Miyashita, O.; Wolynes, P.G.; Onuchic, J.N. Simple Energy Landscape Model for the Kinetics of Functional Transitions in Proteins. J. Phys. Chem. B 2005, 109, 1959–1969. [Google Scholar] [CrossRef]
- Yamamura, H.; Yamada, S.; Kohno, K.; Okuda, N.; Araki, S.; Kobayashi, K.; Katakai, R.; Kano, K.; Kawai, M. Preparation and guest binding of novel β-cyclodextrin dimers linked with various sulfur-containing linker moieties. J. Chem. Soc. Perkin Trans. 1999, 1, 2943–2948. [Google Scholar] [CrossRef]
- Hak, S.C.; Tooru, O.; Shintrao, S.; Nobukiko, Y. Control of rapid phase transition induced by supramolecular complexation of b-cyclodextrin-conjugated poly (e-lysine) with a specific guest. Macromolecules 2003, 36, 5342. [Google Scholar]
- Ghosh, S.; Barve, A.C.; Kumbhar, A.A.; Kumbhar, A.S.; Puranik, V.G.; Datar, P.A.; Sonawane, U.B.; Joshi, R.R. Synthesis, characterization, X-ray structure and DNA photocleavage by cis-dichloro bis(diimine) Co(III) complexes. J. Inorg. Biochem. 2006, 100, 331–343. [Google Scholar] [CrossRef] [PubMed]
- New RRC 1990 Liposomes a Practical Approach; Oxford University Press: London, UK, 1990.
- Subuddhi, U.; Mishra, A.K. Prototropism of 1-hydroxypyrene in liposome suspensions: Implications towards fluorescence probing of lipid bilayers in alkaline medium. Photochem. Photobiol. Sci. 2006, 5, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Leonenko, Z.V.; Finot, E.; Ma, H.; Dahms, T.E.S.; Cramb, D.T. Investigation of temperature-induced phase transitions in DOPC and DPPC phospholipid bilayers using temperature-controlled scanning force microscopy. Biophys. J. 2004, 86, 3783–3793. [Google Scholar] [CrossRef] [PubMed]
- Tumuli, M.S.; Fondler, J.H. Aspects of artificial photosynthesis. Photosensitized electron transfer across bilayers, charge separation, and hydrogen production in anionic surfactant vesicles. J. Am. Chem. Soc. 1981, 103, 2507–2513. [Google Scholar] [CrossRef]
- Batzri, S.; Korn, E.D. Single bilayer liposomes prepared without sonication. Biochim. Biophys. Acta. 1973, 298, 1015–1019. [Google Scholar] [CrossRef]
- Benson, P.; Haim, A. Preparation and Reaction with Nucleophiles. J. Am. Chem. Soc. 1965, 87, 3656–3691. [Google Scholar]
- Arulsamy, N.; Bohle, D.S.; Goodson, P.A.; Jaeger, D.A.; Reddy, V.B. Synthesis, Structure, and Stereochemistry of Double-Chain Surfactant Co(III) Complexes. Inorg. Chem. 2001, 40, 836–842. [Google Scholar] [CrossRef]
- Ismail, A.M. Kinetics and mechanism of amine-catalysed solvolysis of azlactone in water-dioxan mixtures. Indian J. Chem. 2008, 47, 49. [Google Scholar]


| [DPPC] × 105 (mol dm−3) | k × 102, dm3 mol−1 s−1 | |||||
|---|---|---|---|---|---|---|
| 298 K | 303 K | 308 K | 323 K | 328 K | 333 K | |
| 2.0 | 15.0 | 15.2 | 15.5 | 16.4 | 16.8 | 17.3 |
