Itraconazole and Difluorinated-Curcumin Containing Chitosan Nanoparticle Loaded Hydrogel for Amelioration of Onychomycosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solubility Analysis and Analytical Method Development
2.3. Preparation of Chitosan Nanoparticles by Ionotropic Gelation Technique
2.4. Evaluation of Physiochemical Properties of Drug-Loaded Chitosan Nanoparticles
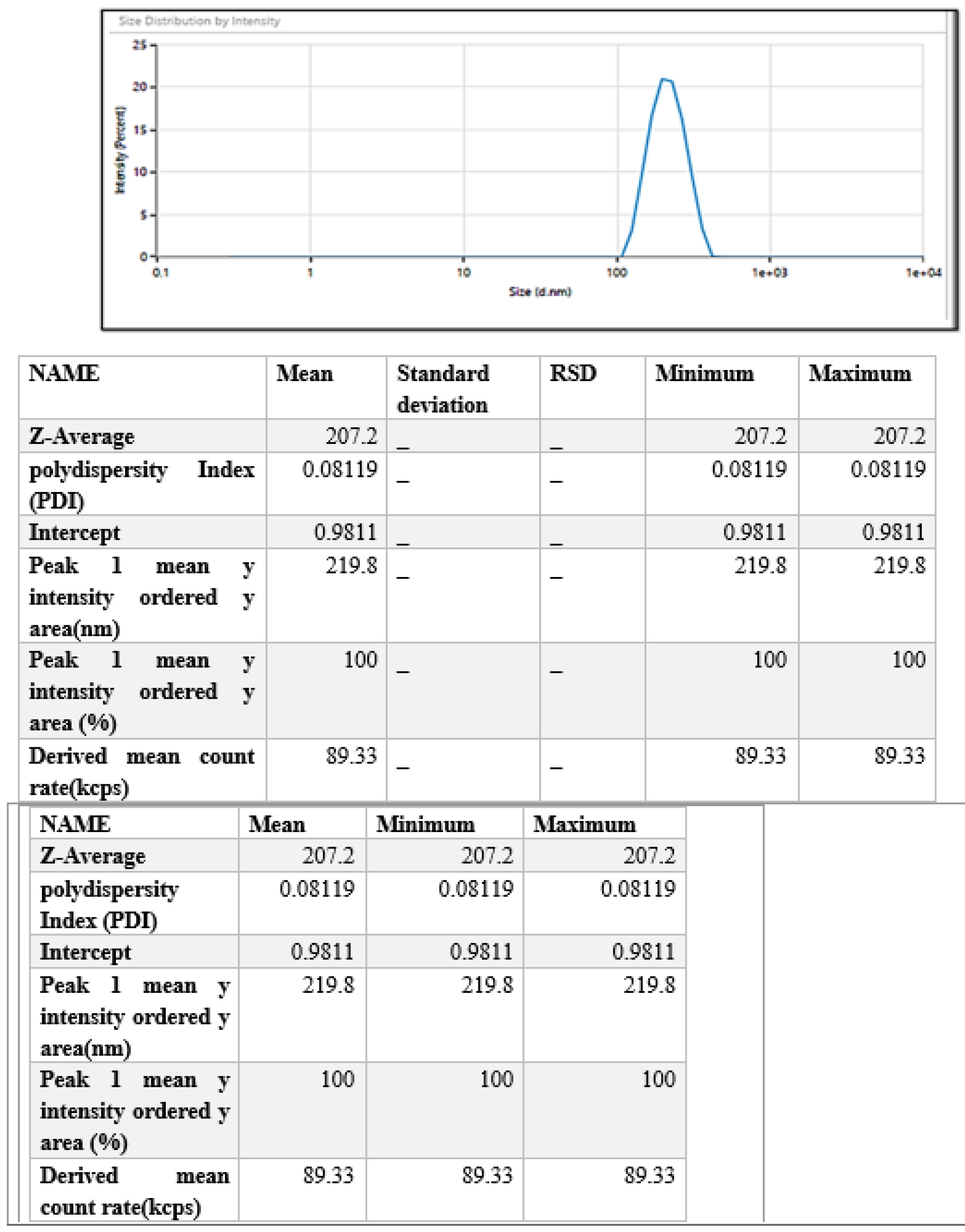
2.4.1. Particle Size, Polydispersity Index (PDI), and Zeta Potential
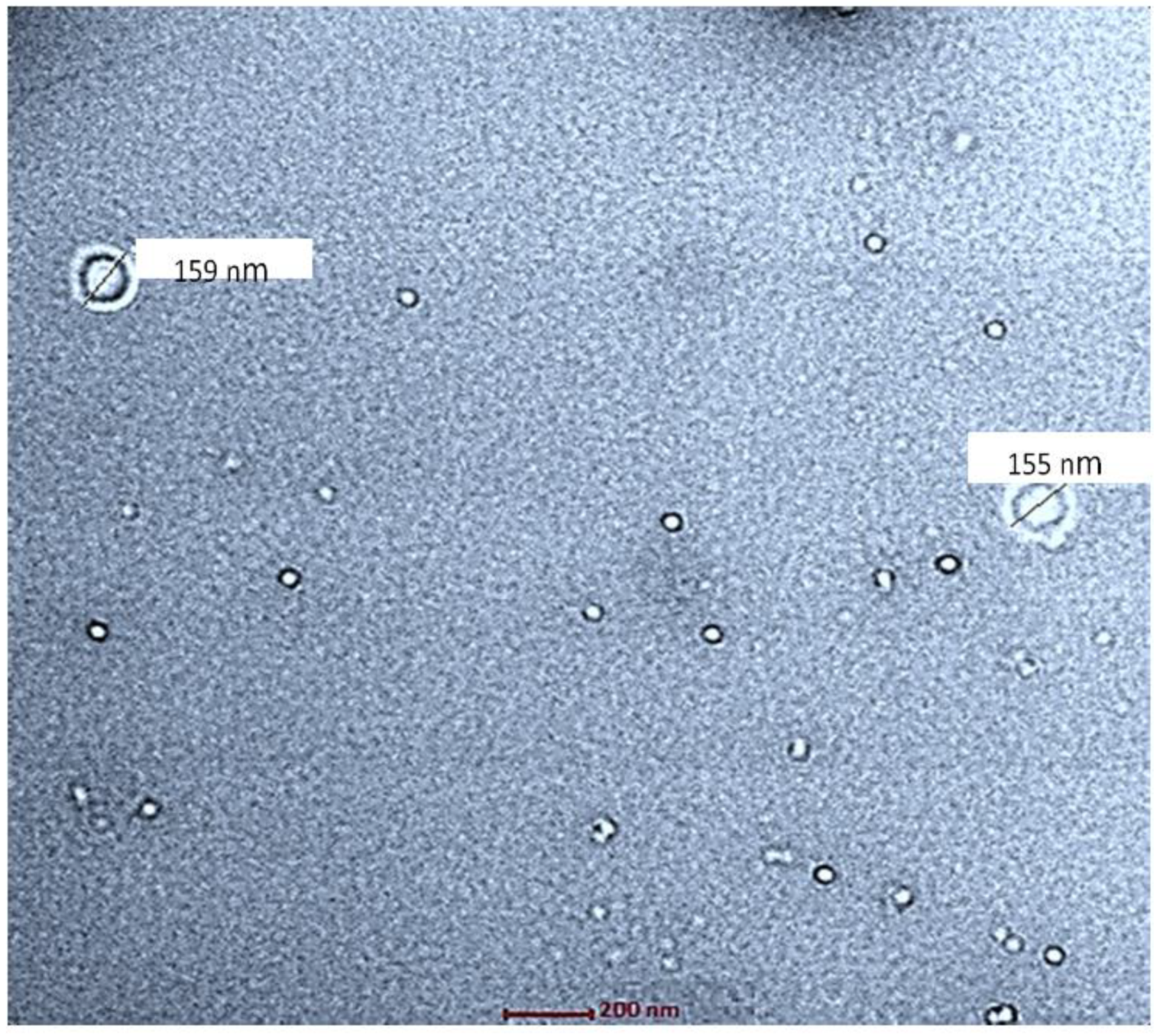
2.4.2. Morphological Examination via Transmission Electron Microscopy
2.4.3. Entrapment Efficiency and Drug Loading Determination
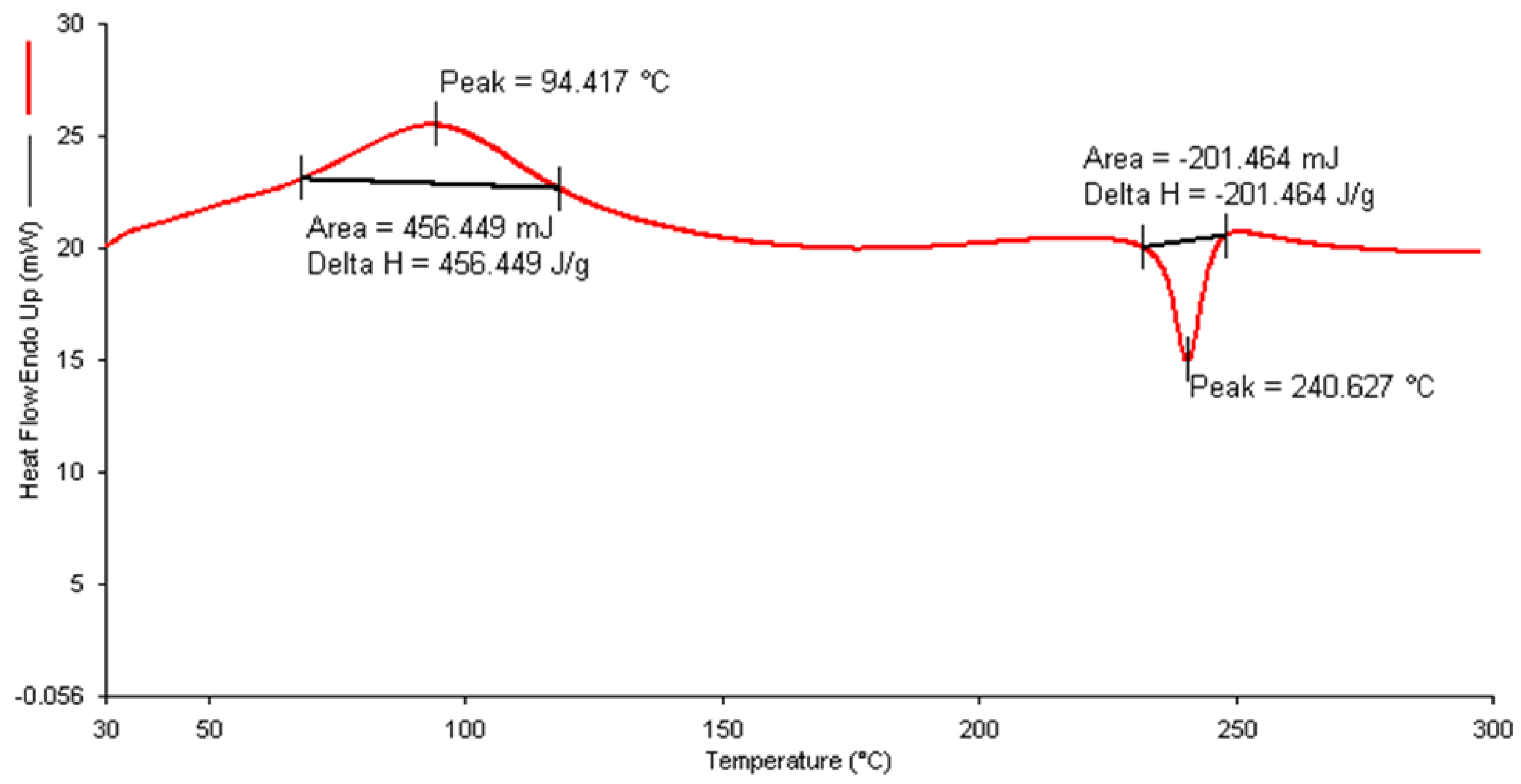
2.4.4. Differential Scanning Calorimetry (DSC)
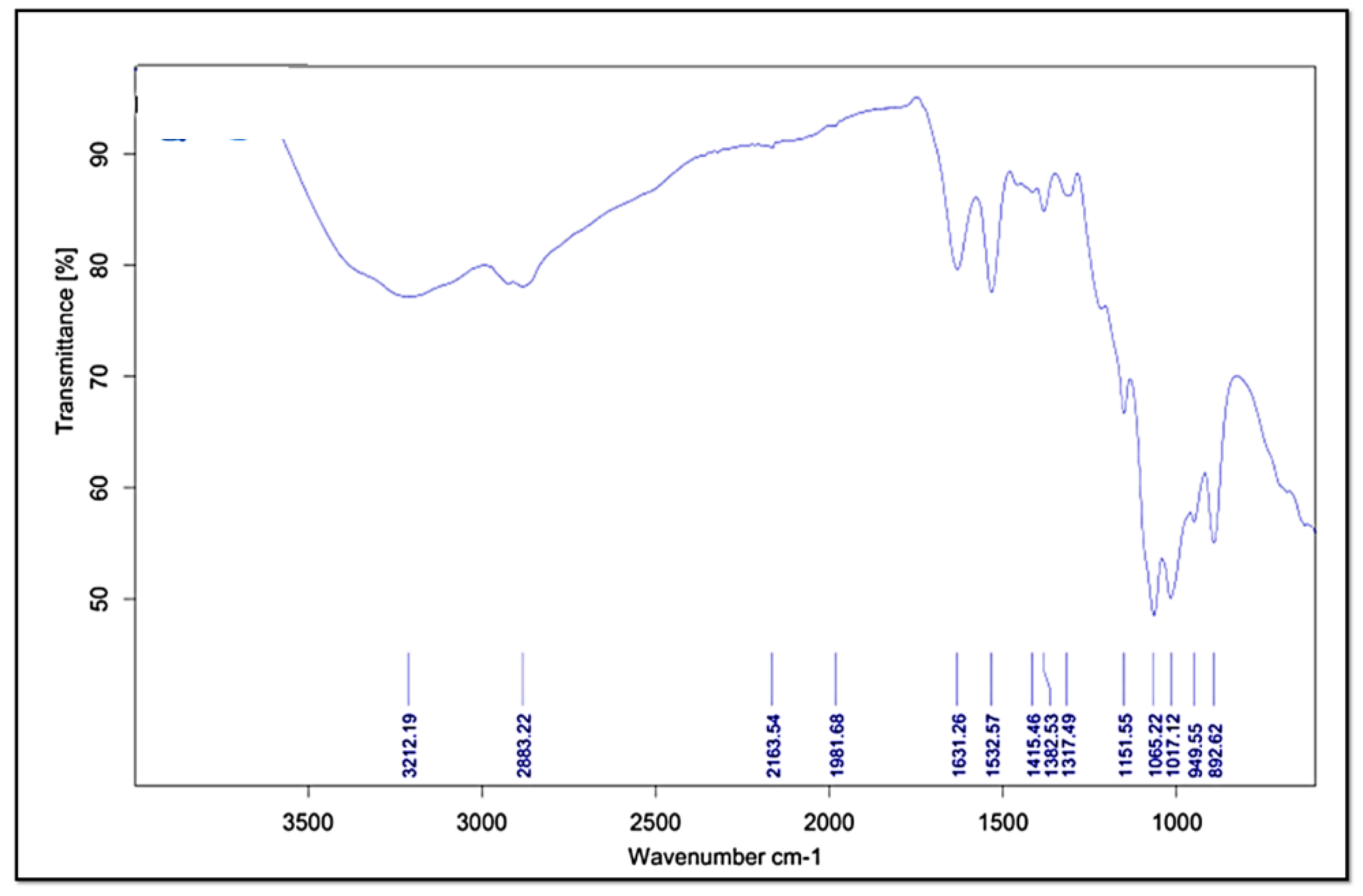
2.4.5. Fourier Transform Infrared Spectroscopy (FT-IR)
2.5. Preparation of ITZ-CDF/CH NPs Loaded Hydrogel
2.6. Evaluation of Physiochemical Properties of Hydrogel
2.6.1. pH Determination
2.6.2. Spreadability Study
2.6.3. Extrudability Study
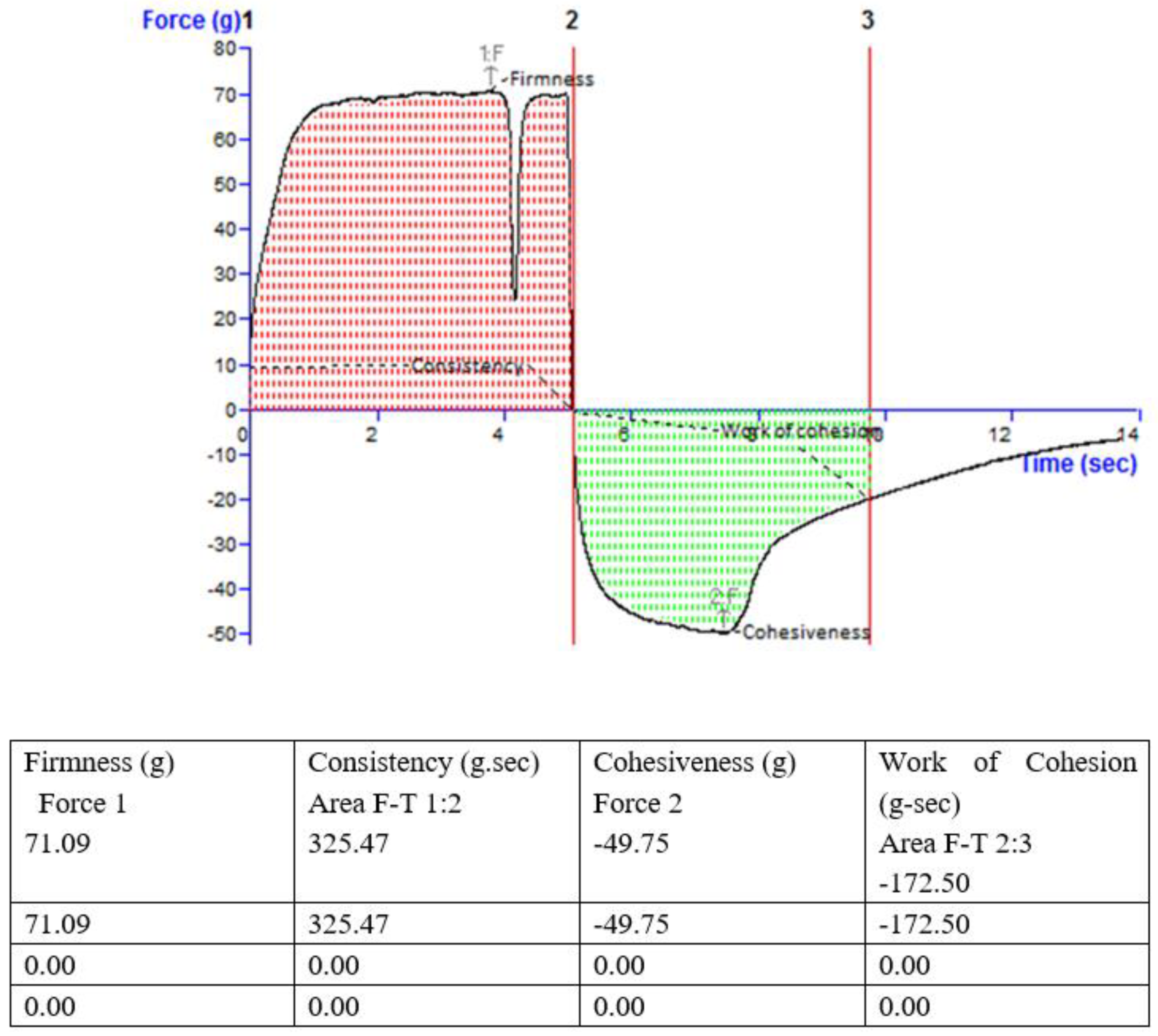
2.6.4. Viscosity and Texture
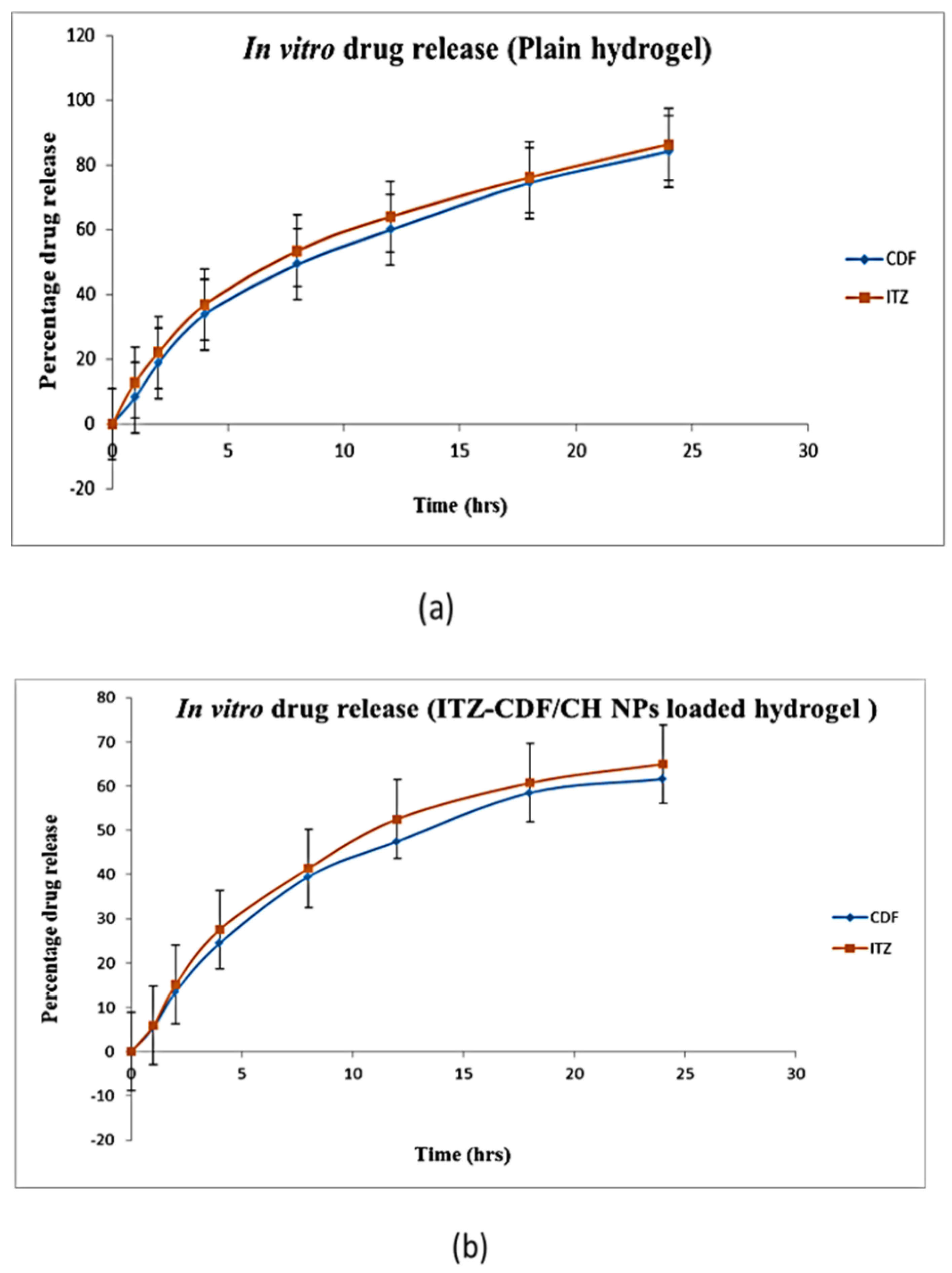
2.7. In Vitro Drug Release Study
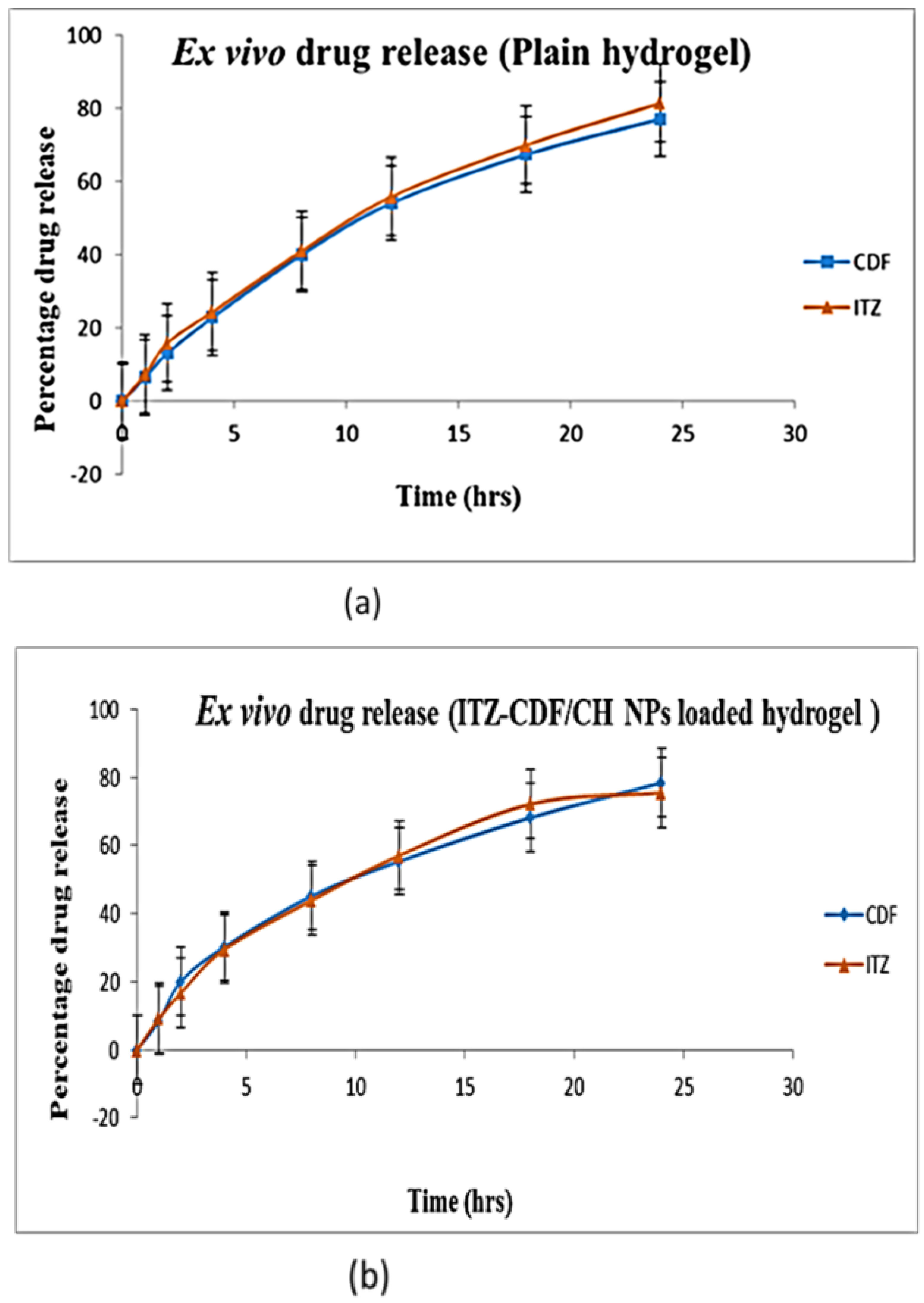
2.8. Transungual Permeation Study
2.9. Nail Clipping Study
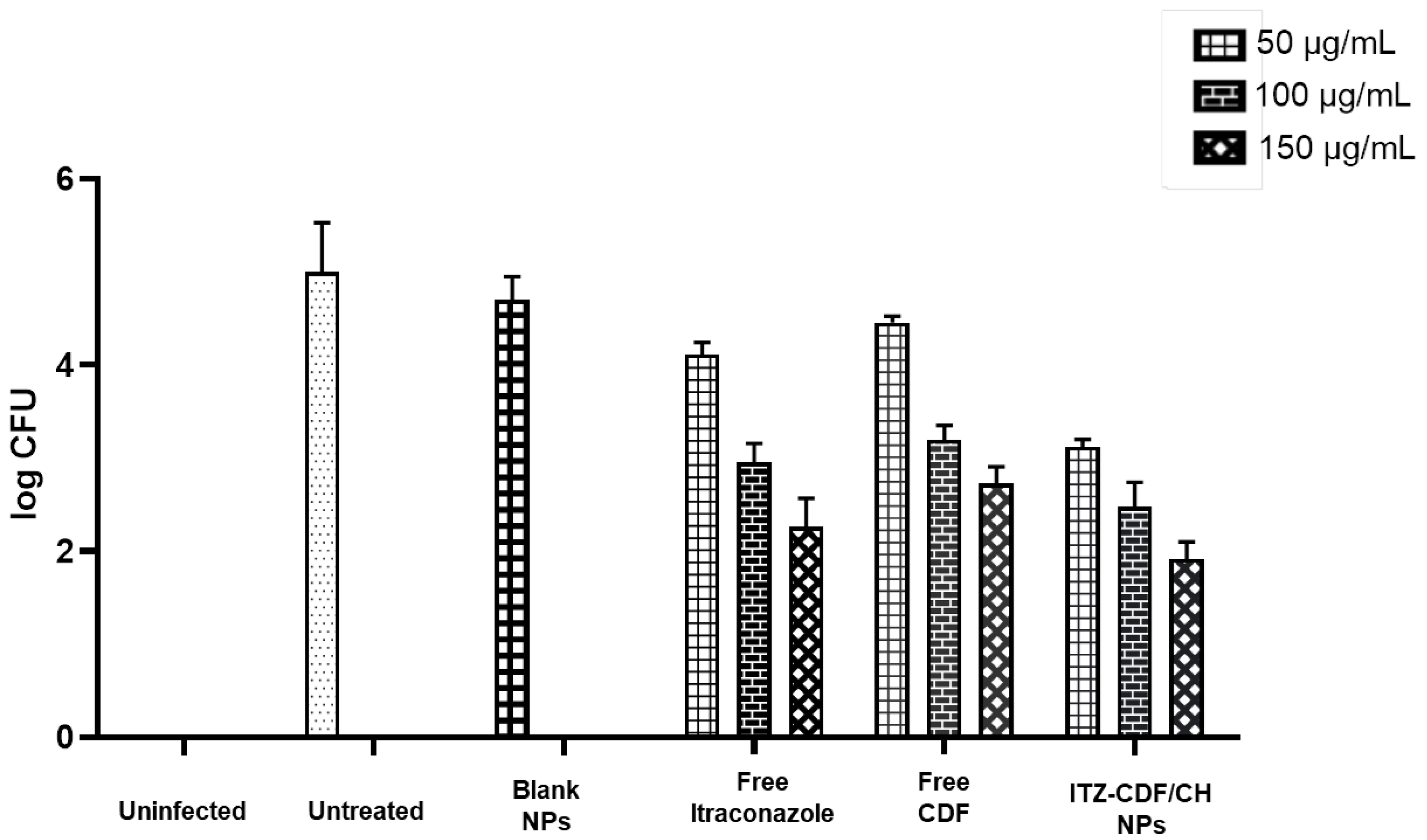
2.10. Antifungal Activity
3. Results and Discussion
3.1. Solubility Analysis and Analytical Method Development
3.2. Preparation of Chitosan Nanoparticles by Ionotropic Gelation Technique
3.3. Evaluation of Physiochemical Properties of Drug-Loaded Chitosan Nanoparticles
3.3.1. Particle Size, Polydispersity Index (PDI), and Zeta Potential Analysis
3.3.2. Morphological Examination via Transmission Electron Microscopy
3.3.3. Entrapment Efficiency and Drug Loading Determination
3.3.4. Differential Scanning Calorimetry (DSC)
3.3.5. Fourier Transform Infrared Spectroscopy (FT-IR)
3.4. Preparation of ITZ-CDF/CH NPs Loaded Hydrogel
