Paleomimetics: A Conceptual Framework for a Biomimetic Design Inspired by Fossils and Evolutionary Processes
Abstract
1. Introduction
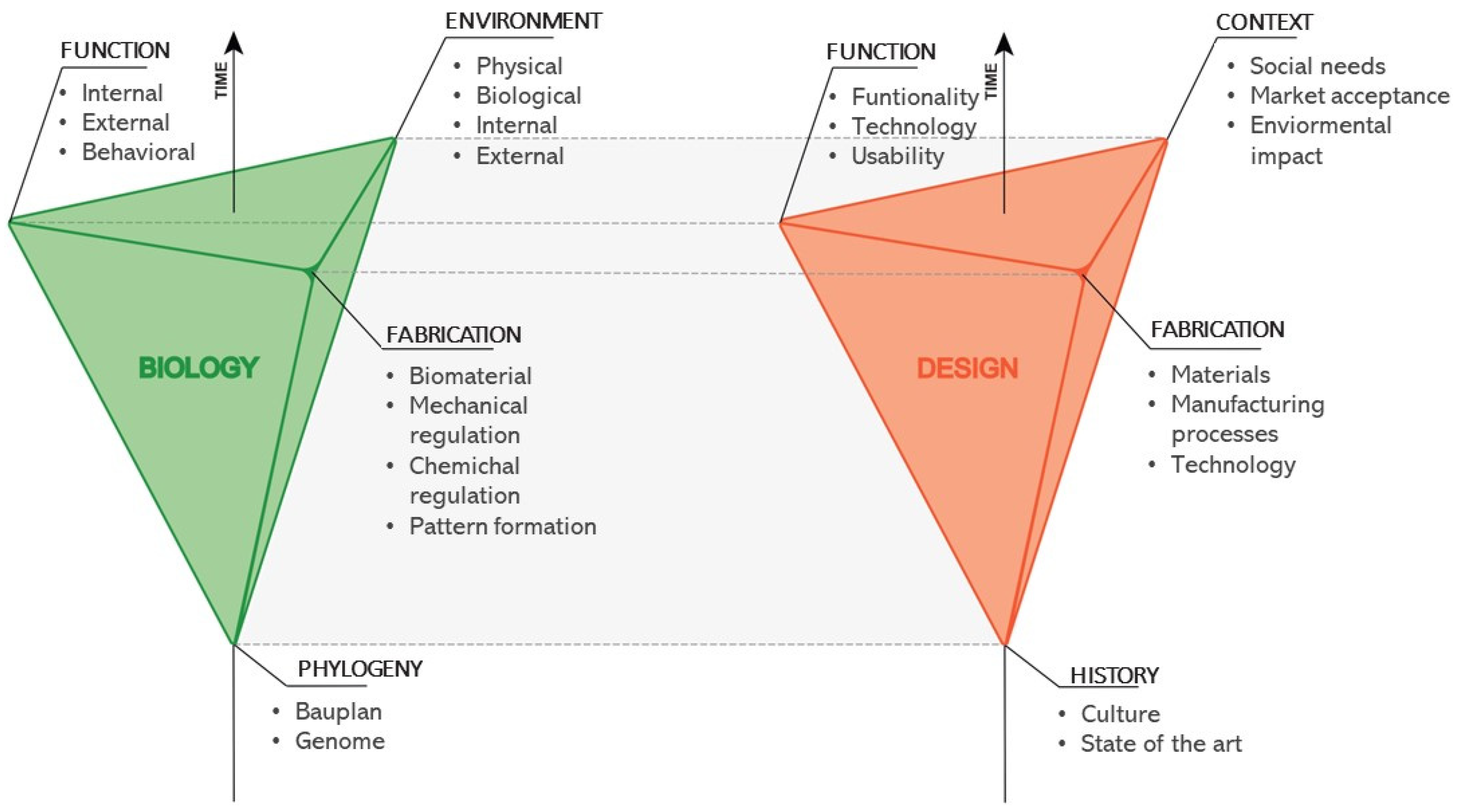
2. Disciplinary Transfer of Knowledge
2.1. From Palaeontology to Biomimetics
2.1.1. Evolutionary Process: Case and Necessity
2.1.2. Evolutionary Constraints
2.1.3. Evolutionary Traps
Trade-Offs
Vestigial Traits
Sexual Traits
Imperfections
2.2. From Biomimetics to Palaeontology
2.2.1. Reverse Biomimetics
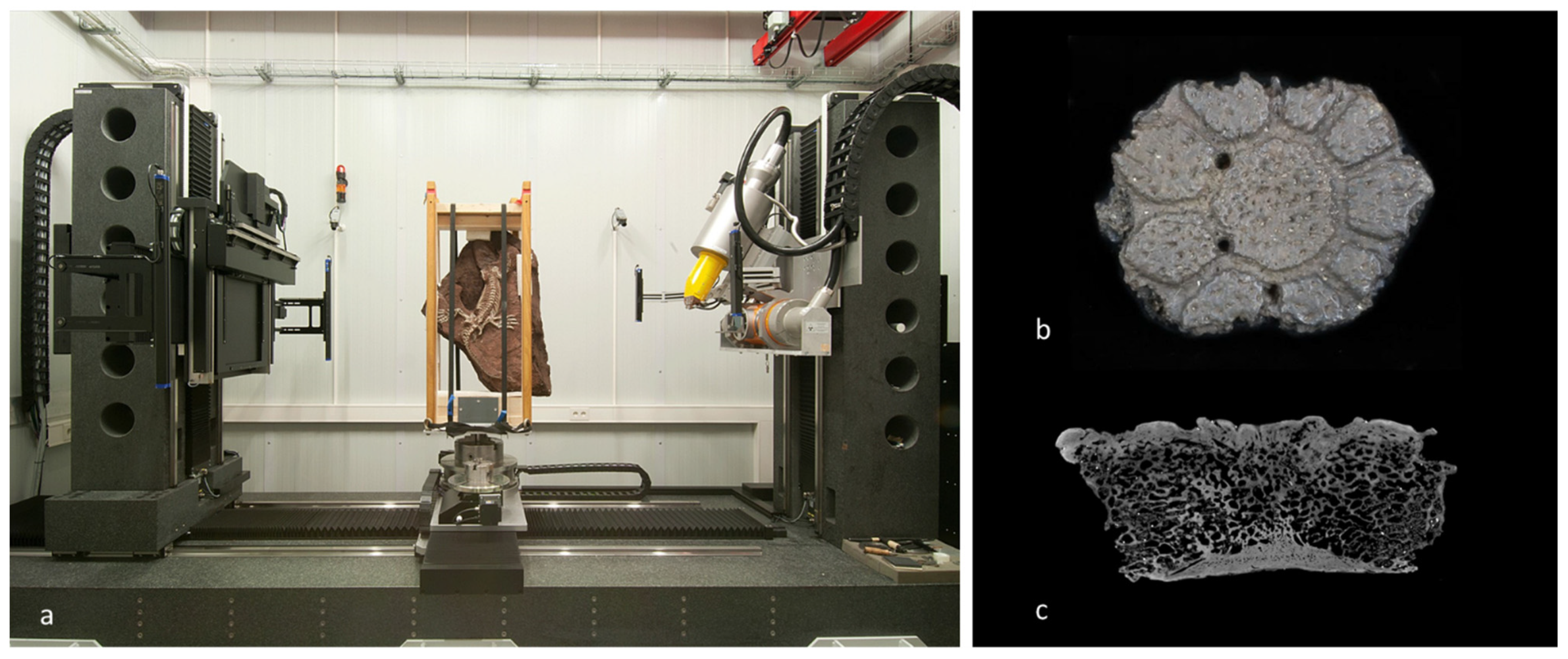
2.2.2. Virtual Palaeontology and Digital Processes
2.2.3. Valorisation of Basic Palaeontological Research
3. Fossil-Inspired Design: The State of the Art for Launching Paleomimetics
4. Paleomimetics: Definition, Methods, and Tools
4.1. Definition
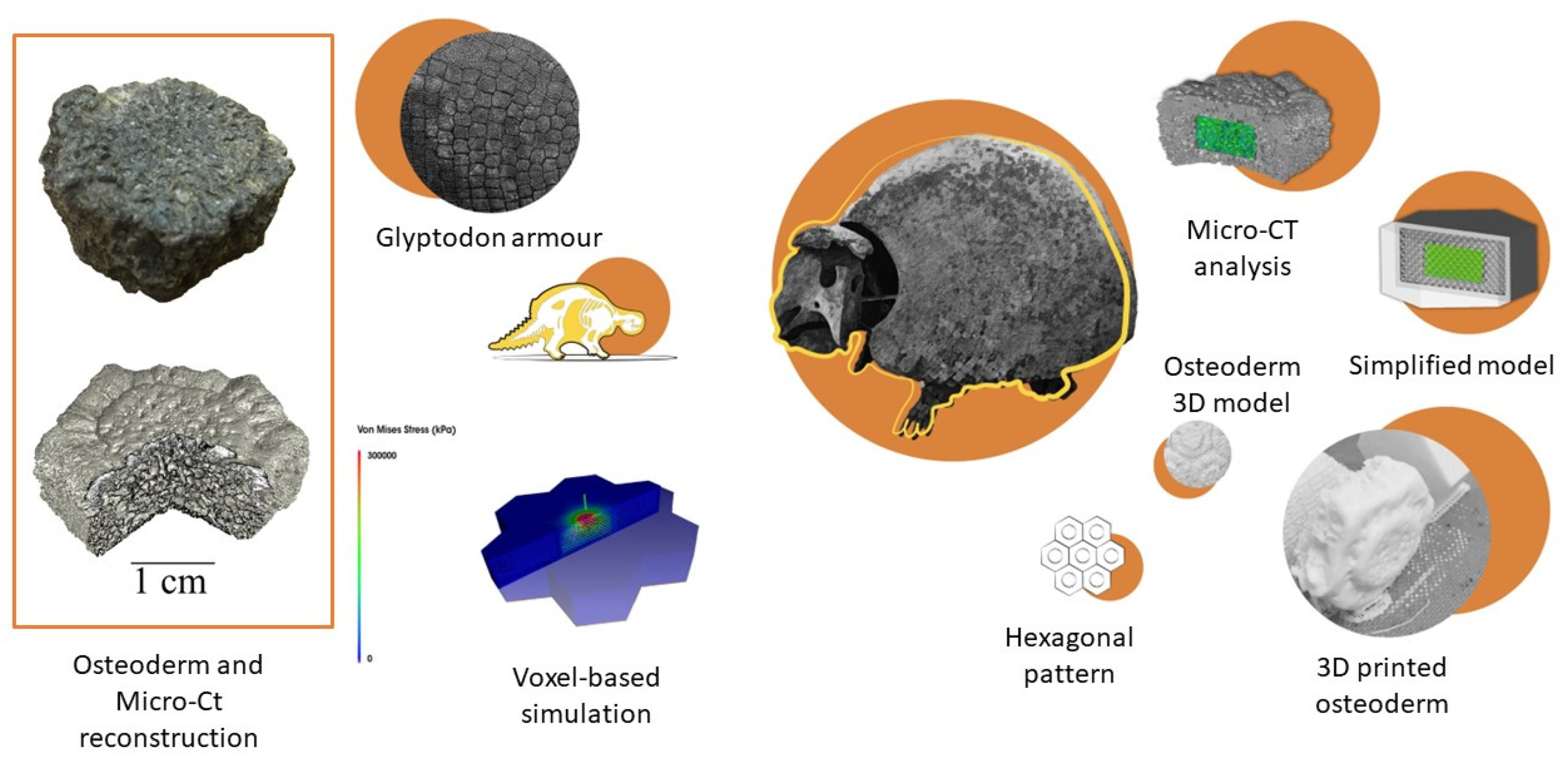
4.2. Methods and Tools
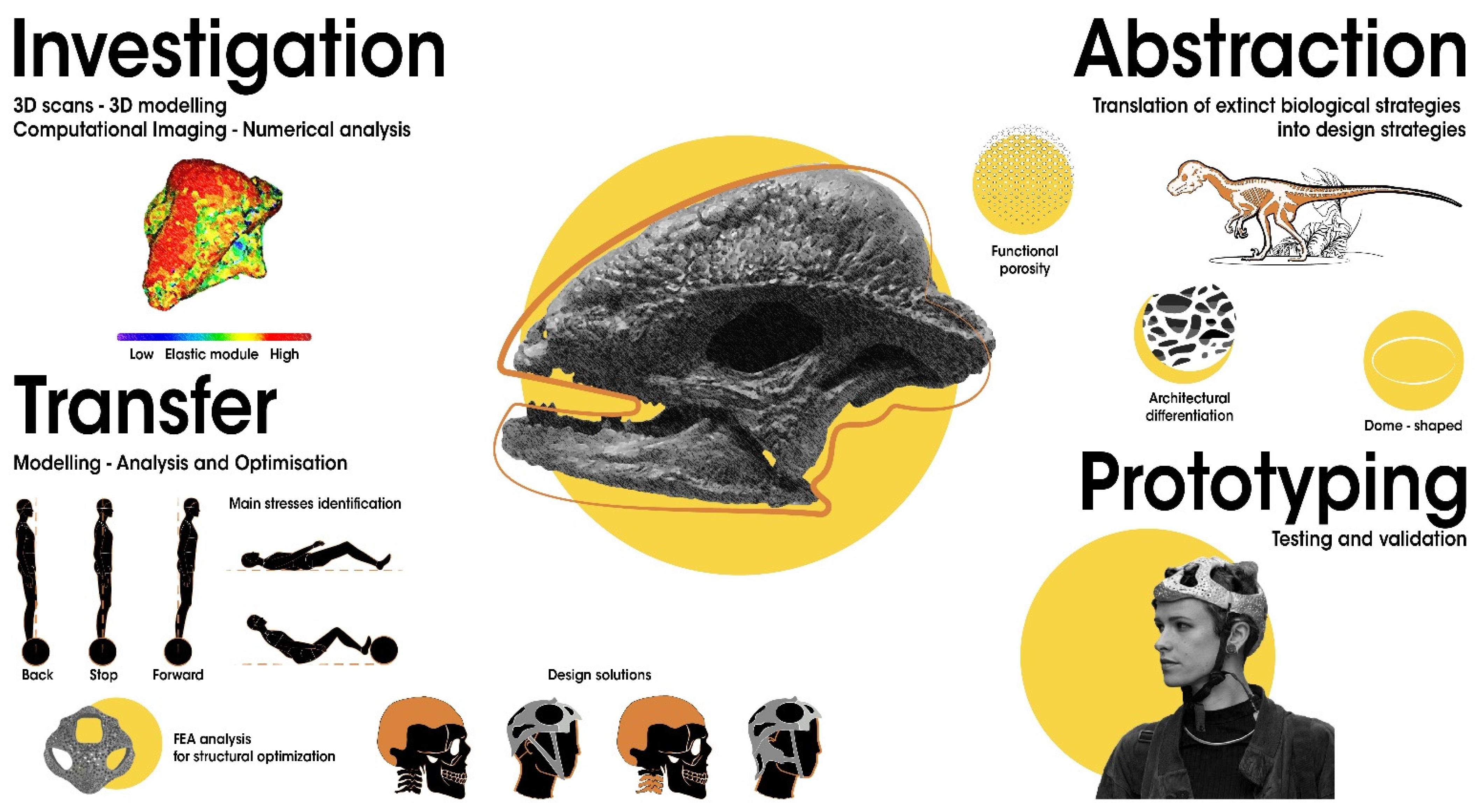
5. Paleomimetic Solutions: Promising Extinct Organismal Adaptations for Design Strategies
- (i)
- In feeding mechanisms, placoderms are an interesting example. They are armoured fishes that lived during the Devonian Period, 415–360 million years ago. The largest of the placoderm was Dunkleosteus terrelli; it was a voracious predator equipped with powerful, bladed jaws characteristic of a novel ability to fragment prey prior to ingestion [121]. Anderson and Westneat [122] developed a biomechanical force and motion model of D. terrelli during feeding, showing a highly kinetic skull driven by a unique four-bar linkage mechanism. Taking into consideration a large sample of D. terrelli (about 6 m in length), they were able to estimate a maximal bite force of over 4400 N at the jaw tip and more than 5300 N at the rear dental plates. The authors declared that “this bite force capability is the greatest of all living or fossil fishes and is among the most powerful bites in animals”. This solution could inspire the development of novel jawed technical devices (e.g., robotic arms, biomedical devices, etc).
- (ii)
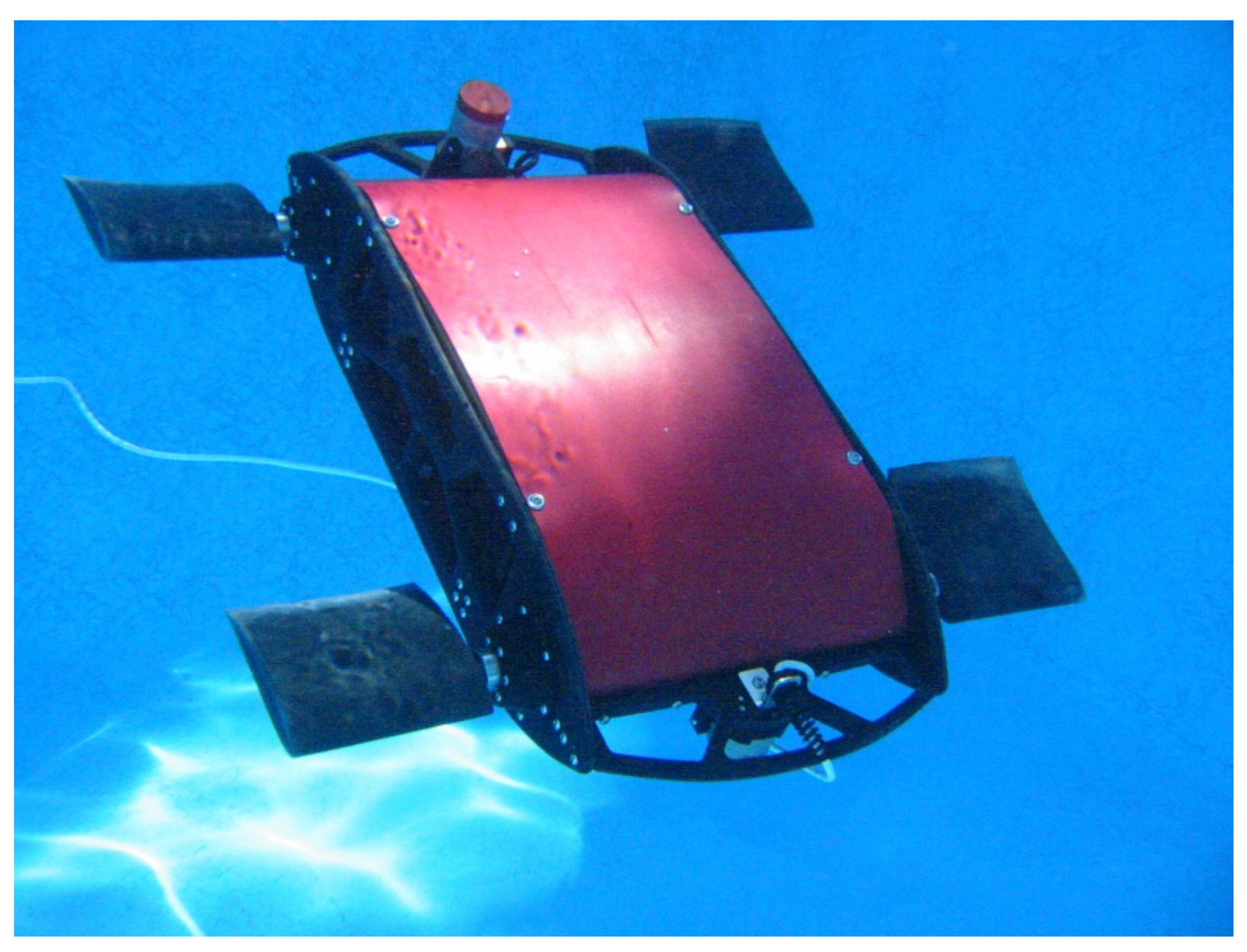
