Biomimetic Implant Surfaces and Their Role in Biological Integration—A Concise Review
Abstract
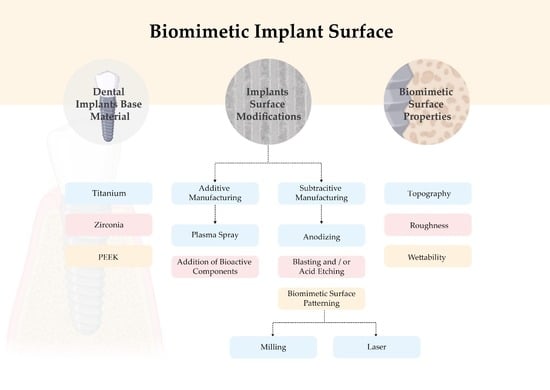
:1. Introduction
- (1)
- The initial tissue response;
- (2)
- Peri-implant osteogenesis;
- (3)
- Peri-implant bone remodelling.
2. Search Strategy and Data Retrieval
3. Dental Implants Base Materials
3.1. Titanium
3.2. Zirconia
3.3. Polyether Ether Eketone
4. Biomimetic Surface Properties
4.1. Topography
4.2. Roughness
4.3. Wettability
5. Implant Surface Modifications
5.1. Biomimetic Surface Modifications—Additive Manufacturing
5.1.1. Plasma Spray
5.1.2. Addition of Bioactive Components
5.2. Biomimetic Surface Modifications—Subtractive Manufacturing
5.2.1. Anodizing
5.2.2. Blasting and/or Acid Etching
5.3. Biomimetic Surface Patterning
5.3.1. Milling
5.3.2. Laser
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Londoño, J.J.; Ramos, A.M.; Correa, S.A.; Mesnard, M. Review of expandable dental implants. Br. J. Oral Maxillo-Facial Surg. 2021, 59, 546–554. [Google Scholar] [CrossRef]
- Esposito, M.; Ardebili, Y.; Worthington, H.V. Interventions for replacing missing teeth: Different types of dental implants. Cochrane Database Syst. Rev. 2019, 2019, CD003815. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Müller, N.; Cionca, N. The epidemiology of peri-implantitis. Clin. Oral Implant. Res. 2012, 23, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Elias, C.N.; Oshida, Y.; Lima, J.H.C.; Muller, C.A. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J. Mech. Behav. Biomed. Mater. 2008, 1, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Gupta, N.; Weber, K.K.; Dental Implants. StatPearls 2021. Available online: https://europepmc.org/article/nbk/nbk470448 (accessed on 13 April 2022).
- Comisso, I.; Arias-Herrera, S.; Gupta, S. Zirconium dioxide implants as an alternative to titanium: A systematic review. J. Clin. Exp. Dent. 2021, 13, e511–e519. [Google Scholar] [CrossRef]
- Bobbio, A. The first endosseous alloplastic implant in the history of man. Bull. Hist. Dent. 1972, 20, 1–6. [Google Scholar]
- Branemark, P. Vital microscopy of bone marrow in rabbit. Scand. J. Clin. Lab. Investig. 1959, 11, 1–82. [Google Scholar]
- Brånermark, P.; Adell, R.; Albrektsson, T.; Lekholm, U.; Lundkivist, S.; Rockler, B. Osseointegrated titanium fixtures in the treatment of edentulousness. Biomaterials 1983, 4, 25–28. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; da Silva Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, G.; Francetti, L.; Barbaro, B.; del Fabbro, M. Novel surfaces and osseointegration in implant dentistry. J. Inves-Tigative Clin. Dent. 2018, 9, e12349. [Google Scholar] [CrossRef]
- McMahon, M.; Ye, S.; Pedrina, J.; Dlugolenski, D.; Stambas, J. Extracellular Matrix Enzymes and Immune Cell Biology. Front. Mol. Biosci. 2021, 8, 703868. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Worthington, H. Interventions for replacing missing teeth: Treatment of peri-implantitis. Cochrane Database Syst. Rev. 2012, 1, CD004970. [Google Scholar] [CrossRef] [PubMed]
- Liñares, A.; Grize, L.; Muñoz, F.; Pippenger, B.E.; Dard, M.; Domken, O.; Blanco-Carrión, J. Histological assessment of hard and soft tissues sur-rounding a novel ceramic implant: A pilot study in the minipig. J. Clin. Periodontol. 2016, 43, 538–546. [Google Scholar] [CrossRef]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prostho-dontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Brånemark, R.; Brånemark, P.I.; Rydevik, B.; Myers, R.R. Osseointegration in skeletal reconstruction and rehabilitation: A review. J. Rehabil. Res. Dev. 2001, 38, 175–181. [Google Scholar]
- Lee, J.W.Y.; Bance, M.L. Physiology of Osseointegration. Otolaryngol. Clin. N. Am. 2019, 52, 231–242. [Google Scholar] [CrossRef]