3.5. Evaluation of Physiochemical Properties of Hydrogel
3.5.1. pH Determination
3.5.2. Spreadability Study
3.5.3. Extrudability Study
3.5.4. Viscosity and Texture
3.6. In Vitro Drug Release Study
3.7. Transungual Permeation Study
3.8. In Vitro Antifungal Activity
3.9. Nail Clipping Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhamoon, R.K.; Popli, H.; Gupta, M. Novel Drug Delivery Strategies for the Treatment of Onychomycosis. Pharm. Nanotechnol. 2019, 7, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Aslam, R.; Hussain, T.; Yousaf, A.M.; Ghori, M.U.; Khan, I.U.; Rizvi, S.A.A.; Shahzad, Y. Onychomycosis: Current Understanding and Strategies for Enhancing Drug Delivery into Human Nail Tissue. Curr. Drug Res. Rev. 2021, 13, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.D.; Mendes, V.; Veiga, F.F.; Negri, M.; Svidzinski, T.I.E. Relevant insights into onychomycosis’ pathogenesis related to the effectiveness topical treatment. Microb. Pathog. 2022, 169, 105640. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.C.; Lam, J.M.; Leong, K.F.; Hon, K.L.; Barankin, B.; Leung, A.A.M.; Wong, A.H.C. Onychomycosis: An Updated Review. Inflamm. Allergy Drug Targets 2020, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.; Patel, R.; Patel, M.; Thanki, K.; Mishra, S. Design and evaluation of microemulsion-based efinaconazole formulations for targeted treatment of onychomycosis through transungual route: Ex vivo and nail clipping studies. Colloids Surf. B Biointerfaces 2021, 201, 111652. [Google Scholar] [CrossRef]
- Puri, V.; Froelich, A.; Shah, P.; Pringle, S.; Chen, K.; Michniak-Kohn, B. Quality by Design Guided Development of Polymeric Nanospheres of Terbinafine Hydrochloride for Topical Treatment of Onychomycosis Using a Nano-Gel Formulation. Pharmaceutics 2022, 14, 2170. [Google Scholar] [CrossRef]
- Flores, F.C.; Rosso, R.S.; Cruz, L.; Beck, R.C.R.; Silva, C.B. An innovative polysaccharide nanobased nail formulation for improvement of onychomycosis treatment. Eur. J. Pharm. Sci. 2017, 100, 56–63. [Google Scholar] [CrossRef]