- In flight mechanisms, the pterosaur provides interesting solutions and some of them have been already incorporated into flight technology (see Section 3). Indeed, Mesozoic pterosaurs were powered fliers with complex and multi-layered membranous wings supported by a single spar (the arm and an elongated fourth finger). Additional smaller membranes to the fore and the rear were also present for flight control [100]. The largest pterosaurs (10 m in wingspan, 250 kg, and 3 m head skulls) vastly exceeded any known flying animal in size and weight. Martin-Silverstone et al. [100] described in detail the key knowledge of flight in extinct and extant organisms suggesting a series of potential solutions for future flight technologies. They highlighted how “fossil forms can provide plethora structures and integrated systems that can contribute to next-generation aircrafts, robots, low-flutter fabrics, and ultra-light structures”. The authors suggested that pterosaur-based wing designs could lead to a novel method for achieving aeroelastic stability by optimising the control of aeroelastic flutter. They emphasised that their “actinofibril orientation, tissue layering, wing shape, and span-wise bone geometry” present in the wing had a crucial role in utilising and controlling aeroelasticity, which could be effectively transferred. New self-launching and landing robots can be inspired by the pterosaur’s ground launch ability; effective over a wide range of body size, this launch model can guide future improvements in unmanned aerial vehicle (UAV) launch, landing, and storage. Additionally, the authors suggested pterosaurs-inspired control surfaces and wings.
6. Conclusions and Future Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Benyus, J.M. Biomimicry: Innovation Inspired by Nature; Morrow: New York, NY, USA, 1997. [Google Scholar]
- Vincent, J.F.; Bogatyreva, O.A.; Bogatyrev, N.R.; Bowyer, A.; Pahl, A.K. Biomimetics: Its practice and theory. J. R. Soc. Interface 2006, 3, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Gebeshuber, I.C.; Drack, M. An attempt to reveal synergies between biology and mechanical engineering. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2008, 222, 1281–1287. [Google Scholar] [CrossRef]