- Ajami, E.; Fu, C.; Wen, H.B.; Bassett, J.; Park, S.J.; Pollard, M. Early Bone Healing on Hydroxyapatite-Coated and Chemically-Modified Hydrophilic Implant Surfaces in an Ovine Model. Int. J. Mol. Sci. 2021, 22, 9361. [Google Scholar] [CrossRef]
- le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Amengual-Peñafiel, L.; Córdova, L.A.; Jara-Sepúlveda, M.C.; Brañes-Aroca, M.; Marchesani-Carrasco, F.; Cartes-Velásquez, R. Osteoimmunology drives dental implant osseointegration: A new paradigm for implant dentistry. Jpn. Dent. Sci. Rev. 2021, 57, 12–19. [Google Scholar] [CrossRef]
- Chawla, A. Control of Macrophage Activation and Function by PPARs. Circ. Res. 2010, 106, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Adachi, T. Cell-fate decision of mesenchymal stem cells toward osteocyte differentiation is committed by spheroid culture. Sci. Rep. 2021, 11, 13204. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Irandoust, S.; Müftü, S. The interplay between bone healing and remodeling around dental implants. Sci. Rep. 2020, 10, 4335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarb, G.A.; Schmitt, A. The longitudinal clinical effectiveness of osseointegrated dental implants in anterior partially edentulous patients. Implant Dent. 1993, 6, 189–196. [Google Scholar] [CrossRef]
- Sykaras, N.; Iacopino, A.M.; Marker, V.A.; Triplett, R.G.; Woody, R.D. Implant materials, designs, and surface topographies: Their effect on osseointegration. A literature review. Int. J. Oral Maxillofac. Implant. 2000, 15, 675–690. [Google Scholar]
- Körtvélyessy, G.; Tarjányi, T.; Baráth, Z.L.; Minarovits, J.; Tóth, Z. Bioactive coatings for dental implants: A review of alternative strategies to prevent peri-implantitis induced by anaerobic bacteria. Anaerobe 2021, 70, 102404. [Google Scholar] [CrossRef]
- Steinemann, S. Titanium–The material of choice? Periodontol 2000 1998, 17, 7–21. [Google Scholar] [CrossRef]
- Huang, Y.S.; McGowan, T.; Lee, R.; Ivanovski, S. 7.23 Dental Implants: Biomaterial Properties Influencing Osseointegration. In Comprehensive Biomaterials II; Elsevier: Amsterdam, The Netherlands, 2017; Volume 7, pp. 444–466. ISBN 9780081006924. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontology 2000 2017, 73, 22–40. [Google Scholar] [CrossRef]
- Sharma, A.; Waddell, J.N.; Li, K.C.; Sharma, L.A.; Prior, D.J.; Duncan, W.J. Is titanium–zirconium alloy a better alternative to pure titanium for oral implant? Composition, mechanical properties, and microstructure analysis. Saudi Dent. J. 2021, 33, 546–553. [Google Scholar] [CrossRef]
- Contaldo, M.; De Rosa, A.; Nucci, L.; Ballini, A.; Malacrinò, D.; La Noce, M.; Inchingolo, F.; Xhajanka, E.; Ferati, K.; Bexheti-Ferati, A.; et al. Titanium Functionalized with Polylysine Homopolymers: In Vitro Enhancement of Cells Growth. Materials 2021, 14, 3735. [Google Scholar] [CrossRef]
- Yin, L.; Nakanishi, Y.; Alao, A.R.; Song, X.F.; Abduo, J.; Zhang, Y. A Review of Engineered Zirconia Surfaces in Biomedical Applications. In Procedia CIRP; Elsevier B.V.: Amsterdam, The Netherlands, 2017; Volume 65, pp. 284–290. [Google Scholar]
- Webber, L.P.; Chan, H.-L.; Wang, H.-L. Will Zirconia Implants Replace Titanium Implants? Appl. Sci. 2021, 11, 6776. [Google Scholar] [CrossRef]
- Sivaraman, K.; Chopra, A.; Narayan, A.I.; Balakrishnan, D. Is zirconia a viable alternative to titanium for oral implant? A critical review. J. Prosthodont. Res. 2018, 62, 121–133. [Google Scholar] [CrossRef]
- Chopra, D.; Jayasree, A.; Guo, T.; Gulati, K.; Ivanovski, S. Advancing dental implants: Bioactive and therapeutic modifications of zirconia. Bioact. Mater. 2021, 13, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Hafezeqoran, A.; Koodaryan, R. Effect of Zirconia Dental Implant Surfaces on Bone Integration: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2017, 2017, 9246721. [Google Scholar] [CrossRef]