- de Doncker, P.; Pande, S.; Richarz, U.; Garodia, N. Itraconazole: What clinicians should know? Indian J. Drugs Dermatol. 2017, 3, 4. [Google Scholar] [CrossRef]
- Tampucci, S.; Terreni, E.; Zucchetti, E.; Burgalassi, S.; Chetoni, P.; Monti, D. Formulations Based on Natural Ingredients for the Treatment of Nail Diseases. Curr. Pharm. Des. 2020, 26, 556–565. [Google Scholar] [CrossRef]
- Lee, W.; Lee, D.G. An antifungal mechanism of curcumin lies in membrane-targeted action within Candida albicans. IUBMB Life 2014, 66, 780–785. [Google Scholar] [CrossRef]
- da Silva, A.P.; Carbinatto, F.M.; Bagnato, V.S.; Inada, N.M. A Promising Strategy for the Treatment of Onychomycosis with Curcumin and Photodynamic Therapy. J. Pharm. Pharmacol. 2015, 3, 434–437. [Google Scholar] [CrossRef]
- Pervaiz, F.; Mushtaq, R.; Noreen, S. Formulation and optimization of terbinafine HCl loaded chitosan/xanthan gum nanoparticles containing gel: Ex-vivo permeation and in-vivo antifungal studies. J. Drug Deliv. Sci. Technol. 2021, 66, 102935. [Google Scholar] [CrossRef]
- Kenechukwu, F.C.; Dias, M.L.; Ricci-Júnior, E. Biodegradable nanoparticles from prosopisylated cellulose as a platform for enhanced oral bioavailability of poorly water-soluble drugs. Carbohydr. Polym. 2021, 256, 117492. [Google Scholar] [CrossRef] [PubMed]
- Bozoğlan, B.K.; Duman, O.; Tunç, S. Smart antifungal thermosensitive chitosan/carboxymethylcellulose/scleroglucan/montmorillonite nanocomposite hydrogels for onychomycosis treatment. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125600. [Google Scholar] [CrossRef]
- Barot, B.S.; Parejiya, P.B.; Patel, H.K.; Gohel, M.C.; Shelat, P.K. Microemulsion-based gel of terbinafine for the treatment of onychomycosis: Optimization of formulation using D-optimal design. AAPS Pharmscitech. 2011, 13, 184–192. [Google Scholar] [CrossRef]