- Bhushan, B. Biomimetics: Lessons from nature—An overview. Philos. Trans. R. Soc. A Philos. T R. Soc. A 2009, 367, 1445–1486. [Google Scholar] [CrossRef] [PubMed]
- Fayemi, P.E.; Wanieck, K.; Zollfrank, C.; Maranzana, N.; Aoussat, A. Biomimetics: Process, tools and practice. Bioinspir. Biomim. 2017, 12, 011002. [Google Scholar] [CrossRef] [PubMed]
- Gruber, P.; Bruckner, D.; Hellmich, C.; Schmiedmayer, H.B.; Stachelberger, H.; Gebeshuber, I.C. Biomimetics—Materials, Structures and Processes: Examples, Ideas and Case Studies; Springer Science & Business Media: Berlin, Germany, 2011. [Google Scholar]
- Fish, F.E. Biomimetics and the Application of the Leading-Edge Tubercles of the Humpback Whale Flipper. In Flow Control through Bio-Inspired Leading-Edge Tubercles; New, D., Ng, B., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Taylor, G.; Thomas, A. Evolutionary Biomechanics: Selection, Phylogeny, and Constraint; OUP Oxford: Oxford, UK, 2014. [Google Scholar]
- Sander, P.M.; Christian, A.; Clauss, M.; Fechner, R.; Gee, C.T.; Griebeler, E.M.; Gunga, H.C.; Hummel, J.; Mallison, H.; Perry, S.F.; et al. Biology of the sauropod dinosaurs: The evolution of gigantism. Biol. Rev. 2011, 86, 117–155. [Google Scholar] [CrossRef] [PubMed]
- Rayfield, E.J. Finite element analysis and understanding the biomechanics and evolution of living and fossil organisms. Annu. Rev. Earth Planet. Sci. 2007, 35, 541–576. [Google Scholar] [CrossRef]
- Du Plessis, A.; Broeckhoven, C. Looking deep into nature: A review of micro-computed tomography in biomimicry. Acta Biomater. 2019, 85, 27–40. [Google Scholar] [CrossRef]
- Weber, G.W. Virtual anthropology. Am. J. Phys. Anthropol. 2015, 156, 22–42. [Google Scholar] [CrossRef]
- Pandolfi, L.; Raia, P.; Fortuny, J.; Rook, L. Editorial: Evolving Virtual and Computational Paleontology. Front. Earth Sci. 2020, 8, 591813. [Google Scholar] [CrossRef]
- Melchionna, M.; Profico, A.; Castiglione, S.; Serio, C.; Mondanaro, A.; Modafferi, M.; Tamagnini, D.; Maiorano, L.; Raia, P.; Witmer, L.M.L.M.; et al. A method for mapping morphological convergence on three-dimensional digital models: The case of the mammalian sabre-tooth. Palaeontology 2021, 64, 573–584. [Google Scholar] [CrossRef]
