Revealing the Wonder of Natural Photonics by Nonlinear Optics
Abstract
1. Introduction
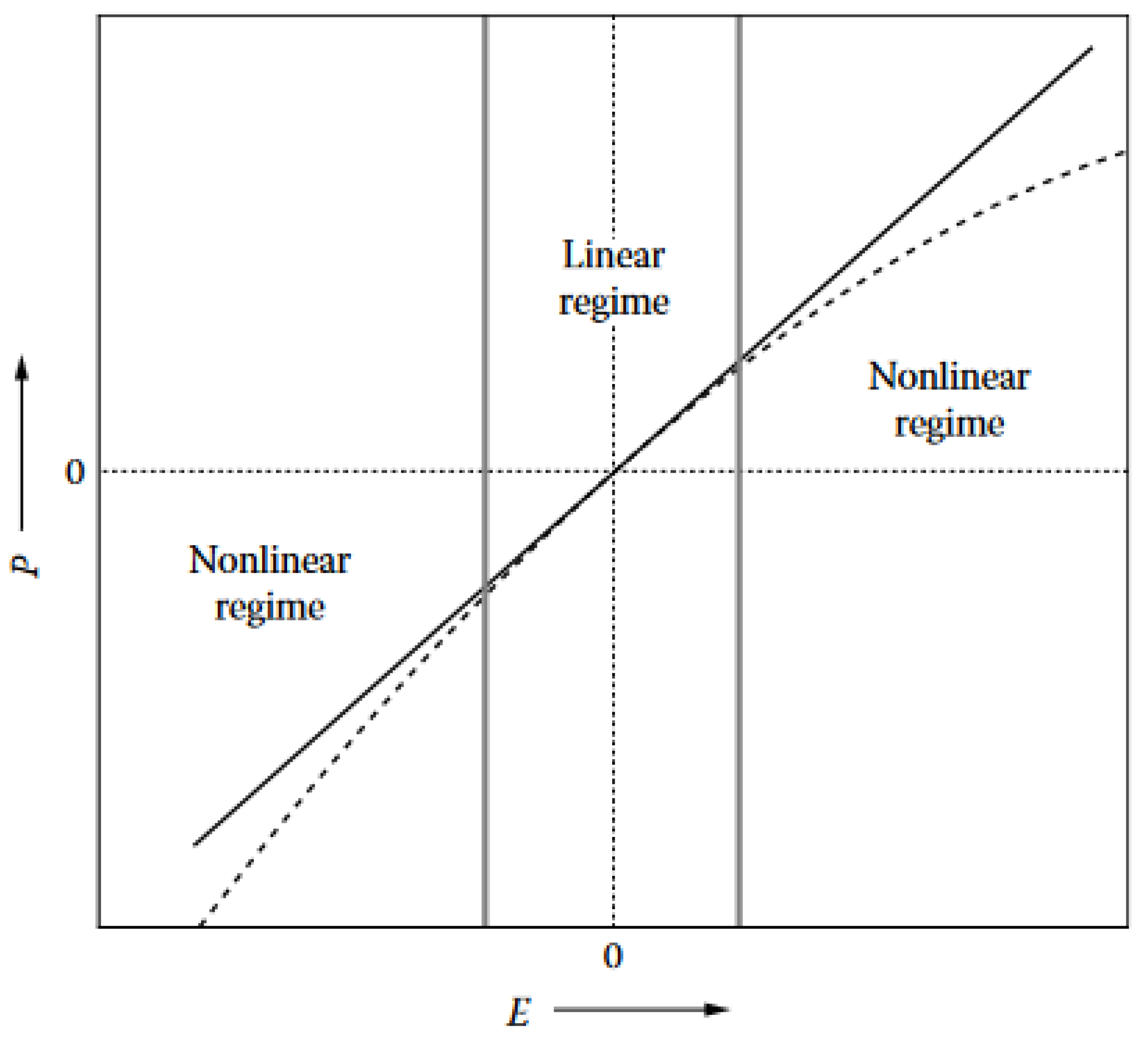
2. Basics of Nonlinear Optics
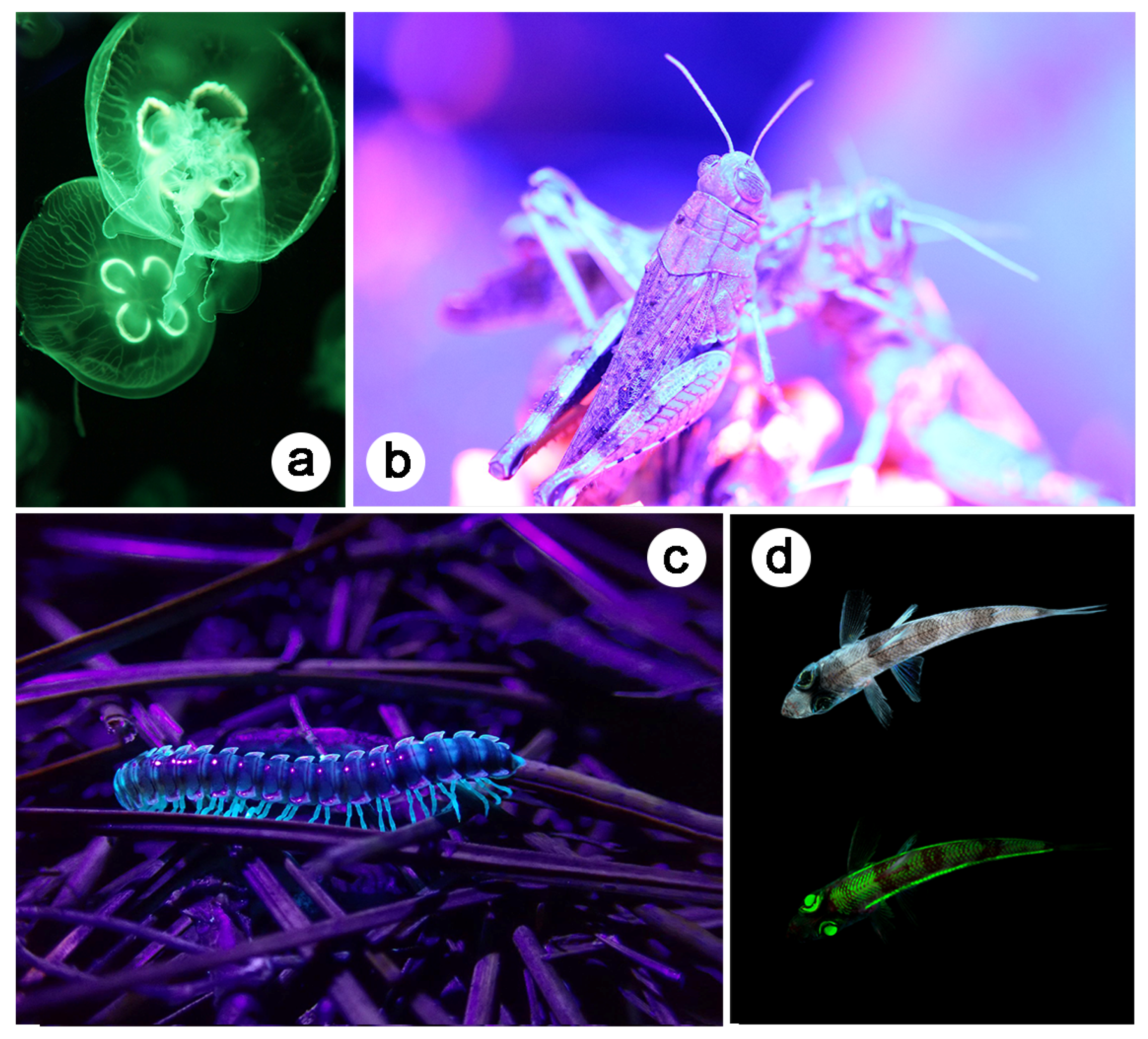
3. Introduction to Natural Photonics
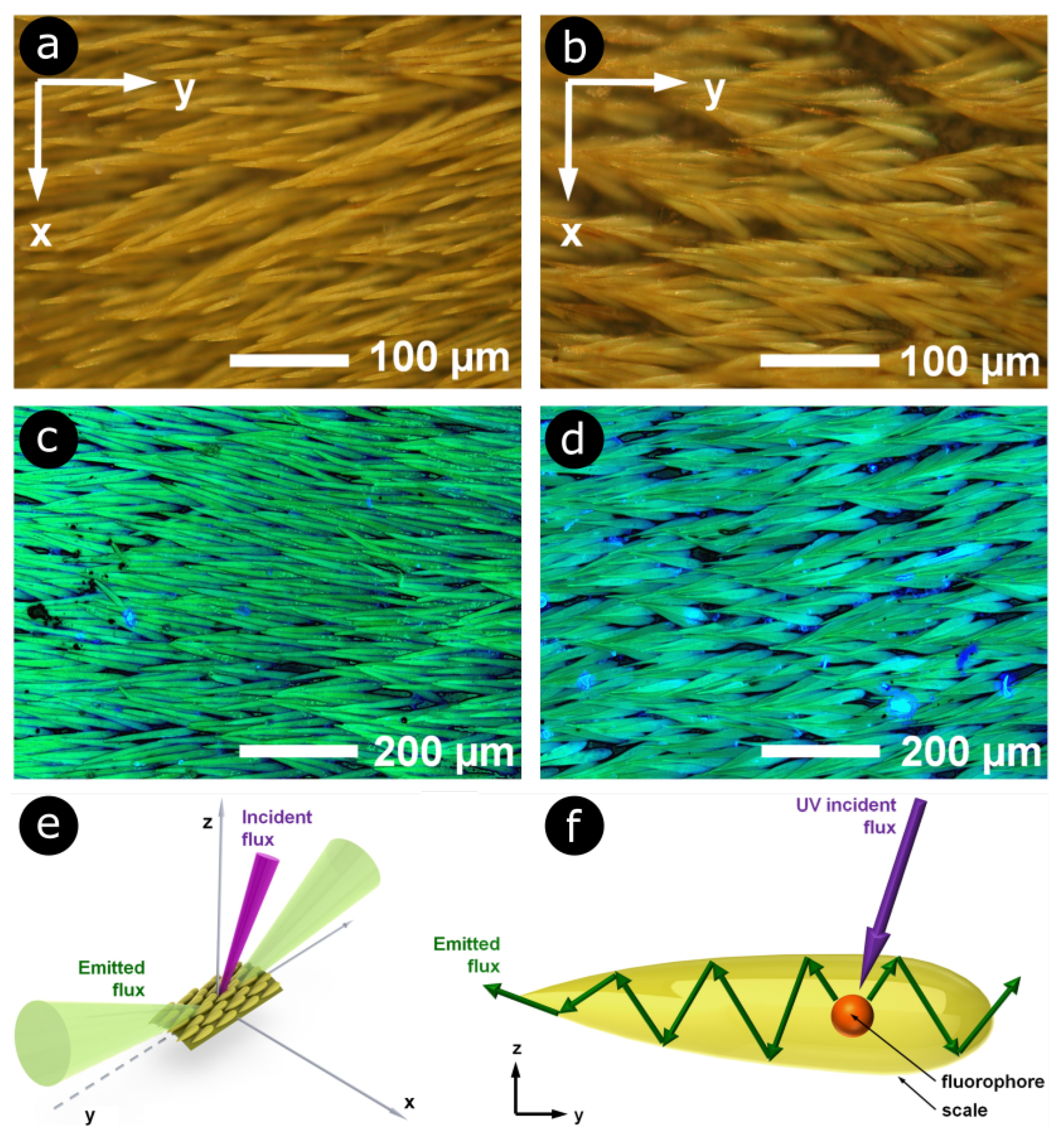
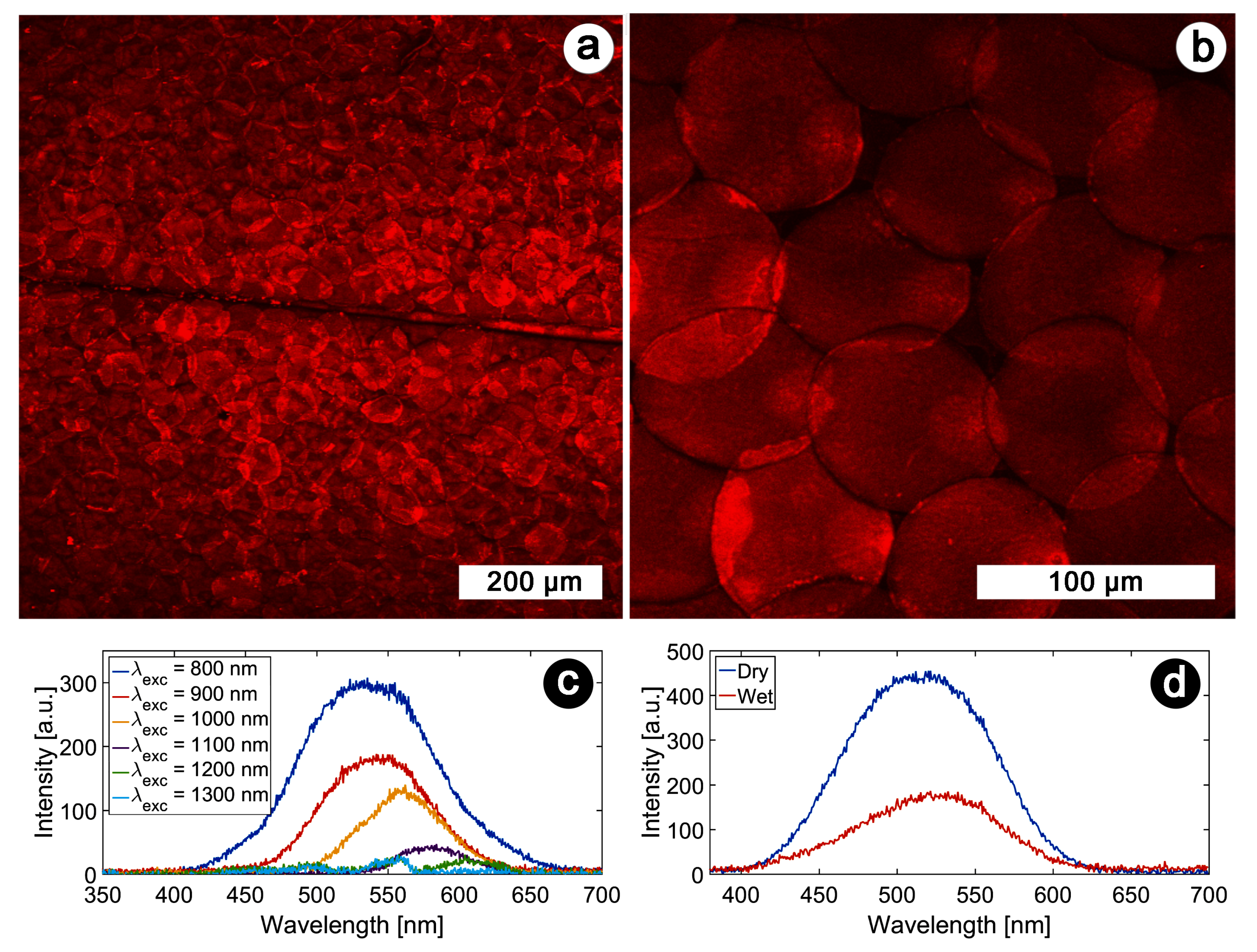
4. Nonlinear Optical Study of Natural Photonic Structures
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kinoshita, S. Structural Colors in the Realm of Nature; World Scientific Publishing Co.: Singapore, 2008. [Google Scholar]
- Mouchet, S.R.; Deparis, O. Natural Photonics and Bioinspiration; Artech House: Boston, MA, USA, 2021. [Google Scholar]
- Hecht, E. Optics; Addison-Wesley Publishing Company: Reading, MA, USA, 2001. [Google Scholar]
- Stratton, J. Electromagnetic Theory; McGraw-Hill: New York, NY, USA, 1941. [Google Scholar]
- Böttcher, C. Theory of Electric Polarization, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1973. [Google Scholar]
- Zernike, F.; Midwinter, J. Applied Nonlinear Optics; Wiley: New York, NY, USA, 1973. [Google Scholar]
- Verbiest, T.; Clays, K.; Rodriguez, V. Second-Order Nonlinear Optical Characterization Techniques: An Introduction; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2009. [Google Scholar]
- Zipfel, W.R.; Williams, R.M.; Webb, W.W. Nonlinear magic: Multiphoton microscopy in the biosciences. Nat. Biotechnol. 2003, 21, 1369. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, C.; Mouchet, S.R.; Verbiest, T.; Kolaric, B. Linear and nonlinear optical effects in biophotonic structures using classical and nonclassical light. J. Biophotonics 2019, 12, e201800262. [Google Scholar] [CrossRef]
- Pathan, A.; Bond, J.; Gaskin, R. Sample preparation for scanning electron microscopy of plant surfaces—Horses for courses. Micron 2008, 39, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Ayache, J.; Beaunier, L.; Boumendil, J.; Ehret, G.; Laub, D. Sample Preparation Handbook for Transmission Electron Microscopy; Springer: New York, NY, USA, 2010. [Google Scholar]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin Pigmentation in Mammalian Skin and Its Hormonal Regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Diversity of human hair pigmentation as studied by chemical analysis of eumelanin and pheomelanin. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Stavenga, D.G.; Giraldo, M.A.; Leertouwer, H.L. Butterfly wing colors: Glass scales of Graphium sarpedon cause polarized iridescence and enhance blue/green pigment coloration of the wing membrane. J. Exp. Biol. 2010, 213, 1731–1739. [Google Scholar] [CrossRef]
- Stavenga, D.G.; Matsushita, A.; Arikawa, K.; Leertouwer, H.L.; Wilts, B.D. Glass scales on the wing of the swordtail butterfly Graphium sarpedon act as thin film polarizing reflectors. J. Exp. Biol. 2012, 215, 657–662. [Google Scholar] [CrossRef]
- Stavenga, D. Thin film and multilayer optics cause structural colors of many insects and birds. Mater. Today Proc. 2014, 1S, 109–121. [Google Scholar] [CrossRef]
- Siddique, R.; Vignolini, S.; Bartels, C.; Wacker, I.; Hölscher, H. Colour formation on the wings of the butterfly Hypolimnas Salmacis Scale Stacking. Sci. Rep. 2016, 6, 36204. [Google Scholar] [CrossRef]
- Vukusic, P.; Sambles, J.R.; Lawrence, C.R.; Wootton, R.J. Limited-view iridescence in the butterfly Ancyluris Meliboeus. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2002, 269, 7–14. [Google Scholar] [CrossRef]