- Tupaki-Sreepurna, A.; Jishnu, B.T.; Thanneru, V.; Sharma, S.; Gopi, A.; Sundaram, M.; Kindo, A.J. An Assessment of In Vitro Antifungal Activities of Efinaconazole and Itraconazole against Common Non-Dermatophyte Fungi Causing Onychomycosis. J. Fungi 2017, 3, 20. [Google Scholar] [CrossRef]
- El-sherif, N.I.; Shamma, R.N.; Abdelbary, G. In-situ gels and nail lacquers as potential delivery systems for treatment of onychomycosis. A comparative study. J. Drug Deliv. Sci. Technol. 2018, 43, 253–261. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Pang, Y.; Qin, A.; Lin, X.; Yang, L.; Wang, Q.; Wang, Z.; Shan, Z.; Li, S.; Wang, J.; Fan, S.; et al. Biodegradable and biocompatible high elastic chitosan scaffold is cell-friendly both in vitro and in vivo. Oncotarget 2017, 8, 35583. [Google Scholar] [CrossRef]
- Zarrinfar, H.; Behnam, M.; Hatamipour, M.; Sahebkar, A. Antifungal Activities of Curcuminoids and Difluorinated Curcumin against Clinical Dermatophyte Isolates. Adv. Exp. Med. Biol. 2021, 1308, 101–107. [Google Scholar] [CrossRef]
- Giri, Y.; Behera, A.; Mohanty, B.; Pattnaik, G.; Habibullah, S.K. Transungual Drug Delivery System for the Topical Treatment of Onychomycosis: A Review. Drug Deliv. Lett. 2022, 12, 2–18. [Google Scholar] [CrossRef]
- Amra, K.; Momin, M. Formulation evaluation of ketoconazole microemulsion-loaded hydrogel with nigella oil as a penetration enhancer. J. Cosmet. Dermatol. 2019, 18, 1742–1750. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-A.; Balakrishnan, P.; Song, C.-K.; Choi, J.-H.; Noh, G.-Y.; Park, C.-G.; Choi, A.-J.; Chung, S.-J.; Shim, C.-K.; Kim, D.-D. Microemulsion-based Hydrogel Formulation of Itraconazole for Topical Delivery. J. Pharm. Investig. 2010, 40, 305–311. [Google Scholar] [CrossRef][Green Version]
- Luong, D.; Kesharwani, P.; Killinger, B.A.; Moszczynska, A.; Sarkar, F.H.; Padhye, S.; Rishi, A.K.; Iyer, A.K. Solubility enhancement and targeted delivery of a potent anticancer flavonoid analogue to cancer cells using ligand decorated dendrimer nano-architectures. J. Colloid Interface Sci. 2016, 484, 33–43. [Google Scholar] [CrossRef]