- Shyam, V.; Friend, L.; Whiteaker, B.; Bense, N.; Dowdall, J.; Boktor, B.; Johny, M.; Reyes, I.; Naser, A.; Sakhamuri, N.; et al. PeTaL (periodic table of life) and physiomimetics. Designs 2019, 3, 43. [Google Scholar] [CrossRef]
- Tihelka, E. Palaeobiotechnology. Geol. Today 2020, 36, 188–191. [Google Scholar] [CrossRef]
- Allasinaz, A. Paleontologia Generale e Sistematica Degli Invertebrati; ECIG: Manchester, UK, 1995. [Google Scholar]
- Seilacher, A.; Gishlick, A.D. Morphodynamics; Taylor & Francis: Oxfordshire, UK, 2015. [Google Scholar]
- Oxman, N. Age of Entanglement. J. Des. Sci. 2016. [Google Scholar] [CrossRef]
- Speck, T.; Speck, O. Process sequences in biomimetic research. In Design and Nature IV; Brebbia, C.A., Ed.; WIT Press: Southampton, UK, 2008; pp. 3–11. [Google Scholar]
- Monod, J. Chance and Necessity: An Essay on the Natural Philosophy of Modern Biology; Vintage Books: New York, NY, USA, 1972. [Google Scholar]
- Darwin, C. On the Origin of Species by Means of Natural Selection, or Preservation of Favoured Races in the Struggle for Life; John Murray: London, UK, 1859. [Google Scholar]
- Hine, R. A Dictionary of Biology; OUP Oxford: Oxford, UK, 2015. [Google Scholar]
- Theißen, G. The proper place of hopeful monsters in evolutionary biology. Theory Biosci. 2006, 124, 349–369. [Google Scholar] [CrossRef]
- Juniper, A. Wabi Sabi: The Japanese Art of Impermanence; Tuttle Publishing: North Clarendon, VT, USA, 2011. [Google Scholar]
- Perricone, V.; Langella, C.; Santulli, C. Sustainable Biomimetics: A Discussion on Differences in Scale, Complexity, and Organization Between the Natural and Artificial World. In Bionics and Sustainable Design; Springer: Singapore, 2022; pp. 171–193. [Google Scholar]
- Oxman, N. Material-Based Design Computation. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2010. [Google Scholar]
- Oxman, N. Structuring materiality: Design fabrication of heterogeneous materials. Archit. Des. 2010, 80, 78–85. [Google Scholar] [CrossRef]
- Coxworth, B. 3D-Printing “Error” Used to Produce High-Tech Textiles. 2020. Available online: https://newatlas.com/3d-printing/defextiles-3d-printed-textiles/ (accessed on 20 February 2022).