- Assal, P.A. The osseointegration of zirconia dental implants. Schweiz Monatsschr. Zahnmed. 2013, 123, 644–654. [Google Scholar]
- Dua, R.; Rashad, Z.; Spears, J.; Dunn, G.; Maxwell, M. Applications of 3D-Printed PEEK via Fused Filament Fabrication: A Systematic Review. Polymers 2021, 13, 4046. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ananth, H.; Kundapur, V.; Mohammed, H.S.; Anand, M.; Amarnath, G.S.; Mankar, S. A Review on Biomaterials in Dental Implan-tology. Intern. J. BIomedIcal Sci. 2015, 11, 113–120. [Google Scholar]
- Parmigiani-Izquierdo, J.M.; Cabaña-Muñoz, M.E.; Merino, J.J.; Sánchez-Pérez, A. Zirconia implants and peek restorations for the replacement of upper molars. Int. J. Implant Dent. 2017, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Zhu, C.; Thouas, G.A. Progress and challenges in biomaterials used for bone tissue engineering: Bioactive glasses and elastomeric composites. Prog. Biomater. 2012, 1, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pezzotti, G.; Marin, E.; Adachi, T.; Lerussi, F.; Rondinella, A.; Boschetto, F.; Zhu, W.; Kitajima, T.; Inada, K.; McEntire, B.J.; et al. Incorporating Si3N4 into PEEK to Produce Anti-bacterial, Osteocondutive, and Radiolucent Spinal Implants. Macromol. Biosci. 2018, 18, 1800033. [Google Scholar] [CrossRef]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases 2015, 3, 52–57. [Google Scholar] [CrossRef]
- Evans, N.T.; Torstrick, F.B.; Lee, C.S.; Dupont, K.M.; Safranski, D.L.; Chang, W.A.; Macedo, A.E.; Lin, A.S.; Boothby, J.M.; Whittingslow, D.C.; et al. High-strength, surface-porous polyeth-er-ether-ketone for load-bearing orthopedic implants. Acta Biomaterialia 2015, 13, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Torstrick, F.B.; Lin, A.S.P.; Potter, D.; Safranski, D.L.; Sulchek, T.A.; Gall, K.; Guldberg, R.E. Porous PEEK improves the bone-implant interface compared to plasma-sprayed titanium coating on PEEK. Biomaterials 2018, 185, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.F. A role for surface topography in creating and maintaining bone at titanium endosseous implants. J. Prosthet. Dent. 2000, 84, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Rompen, E.; Domken, O.; Degidi, M.; Pontes, A.E.F.; Piattelli, A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: A literature review. Clin. Oral Implant. Res. 2006, 17 (Suppl. S2), 55–67. [Google Scholar] [CrossRef] [PubMed]
- Accioni, F.; Vázquez, J.; Merinero, M.; Begines, B.; Alcudia, A. Latest Trends in Surface Modification for Dental Implantology: Innovative Developments and Analytical Applications. Pharmaceutics 2022, 14, 455. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. The Impact of Oral Implants-Past and Future, 1966–2042. J. Can. Dent. Assoc. 2005, 71, 327. [Google Scholar]
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, J.; Scheideler, L.; Altebaeumer, T.; Geis-Gerstorfer, J.; Kern, D. Cellular reactions of osteoblasts to micron- and sub-micron-scale porous structures of titanium surfaces. Cells Tissues Organs 2004, 178, 13–22. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Ketabi, M.; DePorter, D. The effects of laser microgrooves on hard and soft tissue attachment to implant collar surfaces: A literature review and interpretation. Int. J. Periodontics Restor. Dent. 2013, 33, e145–e152. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Kakura, K.; Yamamoto, K.; Kido, H.; Yamazaki, J. Accelerated Osteogenic Differentiation and Bone Formation on Zirconia with Surface Grooves Created with Fiber Laser Irradiation. Clin. Implant Dent. Relat. Res. 2015, 18, 883–894. [Google Scholar] [CrossRef]
- Cervino, G.; Meto, A.; Fiorillo, L.; Odorici, A.; Meto, A.; D’Amico, C.; Oteri, G.; Cicciù, M. Surface Treatment of the Dental Implant with Hyaluronic Acid: An Overview of Recent Data. Int. J. Environ. Res. Public Health 2021, 18, 4670. [Google Scholar] [CrossRef] [PubMed]