- Vukusic, P.; Kelly, R.; Hooper, I. A biological sub-micron thickness optical broadband reflector characterized using both light and microwaves. J. R. Soc. Interface 2009, 6, S193–S201. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vigneron, J.P.; Simonis, P.; Aiello, A.; Bay, A.; Windsor, D.M.; Colomer, J.F.; Rassart, M. Reverse color sequence in the diffraction of white light by the wing of the male butterfly Pierella Luna (Nymphalidae: Satyrinae). Phys. Rev. E 2010, 82, 021903. [Google Scholar] [CrossRef] [PubMed]
- Biró, L.P.; Bálint, Z.; Kertész, K.; Vértesy, Z.; Márk, G.I.; Horváth, Z.E.; Balázs, J.; Méhn, D.; Kiricsi, I.; Lousse, V.; et al. Role of photonic-crystal-type structures in the thermal regulation of a Lycaenid butterfly sister species pair. Phys. Rev. E 2003, 67, 021907. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, S.; Kinoshita, S. Single-scale spectroscopy of structurally colored butterflies: Measurements of quantified reflectance and transmittance. J. Opt. Soc. Am. A 2006, 23, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Noyes, J.A.; Vukusic, P.; Hooper, I.R. Experimental method for reliably establishing the refractive index of buprestid beetle exocuticle. Opt. Express 2007, 15, 4351–4358. [Google Scholar] [CrossRef] [PubMed]
- Kertész, K.; Molnár, G.; Vértesy, Z.; Koós, A.; Horváth, Z.; Márk, G.; Tapasztó, L.; Bálint, Z.; Tamáska, I.; Deparis, O.; et al. Photonic band gap materials in butterfly scales: A possible source of “blueprints”. E-MRS 2007 Spring Conference Symposium A: Sub-wavelength photonics throughout the spectrum: Materials and Techniques. Mater. Sci. Eng. B 2008, 149, 259–265. [Google Scholar] [CrossRef]
- Wilts, B.D.; Leertouwer, H.L.; Stavenga, D.G. Imaging scatterometry and microspectrophotometry of lycaenid butterfly wing scales with perforated multilayers. J. R. Soc. Interface 2009, 6, S185–S192. [Google Scholar] [CrossRef] [PubMed]
- Stavenga, D.G.; Wilts, B.D.; Leertouwer, H.L.; Hariyama, T. Polarized iridescence of the multilayered elytra of the Japanese jewel beetle, Chrysochroa Fulgidissima. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 709–723. [Google Scholar] [CrossRef]
- Vigneron, J.P.; Pasteels, J.M.; Windsor, D.M.; Vértesy, Z.; Rassart, M.; Seldrum, T.; Dumont, J.; Deparis, O.; Lousse, V.; Biró, L.P.; et al. Switchable reflector in the Panamanian tortoise beetle Charidotella Egregia (Chrysomelidae: Cassidinae). Phys. Rev. E 2007, 76, 031907. [Google Scholar] [CrossRef]
- Pasteels, J.M.; Deparis, O.; Mouchet, S.R.; Windsor, D.M.; Billen, J. Structural and physical evidence for an endocuticular gold reflector in the tortoise beetle, Charidotella Ambita. Arthropod Struct. Dev. 2016, 45, 509–518. [Google Scholar] [CrossRef]
- Kertész, K.; Bálint, Z.; Vértesy, Z.; Márk, G.I.; Lousse, V.; Vigneron, J.P.; Rassart, M.; Biró, L.P. Gleaming and dull surface textures from photonic-crystal-type nanostructures in the butterfly Cyanophrys remus. Phys. Rev. E 2006, 74, 021922. [Google Scholar] [CrossRef] [PubMed]
- Michielsen, K.; Stavenga, D. Gyroid cuticular structures in butterfly wing scales: Biological photonic crystals. J. R. Soc. Interface 2008, 5, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Saranathan, V.; Osuji, C.O.; Mochrie, S.G.J.; Noh, H.; Narayanan, S.; Sandy, A.; Dufresne, E.R.; Prum, R.O. Structure, function, and self-assembly of single network gyroid (I4132) Photonic Cryst. Butterfly Wing Scales. Proc. Natl. Acad. Sci. USA 2010, 107, 11676–11681. [Google Scholar] [CrossRef] [PubMed]