- Steadman, P. The Evolution of Designs: Biological Analogy in Architecture and the Applied Arts; Routledge: Oxfordshire, UK, 2008. [Google Scholar]
- Van Wassenbergh, S.; van Manen, K.; Marcroft, T.A.; Alfaro, M.E.; Stamhuis, E.J. Boxfish swimming paradox resolved: Forces by the flow of water around the body promote manoeuvrability. J. R. Soc. Interface 2015, 12, 20141146. [Google Scholar] [CrossRef]
- Adriaens, D. Evomimetics: The biomimetic design thinking 2.0. In Bioinspiration, Biomimetics, and Bioreplication IX 10965; SPIE: Bellingham, WA, USA, 2019; pp. 41–53. [Google Scholar]
- Broeckhoven, C.; du Plessis, A. Escaping the labyrinth of bioinspiration: Biodiversity as key to successful product innovation. Adv. Funct. Mater. 2022, 32, 2110235. [Google Scholar] [CrossRef]
- Hildebrandt, M. Analysis of Vertebrate Structure, 5th ed.; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Ferraguti, M.; Castellacci, C.; Allegrucci, G. Evoluzione: Modelli e Processi; Pearson: London, UK, 2011. [Google Scholar]
- Castiglione, S.; Serio, C.; Tamagnini, D.; Melchionna, M.; Mondanaro, A.; Di Febbraro, M.; Profico, A.; Piras, P.; Barattolo, F.; Raia, P. A new, fast method to search for morphological convergence with shape data. PLoS ONE 2019, 14, e0226949. [Google Scholar] [CrossRef]
- Hendricks, J.R.; Stigall, A.L.; Lieberman, B.S. The Digital Atlas of Ancient Life: Delivering information on paleontology and biogeography via the web. Palaeontol. Electron. 2015, 18.2.3E, 1–9. [Google Scholar]
- Varshney, K.; Chang, S.; Wang, Z.J. The kinematics of falling maple seeds and the initial transition to a helical motion. Nonlinearity 2011, 25, C1. [Google Scholar] [CrossRef]
- Gould, S.J. Evolutionary paleontology and the science of form. Earth Sci. Rev. 1970, 6, 77–119. [Google Scholar] [CrossRef]
- Gould, S.J. The Panda’s Thumb: More Reflections in Natural History; WW Norton & Company: New York, NY, USA, 2010. [Google Scholar]
- Jacob, F. Evolution and tinkering. Science 1977, 196, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An overview on 3D printing technology: Technological, materials, and applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Yan, X.; Chen, H.; Lin, S.; Xiao, S.; Yang, Y. Recent Advancements in Biomimetic 3D Printing Materials with Enhanced mechanical properties. Front. Mater. 2021, 8, 139. [Google Scholar] [CrossRef]
- Sznel, M. The time for Environment-Centered Design Has Come. 2020. Available online: https://uxdesign.cc/the-time-for-environment-centered-design-has-come-770123c8cc61 (accessed on 5 October 2021).
- Gruber, P. Biomimetics in Architecture; Springer: Vienna, Austria, 2011. [Google Scholar]
- Calladine, C.R. Theory of Shell Structures; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Wainwright, S.A.; Biggs, W.D.; Currey, J.D. Mechanical Design in Organisms; Princeton University Press: Princeton, NJ, USA, 1982. [Google Scholar]
- Vogel, S. Cats’ Paws and Catapults: Mechanical Worlds of Nature and People; WW Norton & Company: New York, NY, USA, 2000. [Google Scholar]
- Vogel, S. Comparative Biomechanics: Life’s Physical World; Princeton University Press: Princeton, NJ, USA, 2013. [Google Scholar]
- Perricone, V.; Santulli, C.; Rendina, F.; Langella, C. Organismal Design and Biomimetics: A Problem of Scale. Biomimetics 2021, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Aerts, P.; Van Damme, R.; Vanhooydonck, B.; Zaaf, A.; Herrel, A. Lizard locomotion: How morphology meets ecology. Neth. J. Zool. 2000, 50, 261–278. [Google Scholar] [CrossRef]
- Garland, T. Trade-offs. Curr. Biol. 2014, 24, R60–R61. [Google Scholar] [CrossRef]
- Griffiths, P.E. Functional analysis and proper functions. Br. J. Philos. Sci. 1993, 44, 409–422. [Google Scholar] [CrossRef]
- Müller, G. Vestigial organs and structures. Encycl. Evol. 2002, 2, 1131–1133. [Google Scholar]
- Sadier, A.; Sears, K.E.; Womack, M. Unraveling the heritage of lost traits. J. Exp. Zool. B Mol. Dev. Evol. 2022, 338, 107–118. [Google Scholar] [CrossRef]
- Raia, P.; Boggioni, M.; Carotenuto, F.; Castiglione, S.; Di Febbraro, M.; Di Vincenzo, F.; Melchionna, M.; Mondanaro, A.; Papini, A.; Profico, A.; et al. Unexpectedly rapid evolution of mandibular shape in hominins. Sci. Rep. 2018, 8, 7340. [Google Scholar] [CrossRef]
- Gould, S.J.; Vrba, E.S. Exaptation—A missing term in the science of form. Paleobiology 1982, 8, 4–15. [Google Scholar] [CrossRef]
- Darwin, C. The Descent of Man, and Selection in Relation to Sex; John Murray: London, UK, 1871. [Google Scholar]