- Alsaab, H.; Alzhrani, R.M.; Kesharwani, P.; Sau, S.; Boddu, S.H.; Iyer, A.K. Folate decorated nanomicelles loaded with a potent curcumin analogue for targeting retinoblastoma. Pharmaceutics 2017, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Gajra, B.; Dalwadi, C.; Patel, R. Formulation and optimization of itraconazole polymeric lipid hybrid nanoparticles (Lipomer) using box behnken design. J. Pharm. Sci. 2015, 23, 3. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4312448/ (accessed on 17 October 2022). [CrossRef] [PubMed]
- Kalagatur, N.K.; Ghosh, O.S.N.; Sundararaj, N.; Mudili, V. Antifungal activity of chitosan nanoparticles encapsulated with Cymbopogon martinii essential oil on plant pathogenic fungi Fusarium graminearum. Front. Pharmacol. 2018, 9, 610. [Google Scholar] [CrossRef]
- Bhawana, R.K.; Basniwal, H.S.; Buttar, V.K.; Jain, N. Curcumin nanoparticles: Preparation, characterization, and antimicrobial study. J. Agric. Food Chem. 2011, 59, 2056–2061. [Google Scholar] [CrossRef]
- Kanaze, F.I.; Kokkalou, E.; Niopas, I.; Georgarakis, M.; Stergiou, A.; Bikiaris, D. Thermal analysis study of flavonoid solid dispersions having enhanced solubility. J. Therm. Anal. Calorim. 2006, 83, 283–290. [Google Scholar] [CrossRef]
- Tsirigotis-Maniecka, M.; Gancarz, R.; Wilk, K.A. Polysaccharide hydrogel particles for enhanced delivery of hesperidin: Fabrication, characterization and in vitro evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 48–56. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulgel for Improved Topical Delivery of Retinyl Palmitate: Formulation Design and Stability Evaluation. Nanomaterials 2020, 10, 848. [Google Scholar] [CrossRef]
- Taleuzzaman, M.; Sartaj, A.; Gupta, D.K.; Gilani, S.J.; Mirza, M.A. Phytosomal gel of Manjistha extract (MJE) formulated and optimized with central composite design of Quality by Design (QbD). J. Dispers. Sci. Technol. 2021, 1–9. [Google Scholar] [CrossRef]