- Pouya, C.; Vukusic, P. Electromagnetic characterization of millimetre-scale replicas of the gyroid photonic crystal found in the butterfly Parides Sesostris. Interface Focus 2012, 2, 645–650. [Google Scholar] [CrossRef]
- Mouchet, S.R.; Vigneron, J.P.; Colomer, J.F.; Vandenbem, C.; Deparis, O. Additive photonic colors in the Brazilian diamond weevil: Entimus imperialis. Proc. SPIE 2012, 8480, 848003. [Google Scholar] [CrossRef]
- Mouchet, S.; Colomer, J.F.; Vandenbem, C.; Deparis, O.; Vigneron, J.P. Method for modeling additive color effect in photonic polycrystals with form anisotropic elements: The case of Entimus Imp. Weevil. Opt. Express 2013, 21, 13228–13240. [Google Scholar] [CrossRef][Green Version]
- Prum, R.O.; Torres, R.H.; Williamson, S.W.; Dyck, J. Coherent light scattering by blue feather barbs. Nature 1998, 396, 28–29. [Google Scholar] [CrossRef]
- Prum, R.O.; Torres, R.H. A Fourier Tool for the Analysis of Coherent Light Scattering by Bio-Optical Nanostructures1. Integr. Comp. Biol. 2003, 43, 591–602. [Google Scholar] [CrossRef]
- Prum, R.O.; Torres, R. Structural colouration of avian skin: Convergent evolution of coherently scattering dermal collagen arrays. J. Exp. Biol. 2003, 206, 2409–2429. [Google Scholar] [CrossRef]
- Prum, R.O.; Torres, R.H. Structural colouration of mammalian skin: Convergent evolution of coherently scattering dermal collagen arrays. J. Exp. Biol. 2004, 207, 2157–2172. [Google Scholar] [CrossRef]
- Henze, M.J.; Lind, O.; Wilts, B.D.; Kelber, A. Pterin-pigmented nanospheres create the colours of the polymorphic damselfly Ischnura Elegans. J. R. Soc. Interface 2019, 16, 20180785. [Google Scholar] [CrossRef] [PubMed]
- Mouchet, S.R.; Luke, S.; McDonald, L.T.; Vukusic, P. Optical costs and benefits of disorder in biological photonic crystals. Faraday Discuss. 2020, 223, 9–48. [Google Scholar] [CrossRef] [PubMed]
- Morehouse, N.I.; Vukusic, P.; Rutowski, R. Pterin pigment granules are responsible for both broadband light scattering and wavelength selective absorption in the wing scales of pierid butterflies. Proc. R. Soc. B Biol. Sci. 2007, 274, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Stavenga, D.G.; Stowe, S.; Siebke, K.; Zeil, J.; Arikawa, K. Butterfly wing colours: Scale beads make white pierid wings brighter. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2004, 271, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Stavenga, D.G.; Giraldo, M.A.; Hoenders, B.J. Reflectance and transmittance of light scattering scales stacked on the wings of pierid butterflies. Opt. Express 2006, 14, 4880–4890. [Google Scholar] [CrossRef]
- Ladouce, M.; Barakat, T.; Su, B.L.; Deparis, O.; Mouchet, S.R. Scattering of ultraviolet light by avian eggshells. Faraday Discuss. 2020, 223, 63–80. [Google Scholar] [CrossRef]
- Mouchet, S.R.; Vukusic, P. Structural Colours in Lepidopteran Scales. Adv. Insect Physiol. 2018, 54, 1–53. [Google Scholar] [CrossRef]
- Vigneron, J.P.; Colomer, J.F.m.; Vigneron, N.; Lousse, V. Natural layer-by-layer photonic structure in the squamae of Hoplia Coerulea (Coleoptera). Phys. Rev. E 2005, 72, 061904. [Google Scholar] [CrossRef]