- Williams, R. Unintelligent Design: Why God Isn’t as Smart as She Thinks She Is; Allen & Unwin: London, UK, 2006. [Google Scholar]
- Dawkins, R. The Greatest Show on Earth: The Evidence for Evolution; Simon and Schuster: New York, NY, USA, 2009. [Google Scholar]
- Pievani, T. The Natural History of Imperfection; Raffaello Cortina Editore: Milano, Italy, 2021. [Google Scholar]
- Denny, M.; McFadzean, A. Engineering Animals; Harvard University Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Hone, D.W.; Benton, M.J. The evolution of large size: How does Cope’s Rule work? Trends Ecol. Evol. 2005, 20, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.; Remes, K.; Carole, T.G.; Sander, M. Biology of the Sauropod Dinosaurs: Understanding the Life of Giants; Indiana University Press: Bloomingto, WV, USA, 2011. [Google Scholar]
- Speck, O.; Speck, T. Biomimetics and education in Europe: Challenges, opportunities, and variety. Biomimetics 2021, 6, 49. [Google Scholar] [CrossRef]
- Sutton, M.; Rahman, I.; Garwood, R. Virtual paleontology—An overview. Paleontol. Soc. Pap. 2016, 22, 1–20. [Google Scholar] [CrossRef]
- Speck, O.; Speck, D.; Horn, R.; Gantner, J.; Sedlbauer, K.P. Biomimetic bio-inspired biomorph sustainable? An attempt to classify and clarify biology-derived technical developments. Bioinspir. Biomim. 2017, 12, 011004. [Google Scholar] [CrossRef]
- Yang, Y.; Song, X.; Li, X.; Chen, Z.; Zhou, C.; Zhou, Q.; Chen, Y. Recent progress in biomimetic additive manufacturing technology: From materials to functional structures. Adv. Mater. 2018, 30, 1706539. [Google Scholar] [CrossRef]
- Du Plessis, A.; Broeckhoven, C.; Yadroitsava, I.; Yadroitsev, I.; Hands, C.H.; Kunju, R.; Bhate, D. Beautiful and functional: A review of biomimetic design in additive manufacturing. Addit. Manuf. 2019, 27, 408–427. [Google Scholar] [CrossRef]
- Linder, W. Digital Photogrammetry; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Boehler, W.; Vicent, M.B.; Marbs, A. Investigating laser scanner accuracy. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci.-ISPRS Arch. 2003, 34 Pt 5, 696–701. [Google Scholar]
- Díez Díaz, V.; Mallison, H.; Asbach, P.; Schwarz, D.; Blanco, A. Comparing surface digitization techniques in palaeontology using visual perceptual metrics and distance computations between 3D meshes. Palaeontology 2021, 64, 179–202. [Google Scholar] [CrossRef]
- Profico, A.; Buzi, C.; Castiglione, S.; Melchionna, M.; Piras, P.; Veneziano, A.; Raia, P. Arothron: An R package for geometric morphometric methods and virtual anthropology applications. Am. J. Phys. Anthropol. 2021, 176, 144–151. [Google Scholar]
- Badarnah, L.; Kadri, U. A methodology for the generation of biomimetic design concepts. Archit. Sci. Rev. 2015, 58, 120–133. [Google Scholar] [CrossRef]
- Baltsavias, E.P. A comparison between photogrammetry and laser scanning. ISPRS J. Photogramm. Remote Sens. 1999, 54, 83–94. [Google Scholar] [CrossRef]
- Cattaneo, P.M.; Dalstra, M.; Frich, L.H. A three-dimensional finite element model from computed tomography data: A semi-automated method. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2001, 215, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Hatze, H. Was ist Biomechanik. Leibesüb. Leibeserzieh. 1971, 25, 32–34. [Google Scholar]
- Thilmany, J. Fossils and FEA. Mech. Eng. 2012, 134, 44–47. [Google Scholar] [CrossRef][Green Version]
- Rowe, N.P.; Speck, T. Biomechanics of plant growth forms: The trouble with fossil plants. Rev. Palaeobot. Palynol. 1998, 102, 43–62. [Google Scholar] [CrossRef]
- Bright, J.A. A review of paleontological finite element models and their validity. J. Paleontol. 2014, 88, 760–769. [Google Scholar] [CrossRef]
- Witmer, L.M.; Thomason, J.J. The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils. Funct. Morphol. Vertebr. Paleontol. 1995, 1, 19–33. [Google Scholar]
- Cunningham, J.A.; Rahman, I.A.; Lautenschlager, S.; Rayfield, E.J.; Donoghue, P.C. A virtual world of paleontology. Trends Ecol. Evol. 2014, 29, 347–357. [Google Scholar] [CrossRef]
- Daniel, T.L.; Helmuth, B.S.; Saunders, W.B.; Ward, P.D. Septal complexity in ammonoid cephalopods increased mechanical risk and limited depth. Paleobiology 1997, 24, 470–481. [Google Scholar] [CrossRef]
- Hassan, M.A.; Westermann, G.E.G.; Hewitt, R.A.; Dokainish, M.A. Finite-element analysis of simulated ammonoid septa (extinct Cephalopoda): Septal and sutural complexities do not reduce strength. Paleobiology 2002, 28, 113–126. [Google Scholar] [CrossRef]