- von Wilmowsky, C.; Moest, T.; Nkenke, E.; Stelzle, F.; Schlegel, K.A. Implants in bone: Part I. A current overview about tissue response, surface modifications and future perspectives. Oral Maxillofac. Surg. 2014, 18, 243–257. [Google Scholar] [CrossRef]
- Ventre, M.; Natale, C.F.; Rianna, C.; Netti, P.A. Topographic cell instructive patterns to control cell adhesion, polarization and migration. J. R. Soc. Interface 2014, 11, 20140687. [Google Scholar] [CrossRef] [Green Version]
- Hotchkiss, K.M.; Sowers, K.T.; Olivares-Navarrete, R. Novel in vitro comparative model of osteogenic and inflammatory cell response to dental implants. Dent. Mater. 2019, 35, 176–184. [Google Scholar] [CrossRef]
- Ponche, A.; Bigerelle, M.; Anselme, K. Relative influence of surface topography and surface chemistry on cell response to bone implant materials. Part 1: Physico-chemical effects. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2010, 224, 1471–1486. [Google Scholar] [CrossRef]
- Santos, P.M.D.; Julio, E.N.B.S. A state-of-the-art review on roughness quantification methods for concrete surfaces. Constr. Build. Mater. 2013, 38, 912–923. [Google Scholar] [CrossRef]
- Chen, S.; Feng, R.; Zhang, C.; Zhang, Y. Surface roughness measurement method based on multi-parameter modeling learning. Measurement 2018, 129, 664–676. [Google Scholar] [CrossRef]
- Yamano, S.; Ma, A.K.-Y.; Shanti, R.M.; Kim, S.-W.; Wada, K.; Sukotjo, C. The influence of different implant materials on human gingival fibroblast morphology, proliferation, and gene expression. Int. J. Oral Maxillofac. Implant. 2011, 26, 1247–1255. [Google Scholar]
- Gahlert, M.; Roehling, S.; Sprecher, C.M.; Kniha, H.; Milz, S.; Bormann, K. In vivo performance of zirconia and titanium implants: A histomorphometric study in mini pig maxillae. Clin. Oral Implant. Res. 2011, 23, 281–286. [Google Scholar] [CrossRef]
- Al Qahtani, W.M.; Schille, C.; Spintzyk, S.; Al Qahtani, M.S.; Engel, E.; Geis-Gerstorfer, J.; Rupp, F.; Scheideler, L. Effect of surface modification of zirconia on cell adhesion, metabolic activity and proliferation of human osteoblasts. Biomed. Eng. /Biomed. Tech. 2017, 62, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Cionca, N.; Dena, H.; Mombelli, A. Zirconia dental implants: Where are we now, and where are we heading? Periodontology 2000 2017, 73, 241–258. [Google Scholar] [CrossRef]
- Anselme, K.; Bigerelle, M.; Noel, B.; Dufresne, E.; Judas, D.; Iost, A.; Hardouin, P. Qualitative and quantitative study of human osteoblast adhesion on materials with various surface roughnesses. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. 2000, 49, 155–166. [Google Scholar] [CrossRef]
- Ito, H.; Sasaki, H.; Saito, K.; Honma, S.; Yajima, Y.; Yoshinari, M. Response of osteoblast-like cells to zirconia with different surface topography. Dent. Mater. J. 2013, 32, 122–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staehlke, S.; Oster, P.; Seemann, S.; Kruse, F.; Brief, J.; Nebe, B. Laser Structured Dental Zirconium for Soft Tissue Cell Occupation—Importance of Wettability Modulation. Materials 2022, 15, 732. [Google Scholar] [CrossRef]
- Thalji, G.; Bryington, M.; De Kok, I.J.; Cooper, L.F. Prosthodontic Management of Implant Therapy. Dent. Clin. N. Am. 2014, 58, 207–225. [Google Scholar] [CrossRef]
- Mendonça, G.; Mendonça, D.B.S.; Simões, L.G.P.; Araújo, A.L.; Leite, E.R.; Duarte, W.R.; Aragão, F.J.L.; Cooper, L.F. The effects of implant surface nanoscale features on osteoblast-specific gene expression. Biomaterials 2009, 30, 4053–4062. [Google Scholar] [CrossRef]