- Mouchet, S.; Lobet, M.; Kolaric, B.; Kaczmarek, A.; Van Deun, R.; Vukusic, P.; Deparis, O.; Van Hooijdonk, E. Photonic scales of Hoplia Coerulea Beetle: Any Colour You Like. Mater. Today Proc. 2017, 4, 4979–4986. [Google Scholar] [CrossRef]
- Rassart, M.; Simonis, P.; Bay, A.; Deparis, O.; Vigneron, J.P. Scale coloration change following water absorption in the beetle Hoplia Coerulea (Coleoptera). Phys. Rev. E 2009, 80, 031910. [Google Scholar] [CrossRef]
- Mouchet, S.R.; Su, B.L.; Tabarrant, T.; Lucas, S.; Deparis, O. Hoplia Coerulea, A Porous Nat. Photonic Struct. Template Opt. Vap. Sensor. Proc. SPIE 2014, 9127. [Google Scholar] [CrossRef]
- Mouchet, S.R.; Tabarrant, T.; Lucas, S.; Su, B.L.; Vukusic, P.; Deparis, O. Vapor sensing with a natural photonic cell. Opt. Express 2016, 24, 12267–12280. [Google Scholar] [CrossRef] [PubMed]
- Mouchet, S.; Van Hooijdonk, E.; Welch, V.; Louette, P.; Colomer, J.F.; Su, B.L.; Deparis, O. Liquid-induced colour change in a beetle: The concept of a photonic cell. Sci. Rep. 2016, 6, 19322. [Google Scholar] [CrossRef] [PubMed]
- Mouchet, S.; Van Hooijdonk, E.; Welch, V.; Louette, P.; Tabarrant, T.; Vukusic, P.; Lucas, S.; Colomer, J.F.; Su, B.L.; Deparis, O. Assessment of environmental spectral ellipsometry for characterising fluid-induced colour changes in natural photonic structures. Mater. Today Proc. 2017, 4, 4987–4997. [Google Scholar] [CrossRef]
- Van Hooijdonk, E.; Berthier, S.; Vigneron, J.P. Bio-Inspired approach of the fluorescence emission properties in the scarabaeid beetle Hoplia Coerulea (Coleoptera): Model. Transf.-Matrix Opt. Simulations. J. Appl. Phys. 2012, 112, 114702. [Google Scholar] [CrossRef]
- Mouchet, S.R.; Lobet, M.; Kolaric, B.; Kaczmarek, A.M.; Van Deun, R.; Vukusic, P.; Deparis, O.; Van Hooijdonk, E. Controlled fluorescence in a beetle’s photonic structure and its sensitivity to environmentally induced changes. Proc. R. Soc. Lond. B Biol. Sci. 2016, 283. [Google Scholar] [CrossRef]
- Lagorio, M.G.; Cordon, G.B.; Iriel, A. Reviewing the relevance of fluorescence in biological systems. Photochem. Photobiol. Sci. 2015, 14, 1538–1559. [Google Scholar] [CrossRef]
- Marshall, J.; Johnsen, S. Fluorescence as a means of colour signal enhancement. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef]
- Arnold, K.E.; Owens, I.P.F.; Marshall, N.J. Fluorescent Signaling in Parrots. Science 2002, 295, 92. [Google Scholar] [CrossRef]
- McGraw, K.J.; Toomey, M.B.; Nolan, P.M.; Morehouse, N.I.; Massaro, M.; Jouventin, P. A description of unique fluorescent yellow pigments in penguin feathers. Pigment Cell Res. 2007, 20, 301–304. [Google Scholar] [CrossRef]
- Ladouce, M.; Barakat, T.; Su, B.L.; Deparis, O.; Mouchet, S.R. UV scattering by pores in avian eggshells. Proc. SPIE 2020, 11481, 101–109. [Google Scholar] [CrossRef]
- Cockayne, E.I. The Distribution of Fluorescent Pigments in Lepidoptera. Trans. R. Entomol. Soc. Lond. 1924, 72, 1–19. [Google Scholar] [CrossRef]
- Vukusic, P.; Hooper, I. Directionally Controlled Fluorescence Emission in Butterflies. Science 2005, 310, 1151. [Google Scholar] [CrossRef] [PubMed]
- Israelowitz, M.; Rizvi, S.H.; von Schroeder, H.P. Fluorescence of the “fire-chaser” beetle Melanophila Acuminata. J. Lumin. 2007, 126, 149–154. [Google Scholar] [CrossRef]