- Watson, P.J.; Gröning, F.; Curtis, N.; Fitton, L.C.; Herrel, A.; McCormack, S.W.; Fagan, M.J. Masticatory biomechanics in the rabbit: A multi-body dynamics analysis. J. R. Soc. Interface 2014, 11, 20140564. [Google Scholar] [CrossRef] [PubMed]
- Bates, K.T.; Falkingham, P.L. Estimating maximum bite performance in Tyrannosaurus rex using multi-body dynamics. Biol. Lett. 2012, 8, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Sellers, W.I.; Pond, S.B.; Brassey, C.A.; Manning, P.L.; Bates, K.T. Investigating the running abilities of Tyrannosaurus rex using stress-constrained multibody dynamic analysis. PeerJ 2017, 5, e3420. [Google Scholar] [CrossRef] [PubMed]
- Lautenschlager, S.; Brassey, C.A.; Button, D.J.; Barrett, P.M. Decoupled form and function in disparate herbivorous dinosaur clades. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.A. Computational fluid dynamics as a tool for testing functional and ecological hypotheses in fossil taxa. Palaeontology 2017, 60, 451–459. [Google Scholar] [CrossRef]
- Dynowski, J.F.; Nebelsick, J.H.; Klein, A.; Roth-Nebelsick, A. Computational fluid dynamics analysis of the fossil crinoid Encrinus liliiformis (Echinodermata: Crinoidea). PLoS ONE 2016, 11, e0156408. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Long, J.H.; Schumacher, J.; Livingston, N.; Kemp, M. Four flippers or two? Tetrapodal swimming with an aquatic robot. Bioinspir. Biomim. 2006, 1, 20. [Google Scholar] [CrossRef]
- Ramesh, S.; Sairam, M.; Kumar, S.M.; Harikrishnan, R. The Martian man—A heuristic approach on robots to Mars. In Proceedings of the IEEE International Conference on Robotics and Biomimetics (ROBIO), Guilin, China, 19–23 December 2009. [Google Scholar]
- Germann, D.P.; Schatz, W.; Hotz, P.E. Bivalve burrowing robots: Correlating shell morphology and movement pattern with burrowing efficiency. Des. Nat. V Comp. Des. Nat. Sci. Eng. 2010, 5, 389. [Google Scholar]
- Yan, S.; Hao, J.; Wu, Z. A Dual-Mode Real-Time Lip-Sync System for a Bionic Dinosaur Robot. In Proceedings of the Chinese Automation Congress (CAC), Xi’an, China, 30 November–2 December 2018. [Google Scholar]
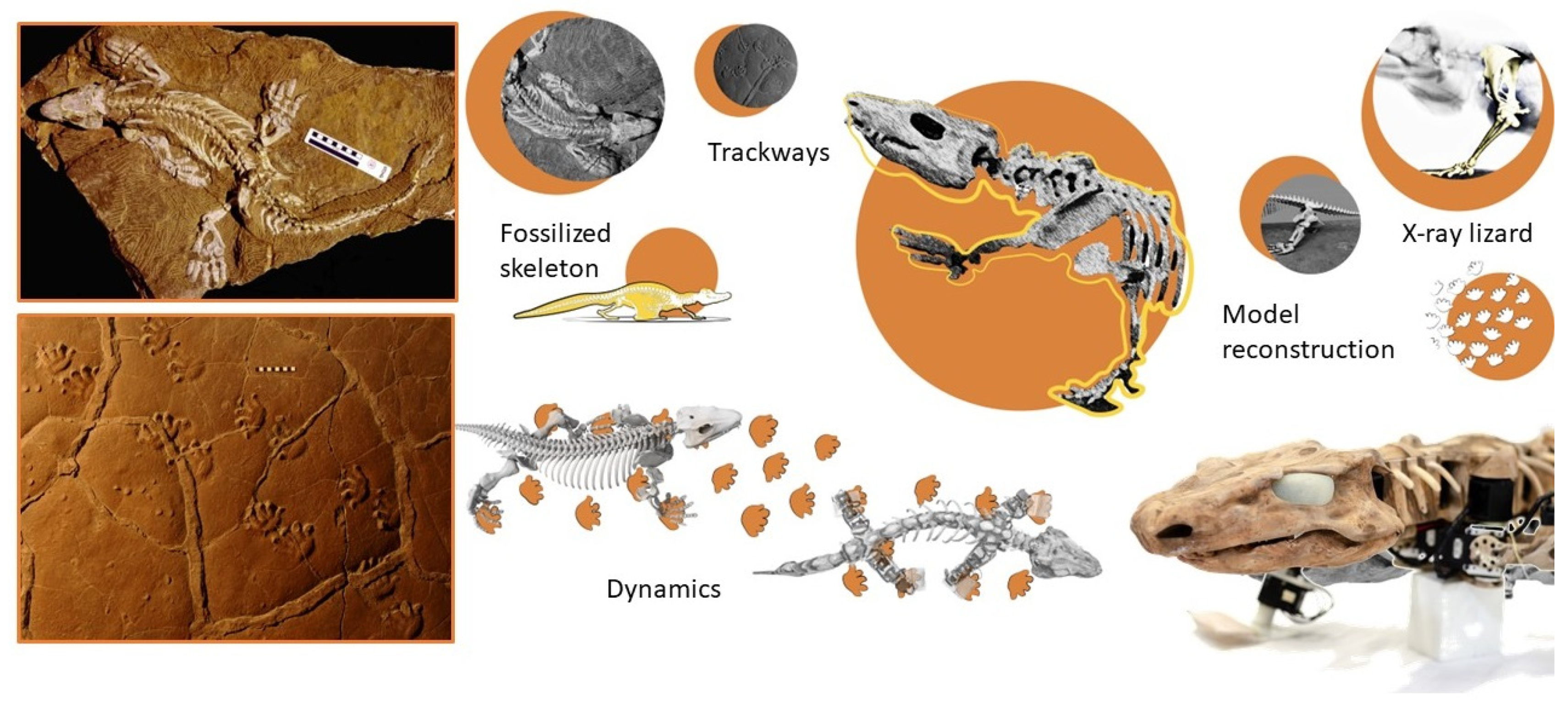
- Nyakatura, J.A.; Melo, K.; Horvat, T.; Karakasiliotis, K.; Allen, V.R.; Andikfar, A.; Andrada, E.; Arnold, P.; Lauströer, J.; Hutchinson, J.R.; et al. Reverse-engineering the locomotion of a stem amniote. Nature 2019, 565, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Kulfan, B. Dr. John McMasters: Following the foot steps of a creative paleoaerodynamicist. In Proceedings of the 47th AIAA Aerospace Sciences Meeting including The New Horizons Forum and Aerospace Exposition, Orlando, FL, USA, 5–8 January 2009. [Google Scholar]
- Zakaria, M.Y.; Taha, H.E.; Hajj, M.R. Design optimization of flapping ornithopters: The pterosaur replica in forward flight. J. Aircr. 2016, 53, 48–59. [Google Scholar] [CrossRef]
- Shahid, F.; Zhao, J.S.; Godefroit, P. Design of flying robots inspired by the evolution of avian flight. Proc. Inst. Mech. Eng. C J. Mech. Eng. Sci. 2019, 233, 7669–7686. [Google Scholar] [CrossRef]
- Martin-Silverstone, E.; Habib, M.B.; Hone, D.W. Volant fossil vertebrates: Potential for bioinspired flight technology. Trends Ecol. Evol. 2020, 35, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Song, H.W.; Talori, Y.S.; Zhao, J.S. Bionic Flapping Mechanism of the Wings of a Cursorial Dinosaur Robot for Estimating Its Lift and Thrust. J. Mech. Robot. 2021, 13, 015002. [Google Scholar] [CrossRef]
- Talori, Y.S.; Liu, Y.F.; Zhao, J.S.; Sullivan, C.; O’Connor, J.K.; Li, Z.H. Winged forelimbs of the small theropod dinosaur Caudipteryx could have generated small aerodynamic forces during rapid terrestrial locomotion. Sci. Rep. 2018, 8, 17854. [Google Scholar] [CrossRef]
- Long, J.H., Jr.; Koob, T.J.; Irving, K.; Combie, K.; Engel, V.; Livingston, N.; Lammert, A.; Schumacher, J. Biomimetic evolutionary analysis: Testing the adaptive value of vertebrate tail stiffness in autonomous swimming robots. J. Exp. Biol. 2006, 209, 4732–4746. [Google Scholar] [CrossRef]
- Roberts, S.F.; Hirokawa, J.; Rosenblum, H.G.; Sakhtah, H.; Gutierrez, A.A.; Porter, M.E.; Long, J.H., Jr. Testing biological hypotheses with embodied robots: Adaptations, accidents, and by-products in the evolution of vertebrates. Front. Robot. AI 2014, 1, 12. [Google Scholar] [CrossRef]
- ISO 18458:2015; Biomimetics—Terminology, Concepts and Methodology. ISO (International Organization for Standardization) TC266; Beuth Verlag: Berlin, Germany, 2015.