- Chakravorty, N.; Hamlet, S.; Jaiprakash, A.; Crawford, R.; Oloyede, A.; Alfarsi, M.; Xiao, Y.; Ivanoviski, S. Pro-osteogenic topographical cues promote early activation of osteoprogenitor differentiation via enhanced TGFβ, Wnt, and Notch signaling. Clin. Oral Implant. Res. 2014, 25, 475–486. [Google Scholar] [CrossRef]
- Andrukhov, O.; Huber, R.; Shi, B.; Berner, S.; Rausch-Fan, X.; Moritz, A.; Spencer, N.D.; Schedle, A. Proliferation, behavior, and differentiation of osteoblasts on surfaces of different microroughness. Dent. Mater. 2016, 32, 1374–1384. [Google Scholar] [CrossRef]
- Matos, G.R.M. Surface Roughness of Dental Implant and Osseointegration. J. Maxillofac. Oral Surg. 2021, 20, 1–4. [Google Scholar] [CrossRef]
- Albouy, J.-P.; Abrahamsson, I.; Persson, L.G.; Berglundh, T. Spontaneous progression of ligatured induced peri-implantitis at implants with different surface characteristics. An experimental study in dogs II: Histological observations. Clin. Oral Implant. Res. 2009, 20, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Wassmann, T.; Kreis, S.; Behr, M.; Buergers, R. The influence of surface texture and wettability on initial bacterial adhesion on titanium and zirconium oxide dental implants. Int. J. Implant Dent. 2017, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Lindahl, C.; Jansåker, A.-M.R.; Persson, G.R. Treatment of peri-implantitis using an Er:YAG laser or an air-abrasive device: A randomized clinical trial. J. Clin. Periodontol. 2010, 38, 65–73. [Google Scholar] [CrossRef]
- Zetterqvist, L.; Feldman, S.; Rotter, B.; Vincenzi, G.; Wennström, J.L.; Chierico, A.; Stach, R.M.; Kenealy, J. A Prospective, Multicenter, Random-ized-Controlled 5-Year Study of Hybrid and Fully Etched Implants for the Incidence of Peri-Implantitis. J. Periodontol. 2010, 81, 493–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junkar, I.; Kulkarni, M.; Drašler, B.; Rugelj, N.; Recek, N.; Drobne, D.; Kovač, J.; Humpolicek, P.; Iglič, A.; Mozetič, M. Enhanced biocompatibility of TiO2surfaces by highly reactive plasma. J. Phys. D Appl. Phys. 2016, 49, 244002. [Google Scholar] [CrossRef] [Green Version]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makowiecki, A.; Hadzik, J.; Błaszczyszyn, A.; Gedrange, T.; Dominiak, M. An evaluation of superhydrophilic surfaces of dental implants—A systematic review and meta-analysis. BMC Oral Health 2019, 19, 79. [Google Scholar] [CrossRef]
- Ghasemi, N.; Behnezhad, M.; Asgharzadeh, M.; Zeinalzadeh, E.; Kafil, H.S. Antibacterial Properties of Aloe vera on Intracanal Medicaments against Enterococcus faecalis Biofilm at Different Stages of Development. Int. J. Dent. 2020, 2020, 8855277. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Maiorani, C.; Preda, C.; Chiesa, A.; Esposito, F.; Pascadopoli, M.; Scribante, A. Management of Gingival Bleeding in Periodontal Patients with Domiciliary Use of Toothpastes Containing Hyaluronic Acid, Lactoferrin, or Paraprobiotics: A Randomized Controlled Clinical Trial. Appl. Sci. 2021, 11, 8586. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Taccardi, D.; Scribante, A. Home Oral Care of Periodontal Patients Using Antimicrobial Gel with Postbiotics, Lactoferrin, and Aloe Barbadensis Leaf Juice Powder vs. Conventional Chlorhexidine Gel: A Split-Mouth Randomized Clinical Trial. Antibiotics 2022, 11, 118. [Google Scholar] [CrossRef]
- Rupp, F.; Gittens, R.A.; Scheideler, L.; Marmur, A.; Boyan, B.D.; Schwartz, Z.; Geis-Gerstorfer, J. A review on the wettability of dental implant surfaces I: Theoretical and experimental aspects. Acta Biomater. 2014, 10, 2894–2906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamlet, S.; Alfarsi, M.; George, R.; Ivanovski, S. The effect of hydrophilic titanium surface modification on macrophage inflam-matory cytokine gene expression. Clin. Oral Implant. Res. 2012, 23, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Bai, J.; Lu, M.; Huang, C.; Geng, D.; Chen, G.; Wang, L.; Qi, J.; Cui, W.; Deng, L. Engineering immunomodulatory and osteoinductive implant surfaces via mussel adhesion-mediated ion coordination and molecular clicking. Nat. Commun. 2022, 13, 160. [Google Scholar] [CrossRef]