- Trzeciak, T.M.; Wilts, B.D.; Stavenga, D.G.; Vukusic, P. Variable multilayer reflection together with long-pass filtering pigment determines the wing coloration of papilionid butterflies of the Nireus Group. Opt. Express 2012, 20, 8877–8890. [Google Scholar] [CrossRef][Green Version]
- Hooijdonk, E.V.; Vandenbem, C.; Berthier, S.; Vigneron, J.P. Bi-Functional photonic structure in the Papilio Nireus (Papilionidae): Model. Scatt.-Matrix Opt. Simulations. Opt. Express 2012, 20, 22001–22011. [Google Scholar] [CrossRef]
- Van Hooijdonk, E.; Berthier, S.; Vigneron, J.P. Contribution of both the upperside and the underside of the wing on the iridescence in the male butterfly Troïdes Magellanus (Papilionidae). J. Appl. Phys. 2012, 112, 074702. [Google Scholar] [CrossRef]
- Welch, V.L.; Van Hooijdonk, E.; Intrater, N.; Vigneron, J.P. Fluorescence in insects. Proc. SPIE 2012, 8480, 848004. [Google Scholar] [CrossRef]
- Wilts, B.D.; Trzeciak, T.M.; Vukusic, P.; Stavenga, D.G. Papiliochrome II pigment reduces the angle dependency of structural wing colouration in Nireus Group Papilionids. J. Exp. Biol. 2012, 215, 796–805. [Google Scholar] [CrossRef]
- Van Hooijdonk, E.; Barthou, C.; Vigneron, J.P.; Berthier, S. Yellow structurally modified fluorescence in the longhorn beetles Celosterna pollinosa sulfurea and Phosphorus virescens (Cerambycidae). J. Lumin. 2013, 136, 313–321. [Google Scholar] [CrossRef]
- Mouchet, S.R.; Kaczmarek, A.M.; Mara, D.; Deun, R.V.; Vukusic, P. Colour and fluorescence emission of Euchroea Auripigmenta Beetle. Proc. SPIE 2019, 10965, 72–82. [Google Scholar] [CrossRef]
- Mouchet, S.R.; Verstraete, C.; Kaczmarek, A.M.; Mara, D.; van Cleuvenbergen, S.; Van Deun, R.; Verbiest, T.; Maes, B.; Vukusic, P.; Kolaric, B. Unveiling the nonlinear optical response of Trictenotoma Child. Longhorn Beetle. J. Biophotonics 2019, 12, e201800470. [Google Scholar] [CrossRef] [PubMed]
- Mouchet, S.R.; Verstraete, C.; Bokic, B.; Mara, D.; Dellieu, L.; Orr, A.G.; Deparis, O.; Deun, R.V.; Verbiest, T.; Vukusic, P.; et al. Naturally occurring fluorescence in transparent insect wings. arXiv 2021, arXiv:2110.06086. [Google Scholar]
- Lawrence, R.F. Fluorescence in Arthropoda. J. Entomol. Soc. S. Afr. 1954, 17, 167–170. [Google Scholar]
- Pavan, M.; Vachon, M. Sur l’existence d’une substance fluorescente dans les téguments des Scorpions (Arachnides). Comptes Rendus L’Académie Sci. 1954, 239, 1700–1702. [Google Scholar]
- Tani, K.; Watari, F.; Uo, M.; Morita, M. Fluorescent Properties of Porcelain-Restored Teeth and Their Discrimination. Mater. Trans. 2004, 45, 1010–1014. [Google Scholar] [CrossRef][Green Version]
- Taboada, C.; Brunetti, A.E.; Pedron, F.N.; Carnevale Neto, F.; Estrin, D.A.; Bari, S.E.; Chemes, L.B.; Peporine Lopes, N.; Lagorio, M.G.; Faivovich, J. Naturally occurring fluorescence in frogs. Proc. Natl. Acad. Sci. USA 2017, 114, 3672–3677. [Google Scholar] [CrossRef]
- Deschepper, P.; Jonckheere, B.; Matthys, J. A Light in the Dark: The Discovery of Another Fluorescent Frog in the Costa Rican Rainforests. Wilderness Environ. Med. 2018, 29, 421–422. [Google Scholar] [CrossRef]
- Mohd Top, M.; Puan, C.L.; Chuang, M.F.; Othman, S.N.; Borzée, A. First record of ultraviolet fluorescence in the Bent-toed Gecko Cyrtodactylus Quadrivirgatus Taylor, 1962 (Gekkonidae: Sauria). Herpetol. Notes 2020, 13, 211–212. [Google Scholar]
- Gruber, D.F.; Gaffney, J.P.; Mehr, S.; DeSalle, R.; Sparks, J.S.; Platisa, J.; Pieribone, V.A. Adaptive Evolution of Eel Fluorescent Proteins from Fatty Acid Binding Proteins Produces Bright Fluorescence in the Marine Environment. PLoS ONE 2015, 10, e0140972. [Google Scholar] [CrossRef]