- Cohen, Y.H.; Reich, Y. Biomimetic Design Method for Innovation and Sustainability; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- AskNature-Innovation Inspired by Nature. Available online: https://asknature.org/ (accessed on 20 January 2022).
- Hamm, C. Evolution of Lightweight Structures: Analyses and Technical Applications; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Parker, S. Evolution: The Whole Story; Thames & Hudson: London, UK, 2015. [Google Scholar]
- Snively, E.; Cox, A. Structural mechanics of pachycephalosaur crania permitted head-butting behavior. Palaeontol. Electron. 2008, 11, 3A. [Google Scholar]
- Snively, E.; Theodor, J.M. Common functional correlates of head-strike behavior in the pachycephalosaur Stegoceras validum (Ornithischia, Dinosauria) and combative artiodactyls. PLoS ONE 2011, 6, e21422. [Google Scholar] [CrossRef] [PubMed]
- Deb, K. Multi-Objective Optimization Using Evolutionary Algorithms; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Mattheck, C.M. Design in Nature: Learning from Trees; Springer Science & Business Media: Berlin, Germany, 1998. [Google Scholar]
- Yang, W.; Chen, I.H.; Mckittrick, J.; Meyers, M.A. Flexible dermal armor in nature. Jom 2012, 64, 475–485. [Google Scholar] [CrossRef]
- Broeckhoven, C.; du Plessis, A.; Hui, C. Functional trade-off between strength and thermal capacity of dermal armor: Insights from girdled lizards. J. Mech. Behav. Biomed. Mater. 2017, 74, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.K.; Wang, H.; Hazell, P.J. From biology to biomimicry: Using nature to build better structures—A review. Constr. Build. Mater. 2022, 320, 126195. [Google Scholar] [CrossRef]
- Du Plessis, A.; Broeckhoven, C.; Yadroitsev, I.; Yadroitsava, I.; le Roux, S.G. Analyzing nature’s protective design: The glyptodont body armor. J. Mech. Behav. Biomed. Mater. 2018, 82, 218–223. [Google Scholar] [CrossRef]
- De Blasio, F.V. Thriving at high hydrostatic pressure: The example of ammonoids (extinct cephalopods). Bioinspiration Biomim. 2006, 1, L1. [Google Scholar] [CrossRef]
- Hildebrand, M. Analysis of Vertebrate Structure, 3rd ed.; Zanichelli: Bologna, Italy, 1992. [Google Scholar]
- Martone, P.T.; Boller, M.; Burgert, I.; Dumais, J.; Edwards, J.; Mach, K.; Rowe, N.; Rueggeberg, M.; Seidel, R.; Speck, T. Mechanics without muscle: Biomechanical inspiration from the plant world. Integr. Comp. Biol. 2010, 50, 888–907. [Google Scholar] [CrossRef]
- Miles, R.S. VI.—Features of Placoderm Diversification and the Evolution of the Arthrodire Feeding Mechanism*. Earth Environ. Sci. Trans. R. Soc. Edinb. 1969, 68, 123–170. [Google Scholar] [CrossRef]
- Anderson, P.S.; Westneat, M.W. Feeding mechanics and bite force modelling of the skull of Dunkleosteus terrelli, an ancient apex predator. Biol. Lett. 2007, 3, 77–80. [Google Scholar] [CrossRef]
- Hart, J.W. Plant Tropisms: And Other Growth Movements; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1990. [Google Scholar]
- Esmon, C.A.; Pedmale, U.V.; Liscum, E. Plant tropisms: Providing the power of movement to a sessile organism. Int. J. Dev. Biol. 2004, 49, 665–674. [Google Scholar] [CrossRef]
- Poppinga, S.; Nestle, N.; Šandor, A.; Reible, B.; Masselter, T.; Bruchmann, B.; Speck, T. Hygroscopic motions of fossil conifer cones. Sci. Rep. 2017, 7, 40302. [Google Scholar] [CrossRef] [PubMed]
- Dodd, J.R.; Stanton, R.J. Paleoecology: Concepts and Applications; John Wiley & Sons: New York, NY, USA, 1991. [Google Scholar]
- Hickman, C.S. Analysis of form and function in fossils. Am. Zool. 1988, 28, 775–793. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perricone, V.; Grun, T.; Raia, P.; Langella, C. Paleomimetics: A Conceptual Framework for a Biomimetic Design Inspired by Fossils and Evolutionary Processes. Biomimetics 2022, 7, 89. https://doi.org/10.3390/biomimetics7030089
Perricone V, Grun T, Raia P, Langella C. Paleomimetics: A Conceptual Framework for a Biomimetic Design Inspired by Fossils and Evolutionary Processes. Biomimetics. 2022; 7(3):89. https://doi.org/10.3390/biomimetics7030089
Chicago/Turabian StylePerricone, Valentina, Tobias Grun, Pasquale Raia, and Carla Langella. 2022. "Paleomimetics: A Conceptual Framework for a Biomimetic Design Inspired by Fossils and Evolutionary Processes" Biomimetics 7, no. 3: 89. https://doi.org/10.3390/biomimetics7030089
APA StylePerricone, V., Grun, T., Raia, P., & Langella, C. (2022). Paleomimetics: A Conceptual Framework for a Biomimetic Design Inspired by Fossils and Evolutionary Processes. Biomimetics, 7(3), 89. https://doi.org/10.3390/biomimetics7030089