- Harawaza, K.; Cousins, B.; Roach, P.; Fernandez, A. Modification of the surface nanotopography of implant devices: A translational perspective. Mater. Today Bio 2021, 12, 100152. [Google Scholar] [CrossRef]
- Sreeharsha, T.; Sharan, S.; Chandra, P.K.; Badola, I.; Jabeen, N.S. Implant surface modifications: A review. Int. J. Appl. Dent. Sci. 2020, 6, 334–338. [Google Scholar]
- Yeo, I.-S.L. Modifications of Dental Implant Surfaces at the Micro- and Nano-Level for Enhanced Osseointegration. Materials 2020, 13, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, G.; Wang, G.; Li, J.J. Advances in implant surface modifications to improve osseointegration. Mater. Adv. 2021, 2, 6901–6927. [Google Scholar] [CrossRef]
- Sun, L.; Berndt, C.C.; Gross, K.A.; Kucuk, A. Material Fundamentals and Clinical Performance of Plasma-Sprayed Hydroxyapatite Coatings: A Review. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2001, 58, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Georgiou, G.; Knowles, J.C.; Koh, Y.H.; Kim, H.E. Calcium phosphates and glass composite coatings on zirconia for en-hanced biocompatibility. Biomaterials 2004, 25, 4203–4213. [Google Scholar] [CrossRef]
- Kligman, S.; Ren, Z.; Chung, C.-H.; Perillo, M.; Chang, Y.-C.; Koo, H.; Zheng, Z.; Li, C. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef]
- Becker, S.T.; Beck-Broichsitter, B.E.; Rossmann, C.M.; Behrens, E.; Jochens, A.; Wiltfang, J. Long-term Survival of Straumann Dental Implants with TPS Surfaces: A Retrospective Study with a Follow-up of 12 to 23 Years. Clin. Implant Dent. Relat. Res. 2015, 18, 480–488. [Google Scholar] [CrossRef]
- Laino, L.; La Noce, M.; Fiorillo, L.; Cervino, G.; Nucci, L.; Russo, D.; Herford, A.S.; Crimi, S.; Bianchi, A.; Biondi, A.; et al. Dental Pulp Stem Cells on Implant Surface: An In Vitro Study. BioMed Res. Int. 2021, 2021, 3582342. [Google Scholar] [CrossRef]
- Åstrand, P.; Anzén, B.; Karlsson, U.; Sahlholm, S.; Svardstrom, P.; Hellem, S. Nonsubmerged Implants in the Treatment of the Edentulous Upper Jaw: A Prospective Clinical and Radiographic Study of ITI Implants—Results after 1 Year. Clin. Implant. Dent Relat. Res. 2000, 2, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Blank, E.; Grischke, J.; Winkel, A.; Eberhard, J.; Kommerein, N.; Doll, K.; Yang, I.; Stiesch, M. Evaluation of biofilm colonization on multi-part dental implants in a rat model. BMC Oral Health 2021, 21, 313. [Google Scholar] [CrossRef]
- Liao, H.; Fatash, B.; Li, J. Stability of hydroxyapatite-coatings on titanium oral implants (IMZ) 2 retrieved cases. Clin. Oral Implant. Res. 1997, 8, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Olivares-Navarrete, R.; Cheng, A.; Anderson, D.M.; McLachlan, T.; Stephan, I.; Geis-Gerstofer, J.; Sandhage, K.H.; Fedorov, A.G.; Rupp, F.; et al. The roles of titanium surface mi-cro/nanotopography and wettability on the differential response of human osteoblast lineage cells. Acta Biomater. 2013, 9, 6268–6277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lellouche, J.; Friedman, A.; Gedanken, A.; Banin, E. Antibacterial and antibiofilm properties of yttrium fluoride nanoparticles. Int. J. Nanomed. 2012, 7, 5611–5624. [Google Scholar] [CrossRef] [Green Version]
- Wyszogrodzka, G.; Marszałek, B.; Gil, B.; Dorozyński, P. Metal-organic frameworks: Mechanisms of antibacterial action and po-tential applications. Drug Discov. Today 2016, 21, 1009–1018. [Google Scholar] [CrossRef]