| 3.0 | 14.7 | 15.0 | 15.4 | 17.0 | 17.5 | 18.0 |
| 4.0 | 14.0 | 14.4 | 15.0 | 17.2 | 17.8 | 18.2 |
| 5.0 | 13.5 | 13.9 | 14.1 | 17.7 | 18.3 | 18.7 |
| 6.0 | 12.7 | 13.0 | 13.4 | 18.3 | 18.6 | 19.1 |
| 7.0 | 11.3 | 11.7 | 12.5 | 18.7 | 19.1 | 19.5 |
| [(BMIM)Br] × 103, mol dm−3 | k × 102, dm−3 mol−1 s−1 | |||||
|---|---|---|---|---|---|---|
| 298 K | 303 K | 308 K | 313 K | 318 K | 323 K | |
| 1.4 | 4.0 | 4.2 | 6.5 | 8.9 | 14.7 | 23.0 |
| 1.6 | 5.2 | 5.5 | 7.7 | 12.0 | 20.6 | 27.4 |
| 1.8 | 6.1 | 7.4 | 8.5 | 25.5 | 31.5 | 33.7 |
| 2.0 | 6.5 | 9.4 | 15.5 | 28.6 | 34.8 | 55.4 |
| 2.2 | 10.5 | 15.2 | 21.3 | 32.1 | 45.3 | 63.7 |
| 2.4 | 11.4 | 20.2 | 24.2 | 35.2 | 58.2 | 77.2 |
| 2.6 | 11.6 | 21.2 | 25.6 | 36.2 | 59.6 | 80.4 |
| [DPPC] × 105 (mol dm−3) | ∆H‡ | −∆S‡ |
|---|---|---|
| 2.0 | 0.14 | 218.2 |
| 3.0 | 0.37 | 212.1 |
| 4.0 | 0.67 | 204.3 |
| 5.0 | 0.99 | 195.7 |
| 6.0 | 1.38 | 185.6 |
| 7.0 | 2.03 | 168.4 |
| [(BMIM)Br]× 103, mol dm−3 | ∆H‡ | −∆S‡ |
|---|---|---|
| 1.4 | 6.62 | 50.2 |
| 1.6 | 6.56 | 49.7 |
| 1.8 | 6.57 | 42.1 |
| 2.0 | 6.67 | 35.0 |
| 2.2 | 6.79 | 34.7 |
| 2.4 | 7.26 | 24.2 |
| 2.6 | 7.96 | 7.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagaraj, K.; Sakthinathan, S.; Chiu, T.-W.; Kamalesu, S.; Lokhandwala, S.; Parekh, N.M.; Karuppiah, C. States of Aggregation and Phase Transformation Behavior of Metallosurfactant Complexes by Hexacyanoferrate(II): Thermodynamic and Kinetic Investigation of ETR in Ionic Liquids and Liposome Vesicles. Biomimetics 2022, 7, 221. https://doi.org/10.3390/biomimetics7040221
Nagaraj K, Sakthinathan S, Chiu T-W, Kamalesu S, Lokhandwala S, Parekh NM, Karuppiah C. States of Aggregation and Phase Transformation Behavior of Metallosurfactant Complexes by Hexacyanoferrate(II): Thermodynamic and Kinetic Investigation of ETR in Ionic Liquids and Liposome Vesicles. Biomimetics. 2022; 7(4):221. https://doi.org/10.3390/biomimetics7040221
Chicago/Turabian StyleNagaraj, Karuppiah, Subramanian Sakthinathan, Te-Wei Chiu, Subramaniam Kamalesu, Snehal Lokhandwala, Nikhil M. Parekh, and Chelladurai Karuppiah. 2022. "States of Aggregation and Phase Transformation Behavior of Metallosurfactant Complexes by Hexacyanoferrate(II): Thermodynamic and Kinetic Investigation of ETR in Ionic Liquids and Liposome Vesicles" Biomimetics 7, no. 4: 221. https://doi.org/10.3390/biomimetics7040221
APA StyleNagaraj, K., Sakthinathan, S., Chiu, T.-W., Kamalesu, S., Lokhandwala, S., Parekh, N. M., & Karuppiah, C. (2022). States of Aggregation and Phase Transformation Behavior of Metallosurfactant Complexes by Hexacyanoferrate(II): Thermodynamic and Kinetic Investigation of ETR in Ionic Liquids and Liposome Vesicles. Biomimetics, 7(4), 221. https://doi.org/10.3390/biomimetics7040221