- Hassan, N.; Singh, M.; Sulaiman, S.; Jain, P.; Sharma, K.; Nandy, S.; Dudeja, M.; Ali, A.; Iqbal, Z. Molecular Docking-Guided Ungual Drug-Delivery Design for Amelioration of Onychomycosis. ACS Omega 2019, 4, 9583–9592. [Google Scholar] [CrossRef] [PubMed]
- Hooda, A.; Sradhanjali, M.; Popsy. Formulation and Evaluation of Novel Solid Lipid Microparticles for the Sustained Release of Ofloxacin. Pharm. Nanotechnol. 2017, 5, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Pangeni, R.; Na, J.; Koo, K.T.; Park, J.W. Preparation and in vivo evaluation of a highly skin- and nail-permeable efinaconazole topical formulation for enhanced treatment of onychomycosis. Drug Deliv. 2019, 26, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M.; Vora, Z.M. Formulation development and optimization of transungual drug delivery system of terbinafine hydrochloride for the treatment of onychomycosis. Drug Deliv. Transl. Res. 2016, 6, 263–275. [Google Scholar] [CrossRef]
- Patel, J.; Patel, A.; Raval, M.; Sheth, N. Formulation and development of a self-nanoemulsifying drug delivery system of irbesartan. J. Adv. Pharm. Technol. Res. 2011, 2, 9. [Google Scholar] [CrossRef]
- Kansagra, H.; Mallick, S. Microemulsion-based antifungal gel of luliconazole for dermatophyte infections: Formulation, characterization and efficacy studies. J. Pharm. Investig. 2015, 46, 21–28. [Google Scholar] [CrossRef]
- Bergonzi, M.C.; Ullah, K.H.; Rasheed, F.; Naz, I.; Haq, N.U.; Fatima, H.; Kanwal, N.; Ur-Rehman, T. Chitosan Nanoparticles Loaded Poloxamer 407 Gel for Transungual Delivery of Terbinafine HCl. Pharmaceutics 2022, 14, 2353. [Google Scholar] [CrossRef]
- Fatima, M.; Monawwar, S.; Mohapatra, S.; Alex, T.S.; Ahmed, A.; Taleuzzaman, M.; Ali, A.; Ansari, M.J.; Mirza, M.A.; Iqbal, Z. In silico drug screening based development of novel formulations for onychomycosis management. Gels 2021, 7, 221. [Google Scholar] [CrossRef]