- Mondal, D.; Nguyen, L.; Oh, I.-H.; Lee, B.-T. Microstructure and biocompatibility of composite biomaterials fabricated from titanium and tricalcium phosphate by spark plasma sintering. J. Biomed. Mater. Res. Part A 2012, 101A, 1489–1501. [Google Scholar] [CrossRef]
- Yazdani, J.; Ahmadian, E.; Sharifi, S.; Shahi, S.; Dizaj, S.M. A short view on nanohydroxyapatite as coating of dental implants. Biomed. Pharmacother. 2018, 105, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Barrè Re, F.; van der Valk, C.M.; Meijer, G.; Dalmeijer, R.A.J.; de Groot, K.; Layrolle, P. Osteointegration of Biomimetic Apatite Coating Applied onto Dense and Porous Metal Implants in Femurs of Goats. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 67, 655–665. [Google Scholar] [CrossRef]
- Habibovic, P.; Li, J.; van der Valk, C.M.; Meijer, G.; Layrolle, P.; van Blitterswijk, C.; de Groot, K. Biological performance of uncoated and octacalcium phosphate-coated Ti6Al4V. Biomaterials 2005, 26, 23–36. [Google Scholar] [CrossRef]
- Bessho, K.; Carnes, D.L.; Cavin, R.; Chen, H.-Y.; Ong, J.L. BMP stimulation of bone response adjacent to titanium implants in vivo. Clin. Oral Implant. Res. 1999, 10, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; De Groot, K.; Hunziker, E.B. BMP-2 liberated from biomimetic implant coatings induces and sustains direct ossification in an ectopic rat model. Bone 2005, 36, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Rocci, A.; Rocci, M.; Rocci, C.; Scoccia, A.; Gargari, M.; Martignoni, M.; Gottlow, J.; Sennerby, L. Immediate loading of Brånemark system TiUnite and machined-surface implants in the posterior mandible, part II: A randomized open-ended 9-year follow-up clinical trial. Int. J. Oral Maxillofac. Implant. 2013, 28, 891–895. [Google Scholar] [CrossRef] [Green Version]
- Traini, T.; Murmura, G.; Sinjari, B.; Perfetti, G.; Scarano, A.; D’Arcangelo, C.; Caputi, S. The Surface Anodization of Titanium Dental Implants Improves Blood Clot Formation Followed by Osseointegration. Coatings 2018, 8, 252. [Google Scholar] [CrossRef] [Green Version]
- Shalabi, M.M.; Gortemaker, A.; Van’t Hof, M.V.; Jansen, J.A.; Creugers, N.H.J. Implant Surface Roughness and Bone Healing: A Systematic Review. J. Dent. Res. 2006, 85, 496–500. [Google Scholar] [CrossRef]
- Al-Nawas, B.; Groetz, K.A.; Goetz, H.; Duschner, H.; Wagner, W. Comparative histomorphometry and resonance frequency analysis of implants with moderately rough surfaces in a loaded animal model. Clin. Oral Implant. Res. 2007, 19, 1–8. [Google Scholar] [CrossRef]
- Cho, S.A.; Park, K.T. The removal torque of titanium screw inserted in rabbit tibia treated by dual acid etching. Biomaterials 2003, 24, 3611–3617. [Google Scholar] [CrossRef]
- Grassi, S.; Piattelli, A.; De Figueiredo, L.C.; Feres, M.; De Melo, L.; Iezzi, G.; Alba, R.C.; Shibli, J.A. Histologic Evaluation of Early Human Bone Response to Different Implant Surfaces. J. Periodontol. 2006, 77, 1736–1743. [Google Scholar] [CrossRef]
- Hirano, T.; Sasaki, H.; Honma, S.; Furuya, Y.; Miura, T.; Yajima, Y.; Yoshinari, M. Proliferation and osteogenic differentiation of human mesenchymal stem cells on zirconia and titanium with different surface topography. Dent. Mater. J. 2015, 34, 872–880. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-K.; Woo, K.M.; Shon, W.-J.; Ahn, J.-S.; Cha, S.; Park, Y.-S. Comparison of peri-implant bone formation around injection-molded and machined surface zirconia implants in rabbit tibiae. Dent. Mater. J. 2015, 34, 508–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunette, D.M. Spreading and orientation of epithelial cells on grooved substrata. Exp. Cell Res. 1986, 167, 203–217. [Google Scholar] [CrossRef]
- Chehroudi, B.; Gould, T.R.; Brunette, D.M. Effects of a grooved epoxy substratum on epithelial cell behavior in vitro and in vivo. J. Biomed. Mater. Res. 1988, 22, 459–473. [Google Scholar] [CrossRef]