- Gruber, D.F.; Loew, E.R.; Deheyn, D.D.; Akkaynak, D.; Gaffney, J.P.; Smith, W.L.; Davis, M.P.; Stern, J.H.; Pieribone, V.A.; Sparks, J.S. Biofluorescence in Catsharks (Scyliorhinidae): Fundamental Description and Relevance for Elasmobranch Visual Ecology. Sci. Rep. 2016, 6, 24751. [Google Scholar] [CrossRef] [PubMed]
- Iriel, A.; Lagorio, M.G. Is the flower fluorescence relevant in biocommunication? Naturwissenschaften 2010, 97, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Iriel, A.; Lagorio, M.G. Implications of reflectance and fluorescence of Rhododendr. Indicum Flowers Biosignaling. Photochem. Photobiol. Sci. 2010, 9, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Purcell, E.M. Spontaneous emission probabilities at radio frequencies. Phys. Rev. 1946, 69, 681. [Google Scholar]
- Yablonovitch, E. Inhibited Spontaneous Emission in Solid-State Physics and Electronics. Phys. Rev. Lett. 1987, 58, 2059–2062. [Google Scholar] [CrossRef]
- Kumazawa, K.; Tanaka, S.; Negita, K.; Tabata, H. Fluorescence from Wing of Morpho Sulkowskyi Butterfly. Jpn. J. Appl. Phys. 1994, 33, 2119–2122. [Google Scholar] [CrossRef]
- Lawrence, C.R.; Vukusic, P.; Sambles, J.R. Grazing-incidence iridescence from a butterfly wing. Appl. Opt. 2002, 41, 437–441. [Google Scholar] [CrossRef]
- Vigneron, J.P.; Kertész, K.; Vértesy, Z.; Rassart, M.; Lousse, V.; Bálint, Z.; Biró, L.P. Correlated diffraction and fluorescence in the backscattering iridescence of the male butterfly Troides Magellanus (Papilionidae). Phys. Rev. E 2008, 78, 021903. [Google Scholar] [CrossRef]
- Van Hooijdonk, E.; Barthou, C.; Vigneron, J.P.; Berthier, S. Detailed experimental analysis of the structural fluorescence in the butterfly Morpho Sulkowskyi (Nymphalidae). J. Nanophotonics 2011, 5, 053525. [Google Scholar] [CrossRef]
- Hooijdonk, E.V.; Barthou, C.; Vigneron, J.P.; Berthier, S. Angular dependence of structural fluorescent emission from the scales of the male butterfly Troïdes Magellanus (Papilionidae). J. Opt. Soc. Am. B 2012, 29, 1104–1111. [Google Scholar] [CrossRef]
- Verbiest, T.; Kauranen, M.; Persoons, A.; Ikonen, M.; Kurkela, J.; Lemmetyinen, H. Nonlinear Optical Activity and Biomolecular Chirality. J. Am. Chem. Soc. 1994, 116, 9203–9205. [Google Scholar] [CrossRef]
- Campagnola, P.J.; Millard, A.C.; Terasaki, M.; Hoppe, P.E.; Malone, C.J.; Mohler, W.A. Three-dimensional high-resolution second-harmonic generation imaging of endogenous structural proteins in biological tissues. Biophys. J. 2002, 82, 493–508. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K.; Harms, G.; Andrian, U.H. Biological Second and Third Harmonic Generation Microscopy. Curr. Protoc. Cell Biol. 2007, 34, 4.15.1–4.15.21. [Google Scholar] [CrossRef] [PubMed]
- Ries, R.S.; Choi, H.; Blunck, R.; Bezanilla, F.; Heath, J.R. Black Lipid Membranes: Visualizing the Structure, Dynamics, and Substrate Dependence of Membranes. J. Phys. Chem. B 2004, 108, 16040–16049. [Google Scholar] [CrossRef]
- Brown, D.J.; Morishige, N.; Neekhra, A.; Minckler, D.S.; Jester, J.V. Application of second harmonic imaging microscopy to assess structural changes in optic nerve head structure ex vivo. J. Biomed. Opt. 2007, 12, 5. [Google Scholar] [CrossRef]