- Ghurghure, S.M.; Ka, K.; Ys, T.; Ma, P. Preparation and in-vitro evaluation of Itraconazole loaded nanosponges for topical drug delivery. Indo Am. J. Pharm. Res. 2019, 9, 1999–2013. [Google Scholar] [CrossRef]
- Hanafy, A.S.; Farid, R.M.; Elgamal, S.S. Complexation as an approach to entrap cationic drugs into cationic nanoparticles administered intranasally for Alzheimer’s disease management: Preparation and detection in rat brain. Drug Dev. Ind. Pharm. 2015, 41, 2055–2068. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.W.; Rajendran, S.; Joshi, M. Synthesis and characterization of chitosan and silver loaded chitosan nanoparticles for bioactive polyester. Carbohydr. Polym. 2011, 83, 438–446. [Google Scholar] [CrossRef]
- Sukirtha, R.; Priyanka, K.M.; Antony, J.J.; Kamalakkannan, S.; Thangam, R.; Gunasekaran, P.; Krishnan, M.; Achiraman, S. Cytotoxic effect of Green synthesized silver nanoparticles using Melia azedarach against in vitro HeLa cell lines and lymphoma mice model. Process Biochem. 2011, 47, 273–279. [Google Scholar] [CrossRef]
- Kavaz, D.; Kirac, F.; Kirac, M.; Vaseashta, A. Low Releasing Mitomycin C Molecule Encapsulated with Chitosan Nanoparticles for Intravesical Installation. J. Biomater. Nanobiotechnol. 2017, 8, 203–219. [Google Scholar] [CrossRef]
- Mahtab, A.; Anwar, M.; Mallick, N.; Naz, Z.; Jain, G.K.; Ahmad, F.J. Transungual Delivery of Ketoconazole Nanoemulgel for the Effective Management of Onychomycosis. AAPS PharmSciTech 2016, 17, 1477–1490. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, N.; Tikoo, K.; Sinha, V.R. Development of mirtazapine loaded solid lipid nanoparticles for topical delivery: Optimization, characterization and cytotoxicity evaluation. Int. J. Pharm. 2020, 586, 119439. [Google Scholar] [CrossRef]
- Gupta, A.K.; Mays, R.R. The Impact of Onychomycosis on Quality of Life: A Systematic Review of the Available Literature. Ski. Appendage Disord. 2018, 4, 208–216. [Google Scholar] [CrossRef]