- Wang, J.H.-C.; Jia, F.; Gilbert, T.W.; Woo, S.L.-Y. Cell orientation determines the alignment of cell-produced collagenous matrix. J. Biomech. 2002, 36, 97–102. [Google Scholar] [CrossRef]
- Mendoza-Arnau, A.; Vallecillo-Capilla, M.-F.; Cabrerizo-Vílchez, M.-Á.; Rosales-Leal, J.-I. Topographic characterisation of dental implants for commercial use. Med. Oral Patol. Oral Cir. Bucal. 2016, 21, e631–e636. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Lu, Q.; Yin, J.; Hu, J.; Wang, Z. Alignment of osteoblast-like cells and cell-produced collagen matrix induced by nanogrooves. Tissue Eng. 2005, 11, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Stangl, R.; Rinne, B.; Kastl, S.; Hendrich, C. The influence of pore geometry in cp Ti-implants—A cell culture investigation. Eur. Cells Mater. 2001, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, D.; Sjostrom, T.; Wilkinson, A.; Smith, C.-A.; Oreffo, R.O.C.; Dalby, M.J.; Su, B. Embossing of micropatterned ceramics and their cellular response. J. Biomed. Mater. Res. Part A 2013, 101, 3247–3255. [Google Scholar] [CrossRef]
- Delgado-Ruíz, R.A.; Moreno, G.G.; Aguilar-Salvatierra, A.; Markovic, A.; Mate-Sánchez, J.E.; Calvo-Guirado, J.L. Human fetal osteoblast behavior on zirconia dental implants and zirconia disks with microstructured surfaces. An experimental in vitro study. Clin. Oral Implant. Res. 2015, 27, e144–e153. [Google Scholar] [CrossRef]
- de Tullio, I.; Berardini, M.; di Iorio, D.; Perfetti, F.; Perfetti, G. Comparative evaluation among laser-treated, machined, and sand-blasted/acid-etched implant surfaces: An in vivo histologic analysis on sheep. Int. J. Implant. Dent. 2020, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef] [Green Version]
- Samant, A. Laser Machining of Structural Ceramics: Computational and Laser Machining of Structural Ceramics: Computational and Experimental Analysis Experimental Analysis. Ph.D. Thesis, University of Tennessee, Knoxville, TN, USA, 2009. [Google Scholar]
- Dumas, V.; Rattner, A.; Vico, L.; Audouard, E.; Dumas, J.C.; Naisson, P.; Bertrand, P. Multiscale grooved titanium processed with femtosecond laser influences mesenchymal stem cell morphology, adhesion, and matrix organization. J. Biomed. Mater. Res. Part A 2012, 100A, 3108–3116. [Google Scholar] [CrossRef]
- Coathup, M.J.; Blunn, G.W.; Mirhosseini, N.; Erskine, K.; Liu, Z.; Garrod, D.R.; Li, L. Controlled laser texturing of titanium results in reliable osteointegration. J. Orthop. Res. 2016, 35, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ruiz, R.A.; Calvo-Guirado, J.L.; Abboud, M.; Ramírez-Fernández, M.P.; de Val, J.E.M.-S.; Negri, B.; Rothamel, D. Histologic and Histomorphometric Behavior of Microgrooved Zirconia Dental Implants with Immediate Loading. Clin. Implant. Dent. Relat. Res. 2014, 16, 856–872. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, M.B.; Silva, N.; Marques, J.F.; Mata, A.; Silva, F.S.; Caramês, J. Biomimetic Implant Surfaces and Their Role in Biological Integration—A Concise Review. Biomimetics 2022, 7, 74. https://doi.org/10.3390/biomimetics7020074
Cruz MB, Silva N, Marques JF, Mata A, Silva FS, Caramês J. Biomimetic Implant Surfaces and Their Role in Biological Integration—A Concise Review. Biomimetics. 2022; 7(2):74. https://doi.org/10.3390/biomimetics7020074
Chicago/Turabian StyleCruz, Mariana Brito, Neusa Silva, Joana Faria Marques, António Mata, Felipe Samuel Silva, and João Caramês. 2022. "Biomimetic Implant Surfaces and Their Role in Biological Integration—A Concise Review" Biomimetics 7, no. 2: 74. https://doi.org/10.3390/biomimetics7020074
APA StyleCruz, M. B., Silva, N., Marques, J. F., Mata, A., Silva, F. S., & Caramês, J. (2022). Biomimetic Implant Surfaces and Their Role in Biological Integration—A Concise Review. Biomimetics, 7(2), 74. https://doi.org/10.3390/biomimetics7020074