- Lis, D.; Guthmuller, J.; Champagne, B.; Humbert, C.; Busson, B.; Tadjeddine, A.; Peremans, A.; Cecchet, F. Selective detection of the antigenic polar heads of model lipid membranes supported on metals from their vibrational nonlinear optical response. Chem. Phys. Lett. 2010, 489, 12–15. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Conboy, J.C. High-Throughput Screening of Drug-Lipid Membrane Interactions via Counter-Propagating Second Harmonic Generation Imaging. Anal. Chem. 2011, 83, 5979–5988. [Google Scholar] [CrossRef]
- Theer, P.; Denk, W.; Sheves, M.; Lewis, A.; Detwiler, P.B. Second-Harmonic Generation Imaging of Membrane Potential with Retinal Analogues. Biophys. J. 2011, 100, 232–242. [Google Scholar] [CrossRef]
- Chen, X.; Nadiarynkh, O.; Plotnikov, S.; Campagnola, P.J. Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nat. Protoc. 2012, 7, 654. [Google Scholar] [CrossRef]
- Akerboom, J.; Carreras Calderón, N.; Tian, L.; Wabnig, S.; Prigge, M.; Tolö, J.; Gordus, A.; Orger, M.B.; Severi, K.E.; Macklin, J.J.; et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front. Mol. Neurosci. 2013, 6, 2. [Google Scholar] [CrossRef]
- Rabasović, M.D.; Pantelić, D.V.; Jelenković, B.M.; Ćurčić, S.B.; Rabasović, M.S.; Vrbica, M.D.; Lazović, V.M.; Ćurčić, B.P.; Krmpot, A.J. Nonlinear microscopy of chitin and chitinous structures: A case study of two cave-dwelling insects. J. Biomed. Opt. 2015, 20, 016010. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lee, S.Y.; Wu, Y.; Brink, K.; Shieh, D.B.; Huang, T.D.; Reisz, R.R.; Sun, C.K. Third-harmonic generation microscopy reveals dental anatomy in ancient fossils. Opt. Lett. 2015, 40, 1354–1357. [Google Scholar] [CrossRef]
- Lis, D.; Cecchet, F. Unique Vibrational Features as a Direct Probe of Specific Antigen–Antibody Recognition at the Surface of a Solid-Supported Hybrid Lipid Bilayer. ChemPhysChem 2016, 17, 2645–2649. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, C.J.; Park, D.; Bruns, O.T.; Piatkevich, K.D.; Fukumura, D.; Jain, R.K.; Bawendi, M.G.; Boyden, E.S.; So, P.T. Wide-field three-photon excitation in biological samples. Light Sci. Appl. 2017, 6, e16255. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, C.; Thoury, M.; Dazzi, A.; Latour, G.; Scheel, M.; Li, J.; Thomas, A.; Moulhérat, C.; Didier, A.; Bertrand, L. In-place molecular preservation of cellulose in5,000-year-old archaeological textiles. Proc. Natl. Acad. Sci. USA 2020, 117, 19670–19676. [Google Scholar] [CrossRef]
- Raoux, C.; Schmeltz, M.; Bied, M.; Alnawaiseh, M.; Hansen, U.; Latour, G.; Schanne-Klein, M.C. Quantitative structural imaging of keratoconic corneas using polarization-resolved SHG microscopy. Biomed. Opt. Express 2021, 12, 4163–4178. [Google Scholar] [CrossRef]
- Schmeltz, M.; Robinet, L.; Heu-Thao, S.; Sintès, J.M.; Teulon, C.; Ducourthial, G.; Mahou, P.; Schanne-Klein, M.C.; Latour, G. Noninvasive quantitative assessment of collagen degradation in parchments by polarization-resolved SHG microscopy. Sci. Adv. 2021, 7, eabg1090. [Google Scholar] [CrossRef]
- Mouchet, S.R.; Verstraete, C.; Mara, D.; Cleuvenbergen, S.V.; Finlayson, E.D.; Deun, R.V.; Deparis, O.; Verbiest, T.; Maes, B.; Vukusic, P.; et al. Nonlinear optical spectroscopy and two-photon excited fluorescence spectroscopy reveal the excited states of fluorophores embedded in a beetle’s elytra. Interface Focus 2019, 9, 20180052. [Google Scholar] [CrossRef]