- Zaslavsky, D.V.; Chuprov, I.N.; Sidikov, A.A.; Khvedelidze, M.G.; Tatarskaya, O.B. Onychomycosis: Features external therapy. Vestn. Dermatol. Venerol. 2016, 92, 90–95. [Google Scholar] [CrossRef]
- Aggarwal, R.; Targhotra, M.; Kumar, B.; Sahoo, P.K.; Chauhan, M.K. Treatment and management strategies of onychomycosis. J. Mycol. Med. 2020, 30, 100949. [Google Scholar] [CrossRef]
- Valdes, B.S.G.; Vilela, C.d.M.; Ascenso, A.; Bordado, J.M.; Ribeiro, H. Topical Formulations for Onychomycosis: A Review. Carrier-Mediated Dermal Delivery; Jenny Stanford Publishing: New York, NY, USA, 2017; pp. 503–553. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kesharwani, P.; Fatima, M.; Singh, V.; Sheikh, A.; Almalki, W.H.; Gajbhiye, V.; Sahebkar, A. Itraconazole and Difluorinated-Curcumin Containing Chitosan Nanoparticle Loaded Hydrogel for Amelioration of Onychomycosis. Biomimetics 2022, 7, 206. https://doi.org/10.3390/biomimetics7040206
Kesharwani P, Fatima M, Singh V, Sheikh A, Almalki WH, Gajbhiye V, Sahebkar A. Itraconazole and Difluorinated-Curcumin Containing Chitosan Nanoparticle Loaded Hydrogel for Amelioration of Onychomycosis. Biomimetics. 2022; 7(4):206. https://doi.org/10.3390/biomimetics7040206
Chicago/Turabian StyleKesharwani, Prashant, Mahak Fatima, Vanshikha Singh, Afsana Sheikh, Waleed H. Almalki, Virendra Gajbhiye, and Amirhossein Sahebkar. 2022. "Itraconazole and Difluorinated-Curcumin Containing Chitosan Nanoparticle Loaded Hydrogel for Amelioration of Onychomycosis" Biomimetics 7, no. 4: 206. https://doi.org/10.3390/biomimetics7040206
APA StyleKesharwani, P., Fatima, M., Singh, V., Sheikh, A., Almalki, W. H., Gajbhiye, V., & Sahebkar, A. (2022). Itraconazole and Difluorinated-Curcumin Containing Chitosan Nanoparticle Loaded Hydrogel for Amelioration of Onychomycosis. Biomimetics, 7(4), 206. https://doi.org/10.3390/biomimetics7040206