- Stoddart, P.R.; Cadusch, P.J.; Boyce, T.M.; Erasmus, R.M.; Comins, J.D. Optical properties of chitin: Surface-enhanced Raman scattering substrates based on antireflection structures on cicada wings. Nanotechnology 2006, 17, 680. [Google Scholar] [CrossRef]
- Garrett, N.L.; Vukusic, P.; Ogrin, F.; Sirotkin, E.; Winlove, C.P.; Moger, J. Spectroscopy on the wing: Naturally inspired SERS substrates for biochemical analysis. J. Biophotonics 2009, 2, 157–166. [Google Scholar] [CrossRef]
- Garrett, N.L. Naturally Inspired SERS Substrates: Datasheet from · Volume: “Raman Spectroscopy for Nanomaterials Characterization”; SpringerMaterials: Cham, Switzerland, 2012. [Google Scholar] [CrossRef]
- Garrett, N.L.; Sekine, R.; Dixon, M.W.A.; Tilley, L.; Bambery, K.R.; Wood, B.R. Bio-Sensing with butterfly wings: Naturally occurring nano-structures for SERS-based malaria parasite detection. Phys. Chem. Chem. Phys. 2015, 17, 21164–21168. [Google Scholar] [CrossRef] [PubMed]
- Palmer, L.D.; Brooks, J.L.; Frontiera, R.R. Probing the coupling of butterfly wing photonic crystals to plasmon resonances with surface-enhanced Raman spectroscopy. J. Mater. Chem. C 2019, 7, 13887–13895. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, J.; Xie, G.; Liu, Z.; Shao, H. Cicada Wings: A Stamp from Nature for Nanoimprint Lithography. Small 2006, 2, 1440–1443. [Google Scholar] [CrossRef]
- Dellieu, L.; Sarrazin, M.; Simonis, P.; Deparis, O.; Vigneron, J.P. A two-in-one superhydrophobic and anti-reflective nanodevice in the grey cicada Cicada Orni (Hemiptera). J. Appl. Phys. 2014, 116, 024701. [Google Scholar] [CrossRef]
- Deparis, O.; Mouchet, S.R.; Dellieu, L.; Colomer, J.F.; Sarrazin, M. Nanostructured Surfaces: Bioinspiration for Transparency, Coloration and Wettability. Mater. Today Proc. 2014, 1S, 122–129. [Google Scholar] [CrossRef]
- Kovačević, A.; Petrović, S.; Mimidis, A.; Stratakis, E.; Pantelić, D.; Kolaric, B. Molding Wetting by Laser-Induced Nanostructures. Appl. Sci. 2020, 10, 6008. [Google Scholar] [CrossRef]
- Gao, T.; Zhu, X.; Wu, X.J.; Zhang, B.; Liu, H.L. Selectively Manipulating Upconversion Emission Channels with Tunable Biological Photonic Crystals. J. Phys. Chem. C 2021, 125, 732–739. [Google Scholar] [CrossRef]
- Zhang, D. Morphology Genetic Materials Templated from Nature Species; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]


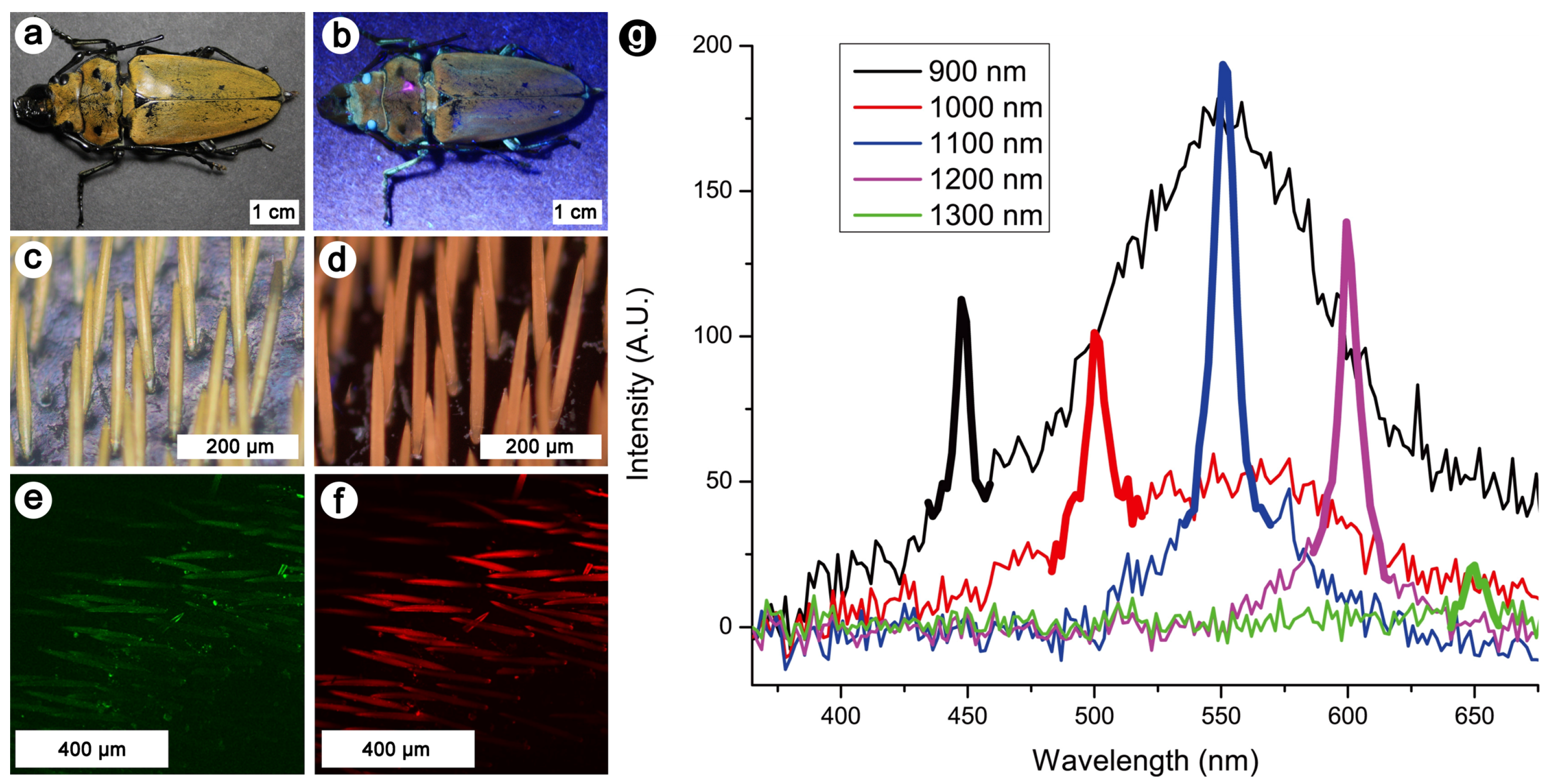
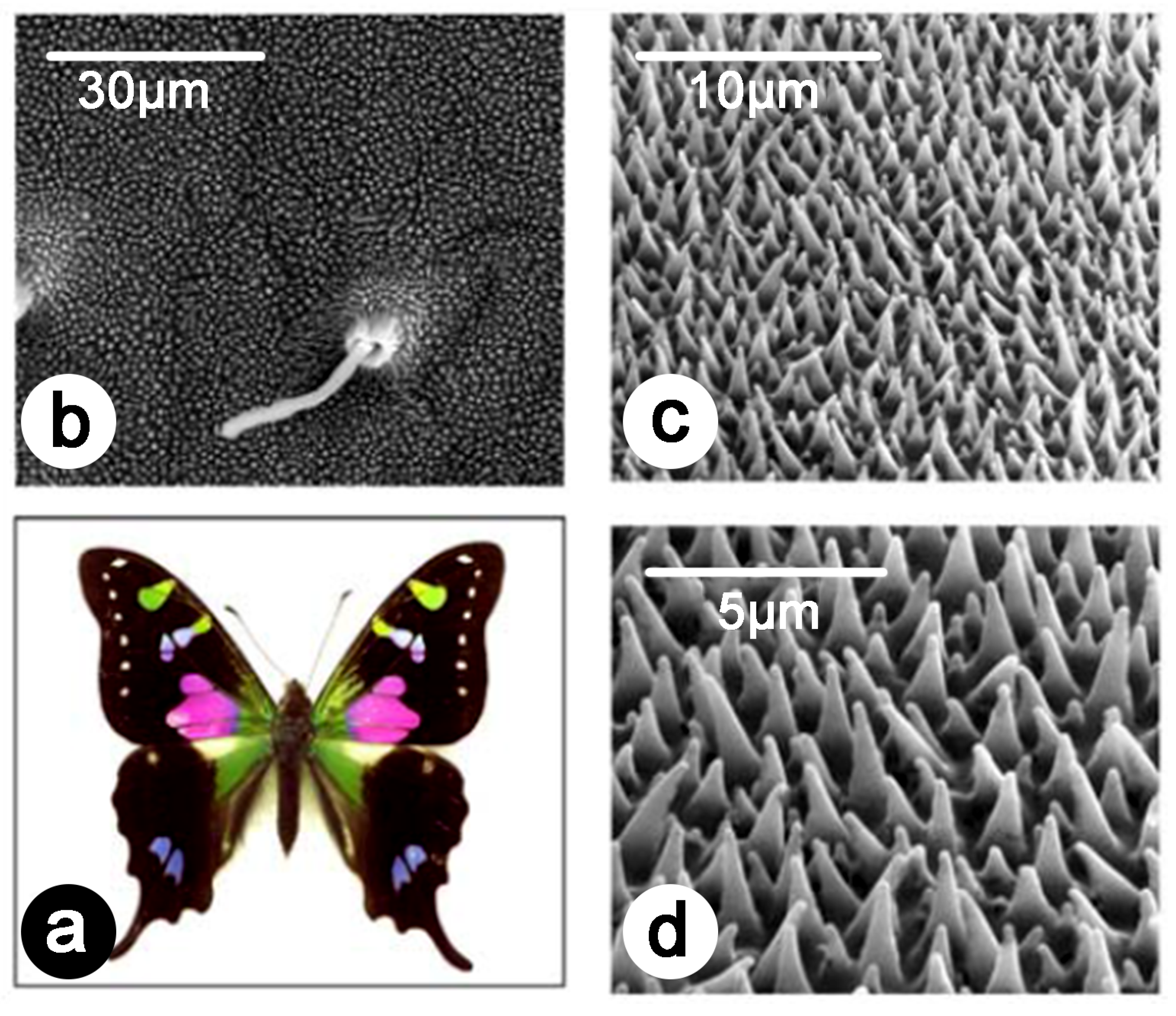
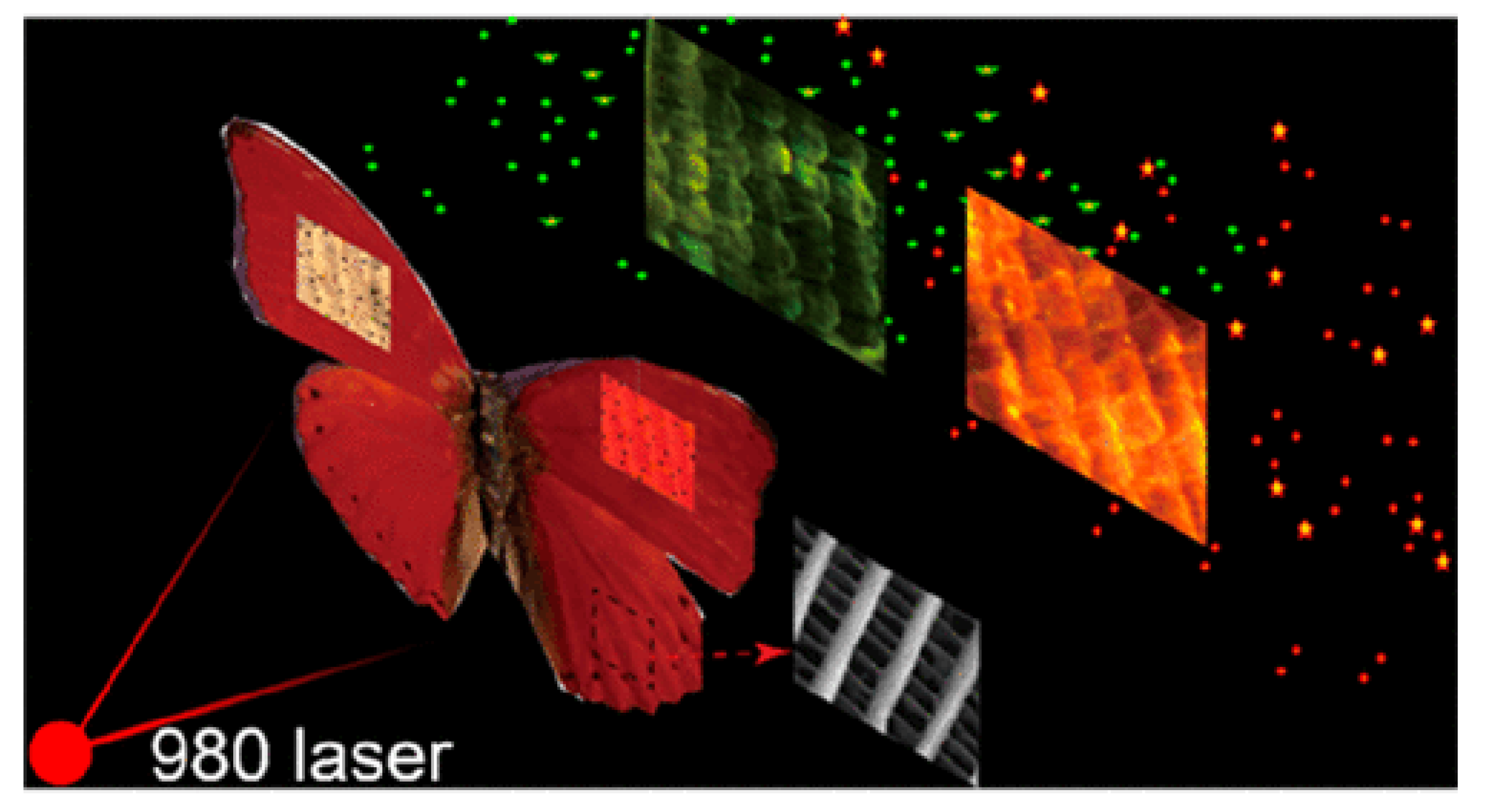
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mara, D.; Bokic, B.; Verbiest, T.; Mouchet, S.R.; Kolaric, B. Revealing the Wonder of Natural Photonics by Nonlinear Optics. Biomimetics 2022, 7, 153. https://doi.org/10.3390/biomimetics7040153
Mara D, Bokic B, Verbiest T, Mouchet SR, Kolaric B. Revealing the Wonder of Natural Photonics by Nonlinear Optics. Biomimetics. 2022; 7(4):153. https://doi.org/10.3390/biomimetics7040153
Chicago/Turabian StyleMara, Dimitrije, Bojana Bokic, Thierry Verbiest, Sébastien R. Mouchet, and Branko Kolaric. 2022. "Revealing the Wonder of Natural Photonics by Nonlinear Optics" Biomimetics 7, no. 4: 153. https://doi.org/10.3390/biomimetics7040153
APA StyleMara, D., Bokic, B., Verbiest, T., Mouchet, S. R., & Kolaric, B. (2022). Revealing the Wonder of Natural Photonics by Nonlinear Optics. Biomimetics, 7(4), 153. https://doi.org/10.3390/biomimetics7040153







